Abstract
Type three secretion systems (T3SSs) are virulence determinants employed by several pathogenic bacteria as molecular syringes to inject effector proteins into host cells. Diarrhea‐producing enteropathogenic Escherichia coli (EPEC) uses a T3SS to colonize the intestinal tract. T3S is a highly coordinated process that ensures hierarchical delivery of three classes of substrates: early (inner rod and needle subunits), middle (translocators), and late (effectors). Translocation of effectors is triggered upon host‐cell contact in response to different environmental cues, such as calcium levels. The T3S substrate specificity switch from middle to late substrates in EPEC is regulated by the SepL and SepD proteins, which interact with each other and form a trimeric complex with the chaperone CesL. In this study, we investigated the link between calcium concentration and secretion regulation by the gatekeeper SepL. We found that calcium depletion promotes late substrate secretion in a translocon‐independent manner. Furthermore, the stability, formation, and subcellular localization of the SepL/SepD/CesL regulatory complex were not affected by the absence of calcium. In addition, we demonstrate that SepL interacts in a calcium‐independent manner with the major export gate component EscV, which in turn interacts with both middle and late secretion substrates, providing a docking site for T3S. These results suggest that EscV serves as a binding platform for both the SepL regulatory protein and secreted substrates during the ordered assembly of the T3SS.
Keywords: EPEC, EscV, injectisome, secretion hierarchy, SepL, T3SS
1. INTRODUCTION
Type III secretion systems (T3SSs) are essential virulence determinants of many animal and plant bacterial pathogens, as they serve to inject virulence proteins, or effectors, directly into the host cell cytoplasm in a process called translocation (Buttner, 2012; Gaytan, Martinez‐Santos, Soto, & Gonzalez‐Pedrajo, 2016). These effectors modify host cell physiology for the benefit of the bacteria (Dean, 2011; Tosi, Pflug, Discola, Neves, & Dessen, 2013). T3SSs or injectisomes are employed by pathogenic bacteria that threaten human health, including enteropathogenic Escherichia coli (EPEC), which colonizes the small intestine and produces a singular histopathological modification called the attaching and effacing (A/E) lesion. This alteration is characterized by the effacement of epithelial microvilli, the intimate attachment of the bacteria to the host cell, and finally the development of an actin‐rich pedestal‐like structure beneath the adherence site (Kaper, Nataro, & Mobley, 2004). The effectors required for the formation of the A/E lesion are encoded in a pathogenicity island known as locus of enterocyte effacement (LEE) (McDaniel, Jarvis, Donnenberg, & Kaper, 1995; McDaniel & Kaper, 1997), which also contains all the genes necessary to assemble a functional T3SS (Jarvis et al., 1995; Pallen, Beatson, & Bailey, 2005). In addition to the seven effectors encoded in the LEE, there are many others encoded by genes scattered through the genome (Nles: Non‐LEE encoded effectors) that are also translocated by the T3SS (Dean & Kenny, 2009; Deng et al., 2012; Iguchi et al., 2009).
The injectisome of EPEC consists of an outer (EscC) and a pair of inner (EscJ and EscD) membrane rings that are interconnected through a periplasmic inner rod (EscI), forming a core structure, the so‐called basal body, that spans both bacterial membranes (Ogino et al., 2006; Sal‐Man, Deng, & Finlay, 2012; Spreter et al., 2009; Yip et al., 2005). The export apparatus resides within the inner membrane ring and is formed by five highly conserved proteins, named EscR, EscS, EscT, EscU, and EscV (Moraes, Spreter, & Strynadka, 2008). The cytoplasmic side of the basal body is associated to a ring‐shaped oligomeric structure (EscQ and EscK), which functions as a substrate recognition platform, and provides a docking site for the ATPase complex (EscN, EscO, and EscL) (Andrade, Pardo, Espinosa, Perez‐Hernandez, & Gonzalez‐Pedrajo, 2007; Biemans‐Oldehinkel, Sal‐Man, Deng, Foster, & Finlay, 2011; Romo‐Castillo et al., 2014; Soto et al., 2017; Zarivach et al., 2008). Moreover, the extracellular part of the injectisome comprises a 23 nm in length needle‐like structure (EscF), which is extended by a long filament (EspA) (Knutton et al., 1998; Monjaras Feria et al., 2012; Ogino et al., 2006; Sekiya et al., 2001; Wilson, Shaw, Daniell, Knutton, & Frankel, 2001). Upon host cell contact, the filament serves as a scaffold for the assembly of the translocation pore (EspB and EspD) (Chatterjee, Caballero‐Franco, Bakker, Totten, & Jardim, 2015; Luo & Donnenberg, 2011).
Notably, although there is a temporal regulation of LEE gene expression in which the LEE1 operon is expressed first, the genes encoding all secreted proteins are expressed simultaneously (Yerushalmi, Litvak, Gur‐Arie, & Rosenshine, 2014). Therefore, T3S regulators are involved in setting up a hierarchy of secretion to ensure that the structural proteins that make up the T3SS are secreted prior to effectors (Gaytan et al., 2016; Portaliou, Tsolis, Loos, Zorzini, & Economou, 2016). Depending on the timing of secretion, the T3SS‐dependent substrates are classified as early (EscI and EscF), middle or translocators (EspA, EspB, and EspD), and late substrates or effectors (Deane, Abrusci, Johnson, & Lea, 2010). Coordinated secretion of middle and late substrates has been suggested to be controlled by the LEE‐encoded proteins SepL and SepD (Deng et al., 2004, 2005; O'Connell et al., 2004; Wang, Roe, McAteer, Shipston, & Gally, 2008). Deletion of sepL or sepD completely abolishes translocator secretion and significantly increases effector secretion (Deng et al., 2004, 2005; Wang et al., 2008). SepL belongs to a family of proteins whose members include MxiC from Shigella (Botteaux, Sory, Biskri, Parsot, & Allaoui, 2009), InvE and SsaL from Salmonella pathogenicity islands 1 and 2, respectively (Coombes, Brown, Valdez, Brumell, & Finlay, 2004; Kubori & Galan, 2002), CopN from Chlamydia (Silva‐Herzog et al., 2011), YopN/TyeA from Yersinia (Forsberg, Viitanen, Skurnik, & Wolf‐Watz, 1991; Iriarte et al., 1998), and PopN/Pcr1 from Pseudomonas (Yang et al., 2007). These proteins, known as gatekeepers, prevent premature effector secretion before host cell contact is established. In most systems, the regulatory function of gatekeepers relies on their ability to disengage from the T3SS base once there is an activation signal upon host cell contact, and subsequently either be secreted, as is the case of YopN, PopN, CopN, and MxiC (Archuleta & Spiller, 2014; Botteaux et al., 2009; Cheng, Kay, & Schneewind, 2001; Cherradi et al., 2013; Lee, Zmina, Stopford, Toska, & Rietsch, 2014), or degraded, as is the case of SsaL (Yu, McGourty, Liu, Unsworth, & Holden, 2010). However, although SepL has been shown to contain a T3S‐signal at its N‐terminal domain, the full‐length protein is not secreted (Deng et al., 2004, 2005; Younis et al., 2010).
SepL forms a trimeric complex with SepD and CesL (Younis et al., 2010). SepD has been proposed to be a homolog of SpiC from Salmonella SPI‐2, and CesL a homolog of SycN from Yersinia, and both SpiC and SycN are known to be involved in regulation of secretion hierarchy (Cheng et al., 2001; Day & Plano, 1998; Schubot et al., 2005; Younis et al., 2010; Yu, Liu, & Holden, 2004; Yu et al., 2010). Moreover, CesL was suggested to function as a SepL chaperone (Younis et al., 2010), and deletion of the cesL (orf12) gene in the A/E pathogen Citrobacter rodentium abolishes secretion of both translocators and effectors (Deng et al., 2004). Thus, the SepL/SepD/CesL complex resembles the YopN‐TyeA/YscB/SycN complex from Yersinia and the SsaL/SpiC/SsaM complex from Salmonella SPI‐2 (Younis et al., 2010). These complexes trigger effector secretion by different mechanisms. In the case of Yersinia, substrate secretion switching occurs upon YopN secretion, whereas in Salmonella it requires the switch protein complex dissociation and the subsequent degradation of its components. In both cases, the switching event occurs in response to environmental cues detected upon host membrane contact, for example, calcium depletion and a pH change, respectively (Cheng et al., 2001; Day & Plano, 1998; Yu et al., 2010). In EPEC, calcium depletion from the bacterial growth media has an effect on substrate secretion similar to that observed in a sepL or sepD null mutants (Deng et al., 2005; Ide, Michgehl, Knappstein, Heusipp, & Schmidt, 2003). Yet, the molecular mechanism by which the switch protein complex participates in the regulation of substrate secretion in the absence of calcium is still poorly understood. In this work, we investigated the effect of calcium depletion in the gatekeeper‐dependent triggering of effector secretion and uncovered the existence of novel protein interactions.
2. MATERIALS AND METHODS
2.1. Bacterial strains and growth conditions
All bacterial strains and plasmids used in this study are listed in Table 1. For overnight (O/N) cultures, bacteria were aerobically grown in Lysogeny Broth (LB) at 37°C with constant shaking at 250 rpm. Bacterial growth conditions required for induction of T3S and overproduction of recombinant proteins are indicated below. When necessary, bacterial cultures were supplemented with the appropriate antibiotics at the following concentrations: streptomycin (Sm, 25 μg/ml), kanamycin (Km, 50 μg/ml), ampicillin (Ap, 100 μg/ml), chloramphenicol (Cm, 25 μg/ml), or tetracycline (Tc, 25 μg/ml).
Table 1.
Strains and plasmids used in this study
| Strain/plasmid | Description | Reference |
|---|---|---|
| Escherichia coli | ||
| EPEC E2348/69 | WT EPEC O127:H6 strain; Smr | Levine et al. (1978) |
| ∆escN mutant | E2348/69 carrying an in‐frame deletion of escN; Smr | Gauthier et al. (2003) |
| ∆espA mutant | E2348/69 carrying an in‐frame deletion of espA; Smr Kmr | Gift of the Navarro F. Lab |
| ∆espB mutant | E2348/69 carrying an in‐frame deletion of espB; Smr Kmr | Gift of the Xicohtencatl J. Lab |
| ∆espD mutant | E2348/69 carrying an in‐frame deletion of espD; Smr Kmr | Gift of the Xicohtencatl J. Lab |
| ∆sepL mutant | E2348/69 carrying an in‐frame deletion of sepL; Smr | Gift of the Puente JL Lab |
| ∆escU mutant | E2348/69 carrying an in‐frame deletion of escU; Smr Kmr | Soto et al. (2017) |
| JPEP39 (∆grlR mutant) | E2348/69 carrying an in‐frame deletion of grlR; Smr | Garcia‐Angulo et al. (2012) |
| EPEC sepL‐3FLAG::km | E2348/69 expressing 3‐FLAG‐tagged sepL; Smr, Kmr | This study |
| EPEC cesL‐2HA::km | E2348/69 expressing 2‐HA‐tagged cesL; Smr, Kmr | This study |
| EPEC sepL‐3FLAG cesL‐2HA::km | E2348/69 expressing 3‐FLAG‐tagged sepL and 2‐HA‐tagged cesL; Smr, Kmr | This study |
| BL21(DE3)/pLysS | Strain used for expression of pET19b constructs; Cmr | Novagen |
| XL1‐Blue | Strain used for cloning; Tcr | Stratagene |
| Salmonella | ||
| JR501 | Strain used to convert plasmids to Salmonella compatibility | Ryu & Hartin (1990) |
| SJW1368 | Strain used for expression of pTrc99A_FF4 constructs; flagellar master operon mutant, ∆(cheW‐flhD) | Ohnishi et al. (1994) |
| Plasmids | ||
| pTrc99A_FF4 | Modified pTrc99A expression vector under the control of the trc promoter; Apr | Ohnishi, Fan, Schoenhals, Kihara, & Macnab (1997) |
| pET19b | Expression vector under the control of the T7 promoter; Apr | Novagen |
| pACTrc | Expression vector under the control of the trc promoter; Cmr | Gift of the Fraser GM Lab |
| pKD46 | Red recombinase system plasmid under the control of the araB promoter; Apr | Datsenko & Wanner (2000) |
| pSUB11 | Template plasmid for amplification of 3‐FLAG‐kanamycin‐resistance cassette; Apr, Kmr | Uzzau et al., (2001) |
| pSU315 | Template plasmid for amplification of HA‐kanamycin‐resistance cassette; Apr, Kmr | Uzzau et al. (2001) |
| pFLP2 | Flp recombinase expression plasmid; Apr | Hoang et al. (1998) |
| pMTpL | sepL cloned into pTrc99A_FF4 | This study |
| pMTpL∆C75 | sepL lacking codons 277 to 351 cloned into pTrc99A_FF4 | This study |
| pMTpL∆C11 | sepL lacking codons 341 to 351 cloned into pTrc99A_FF4 | This study |
| pKEeVc | escV codons 335 to 675 cloned into pET19b | This study |
| pKTeDN | escD codons 1 to 120 cloned into pTrc99A_FF4 | This study |
| pKEeDN | escD codons 1 to 120 cloned into pET19b | This study |
| pJEeI | escI cloned into pET19b | Monjaras Feria et al. (2012) |
| pETeI | escI cloned into pTrc99A_FF4 | This study |
| pSLo4 | escK cloned into pMAL‐c2x | Soto et al. (2017) |
| pJHeH | espH with its native RBS cloned into pTOPO‐2HA | Monjaras Feria et al. (2012) |
| pJHnC | nleC with its native RBS cloned into pTOPO‐2HA | Monjaras Feria et al. (2012) |
| pJHeI | escI with its native RBS cloned into pTOPO‐2HA | Monjaras Feria et al. (2012) |
| pJHnH2 | nleH2 with its native RBS cloned into pTOPO‐2HA | Monjaras Feria et al. (2012) |
| pATpD | sepD cloned into pTrc99A_FF4 | This study |
| pMEcL | cesL cloned into pET19b | This study |
| pMTBISpDcL | sepD and his‐cesL cloned into pTrc99A_FF4 | This study |
| pMATpL | sepL cloned into pACTrc | This study |
| pMATpL∆C11 | sepL lacking codons 341 to 351 cloned into pACTrc | This study |
2.2. Plasmid construction and oligonucleotides
The sequences of the oligonucleotides used in this study are listed in Table 2. The sepL, sepD, and cesL genes were amplified from chromosomal DNA of the wild‐type EPEC strain E2348/69 (WT), using the following primer pairs: sepL_Fw/sepL_Rv, sepL_Fw/sepL∆C75_Rv, sepL_Fw/sepL∆C11_Rv sepD_Fw/sepD_Rv, and cesL_Fw/cesL_Rv. The resulting PCR products containing the NdeI and BamHI restriction sites were cloned into pTrc99A_FF4 to generate plasmids pMTpL, pMTpL∆C75, pMTpL∆C11, and pATpD, or into pET19b to generate plasmid pMEcL. To construct plasmids pMATpL and pMATpL∆C11, sepL or sepL lacking the last 11 codons were subcloned from plasmids pMTpL and pMTpL∆C11, respectively, into the NdeI/BamHI sites of the pACTrc vector. The pKEeVc plasmid, encoding the cytoplasmic region of EscV, was constructed using primers escV_CtermFw and escV_CtermRv, to amplify a PCR fragment that was cloned into the NdeI/BamHI sites of the pET19b vector. To generate the bicistronic plasmid pMTBISpDcL, the pMEcL plasmid was double digested with XbaI and PstI, and the resulting fragment was cloned into the XbaI/PstI sites of the pATpD plasmid. The pKTeDN plasmid was constructed by amplifying the cytoplasmic region of EscD (codons 1 to 120) using primers escDN_Fw and escDN_Rv. The obtained PCR fragment was cloned into the NdeI/BamHI sites of the pTrc99A_FF4 vector. To generate plasmid pKEeDN, the pKTeDN construct was double digested with NdeI and BamHI and the resulting fragment was cloned into pET19b. The pJEeI plasmid (Monjaras Feria et al., 2012) was double digested with NdeI and BamHI, and the resulting fragment was cloned into the NdeI/BamHI sites of the pTrc99A_FF4 vector to generate plasmid pETeI. DNA fragments cloned from PCR‐amplified material were sequenced to verify that no undesired base changes had been introduced.
Table 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence 5′ – 3′ |
|---|---|
| sepL_Fw | AGTTTCATATGGCTAATGGTATTG |
| sepL_Rv | CTATAAAAAAAAGGATCCTCACAT |
| sepL∆C75_Rv | TAGCATGGATCCTCAAATGACATC |
| sepL∆C11_Rv | AATCTATGGATCCTCAAATCATTA |
| sepD_Fw | TAATACATATGAACAATAATAATG |
| sepD_Rv | AAAAACTTATTGGATCCATTACAC |
| cesL_Fw | AGAGCCTGCATATGAATCTTTTAG |
| cesL_Rv | ATTTAAGAGGATCCTCATGATGTC |
| escV_CtermFw | AATAATAAGGATCATATGGGAGCTGATTTG |
| escV_CtermRv | GTGGGTATGGATCCAATACAGAATC |
| escD_Fw | GGATGAATAAAATTTACATATGTTATCCTCATATAA |
| escD_Nterm_Rv | CTCGCCAGGATCCGGCGTTATTTGC |
| sepL‐3FLAG_Fw | ATACATTATTAATGATTGGTAAAGTGATAGATTATAAGGAGGATGTTATGGACTACAAAGACCATGACGG |
| sepL‐FLAG_Rv | CCTCTTCATAATCTTTCTTAGCATGACAAAAACTATAAAAAAAAACAATAATGAATATCCTCCTTAGTTC |
| cesL‐2HA_Fw | CTTTTCAACAGCATGTGCAGATTATTGAGCGCGTTCGCAGGATGACATCATATCCGTATGATGTGCCGGACTATGCGTATCCGTATGATGTTCCTGAT |
| cesL‐2HA_Rv | AAGATCGTGATATGACTCTGCTTTTTTAAATATATTTAAGAGTTTATTCATATGAATATCCTCCTTAGTTC |
2.3. Epitope tagging of chromosomal genes
A modification of the λ‐Red recombinase system was used for chromosomal epitope tagging as described in (Uzzau, Figueroa‐Bossi, Rubino, & Bossi, 2001). The EPEC strains expressing a 3xFLAG‐tagged version of sepL (EPEC sepL‐3FLAG::km) or a 2xHA version of cesL (EPEC cesL‐2HA::km), were constructed by amplifying the kanamycin cassette from either plasmid pSUB11, using the primer pair sepL‐3FLAG_Fw and sepL‐3FLAG_Rv, or from plasmid pSU315, using the primer pair cesL‐2HA_Fw and cesL‐2HA_Rv. The resulting PCR products were electroporated into EPEC WT strain carrying plasmid pKD46, which was grown at 30°C in LB medium containing 100 mmol/L of L‐arabinose to induce Red recombinase expression. Transformant cells were grown at 37°C to eliminate the pKD46 plasmid. Recombinant EPEC sepL‐3FLAG::km and EPEC cesL‐2HA::km strains were selected on LB plates supplemented with 300 μg/mL Km and tested for Ap sensitivity. To generate the double‐tagged EPEC sepL‐3FLAG cesL‐2HA::km strain, the kanamycin cassette of the EPEC sepL‐3FLAG::km strain was excised using the helper plasmid pFLP2 (Hoang, Karkhoff‐Schweizer, Kutchma, & Schweizer, 1998), and the homologous recombination of cesL‐2HA::km was performed as described above.
2.4. Type III protein secretion assay
To induce EPEC T3S, preequilibrated regular Dulbecco's modified Eagle's medium (DMEM, Gibco, 12100‐046) was inoculated with 1/100 of an overnight (O/N) culture of LB and grown under static conditions at 37°C in a 5% CO2 atmosphere. At an optical density at 600 nm (OD600) of 0.8 to 1, the culture was centrifuged (19,800g for 10 min) to separate secreted proteins from the bacterial cells. The resulting pellet was resuspended in SDS‐PAGE sample buffer and normalized according to the OD600. The supernatant, containing secreted proteins, was precipitated overnight at 4°C with 10% trichloroacetic acid. Precipitated proteins were collected by centrifugation at 19,800g for 30 min, and protein pellets were resuspended in SDS‐PAGE sample buffer, normalized according to the OD600 and neutralized by adding a saturated Tris solution. Protein secretion profiles were analyzed by 15% SDS‐PAGE and immunoblotting. Calcium‐free DMEM (Gibco, 21068‐028) was used to evaluate the effect of calcium on the EPEC secretion profile. The calcium‐free DMEM solution was supplemented with L‐Glutamine and HEPES to a final concentration of 4 mmol/L and 100 mmol/L, respectively.
2.5. Immunoblotting
Samples subjected to SDS‐PAGE were transferred onto nitrocellulose or polyvinylidene fluoride (PVDF) membranes and blocked O/N at 4°C with Tris‐buffered saline (TBS; 20 mmol/L Tris‐HCl, pH 7.5, 150 mmol/L NaCl) containing 0.1% (v/v) Tween 20 and 5% (w/v) nonfat dry milk. After washing with TBS‐Tween, membranes were probed against rabbit‐raised polyclonal anti‐Tir, anti‐Map, anti‐EspA, anti‐EscJ, anti‐EspD, anti‐EscI, anti‐SepD, or anti‐SepL antibodies, and monoclonal anti‐DnaK (MBL International), anti‐FLAG (SIGMA) or HRP‐conjugated anti‐HA (Roche) antibodies. Secondary antibodies used in this study (horseradish peroxidase (HRP)‐conjugated goat antirabbit and goat antimouse), were obtained from Santa Cruz Biotechnology. Protein detection was performed with the Immobilon Western Chemiluminescent HRP Substrate kit (Millipore). Animals were handled and cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals (A5281‐01) and with the approval of the local Animal Use and Care Committee (CICUAL) of the Instituto de Fisiología Celular, UNAM for protocol BGP70‐15.
2.6. Protein overproduction and pull‐down assays
Salmonella SJW1368 strain, which lacks the flagellar master operon and has been extensively used for expression of pTrc99A‐based plasmids (Gonzalez‐Pedrajo, Minamino, Kihara, & Namba, 2006; Ohnishi, Ohto, Aizawa, Macnab, & Iino, 1994; Okabe, Minamino, Imada, Namba, & Kihara, 2009), was transformed with plasmids pMTpL, pMTpL∆C75, or pMTpL∆C11 and E. coli BL21 (DE3)/pLysS (BDP) strain with plasmid pKEeVc, for protein overproduction. O/N cultures of the transformed strains were used to inoculate fresh LB containing appropriate antibiotics, and cultures were grown at 37°C with shaking. At an OD600 of 0.8, protein production was induced by the addition of isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) to a final concentration of 0.1 mmol/L, and bacterial growth was continued for 4 hr at 30°C. The bacterial cells were harvested by centrifugation at 15,500g for 10 min, and the pellets were resuspended in binding buffer (20 mmol/L Tris‐HCl pH 8, 0.5 mol/L NaCl) containing 20 mmol/L imidazole and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF) and lysed by sonication. Cell lysates were centrifuged (15,500g for 30 min) and the supernatants (cleared lysates) containing soluble proteins were recovered. The cleared lysate of the His‐EscVc recombinant protein was mixed with the cleared lysate of SepL, SepL∆C75, or SepL∆C11 and incubated for 2 hr at 4°C. Mixed lysates were loaded onto a polypropylene column (QIAGEN) containing 100 μl of preequilibrated Ni‐nitrilotriacetic acid (Ni‐NTA) agarose beads. The resin was washed with 10 column volumes of binding buffer containing increasing concentrations of imidazole and bound proteins were eluted with 500 mmol/L imidazole. To purify the SepL/SepD/CesL protein complex, Salmonella SJW1368 cells were cotransformed with the bicistronic plasmid pMTBISpDcL and the plasmid pMATpL or pMATpL∆C11. Protein production was induced by the addition of 1 mmol/L IPTG. The cleared lysate was prepared as described above, incubated with 100 μL of Ni‐NTA for 2 hr at 4°C and then loaded onto a polypropylene column. After extensive washing, proteins were eluted with 500 mmol/L imidazole. To assess the effect of calcium on the EscVc/SepL and SepL/SepD/CesL interactions, 2 mmol/L CaCl2 was added to all buffers used in the pull‐down assays.
To evaluate the interaction of EscVc and EscDN with T3‐secreted proteins, a modified version of the method employed by Thomas et al. (2005) to identify cognate substrates of the CesT chaperone was used. Briefly, the cleared lysate of His‐EscVc or His‐EscDN was incubated with Ni‐NTA agarose beads for 2 hr and the resin was packed into a polypropylene column. After washing with 20 mmol/L imidazole‐binding buffer, the mixed supernatant of mutant strains ∆sepL and ∆grlR grown under T3S‐inducing conditions was loaded onto the column, washed with five column volumes of binding buffer containing increasing concentrations of imidazole, and eluted with 500 mmol/L imidazole. All resulting samples were subjected to SDS‐PAGE and immunoblotting.
2.7. Subcellular fractionation
Subcellular fractionation was carried out as previously reported (Gauthier, Puente, & Finlay, 2003) with slight modifications. The EPEC sepL‐3FLAG cesL‐2HA::km strain was grown under T3S inducing conditions in regular DMEM or DMEM without calcium until an OD600 of 1. Cells were harvested by centrifugation (15,500g for 10 min), the cell pellet was washed once with phosphate‐buffered saline and then resuspended in 20 mmol/L Tris‐HCl pH 7.5 containing 1 mmol/L PMSF. Cells were disrupted by sonication and unbroken cells were removed by centrifugation at 19,800g for 10 min. The cleared lysate was subjected to ultracentrifugation at 90,000g for 1 hr. The supernatant (containing the cytoplasmic proteins) was collected into a clean tube, and the membrane‐containing pellet was washed with 20 mmol/L Tris‐HCl pH 7.5. Both fractions were ultracentrifuged once again as previously described, and the cytoplasmic and membrane fractions were collected. Total protein concentration of the samples was quantified using the DC protein assay (Bio‐Rad), and proteins were analyzed by immunoblotting as described above.
2.8. Protein stability assay
The EPEC sepL‐3FLAG cesL‐2HA::km strain was grown in calcium‐free DMEM added with 1.8 mmol/L CaCl2 under T3S inducing conditions. When an OD600 of 1 was reached, cells were harvested by centrifugation at 4,600g for 10 min, washed with and resuspended in either calcium‐free DMEM or calcium‐free DMEM added with 1.8 mmol/L CaCl2, containing 50 μg/ml Cm in order to stop protein synthesis. Bacterial growth was continued and samples were taken every 30 min during 3 hr. Samples were normalized according to the OD600 and proteins were analyzed by immunoblotting as described above.
2.9. Coimmunoprecipitation
50 mL of DMEM without calcium or the same medium added with 1.8 mmol/L CaCl2 was inoculated with O/N cultures of the EPEC sepL‐3FLAG cesL‐2HA::km or EPEC cesL‐2HA::km strains. Cell cultures were grown under static conditions at 37°C in a 5% CO2 atmosphere. At an OD600 of 1, cultures were harvested by centrifugation, cell pellets were washed with HEPES 20 mmol/L, NaCl 250 mmol/L pH 7.4, resuspended in the same buffer and lysed by sonication. Cell lysates were incubated with Triton X‐100 [0.1% (v/v)] for 15 min and then cleared by centrifugation at 19,800g for 30 min. Supernatants were carefully collected and the crosslinker dithiobis succinimidyl propionate was added to a final concentration of 1 mmol/L. After 1 hr incubation at room temperature, the crosslinking reaction was stopped by the addition of 20 mmol/L Tris pH 7.5. Cross‐linked samples were mixed with 40 μl of preequilibrated ANTI‐FLAG M2 Affinity Gel beads (SIGMA) and incubated O/N at 4°C with shaking. SepL‐3FLAG coupled beads were centrifuged at 13,500g for 2 min and washed three times with HEPES 20 mmol/L, NaCl 250 mmol/L pH 7.4. Finally, the beads were resuspended in 20 μl of SDS‐PAGE sample buffer, containing 2 μl of β‐mercaptoethanol and boiled for 5 min. Protein samples were resolved by SDS‐PAGE and analyzed by immunoblotting as described above.
3. RESULTS
3.1. Calcium removal from the culture media solely affects effector secretion
Previous studies have implicated calcium as an important player in the regulation of T3 substrate secretion in EPEC (Deng et al., 2005; Ide et al., 2003; Kenny, Abe, Stein, & Finlay, 1997; Shaulov, Gershberg, Deng, Finlay, & Sal‐Man, 2017). Therefore, we examined the secretion profile of EPEC wild‐type strain E2348/69 grown under T3S inducing conditions in regular DMEM (containing 1.8 mmol/L CaCl2) or calcium‐free DMEM. We confirmed that the secretion of the effector Tir is increased in the absence of calcium (Figure 1a), and extended this finding to other LEE and Nle effectors as judged by the enhanced secretion of Map, EspH‐2HA, NleH2‐2HA, and NleC‐2HA into the supernatant (Figure 1a and b). Addition of 1.8 mmol/L CaCl2 to calcium‐free DMEM restored effector secretion to the levels seen with regular DMEM (Figure 1), corroborating that the observed effect is due to the absence of calcium. For comparison, the secretion profiles of the sepL null mutant, which entirely deregulates effector secretion, and that of the secretion‐deficient escU null mutant, are shown (Figure 1a). In addition, our results demonstrated that protein production was not affected by the different culture media used or in the mutant strains (Figure 1a lower panel and b right panel). Furthermore, in contrast to previous reports (Deng et al., 2005; Ide et al., 2003), we did not observe a significant decrease in translocator secretion (Figure 1a). Therefore, to provide a more quantitative measure of the overall change in the secretion of effectors and translocators, we performed a statistical analysis on the secretion data of several independent experiments by quantifying the Coomassie‐stained protein band intensities of the translocator EspA and the effector Tir. The values were normalized relative to the corresponding band intensity of the autotransporter EspC, which is secreted through the type V secretion system and thus serves as a loading control (Mellies et al., 2001; Stein, Kenny, Stein, & Finlay, 1996). This analysis showed no significant differences in EspA translocator secretion in the different media used in the assay, whereas there was a clear 11‐fold increase in Tir effector secretion in the absence of calcium (Figure 1c). Moreover, we investigated the effect of calcium on the secretion of an early substrate, namely, the inner rod component EscI. As shown in Figure 1b, secretion of EscI‐2HA was not altered by different calcium levels in the growth media. It is worth mentioning that although overproduction of EscI‐2HA caused a decrease in T3 secretion, EPEC was still responsive to calcium depletion as demonstrated by an increased secretion of Tir (Figure S1A). Besides, overproduction of an untagged version of EscI, which did not interfere with T3 secretion, further demonstrated that calcium levels had no effect on the secretion of this early substrate (Figure S1B). Overall, our results suggest that only late substrate secretion is significantly affected by calcium depletion from the growth media. Thus, we next decided to investigate the mechanism that triggers effector secretion under this condition.
Figure 1.
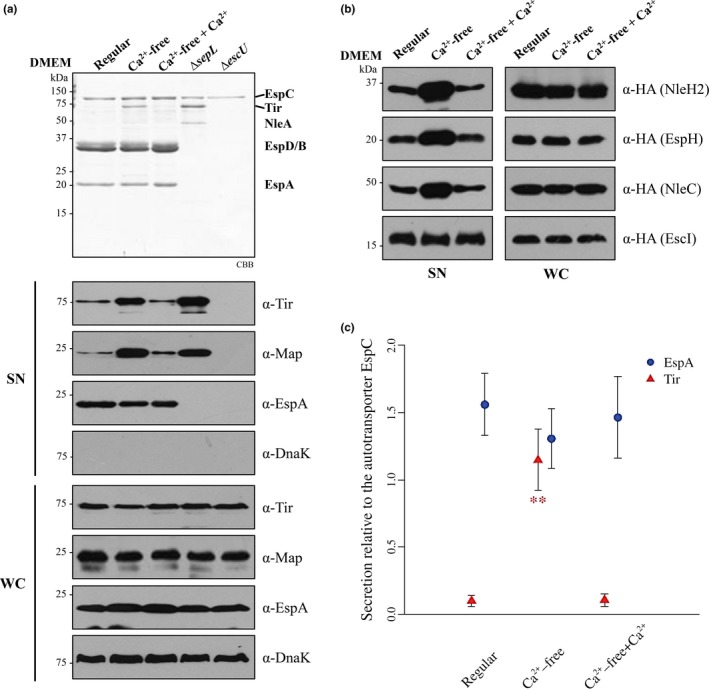
Calcium depletion from the bacterial growth media enhances effector secretion. (a) T3 secretion profiles of the EPEC wild‐type strain (WT) grown in regular DMEM containing 1.8 mmol/L of CaCl2 (Regular), calcium‐free DMEM (Ca2+‐free) or calcium‐free DMEM supplemented with 1.8 mmol/L of CaCl2 (Ca2+‐free + Ca2+), and the ∆sepL and ∆escU mutant strains, visualized by SDS‐PAGE stained with Coomassie brilliant blue (CBB) (upper panel). The presence of DnaK, Tir, Map, and EspA in the supernatants (SN) and whole‐cell lysates (WC) was examined by immunoblotting, using anti‐DnaK, anti‐Tir, anti‐Map, and anti‐EspA antibodies (lower panels). (b) Protein secretion profiles of EPEC WT strain overproducing HA‐tagged T3 substrates, grown in the presence or absence of calcium as described in (a). Immunodetection of LEE and non‐LEE‐encoded effectors (NleH2, EspH, and NleC) and the inner rod protein EscI, was performed in the supernatants (SN) and whole‐cell lysates (WC) using specific antibodies against the HA tag. The results shown are representative of three independent experiments. (c) Relative abundance of EspA and Tir proteins in the supernatant of EPEC wild‐type strain grown in the presence or absence of calcium as described in (a). CBB‐stained protein bands were quantified from six independent secretion assays by gel densitometry using the ImageJ software (Schneider, Rasband, & Eliceiri, 2012). The secretion level of EspA and Tir proteins was normalized relative to the secretion level of the EspC autotransporter band. The average and the standard deviation of normalized data are displayed. Significant statistical differences compared with the regular DMEM condition are denoted by asterisks. A p value < .05 was considered statistically significant. ** p = .002, Wilcoxon‐test
3.2. Calcium sensing is translocon‐independent
It has been previously shown that effector secretion is elicited upon host cell contact, and that different environmental cues are involved in the activation of this secretion stage, for example, a pH shift in Salmonella and low‐calcium in Yersinia, Pseudomonas, and A/E pathogens (Beuzon, Banks, Deiwick, Hensel, & Holden, 1999; Deng et al., 2005; Kim et al., 2005; Lee, Mazmanian, & Schneewind, 2001; Mills, Baruch, Charpentier, Kobi, & Rosenshine, 2008; Rosqvist, Magnusson, & Wolf‐Watz, 1994; Yu et al., 2010). These in vitro conditions are proposed to mimic those found during the bacterial infection process. Therefore, we reasoned that the translocon proteins in EPEC, which establish contact with host cells, could be involved in sensing calcium‐level changes. This hypothesis was evaluated by comparing the secretion profile of the EPEC wild‐type strain with that of the isogenic translocator null mutants espA, espB, and espD, when grown in the presence or absence of calcium. Translocator null mutants were still responsive to calcium depletion, as shown by an increased secretion of the effector Tir in calcium‐free medium (Figure 2). Tir production was not affected in the different culture media or in the mutant strains (Figure 2 lower panel). Thus, although translocators are the proteins that make direct contact with host cells, they appear not to be required for in vitro calcium sensing.
Figure 2.
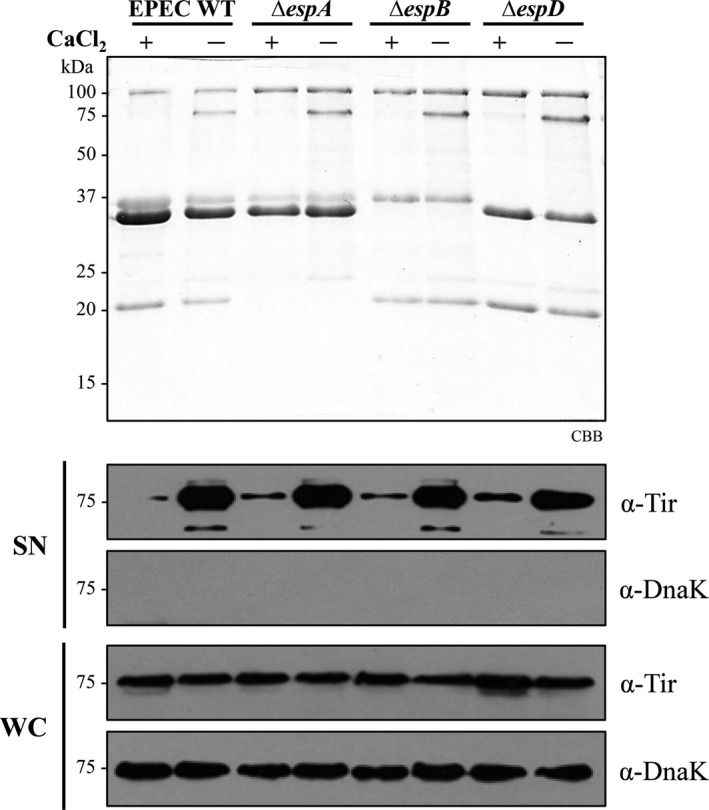
The translocon components EspA, EspB, and EspD are dispensable for calcium sensing. The secretion profiles of the EPEC wild‐type strain (WT) and the ∆espA, ∆espB, and ∆espD null mutants grown under T3S inducing conditions in the presence or absence of calcium were analyzed by Coomassie brilliant blue stained SDS‐PAGE (upper panel). Immunodetection of secreted proteins (SN) and whole‐cell lysates (WC) was performed using specific antibodies against Tir and DnaK (bottom panel)
3.3. The SepL/SepD/CesL complex is insensitive to calcium concentration changes
It has been reported that the SsaL/SpiC/SsaM complex of the S. enterica SPI‐2 T3SS prevents premature effector secretion at pH 5.0, a condition found within host cell vacuoles. However, upon T3SS vacuolar membrane contact, the detection of a pH increase to 7.2 in the cytoplasm leads to dissociation of the protein complex and degradation of its components, resulting in effector secretion (Yu et al., 2010). Since the proteins forming the SsaL/SpiC/SsaM complex in Salmonella are homologous to the ones forming the SepL/SepD/CesL complex in EPEC, and both protein complexes participate in the substrate specificity switch from translocators to effectors without secretion of the gatekeeper protein (Coombes et al., 2004; Younis et al., 2010; Yu et al., 2010), we argued that the mechanism by which these complexes respond to environmental cues that induce effector secretion could be similar. Therefore, we examined the effect of calcium depletion on the stability, formation, and subcellular localization of the SepL/SepD/CesL complex. To this end, we constructed a sepL‐3FLAG cesL‐2HA chromosomally tagged strain and showed that its T3 secretion profile is comparable to that of the wild‐type strain (Figure S2). However, we were unable to obtain a functional SepD chromosomally tagged strain, because the C‐terminal tag affected the protein secretion profile (data not shown). Then, we evaluated the effect of calcium depletion on the protein stability of SepL‐3FLAG and CesL‐2HA. Stability assays were performed as described under Materials and Methods, inhibiting de novo protein synthesis with chloramphenicol in cells grown in the presence or absence of calcium. As shown in Figure 3a, the stability profile of SepL and CesL was similar under both conditions tested, indicating that neither of these proteins is degraded in response to a drop in calcium concentration. Subsequently, we immunoprecipitated SepL‐3FLAG from the EPEC sepL‐3FLAG cesL‐2HA strain grown in the presence or absence of calcium. The results showed that CesL‐2HA and SepD were coprecipitated at similar levels in both conditions (Figure 3b left panel), indicating that calcium removal from the medium does not result in SepL/SepD/CesL complex dissociation. Specificity controls demonstrated that neither the CesL‐2HA nor SepD proteins bound nonspecifically to the anti‐FLAG beads (Figure 3b right panel), and that a non‐T3SS‐related protein, the chaperone DnaK, was not coprecipitated with SepL‐3FLAG and did not bind the anti‐FLAG beads (Figure 3b bottom panels). Furthermore, to test the possibility that the localization of the SepL/SepD/CesL complex, and hence, its association with the T3S base components might be altered, we carried out a subcellular fractionation of the EPEC sepL‐3FLAG cesL‐2HA strain grown in the presence or absence of calcium. The results showed that both SepL and CesL were similarly distributed in the cytoplasm and cosedimented with the membrane fraction under the two different growth conditions (Figure 3c), indicating that calcium depletion does not affect the localization of the SepL/SepD/CesL complex.
Figure 3.
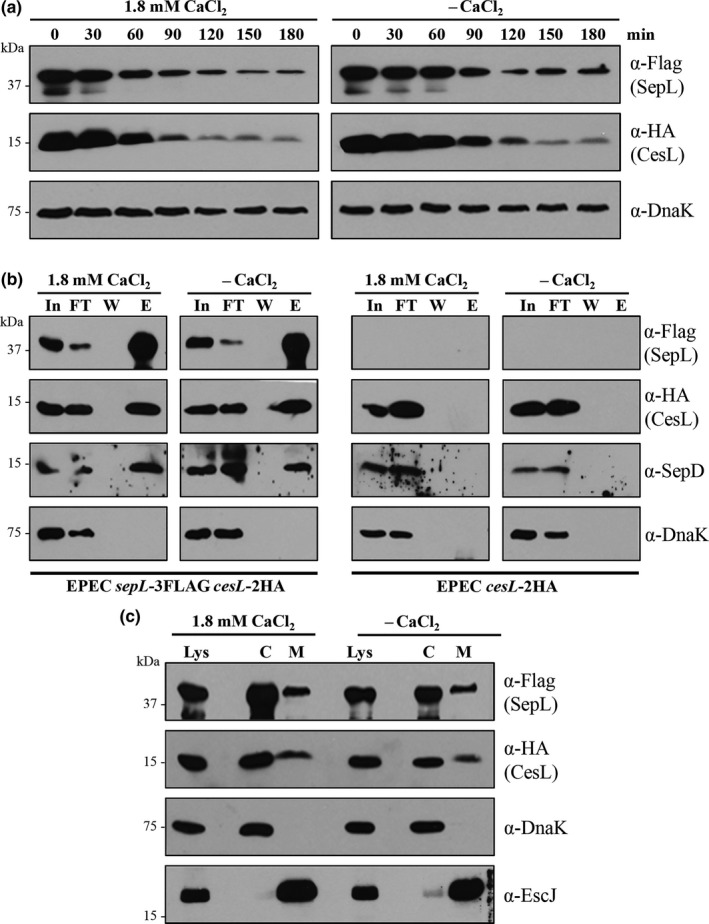
The SepL/SepD/CesL complex does not participate in the calcium‐absence response. (a) Immunoblot analysis of whole‐cell lysates of the EPEC sepL‐3FLAG cesL‐2HA strain grown under T3S inducing conditions in the presence or absence of calcium. Intracellular protein stability of SepL‐3FLAG and CesL‐2HA was examined every 30 min during 3 hr after the addition of chloramphenicol, by immunodetection using anti‐FLAG and anti‐HA antibodies. Anti‐DnaK was used as a loading control. (b) Immunoprecipitation of SepL‐3FLAG from the EPEC sepL‐3FLAG cesL‐2HA and EPEC cesL‐2HA strains grown in the presence or absence of calcium. Coimmunoprecipitation of SepD, CesL‐2HA, and DnaK with SepL‐3FLAG was corroborated with specific antibodies against SepD, HA‐tag, and DnaK. (c) The EPEC sepL‐3FLAG cesL‐2HA strain grown in the presence or absence of calcium was fractionated into cytoplasmic (C) and membrane (M) fractions. Immunodetection of SepL‐3FLAG and CesL‐2HA in the cell lysate (Lys) and in the cytoplasmic and membrane fractions was performed using antibodies against the FLAG and HA tags. To confirm proper fractionation, samples were probed with anti‐DnaK and anti‐EscJ antibodies as cytoplasmic and membrane markers, respectively
Moreover, it has been suggested that environmental signals can diffuse through the needle channel to a sensor protein at the base of the secretion apparatus (Notti & Stebbins, 2016; Portaliou et al., 2016; Shaulov et al., 2017; Yu et al., 2010). Therefore, we decided to investigate whether calcium directly affected the formation of the SepL/SepD/CesL trimeric complex. To this end, in vitro pull‐down assays were performed in the presence or absence of calcium as described in 2. The results showed no differences in the copurification assays with or without calcium (Figure S3). This is consistent with a previous report showing that calcium did not affect the SepL‐SepD interaction (Deng et al., 2005). Overall, these findings indicate that the mechanism by which effector secretion increases upon calcium removal from the growth media does not involve changes in the stability, formation, or localization of the SepL/SepD/CesL secretion regulatory complex.
3.4. SepL interacts with EscV, a T3SS component engaged in substrate recognition
The gatekeeper protein SepL and its homologs in different bacteria have been shown to interact with diverse T3SS components (Archuleta & Spiller, 2014; Botteaux et al., 2009; Cherradi et al., 2013; Kubori & Galan, 2002; Lee et al., 2014; Roehrich et al., 2017; Shen & Blocker, 2016; Wang et al., 2008). On the basis of our previous results, we reasoned that T3SS proteins involved in substrate recognition or targeting might be prone to calcium‐mediated control.
Homologous proteins of the EPEC export apparatus component EscV have been implicated in the recruitment of T3 secretion substrates and chaperones, in both the flagellar and virulence T3SSs. In Xanthomonas spp., the cytoplasmic domain of HrcV was shown to bind early and late substrates, as well as chaperones (Alegria et al., 2004; Buttner, Lorenz, Weber, & Bonas, 2006; Hartmann & Buttner, 2013), whereas the cytoplasmic domain of the flagellar export gate protein FlhA, binds different chaperone/substrate complexes (Bange et al., 2010; Kinoshita, Hara, Imada, Namba, & Minamino, 2013). To investigate whether EscV might play a role in substrate recognition, we tested the ability of the C‐terminal cytoplasmic domain of EscV (EscVc) to interact with secreted proteins. To this aim, His‐EscVc was coupled to Ni‐NTA agarose beads and incubated with the combined supernatants of the secretion assays of a ∆sepL mutant, which hypersecretes effectors, and a ∆grlR mutant, which produces and secretes more effectors and translocators (Deng et al., 2004, 2005; Wang et al., 2008). After extensive washing, proteins were eluted and analyzed by SDS‐PAGE and immunoblotting. The results showed that His‐EscVc interacts with both middle (EspA and EspD) and late (Tir) substrates (Figure 4 left panel). As a control, substrate binding to EscD, which is also an inner membrane protein with a cytoplasmic domain (EscDN), was evaluated under the same conditions and no interaction was observed (Figure 4 middle panel). In addition, we discarded the possibility of substrate nonspecific binding to the Ni‐NTA resin (Figure 4 right panel). These results suggest that the major export gate component of the EPEC injectisome, EscV, provides a docking site for substrates prior to their T3‐dependent secretion.
Figure 4.
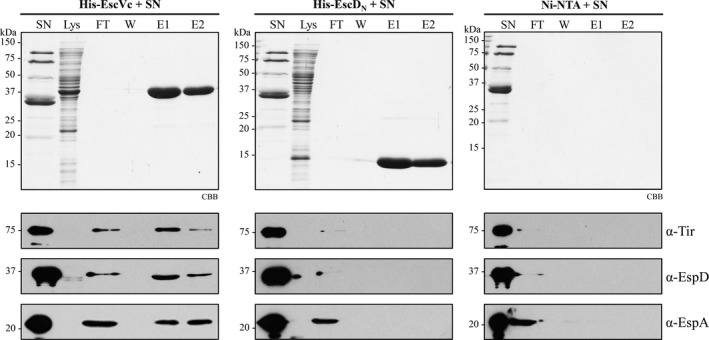
EscVc directly interacts with secreted proteins. Pull‐down assay of His‐EscVc (left panels) and His‐EscDN (middle panels) with secreted proteins, performed by nickel affinity chromatography. The cleared lysate containing His‐EscVc or His‐EscDN (Lys), was incubated with Ni‐NTA beads and loaded into a column. The proteins secreted into the supernatant by the ∆sepL and ∆grlR null mutant strains (SN) were passed through the His‐EscVc or His‐EscDN‐coupled resin in the column and the flow through (FT) was collected. After extensive washing (W), proteins were eluted (E1 and E2). Samples were visualized by SDS‐PAGE stained with Coomassie brilliant blue (CBB) (upper panels). Detection of copurified proteins was performed by immunoblotting with specific antibodies against Tir, EspD, and EspA (lower panels). As an additional control, the nonspecific binding of Tir, EspD, and EspA to the Ni‐NTA beads was analyzed by SDS‐PAGE stained with CBB and immunoblotting (right panels)
Next, we investigated a possible link between the gatekeeper SepL and the EscV protein, by evaluating a physical interaction between these proteins and its calcium‐dependence. For this purpose, we performed a nickel affinity copurification assay by mixing the cleared lysates of cells producing His‐EscVc and untagged SepL, in the presence or absence of calcium. Affinity resin‐bound proteins were eluted and analyzed by SDS‐PAGE and immunoblotting. The result showed that SepL directly interacts with the cytoplasmic domain of EscV and that this interaction is not dependent on the presence of calcium (Figure 5a). SepL binding to the Ni‐NTA resin was not detected (Figure 5a lower panel). As an additional control, we showed that under the conditions tested, His‐EscVc does not bind to MBP‐EscK (data not shown), as previously observed in a yeast two hybrid screen (Soto et al., 2017), corroborating the specificity of the EscV‐SepL interaction.
Figure 5.
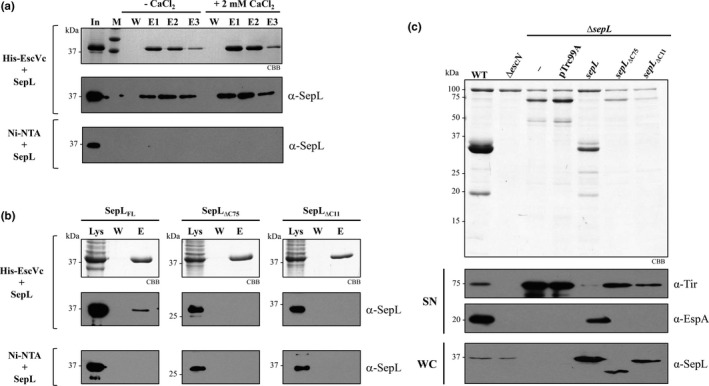
SepL interacts with the export gate component EscV. (a) Pull‐down assays of His‐EscVc and SepL (His‐EscVc + SepL) performed by nickel affinity chromatography in the presence or absence of 2 mmol/L CaCl2. Cleared lysates containing His‐EscVc or untagged SepL were mixed and incubated for 2 hr (In) in the absence or presence of calcium. Samples were loaded into columns packed with Ni‐NTA resin, washed (W) and eluted (E1, E2, and E3). His‐EscVc was visualized by SDS‐PAGE stained with Coomassie brilliant blue (CBB). The molecular mass markers (M) of 37 and 50 kDa are shown. Detection of copurified SepL was performed by immunoblotting with polyclonal antibodies against SepL (middle panel). As a negative control, SepL was loaded into a Ni‐NTA column (Ni‐NTA + SepL) and treated under the same conditions described above (lower panel). (b) Pull‐down assays of His‐EscVc and C‐terminal truncated versions of SepL (SepL∆C75 and SepL∆C11) performed by nickel affinity chromatography. Cleared lysates containing His‐EscVc and full‐length SepL (SepLFL), SepL∆C75, or SepL∆C11 were mixed and incubated for 2 hr (Lys). Samples were loaded into columns packed with Ni‐NTA resin, washed (W), and eluted (E). His‐EscV was visualized by SDS‐PAGE stained with CBB. Copurified proteins were detected by immunoblotting using polyclonal anti‐SepL antibodies (middle panels). As negative controls, the nonspecific binding of SepLFL, SepL∆C75, and SepL∆C11 to the Ni‐NTA resin was analyzed by immunoblotting (lower panels). (c) Protein secretion profiles of EPEC wild‐type (WT), ∆escN and ∆sepL mutant strains, and the ∆sepL strain carrying the empty vector pTrc99A_FF4 (pTrc99A), or the pTrc99A_FF4‐based plasmids expressing sepL or the C‐terminal truncated versions sepL ∆C75 and sepL ∆C11. Secreted proteins were visualized by SDS‐PAGE stained with CBB (upper panel) and detected by immunoblotting in the supernatants (SN) with specific antibodies against Tir and EspA. The production of SepL and its truncated versions in whole‐cell lysates (WC) was examined by immunoblotting with polyclonal antibodies against SepL
To further dissect the SepL interacting region with EscVc, we generated SepL truncated protein versions. In P. aeruginosa, the EscV homolog PcrD interacts with the Pcr1 protein, which is homologous to the C‐terminal domain of SepL (Lee et al., 2014). Therefore, we generated two C‐terminal truncated versions of SepL (SepL∆C75 and SepL∆C11) and assessed their ability to interact with His‐EscVc by in vitro pull‐down assays. We found that in contrast to full‐length SepL, neither SepL∆C75 nor SepL∆C11 interacted with His‐EscVc (Figure 5b). In addition, we demonstrated that the structural integrity of the SepL∆C11 truncated protein is not affected, since it is able to form the SepL∆C11/SepD/CesL ternary complex (Figure S4). These results indicate that the last 11 amino acids of SepL are crucial for its interaction with EscVc. Moreover, we further evaluated the ability of the SepL truncated versions to complement the phenotype of the ∆sepL null strain. Neither SepL∆C75 nor SepL∆C11 could restore the secretion of translocators, and only partially reduced the hypersecretion of effectors of the sepL mutant (Figure 5c). Therefore, our results highlight the importance of the extreme C‐terminal region of SepL for substrate secretion regulation.
4. DISCUSSION
Injectisome assembly demands orderly secretion of T3 substrates. Secretion of translocators prior to effectors ensures that the latter are translocated directly into the host cell cytoplasm. In EPEC, this secretion hierarchy is proposed to be controlled by the gatekeeper SepL (Deng et al., 2004, 2005; Wang et al., 2008), and to respond to environmental cues such as calcium concentration (Deng et al., 2005; Shaulov et al., 2017). However, the exact mechanism underlying this specificity‐switching process is still poorly understood. In this work, we evaluated the link between calcium sensing and SepL hierarchical regulation.
Previous reports showed that calcium depletion from the growth medium in A/E pathogens has a differential effect on T3 substrate secretion, that is, reduced secretion of translocators and increased secretion of effectors (Deng et al., 2005; Ide et al., 2003). In accordance, we showed that the absence of calcium in the EPEC growth medium considerably increased the secretion of several LEE and Nle effectors. However, although the secretion of translocators appears to be slightly reduced in the absence of calcium, our statistical analysis demonstrated that the secretion of middle substrates is not significantly affected under this condition (Figure 1). The dissimilar results on the effect of calcium on translocator secretion could be attributed to either differences in the experimental setups for the secretion assay or to the use of chelators to eliminate calcium (Deng et al., 2005; Ide et al., 2003), given that EGTA and BAPTA bind in addition to calcium other multivalent cations, which in turn could also affect T3‐dependent secretion (Gode‐Potratz, Chodur, & McCarter, 2010; Kenny et al., 1997; Sarty et al., 2012). In agreement with our results, a previous report in P. aeruginosa showed that in vitro calcium levels influence effector but not translocator secretion (Cisz, Lee, & Rietsch, 2008). Hence, it is possible that secretion of translocators and effectors can be promoted by different environmental signals as recently suggested (Roehrich et al., 2017). Additionally, we extended our analysis to investigate the effect of calcium absence on the secretion of an early substrate, the inner rod protein EscI, and demonstrated that its secretion is not affected under this condition. This result supports our proposal that the calcium signal exclusively regulates the substrate specificity switch promoting late substrate secretion.
Moreover, it has been proposed that physical contact between the bacteria and host cells along with the assembly of the translocation pore, allows for detection of lowered calcium levels in the eukaryotic cell cytoplasm, triggering effector secretion (Deng et al., 2005; Lee et al., 2001). Accordingly, the translocon components would be expected to be involved in signal sensing as has been previously reported (Armentrout & Rietsch, 2016; Cisz et al., 2008; Roehrich, Guillossou, Blocker, & Martinez‐Argudo, 2013; Urbanowski, Brutinel, & Yahr, 2007). Here we demonstrated that the translocator proteins EspA, EspB, and EspD are not implicated in transducing the signal that triggers effector secretion upon calcium depletion in vitro (Figure 2). This finding is in agreement with previous reports in S. enterica and P. aeruginosa, where mutants lacking the translocators are still able to trigger effector secretion upon changes in chemical cues (Cisz et al., 2008; Yu et al., 2010). The apparent discrepancy regarding the requirement of translocators could be explained by the nature of the triggering signal. While translocators have been shown to be essential for effector translocation upon host cell contact (Armentrout & Rietsch, 2016; Cisz et al., 2008; Urbanowski et al., 2007), they are not required for induction of effector secretion under calcium depletion in vitro (Cisz et al., 2008; Lee, Stopford, Svenson, & Rietsch, 2010). Thus, under in vitro calcium‐depleted conditions in EPEC, either the needle plays an active role in detecting and conveying the environmental signal as has been demonstrated for S. flexneri and Y. pestis (Deane et al., 2006; Kenjale et al., 2005; Torruellas, Jackson, Pennock, & Plano, 2005); or as recently suggested, diffusion of chemical signals through the needle channel might alter the local concentration of different ions at the base of the secretion apparatus (Notti & Stebbins, 2016; Portaliou et al., 2016; Shaulov et al., 2017; Yu et al., 2010), resulting in modifications at the T3SS basal machinery that trigger effector secretion. Nonetheless, further studies are required to elucidate the mechanistic basis of physiological signal sensing and transmission.
The deregulation of effector secretion upon calcium depletion somewhat mimics the hypersecretion phenotype seen in the absence of the SepL and SepD proteins (Deng et al., 2005). SepL and SepD form a ternary complex with CesL that is homologous to the S. enterica SsaL/SpiC/SsaM complex (Younis et al., 2010). Besides, in contrast to other gatekeeper proteins, SepL and SsaL are not secreted, share more than 40% sequence similarity and SepL partially complements an ssaL null mutant (Coombes et al., 2004; Younis et al., 2010). For these reasons, we hypothesized that the SepL/SepD/CesL complex regulates the switch from middle to late substrates in response to environmental calcium in the same manner as the SsaL/SpiC/SsaM complex does in response to pH (Yu et al., 2010). Nevertheless, our results demonstrated that the stability, localization, and association of the SepL/SepD/CesL complex are not affected by calcium changes (Figure 3 and S3), indicating that this gatekeeper complex is not prone to direct or indirect calcium regulation. These data prompted us to investigate whether the interaction with other T3SS components might be involved in the calcium‐signaling pathway.
Gatekeepers are known to interact with several T3SS components (Archuleta & Spiller, 2014; Botteaux et al., 2009; Cherradi et al., 2013; Day & Plano, 1998; Kubori & Galan, 2002; Lee et al., 2014; Roehrich et al., 2017; Shen & Blocker, 2016; Silva‐Herzog et al., 2011; Stone, Johnson, Bulir, Gilchrist, & Mahony, 2008). In EPEC, besides the SepL/SepD/CesL complex, SepL binds to the effector Tir, the inner membrane ring‐forming protein EscD (Wang et al., 2008), and to the switch protein EscP (Shaulov et al., 2017). Among the gatekeepers interacting partners, those involved in substrate recognition are perfect targets for secretion regulation. Substrate docking sites have been found in the cytoplasmic domain of the export apparatus component SctV and its flagellar homolog FlhA (Bange et al., 2010; Hartmann & Buttner, 2013; Kinoshita et al., 2013; Minamino et al., 2012). Noteworthy, in the flagellar T3SS, the distinct binding affinities of FlhA for different substrate/chaperone complexes seem to coordinate the order of substrate secretion (Kinoshita et al., 2013). In this study, we demonstrated that the cytoplasmic domain of the major export gate component EscV (EscVc) is indeed capable of interacting with both middle and late T3 substrates (Figure 4). In addition, we showed that SepL interacts with EscVc, even though the interplay between these components was not regulated directly by calcium (Figure 5a). In this same regard, the SepL homologs in P. aeruginosa (PopN/Pcr1) and S. flexneri (MxiC) have been found to interact with SctV (PcrD and MxiA, respectively) (Lee et al., 2014; Shen & Blocker, 2016). In agreement with our result, the Pcr1‐PcrD interaction was not sensitive to calcium removal from the growth media (Lee et al., 2014). Interestingly, a single point mutation in PcrD and MxiA that interferes with their binding to Pcr1 and MxiC, respectively, caused a deregulation of effector secretion (Lee et al., 2014; Shen & Blocker, 2016), suggesting that the SctV‐gatekeeper protein interaction is relevant for T3S regulation.
The SepL region involved in the interaction with EscV was mapped to the last 11 amino acids (Figure 5b), and we showed that this protein version, SepL∆C11, is unable to rescue the phenotype of a sepL null mutant (Figure 5c). This result is similar to previous observations in enterohemorrhagic E. coli, where complementation of a sepL mutant with a SepL∆C11 protein version did not recover the secretion of translocators to wild‐type levels, and only marginally reduced the boosted secretion of effectors (Wang et al., 2008). These results are also in agreement with recent observations in Shigella showing that the C‐terminal region of the gatekeeper MxiC is essential for substrate secretion regulation (Roehrich et al., 2017). Remarkably, the last 11 residues of SepL are also involved in EscD and Tir binding (Wang et al., 2008), suggesting a plausible model for SepL control of substrate secretion that could involve its dynamic interactions with EscD, Tir, and EscV. The interaction of SepL with Tir was proposed to delay effector secretion (since Tir secretion is required for the secretion of other effectors (Thomas, Deng, Baker, Puente, & Finlay, 2007)), while its interaction with EscD was suggested to release Tir and promote effector secretion (Wang et al., 2008). This is supported by the finding that overexpression of escD leads to hypersecretion of Tir (Ogino et al., 2006). In addition, since SepL resembles a T3 effector but without being secreted (Younis et al., 2010), it could function as a Trojan horse that, after its recognition as a T3 substrate, blocks a substrate acceptor site on EscV, or modifies the affinity of this export gate component for different substrates. Nonetheless, whether the SepL–EscV interaction participates in secretion regulation remains to be investigated.
Furthermore, it was recently shown that SepL interacts with the molecular ruler protein EscP, and that the SepL/EscP complex is stabilized by calcium, preventing effector secretion. The model proposed that once the translocation pore is assembled in the host cell membrane, the calcium flux to the T3SS base is reduced, resulting in dissociation of the SepL/EscP complex and effector secretion (Shaulov et al., 2017). Thus, the EscP switch protein, which has been shown to play a role in effector secretion control (Monjaras Feria et al., 2012), emerges as an important player in the in vitro calcium‐dependent SepL secretion regulatory pathway (Shaulov et al., 2017). Besides, EscP was previously shown to bind to the cognate Tir chaperone CesT, and suggested to act, together with Tir and the SepL/SepD complex, in the restriction of effector secretion (Monjaras Feria et al., 2012). Overall, the intricate interaction network in which SepL participates may reflect the different regulation layers required to fine‐tune the timing of substrate secretion (Figure 6). However, based on previous observations and our results (i.e., neither the translocon senses the absence of calcium nor the SepL/SepD/CesL switch complex is affected by calcium changes), we suggest that the molecular mechanism triggering effector secretion upon in vitro calcium removal could be different from the one triggering effector translocation upon host cell contact, as outlined below.
Figure 6.
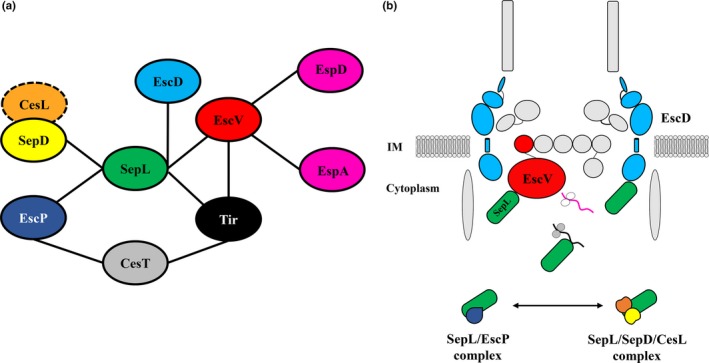
SepL protein–protein interaction network involved in middle and late substrate secretion regulation. (a) The SepL protein binds directly to five different T3SS components: SepD, EscP, Tir, EscD, and EscV. Although SepL forms a ternary complex with SepD and CesL (Younis et al., 2010), no direct interaction between CesL (dashed circle) and SepL or SepD has been reported. The effector Tir binds to its cognate chaperone CesT (Elliott et al., 1999), which in turn has been demonstrated to interact with EscP (Monjaras Feria et al., 2012). Finally, the interaction between EscV and different T3S substrates is also displayed. (b) Schematic representation of the EPEC T3SS base showing the SepL binding partners described so far. SepL [green] interacts with EscD [cyan], Tir [black] (Wang et al., 2008), and EscV [red] (this work). In addition, SepL was reported to form two mutually exclusive complexes: the SepL‐EscP [blue] complex, which was proposed to regulate substrate secretion in response to calcium changes in vitro (Shaulov et al., 2017); and the SepL‐SepD [yellow]‐CesL [orange] complex (Younis et al., 2010), whose role in substrate secretion regulation remains to be determined
It is noteworthy that in vitro calcium depletion acts as a common environmental cue that promotes effector secretion in phylogenetically distant bacteria (e.g., Yersinia, Pseudomonas, Chlamydia, Vibrio, and A/E pathogens) (Cisz et al., 2008; Deng et al., 2005; Gode‐Potratz et al., 2010; Ide et al., 2003; Jamison & Hackstadt, 2008; Kim et al., 2005; Michiels, Wattiau, Brasseur, Ruysschaert, & Cornelis, 1990; Sarty et al., 2012; Shaulov et al., 2017), probably by inducing conformational changes in the needle structure that mimic those adopted upon host cell contact (Torruellas et al., 2005). However, by increasing host intracellular calcium levels, Cisz et al. (2008) demonstrated that the in vivo translocation of effectors is not influenced by calcium concentration changes. Therefore, a distinction should be made between induction of effector secretion in vitro and upon host cell contact. While the former can promote effector secretion in a translocator‐independent mechanism (Figure 2) that involves the SepL/EscP protein complex in EPEC (Shaulov et al., 2017), the mechanism participating in effector translocation in vivo is still largely unknown. Finally, our data revealed EscV as a new player that serves as a docking site for both SepL and T3 substrates. Nevertheless, whether EscV acts in concert with the SepL/SepD/CesL regulatory switch complex to ensure the proper timing of substrate secretion remains to be determined.
CONFLICT OF INTEREST
None declared.
Supporting information
ACKNOWLEDGMENTS
This work was supported by grants from Dirección General de Asuntos del Personal Académico, PAPIIT, UNAM (IN209617) (to B.G.‐P.), and Consejo Nacional de Ciencia y Tecnología (CONACyT) 180460 (to B.G.‐P.) and 178033 (to D.G.). M.O.G. was supported by fellowship 262002 from CONACyT.
We are grateful to José Luis Puente, Juan Xicohtencatl, and Fernando Navarro for the kind donation of the ∆sepL, ∆espB, ∆espD, and ∆espA strains and the anti‐EspD antibody. We thank Ana Karen Mojica Ávila for help with plasmid construction and María Fernanda Rosales Larios for the anti‐Map antibody. We are indebted to Miguel Ángel Díaz Guerrero for help with protein interaction experiments. We thank Claudia Rivera Cerecedo and Héctor Malagón Rivero (Bioterio, IFC, UNAM); and Laura Ongay, Guadalupe Códiz and Minerva Mora (Unidad de Biología Molecular, IFC, UNAM) for excellent technical assistance. We acknowledge Tohru Minamino and Keiichi Namba for continuous advice and support.
Gaytán MO, Monjarás Feria J, Soto E, et al. Novel insights into the mechanism of SepL‐mediated control of effector secretion in enteropathogenic Escherichia coli . MicrobiologyOpen. 2018;7:e571 10.1002/mbo3.571
REFERENCES
- Alegria, M. C. , Docena, C. , Khater, L. , Ramos, C. H. , da Silva, A. C. , & Farah, C. S. (2004). New protein‐protein interactions identified for the regulatory and structural components and substrates of the type III Secretion system of the phytopathogen Xanthomonas axonopodis Pathovar citri. Journal of Bacteriology, 186, 6186–6197. 10.1128/JB.186.18.6186-6197.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade, A. , Pardo, J. P. , Espinosa, N. , Perez‐Hernandez, G. , & Gonzalez‐Pedrajo, B. (2007). Enzymatic characterization of the enteropathogenic Escherichia coli type III secretion ATPase EscN. Archives of Biochemistry and Biophysics, 468, 121–127. 10.1016/j.abb.2007.09.020 [DOI] [PubMed] [Google Scholar]
- Archuleta, T. L. , & Spiller, B. W. (2014). A gatekeeper chaperone complex directs translocator secretion during type three secretion. PLoS Pathogens, 10, e1004498 10.1371/journal.ppat.1004498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentrout, E. I. , & Rietsch, A. (2016). The Type III secretion translocation pore senses host cell contact. PLoS Pathogens, 12, e1005530 10.1371/journal.ppat.1005530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange, G. , Kummerer, N. , Engel, C. , Bozkurt, G. , Wild, K. , & Sinning, I. (2010). FlhA provides the adaptor for coordinated delivery of late flagella building blocks to the type III secretion system. Proceedings of the National Academy of Sciences United States of America, 107, 11295–11300. 10.1073/pnas.1001383107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzon, C. R. , Banks, G. , Deiwick, J. , Hensel, M. , & Holden, D. W. (1999). pH‐dependent secretion of SseB, a product of the SPI‐2 type III secretion system of Salmonella typhimurium . Molecular Microbiology, 33, 806–816. 10.1046/j.1365-2958.1999.01527.x [DOI] [PubMed] [Google Scholar]
- Biemans‐Oldehinkel, E. , Sal‐Man, N. , Deng, W. , Foster, L. J. , & Finlay, B. B. (2011). Quantitative proteomic analysis reveals formation of an EscL‐EscQ‐EscN type III complex in enteropathogenic Escherichia coli . Journal of Bacteriology, 193, 5514–5519. 10.1128/JB.05235-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteaux, A. , Sory, M. P. , Biskri, L. , Parsot, C. , & Allaoui, A. (2009). MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Molecular Microbiology, 71, 449–460. 10.1111/j.1365-2958.2008.06537.x [DOI] [PubMed] [Google Scholar]
- Buttner, D. (2012). Protein export according to schedule: Architecture, Assembly, and regulation of type III secretion systems from plant‐ and animal‐Pathogenic Bacteria. Microbiology and Molecular Biology Reviews, 76, 262–310. 10.1128/MMBR.05017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner, D. , Lorenz, C. , Weber, E. , & Bonas, U. (2006). Targeting of two effector protein classes to the type III secretion system by a HpaC‐ and HpaB‐dependent protein complex from Xanthomonas campestris pv. vesicatoria. Molecular Microbiology, 59, 513–527. 10.1111/j.1365-2958.2005.04924.x [DOI] [PubMed] [Google Scholar]
- Chatterjee, A. , Caballero‐Franco, C. , Bakker, D. , Totten, S. , & Jardim, A. (2015). Pore‐forming activity of the Escherichia coli type III secretion system protein EspD. Journal of Biological Chemistry, 290, 25579–25594. 10.1074/jbc.M115.648204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, L. W. , Kay, O. , & Schneewind, O. (2001). Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica . Journal of Bacteriology, 183, 5293–5301. 10.1128/JB.183.18.5293-5301.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherradi, Y. , Schiavolin, L. , Moussa, S. , Meghraoui, A. , Meksem, A. , Biskri, L. , … Botteaux, A. (2013). Interplay between predicted inner‐rod and gatekeeper in controlling substrate specificity of the type III secretion system. Molecular Microbiology, 87, 1183–1199. 10.1111/mmi.12158 [DOI] [PubMed] [Google Scholar]
- Cisz, M. , Lee, P. C. , & Rietsch, A. (2008). ExoS controls the cell contact‐mediated switch to effector secretion in Pseudomonas aeruginosa . Journal of Bacteriology, 190, 2726–2738. 10.1128/JB.01553-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes, B. K. , Brown, N. F. , Valdez, Y. , Brumell, J. H. , & Finlay, B. B. (2004). Expression and secretion of Salmonella pathogenicity island‐2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. Journal of Biological Chemistry, 279, 49804–49815. 10.1074/jbc.M404299200 [DOI] [PubMed] [Google Scholar]
- Datsenko, K. A. , & Wanner, B. L. (2000). One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proceedings of the National Academy of Sciences United States of America, 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, J. B. , & Plano, G. V. (1998). A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Molecular Microbiology, 30, 777–788. 10.1046/j.1365-2958.1998.01110.x [DOI] [PubMed] [Google Scholar]
- Dean, P. (2011). Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiology Reviews, 35, 1100–1125. 10.1111/j.1574-6976.2011.00271.x [DOI] [PubMed] [Google Scholar]
- Dean, P. , & Kenny, B. (2009). The effector repertoire of enteropathogenic E. coli: Ganging up on the host cell. Current Opinion in Microbiology, 12, 101–109. 10.1016/j.mib.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane, J. E. , Abrusci, P. , Johnson, S. , & Lea, S. M. (2010). Timing is everything: The regulation of type III secretion. Cellular and Molecular Life Sciences, 67, 1065–1075. 10.1007/s00018-009-0230-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane, J. E. , Roversi, P. , Cordes, F. S. , Johnson, S. , Kenjale, R. , Daniell, S. , … Lea, S. M. (2006). Molecular model of a type III secretion system needle: implications for host‐cell sensing. Proceedings of the National Academy of Sciences United States of America, 103, 12529–12533. 10.1073/pnas.0602689103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. , Li, Y. , Hardwidge, P. R. , Frey, E. A. , Pfuetzner, R. A. , Lee, S. , … Finlay, B. B. (2005). Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infection and Immunity, 73, 2135–2146. 10.1128/IAI.73.4.2135-2146.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. , Puente, J. L. , Gruenheid, S. , Li, Y. , Vallance, B. A. , Vazquez, A. , … Finlay, B. B. (2004). Dissecting virulence: Systematic and functional analyses of a pathogenicity island. Proceedings of the National Academy of Sciences United States of America, 101, 3597–3602. 10.1073/pnas.0400326101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. , Yu, H. B. , de Hoog, C. L. , Stoynov, N. , Li, Y. , Foster, L. J. , & Finlay, B. B. (2012). Quantitative proteomic analysis of type III secretome of enteropathogenic Escherichia coli reveals an expanded effector repertoire for attaching/effacing bacterial pathogens. Molecular & Cellular Proteomics: MCP, 11, 692–709. 10.1074/mcp.M111.013672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, S. J. , Hutcheson, S. W. , Dubois, M. S. , Mellies, J. L. , Wainwright, L. A. , Batchelor, M. , … Kaper, J. B. (1999). Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli . Molecular Microbiology, 33, 1176–1189. [DOI] [PubMed] [Google Scholar]
- Forsberg, A. , Viitanen, A. M. , Skurnik, M. , & Wolf‐Watz, H. (1991). The surface‐located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis . Molecular Microbiology, 5, 977–986. 10.1111/j.1365-2958.1991.tb00773.x [DOI] [PubMed] [Google Scholar]
- Garcia‐Angulo, V. A. , Martinez‐Santos, V. I. , Villasenor, T. , Santana, F. J. , Huerta‐Saquero, A. , Martinez, L. C. , … Puente, J. L. (2012). A distinct regulatory sequence is essential for the expression of a subset of nle genes in attaching and effacing Escherichia coli . Journal of Bacteriology, 194, 5589–5603. 10.1128/JB.00190-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, A. , Puente, J. L. , & Finlay, B. B. (2003). Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infection and Immunity, 71, 3310–3319. 10.1128/IAI.71.6.3310-3319.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan, M. O. , Martinez‐Santos, V. I. , Soto, E. , & Gonzalez‐Pedrajo, B. (2016). Type three secretion system in attaching and effacing pathogens. Frontiers in Cellular and Infection Microbiology, 6, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gode‐Potratz, C. J. , Chodur, D. M. , & McCarter, L. L. (2010). Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus . Journal of Bacteriology, 192, 6025–6038. 10.1128/JB.00654-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Pedrajo, B. , Minamino, T. , Kihara, M. , & Namba, K. (2006). Interactions between C ring proteins and export apparatus components: A possible mechanism for facilitating type III protein export. Molecular Microbiology, 60, 984–998. 10.1111/j.1365-2958.2006.05149.x [DOI] [PubMed] [Google Scholar]
- Hartmann, N. , & Buttner, D. (2013). The inner membrane protein HrcV from Xanthomonas spp. is involved in substrate docking during type III secretion. Molecular Plant‐Microbe Interactions, 26, 1176–1189. 10.1094/MPMI-01-13-0019-R [DOI] [PubMed] [Google Scholar]
- Hoang, T. T. , Karkhoff‐Schweizer, R. R. , Kutchma, A. J. , & Schweizer, H. P. (1998). A broad‐host‐range Flp‐FRT recombination system for site‐specific excision of chromosomally‐located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene, 212, 77–86. 10.1016/S0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- Ide, T. , Michgehl, S. , Knappstein, S. , Heusipp, G. , & Schmidt, M. A. (2003). Differential modulation by Ca2 + of type III secretion of diffusely adhering enteropathogenic Escherichia coli . Infection and Immunity, 71, 1725–1732. 10.1128/IAI.71.4.1725-1732.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi, A. , Thomson, N. R. , Ogura, Y. , Saunders, D. , Ooka, T. , Henderson, I. R. , … Frankel, G. (2009). Complete genome sequence and comparative genome analysis of enteropathogenic Escherichia coli O127:H6 strain E2348/69. Journal of Bacteriology, 191, 347–354. 10.1128/JB.01238-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriarte, M. , Sory, M. P. , Boland, A. , Boyd, A. P. , Mills, S. D. , Lambermont, I. , & Cornelis, G. R. (1998). TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO Journal, 17, 1907–1918. 10.1093/emboj/17.7.1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison, W. P. , & Hackstadt, T. (2008). Induction of type III secretion by cell‐free Chlamydia trachomatis elementary bodies. Microbial Pathogenesis, 45, 435–440. 10.1016/j.micpath.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis, K. G. , Giron, J. A. , Jerse, A. E. , McDaniel, T. K. , Donnenberg, M. S. , & Kaper, J. B. (1995). Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proceedings of the National Academy of Sciences United States of America, 92, 7996–8000. 10.1073/pnas.92.17.7996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper, J. B. , Nataro, J. P. , & Mobley, H. L. (2004). Pathogenic Escherichia coli . Nature Reviews Microbiology, 2, 123–140. 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- Kenjale, R. , Wilson, J. , Zenk, S. F. , Saurya, S. , Picking, W. L. , Picking, W. D. , & Blocker, A. (2005). The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. Journal of Biological Chemistry, 280, 42929–42937. 10.1074/jbc.M508377200 [DOI] [PubMed] [Google Scholar]
- Kenny, B. , Abe, A. , Stein, M. , & Finlay, B. B. (1997). Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infection and Immunity, 65, 2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Ahn, K. , Min, S. , Jia, J. , Ha, U. , Wu, D. , & Jin, S. (2005). Factors triggering type III secretion in Pseudomonas aeruginosa . Microbiology, 151, 3575–3587. 10.1099/mic.0.28277-0 [DOI] [PubMed] [Google Scholar]
- Kinoshita, M. , Hara, N. , Imada, K. , Namba, K. , & Minamino, T. (2013). Interactions of bacterial flagellar chaperone‐substrate complexes with FlhA contribute to co‐ordinating assembly of the flagellar filament. Molecular Microbiology, 90, 1249–1261. 10.1111/mmi.12430 [DOI] [PubMed] [Google Scholar]
- Knutton, S. , Rosenshine, I. , Pallen, M. J. , Nisan, I. , Neves, B. C. , Bain, C. , … Frankel, G. (1998). A novel EspA‐associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO Journal, 17, 2166–2176. 10.1093/emboj/17.8.2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubori, T. , & Galan, J. E. (2002). Salmonella type III secretion‐associated protein InvE controls translocation of effector proteins into host cells. Journal of Bacteriology, 184, 4699–4708. 10.1128/JB.184.17.4699-4708.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, V. T. , Mazmanian, S. K. , & Schneewind, O. (2001). A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. Journal of Bacteriology, 183, 4970–4978. 10.1128/JB.183.17.4970-4978.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P. C. , Stopford, C. M. , Svenson, A. G. , & Rietsch, A. (2010). Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV. Molecular Microbiology, 75, 924–941. 10.1111/j.1365-2958.2009.07027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P. C. , Zmina, S. E. , Stopford, C. M. , Toska, J. , & Rietsch, A. (2014). Control of type III secretion activity and substrate specificity by the cytoplasmic regulator PcrG. Proceedings of the National Academy of Sciences United States of America, 111, E2027–E2036. 10.1073/pnas.1402658111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, M. M. , Bergquist, E. J. , Nalin, D. R. , Waterman, D. H. , Hornick, R. B. , Young, C. R. , & Sotman, S. (1978). Escherichia coli strains that cause diarrhoea but do not produce heat‐labile or heat‐stable enterotoxins and are non‐invasive. Lancet, 1, 1119–1122. 10.1016/S0140-6736(78)90299-4 [DOI] [PubMed] [Google Scholar]
- Luo, W. , & Donnenberg, M. S. (2011). Interactions and predicted host membrane topology of the enteropathogenic Escherichia coli translocator protein EspB. Journal of Bacteriology, 193, 2972–2980. 10.1128/JB.00153-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel, T. K. , Jarvis, K. G. , Donnenberg, M. S. , & Kaper, J. B. (1995). A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proceedings of the National Academy of Sciences United States of America, 92, 1664–1668. 10.1073/pnas.92.5.1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel, T. K. , & Kaper, J. B. (1997). A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K‐12. Molecular Microbiology, 23, 399–407. 10.1046/j.1365-2958.1997.2311591.x [DOI] [PubMed] [Google Scholar]
- Mellies, J. L. , Navarro‐Garcia, F. , Okeke, I. , Frederickson, J. , Nataro, J. P. , & Kaper, J. B. (2001). espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infection and Immunity, 69, 315–324. 10.1128/IAI.69.1.315-324.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels, T. , Wattiau, P. , Brasseur, R. , Ruysschaert, J. M. , & Cornelis, G. (1990). Secretion of Yop proteins by Yersiniae. Infection and Immunity, 58, 2840–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, E. , Baruch, K. , Charpentier, X. , Kobi, S. , & Rosenshine, I. (2008). Real‐time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli . Cell Host & Microbe, 3, 104–113. 10.1016/j.chom.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Minamino, T. , Kinoshita, M. , Hara, N. , Takeuchi, S. , Hida, A. , Koya, S. , … Namba, K. (2012). Interaction of a bacterial flagellar chaperone FlgN with FlhA is required for efficient export of its cognate substrates. Molecular Microbiology, 83, 775–788. 10.1111/j.1365-2958.2011.07964.x [DOI] [PubMed] [Google Scholar]
- Monjaras Feria, J. , Garcia‐Gomez, E. , Espinosa, N. , Minamino, T. , Namba, K. , & Gonzalez‐Pedrajo, B. (2012). Role of EscP (Orf16) in injectisome biogenesis and regulation of type III protein secretion in enteropathogenic Escherichia coli . Journal of Bacteriology, 194, 6029–6045. 10.1128/JB.01215-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes, T. F. , Spreter, T. , & Strynadka, N. C. (2008). Piecing together the type III injectisome of bacterial pathogens. Current Opinion in Structural Biology, 18, 258–266. 10.1016/j.sbi.2007.12.011 [DOI] [PubMed] [Google Scholar]
- Notti, R. Q. , & Stebbins, C. E . (2016). The structure and function of type III secretion systems. Microbiology Spectrum, 4(1). 10.1128/microbiolspec.VMBF-0004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, C. B. , Creasey, E. A. , Knutton, S. , Elliott, S. , Crowther, L. J. , Luo, W. , … Donnenberg, M. S. (2004). SepL, a protein required for enteropathogenic Escherichia coli type III translocation, interacts with secretion component SepD. Molecular Microbiology, 52, 1613–1625. 10.1111/j.1365-2958.2004.04101.x [DOI] [PubMed] [Google Scholar]
- Ogino, T. , Ohno, R. , Sekiya, K. , Kuwae, A. , Matsuzawa, T. , Nonaka, T. , … Abe, A. (2006). Assembly of the type III secretion apparatus of enteropathogenic Escherichia coli . Journal of Bacteriology, 188, 2801–2811. 10.1128/JB.188.8.2801-2811.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, K. , Fan, F. , Schoenhals, G. J. , Kihara, M. , & Macnab, R. M. (1997). The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: Putative components for flagellar assembly. Journal of Bacteriology, 179, 6092–6099. 10.1128/jb.179.19.6092-6099.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, K. , Ohto, Y. , Aizawa, S. , Macnab, R. M. , & Iino, T. (1994). FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium . Journal of Bacteriology, 176, 2272–2281. 10.1128/jb.176.8.2272-2281.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe, M. , Minamino, T. , Imada, K. , Namba, K. , & Kihara, M. (2009). Role of the N‐terminal domain of FliI ATPase in bacterial flagellar protein export. FEBS Letters, 583, 743–748. 10.1016/j.febslet.2009.01.026 [DOI] [PubMed] [Google Scholar]
- Pallen, M. J. , Beatson, S. A. , & Bailey, C. M. (2005). Bioinformatics analysis of the locus for enterocyte effacement provides novel insights into type‐III secretion. BMC Microbiology, 5, 9 10.1186/1471-2180-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaliou, A. G. , Tsolis, K. C. , Loos, M. S. , Zorzini, V. , & Economou, A. (2016). Type III Secretion: Building and Operating a Remarkable Nanomachine. Trends in Biochemical Sciences, 41, 175–189. 10.1016/j.tibs.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Roehrich, A. D. , Bordignon, E. , Mode, S. , Shen, D. K. , Liu, X. , Pain, M. , … Blocker, A. J. (2017). Steps for shigella gatekeeper Protein MxiC function in hierarchical type III secretion regulation. Journal of Biological Chemistry, 292, 1705–1723. 10.1074/jbc.M116.746826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrich, A. D. , Guillossou, E. , Blocker, A. J. , & Martinez‐Argudo, I. (2013). Shigella IpaD has a dual role: Signal transduction from the type III secretion system needle tip and intracellular secretion regulation. Molecular Microbiology, 87, 690–706. 10.1111/mmi.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo‐Castillo, M. , Andrade, A. , Espinosa, N. , Monjaras Feria, J. , Soto, E. , Diaz‐Guerrero, M. , & Gonzalez‐Pedrajo, B. (2014). EscO, a functional and structural analog of the flagellar FliJ protein, is a positive regulator of EscN ATPase activity of the enteropathogenic Escherichia coli injectisome. Journal of Bacteriology, 196, 2227–2241. 10.1128/JB.01551-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist, R. , Magnusson, K. E. , & Wolf‐Watz, H. (1994). Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO Journal, 13, 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, J. , & Hartin, R. J. (1990). Quick transformation in Salmonella typhimurium LT2. BioTechniques, 8, 43–45. [PubMed] [Google Scholar]
- Sal‐Man, N. , Deng, W. , & Finlay, B. B. (2012). EscI: A crucial component of the type III secretion system forms the inner rod structure in enteropathogenic Escherichia coli . The Biochemical Journal, 442, 119–125. 10.1042/BJ20111620 [DOI] [PubMed] [Google Scholar]
- Sarty, D. , Baker, N. T. , Thomson, E. L. , Rafuse, C. , Ebanks, R. O. , Graham, L. L. , & Thomas, N. A. (2012). Characterization of the type III secretion associated low calcium response genes of Vibrio parahaemolyticus RIMD2210633. Canadian Journal of Microbiology, 58, 1306–1315. 10.1139/w2012-109 [DOI] [PubMed] [Google Scholar]
- Schneider, C. A. , Rasband, W. S. , & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubot, F. D. , Jackson, M. W. , Penrose, K. J. , Cherry, S. , Tropea, J. E. , Plano, G. V. , & Waugh, D. S. (2005). Three‐dimensional structure of a macromolecular assembly that regulates type III secretion in Yersinia pestis. Journal of Molecular Biology, 346, 1147–1161. 10.1016/j.jmb.2004.12.036 [DOI] [PubMed] [Google Scholar]
- Sekiya, K. , Ohishi, M. , Ogino, T. , Tamano, K. , Sasakawa, C. , & Abe, A. (2001). Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA‐sheath‐like structure. Proceedings of the National Academy of Sciences United States of America, 98, 11638–11643. 10.1073/pnas.191378598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulov, L. , Gershberg, J. , Deng, W. , Finlay, B. B. , & Sal‐Man, N . (2017). The ruler protein escp of the enteropathogenic Escherichia coli Type III secretion system is involved in calcium sensing and secretion hierarchy regulation by interacting with the gatekeeper protein sepl. MBio, 8, pii: e01733–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, D. K. , & Blocker, A. J. (2016). MxiA, MxiC and IpaD regulate substrate selection and secretion mode in the T3SS of Shigella flexneri. PLoS ONE, 11, e0155141 10.1371/journal.pone.0155141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva‐Herzog, E. , Joseph, S. S. , Avery, A. K. , Coba, J. A. , Wolf, K. , Fields, K. A. , & Plano, G. V. (2011). Scc1 (CP0432) and Scc4 (CP0033) function as a type III secretion chaperone for CopN of Chlamydia pneumoniae. Journal of Bacteriology, 193, 3490–3496. 10.1128/JB.00203-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto, E. , Espinosa, N. , Diaz‐Guerrero, M. , Gaytan, M. O. , Puente, J. L. , & Gonzalez‐Pedrajo, B. (2017). Functional characterization of EscK (Orf4), a sorting platform component of the enteropathogenic Escherichia coli injectisome. Journal of Bacteriology, 199, e00538–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreter, T. , Yip, C. K. , Sanowar, S. , Andre, I. , Kimbrough, T. G. , Vuckovic, M. , … Strynadka, N. C. (2009). A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nature Structural & Molecular Biology, 16, 468–476. 10.1038/nsmb.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. , Kenny, B. , Stein, M. A. , & Finlay, B. B. (1996). Characterization of EspC, a 110‐kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease‐like family of secreted proteins. Journal of Bacteriology, 178, 6546–6554. 10.1128/jb.178.22.6546-6554.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, C. B. , Johnson, D. L. , Bulir, D. C. , Gilchrist, J. D. , & Mahony, J. B. (2008). Characterization of the putative type III secretion ATPase CdsN (Cpn0707) of Chlamydophila pneumoniae . Journal of Bacteriology, 190, 6580–6588. 10.1128/JB.00761-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, N. A. , Deng, W. , Baker, N. , Puente, J. , & Finlay, B. B. (2007). Hierarchical delivery of an essential host colonization factor in enteropathogenic Escherichia coli . Journal of Biological Chemistry, 282, 29634–29645. 10.1074/jbc.M706019200 [DOI] [PubMed] [Google Scholar]
- Thomas, N. A. , Deng, W. , Puente, J. L. , Frey, E. A. , Yip, C. K. , Strynadka, N. C. , & Finlay, B. B. (2005). CesT is a multi‐effector chaperone and recruitment factor required for the efficient type III secretion of both LEE‐ and non‐LEE‐encoded effectors of enteropathogenic Escherichia coli . Molecular Microbiology, 57, 1762–1779. 10.1111/j.1365-2958.2005.04802.x [DOI] [PubMed] [Google Scholar]
- Torruellas, J. , Jackson, M. W. , Pennock, J. W. , & Plano, G. V. (2005). The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Molecular Microbiology, 57, 1719–1733. 10.1111/j.1365-2958.2005.04790.x [DOI] [PubMed] [Google Scholar]
- Tosi, T. , Pflug, A. , Discola, K. F. , Neves, D. , & Dessen, A. (2013). Structural basis of eukaryotic cell targeting by type III secretion system (T3SS) effectors. Research in Microbiology, 164, 605–619. 10.1016/j.resmic.2013.03.019 [DOI] [PubMed] [Google Scholar]
- Urbanowski, M. L. , Brutinel, E. D. , & Yahr, T. L. (2007). Translocation of ExsE into Chinese hamster ovary cells is required for transcriptional induction of the Pseudomonas aeruginosa type III secretion system. Infection and Immunity, 75, 4432–4439. 10.1128/IAI.00664-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzau, S. , Figueroa‐Bossi, N. , Rubino, S. , & Bossi, L. (2001). Epitope tagging of chromosomal genes in Salmonella. Proceedings of the National Academy of Sciences United States of America, 98, 15264–15269. 10.1073/pnas.261348198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Roe, A. J. , McAteer, S. , Shipston, M. J. , & Gally, D. L. (2008). Hierarchal type III secretion of translocators and effectors from Escherichia coli O157:H7 requires the carboxy terminus of SepL that binds to Tir. Molecular Microbiology, 69, 1499–1512. 10.1111/j.1365-2958.2008.06377.x [DOI] [PubMed] [Google Scholar]
- Wilson, R. K. , Shaw, R. K. , Daniell, S. , Knutton, S. , & Frankel, G. (2001). Role of EscF, a putative needle complex protein, in the type III protein translocation system of enteropathogenic Escherichia coli . Cellular Microbiology, 3, 753–762. 10.1046/j.1462-5822.2001.00159.x [DOI] [PubMed] [Google Scholar]
- Yang, H. , Shan, Z. , Kim, J. , Wu, W. , Lian, W. , Zeng, L. , … Jin, S. (2007). Regulatory role of PopN and its interacting partners in type III secretion of Pseudomonas aeruginosa . Journal of Bacteriology, 189, 2599–2609. 10.1128/JB.01680-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerushalmi, G. , Litvak, Y. , Gur‐Arie, L. , & Rosenshine, I. (2014). Dynamics of expression and maturation of the type III secretion system of enteropathogenic Escherichia coli . Journal of Bacteriology, 196, 2798–2806. 10.1128/JB.00069-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip, C. K. , Kimbrough, T. G. , Felise, H. B. , Vuckovic, M. , Thomas, N. A. , Pfuetzner, R. A. , … Strynadka, N. C. (2005). Structural characterization of the molecular platform for type III secretion system assembly. Nature, 435, 702–707. 10.1038/nature03554 [DOI] [PubMed] [Google Scholar]
- Younis, R. , Bingle, L. E. , Rollauer, S. , Munera, D. , Busby, S. J. , Johnson, S. , … Pallen, M. J. (2010). SepL resembles an aberrant effector in binding to a class 1 type III secretion chaperone and carrying an N‐terminal secretion signal. Journal of Bacteriology, 192, 6093–6098. 10.1128/JB.00760-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. J. , Liu, M. , & Holden, D. W. (2004). SsaM and SpiC interact and regulate secretion of Salmonella pathogenicity island 2 type III secretion system effectors and translocators. Molecular Microbiology, 54, 604–619. 10.1111/j.1365-2958.2004.04297.x [DOI] [PubMed] [Google Scholar]
- Yu, X. J. , McGourty, K. , Liu, M. , Unsworth, K. E. , & Holden, D. W. (2010). pH sensing by intracellular Salmonella induces effector translocation. Science, 328, 1040–1043. 10.1126/science.1189000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarivach, R. , Deng, W. , Vuckovic, M. , Felise, H. B. , Nguyen, H. V. , Miller, S. I. , … Strynadka, N. C. (2008). Structural analysis of the essential self‐cleaving type III secretion proteins EscU and SpaS. Nature, 453, 124–127. 10.1038/nature06832 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


