Abstract
We describe here the identification and properties of SCH-C (SCH 351125), a small molecule inhibitor of HIV-1 entry via the CCR5 coreceptor. SCH-C, an oxime–piperidine compound, is a specific CCR5 antagonist as determined in multiple receptor binding and signal transduction assays. This compound specifically inhibits HIV-1 infection mediated by CCR5 in U-87 astroglioma cells but has no effect on infection of CXCR4-expressing cells. SCH-C has broad and potent antiviral activity in vitro against primary HIV-1 isolates that use CCR5 as their entry coreceptor, with mean 50% inhibitory concentrations ranging between 0.4 and 9 nM. Moreover, SCH-C strongly inhibits the replication of an R5-using HIV-1 isolate in SCID-hu Thy/Liv mice. SCH-C has a favorable pharmacokinetic profile in rodents and primates with an oral bioavailability of 50–60% and a serum half-life of 5–6 h. On the basis of its novel mechanism of action, potent antiviral activity, and in vivo pharmacokinetic profile, SCH-C is a promising new candidate for therapeutic intervention of HIV infection.
Despite the tremendous progress that has been made over the past decade in antiretroviral research and therapy to reduce morbidity and mortality in HIV-1-infected individuals, there remains a need for more potent and less toxic therapies. The emergence of viral resistance to protease and reverse transcriptase inhibitors, combined with the difficulty of adhering to complicated dosing regimens that have associated side effects, has meant that an increasing number of patients are now failing to achieve sustained suppression of viral replication (1–4).
The process of HIV-1 entry into target cells is an attractive target for antiviral intervention (5–7). Inhibitors that target this stage of the viral life cycle are highly unlikely to share cross-resistance with the existing protease and reverse transcriptase inhibitors, which interfere with later events in viral replication. The receptor-mediated events that drive membrane fusion are increasingly well understood from a molecular perspective, and several individual stages have been identified as being susceptible to pharmacological intervention (7). The potential for entry inhibitors to be useful drugs has been established by the T-20 peptide, which blocks the conformational changes in HIV-1 gp41 that are necessary for virus–cell fusion (8). This peptide can cause reductions in plasma viremia when delivered i.v. or s.c. to HIV-1-infected individuals (9, 10). Other entry inhibitors are also in preclinical or early clinical development (5–7, 11).
One stage in the HIV-1 entry process that is a particularly plausible site for pharmacological intervention is the CD4-dependent interaction between HIV-1 gp120 and the CCR5 chemokine receptor, which serves as a viral coreceptor (12–16). The CCR5 receptor is a member of the G protein-coupled receptor superfamily. The natural genetic absence of CCR5 from humans (the CCR5-Δ32 homozygous genotype) has little impact on health while being strongly protective against HIV-1 infection (17). The reduced expression of CCR5 due to heterozygosity for the defective CCR5-Δ32 allele is associated with a reduction in the rate of disease progression in HIV-1-infected individuals (18–20). Furthermore, the experimental knockout of CCR5 in mice has a benign phenotype with only a subtle impact on immune function (21–23). Hence, an antagonist that is specific for CCR5 may cause few, if any, mechanism-based side effects. From an antiviral perspective, CCR5 is the coreceptor used by the most commonly transmitted HIV-1 strains, which predominate during the early stages of HIV-1 infection—the so-called macrophage-tropic viruses (now designated R5 strains) (24). Several inhibitors of CCR5-mediated HIV-1 entry have been shown to prevent R5 virus infection in vitro. These include modified chemokines (25, 26), monoclonal antibodies to CCR5 (27, 28), and a small molecule antagonist, TAK-779 (29), that binds within a cavity in the transmembrane domains of CCR5 (30).
The potential for CCR5 antagonists as antivirals for HIV-1 infection motivated our group to establish a drug development program for this target. Here, we report on SCH-C, a small molecule antagonist that is specific for CCR5 and displays potent antiviral activity against HIV-1 strains from multiple genetic subtypes in vitro. Moreover, SCH-C strongly inhibits HIV-1 replication in the SCID-hu Thy/Liv mouse model and has excellent pharmacological properties in rats and primates.
Materials and Methods
RANTES Inhibition Assay.
Membranes from Chinese hamster ovary (CHO) cells expressing human CCR5 (Biosignal, Montreal) were incubated with SCH-C (10–0.3 nM) or MIP-1β in the presence of 50 pM 125I-RANTES (NEN) in buffer (50 mM Hepes, pH 7.4/5 mM MgCl2/1 mM CaCl2/0.2% BSA) for 1 h at 25°C. Reaction mixtures were filtered through GFC filters (Packard) and washed six times with cold wash buffer (10 mM Hepes, pH 7.4/150 mM NaCl). Bound RANTES was quantitated by liquid scintillation counting. Ki values were calculated from the experimentally determined IC50 and KD values by using GRAPHPAD PRISM software (Intuitive Software for Science, San Diego).
Saturation Binding Analysis.
A murine cell line, Ba/F3, was transfected with a pME185neo-CCR5 expression plasmid and stable transfectants selected by resistance to G418 (B550 cells). B550 cells (5 × 105) were incubated in binding buffer (PBS/1 mM CaCl2/5 mM MgCl2/0.5% BSA/0.02% NaN3) with serially diluted [3H]SCH-C (43.4 Ci/mM) +/− unlabeled SCH-C (10 μM) for 2 h at room temperature. Cells were isolated by using a TOMTEC (Wallac, Gaithersburg, MD) and washed twice with PBS containing 1 mM CaCl2 and 5 mM MgCl2. Radioactivity was measured, and dissociation (KD) and maximal binding (Bmax) values were calculated by using an EXCEL-based program written by W. Gonsiorek (personal communication).
Calcium Flux Assay.
B550 cells were washed and resuspended at 1 × 107/ml in RPMI 1640 medium with 10% FBS and then loaded with 3 mg of Fura 2AM (Sigma) for 1 h at 25°C with gentle shaking. The cells were centrifuged and resuspended in Hanks' balanced salt solution supplemented with 1% FBS, transferred to a cuvette where 1.6 ml of warm flux buffer (HBSS/10 mM Hepes/1.6 mM CaCl2) was added. The cuvettes were placed in an LS-50B luminescence fluorimeter (Perkin–Elmer) preequilibrated at 37°C. SCH-C was added at 0.01, 0.1, and 1.0 μM and before addition of RANTES at a final concentration of 10 nM. Changes in intracellular calcium over time were measured as the ratio of fluorescence intensities at 340 nm and 380 nm.
Chemotaxis Assay.
Chemotaxis buffer (1% FBS/15 mM glycylglycine/140 mM NaCl) containing 0.01 nM MIP-1β was added to the bottom compartment of a 24-well 5-μm Transwell plate (Costar). About 5 × 106 B550 cells, pretreated with SCH-C (1 μM–0.01 nM) or 0.01% DMSO in RPMI 1640 medium, were seeded into the top well. The plates were incubated at 37°C for 1.5 h, the top chambers were removed, and the number of cells migrating to the lower chamber were counted.
[35S]Guanosine 5′-[γ-thio]Triphosphate (GTPγS)-Binding Assay.
[35S]GTP[γS]-binding assays by using CHO-CCR5 cell membranes (20 μg, 200-μl reaction volume) were performed as described by Fawzi et al. (31).
Virus Stocks and Reagents.
Luciferase reporter viruses (ADA, YU-2) were generated as described by Connor et al. (32). Primary HIV-1 isolates were obtained from the National Institutes of Health AIDS Reference and Reagent Program, Division of AIDS, and the World Health Organization, with the exception of HIV-1 Ba-L, YU-2, ADA-M, JR-Fl, P-17, and 2067cl 3, which have been described (33). Viral stocks were propagated in phytohemagglutinin (5 μg/ml) and IL-2 (50 units/ml)-stimulated peripheral blood mononuclear cells (PBMC) obtained from healthy donors. For in vitro antiviral assays, the Ba-L (Gladstone) stock was prepared in PBMC from a seed stock of Ba-L passaged exclusively in macrophage cultures that was used in the mouse infection study.
Infection of U87 Astroglioma Cells.
U-87 astroglioma cells expressing human CD4 and CCR5 or CXCR4 were obtained from Dan Littman (New York University, New York). Cells were seeded into 96-well plates (7 × 103/well) and pretreated with compounds or media (DMEM, 10% FCS) at 37°C for 1 h in 100 μl. The medium was aspirated and replaced with 20 μl of fresh medium containing compound. The cells were infected with 5–20 μl of luciferase virus supernatant for 3 h at 37°C, then washed with PBS and incubated in culture medium containing SCH-C for 3 days. Luciferase activity was measured in cell lysates by using the luciferase assay system (Promega). For replication assays, cells were treated with 1 μM SCH-C or AMD3100, then infected with the R5X4 viruses HIV-1 P17 or HIV-1 2073cl.3 as described above. Viral replication was measured on day 4 by using a p24 antigen ELISA (DuPont/NEN).
HIV-1 Replication in PBMC Cultures.
Ficoll-purified PBMC were stimulated in vitro with 5 μg/ml phytohemagglutinin and 50 units/ml IL-2 for 3 days. The cells were resuspended at 4 × 106/ml in complete medium (RPMI, 10% FBS/50 units/ml IL-2), seeded into 96-well plates (2 × 105/well), incubated with inhibitor for 1 h at 37°C, and infected in triplicate with 25–100 tissue culture 50% infective dose (TCID50) per well of an HIV-1 primary isolate for 3–4 h. The cells were washed twice in PBS to remove residual virus and cultured in the presence of inhibitor for 4–6 days. HIV-1 replication was quantitated by measurement of extracellular p24 antigen by ELISA. The IC50 and IC90 values for each virus were determined by using GRAPHPAD PRISM software.
SCID-hu Thy/Liv Mouse Model.
Homozygous C.B-17 scid/scid mice were implanted with human fetal thymus and liver as described (34, 35). Groups of seven mice were dosed twice daily by oral gavage with SCH-C in vehicle (0.4% methylcellulose) at 3, 10, and 30 mg/kg per day, or with ddI at 50 mg/kg per day by twice-daily i.p. injection of 200 μl per dose. Implants were inoculated with 2,000 TCID50 of HIV-1 Ba-L 1 day after the initiation of dosing. Mice were dosed daily until the implants were collected 28 days after inoculation for analysis of cell-associated viral RNA by branched DNA assay and p24 by ELISA (34, 36). Thymocyte subpopulations were analyzed by flow cytometry by resuspending 3 × 106 implant cells in 50 μl of an antibody mixture containing allophycocyanin-conjugated anti-CD4, peridinin chlorophyll protein-conjugated anti-CD8, and FITC-conjugated anti-HLA-ABC antibody, W6/32, or a similar mixture containing isotype control antibodies. The cells were incubated for 30 min, washed with 2% FBS in PBS, fixed with 1 ml of 1% paraformaldehyde, and analyzed on a FACSCalibur instrument (Becton Dickinson). All results are expressed as the mean ± SE for each group. Nonparametric statistical analyses were performed by using the Mann–Whitney u test (STATVIEW 5.0, Abacus Concepts, Berkeley, CA). P values < 0.05 were considered statistically significant.
Pharmacokinetic Analysis.
Groups of three fasted male Sprague–Dawley rats were dosed with [3H]SCH-C at 10 mg/kg by either i.v. injection or oral gavage. For the monkey studies, three fasted male cynomolgus monkeys were dosed at 2 mg/kg. The compound was dissolved in 0.4% methylcellulose for oral dosing and in 20% hydroxypropyl-β-cyclodextrin for i.v. dosing. After compound administration, plasma samples were collected periodically over 24 h. The plasma samples were subjected to protein precipitation, and the supernatant was injected into the HPLC-MS/MS system. Parent compound was quantitated against a standard curve, area under the curve (AUC) was calculated from the concentration vs. time profiles, and the pharmacokinetic parameters were calculated by using the Watson LIMS system. Total radioactivity in the plasma samples was measured by scintillation counting and a concentration equivalent vs. time profile was determined. Absorption was calculated from the relative AUC ratios of the i.v. and oral gavage total radioactivity concentration equivalents vs. time profiles.
Results
Identification of SCH-C.
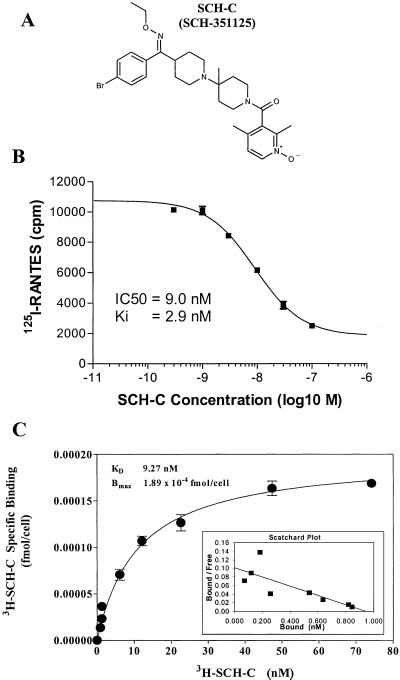
Because the natural ligands for CCR5 include the CC-chemokine RANTES, we used a high-throughput screen to identify inhibitors of RANTES binding to CCR5, on the assumption that such compounds also might be able to block HIV-1 binding to the same receptor. In the screening assay, [125I]RANTES binding to CHO cell membranes expressing CCR5 was measured in the presence and absence of test compounds. Leads from the initial screen were optimized by medicinal chemistry to increase their antiviral potency, receptor specificity, and pharmacokinetic parameters (37). The result of this structure activity relationship study was the synthesis of SCH-C, a small nonpeptidic molecule with potent antiviral activity and high affinity, specific receptor binding. SCH-C is an oxime–piperidine compound with a molecular weight of 557.5 (Fig. 1A). The tartarate salt of this compound is a white, amorphous solid with a solubility of >200 mg/ml.
Figure 1.
Structure and receptor-binding properties of SCH-C. (A) The structure of SCH-C. (B) SCH-C inhibits RANTES binding to CCR5. SCH-C was titrated over the concentration range indicated in the presence of [125I]RANTES, and the binding of RANTES to human CCR5 expressed on CHO-cell membranes was measured (■). (C) SCH-C binds with high affinity to CCR5. [3H]SCH-C was titrated over the concentration range indicated; the amount bound to human CCR5 on B550 cells was measured, and the Bmax and KD values were calculated from the Scatchard plot (Inset). The value of each assay point is an average of triplicates (●).
Receptor-Binding Properties of SCH-C and Determination of Antagonist Activity.
In the standard assay used to screen compounds for RANTES-blocking activity, SCH-C inhibited, in a dose-dependent manner, the binding of [125I]RANTES to the CHO cell membranes expressing human CCR5. The Ki value for SCH-C in this assay is 2.9 nM (Fig. 1B). To determine the maximal binding (Bmax) and KD of SCH-C, a saturation binding analysis was performed by using B550 cells, a Ba/F3 murine pro-B cell line that stably expresses human CCR5. This analysis showed that SCH-C binds with high affinity to CCR5, with a KD of 9.27 nM and a Bmax value of 1.98 × 10−4 fmol/cell (Fig. 1C). A counterscreen was performed with SCH-C against a panel of over 80 different human receptors to determine the compound's specificity for CCR5. The results of these studies revealed that SCH-C did not show significant binding or inhibition of ligand binding to any of the receptors tested. These included the closely related chemokine receptors CCR1, CCR2, CCR3, and CCR7, which were inhibited by 0.0%, 12%, 2.0%, and 0.0%, respectively, by SCH-C assayed at concentrations of 1.8 μM or higher in a ligand-binding assay.
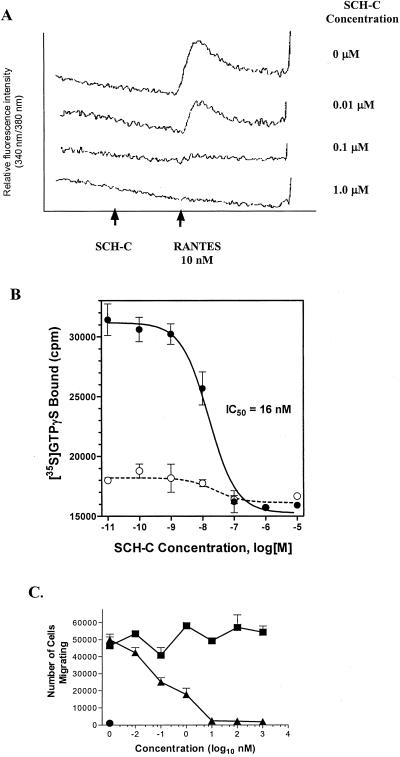
To determine whether SCH-C is a receptor antagonist, several functional assays were used, including measurements of calcium flux, GTPγS binding, and chemotaxis. In the calcium flux assay, B-550 cells were loaded with the calcium-sensitive dye Fura-2AM before addition of the CCR5 ligand, RANTES. Compounds with CCR5 agonist properties induce an intracellular calcium increase, whereas antagonists do not themselves induce signaling but block induction by natural ligands (RANTES, MIP-1α, MIP-1β). The results clearly show that SCH-C is a CCR5 antagonist (Fig. 2A).
Figure 2.
SCH-C is a CCR5 antagonist. (A) SCH-C antagonizes the calcium response to RANTES but does not itself induce a calcium signal. SCH-C at the concentrations indicated was added to B-550 cells loaded with the calcium-sensitive dye FURA-2 before addition of RANTES (10 nM). (B) SCH-C inhibits agonist-induced [35S]GTPγS binding to the CCR5 receptor. SCH-C at the concentrations indicated was added to membrane preparations from CHO cells expressing hCCR5 in the presence (●) or absence (○) of MIP-1β (10 nM), and the amount of membrane-bound [35S]GTPγS was determined. (C) SCH-C inhibits chemotaxis induced by MIP-1β. SCH-C (▴) at the concentrations indicated, or the equivalent concentration of DMSO (■), was added to B550 cells in the presence of MIP-1β (0.01 nM), and the extent of cell migration was determined. Spontaneous migration in the absence of chemokine was also measured (●).
The antagonist properties of SCH-C were further confirmed by using a second membrane-binding assay that measures receptor activation by CCR5 ligands, specifically the binding of [35S]GTPγS to receptor-coupled G proteins that occurs in response to receptor activation by an agonist. In this assay, SCH-C causes no CCR5 activation itself but inhibits the functional response to MIP-1β in a concentration-dependent manner, with an IC50 of ≈16 nM (Fig. 2B). Similarly, SCH-C inhibited RANTES induced GTPγS binding with a similar IC50 concentration (data not shown). Using a functional chemotaxis assay in which B550 cells migrate across a membrane in response to compounds or chemokines (e.g., RANTES, MIP-1β), we found that SCH-C-inhibited MIP-1β induced chemotaxis with an IC50 value of less than 1 nM (Fig. 2C). Furthermore, the compound had no chemoattractant activity itself (data not shown). Collectively, the data from these studies clearly demonstrate that SCH-C is a CCR5 receptor antagonist.
In Vitro Antiviral Activity of SCH-C.
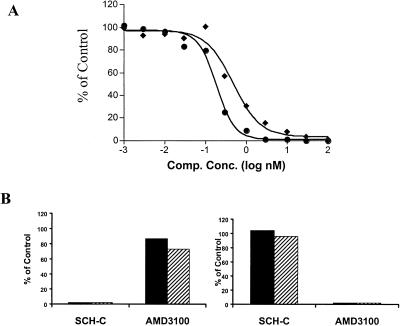
The ability of SCH-C to inhibit HIV-1 entry and replication was determined in vitro using several different assays. In the first of these, the entry of envelope-pseudotyped HIV-1 into U87 astroglioma cells expressing CD4 and CCR5 (U87-CD4-CCR5) was measured in a single-cycle infection assay with a luciferase reporter-gene readout. SCH-C inhibits the entry of two different HIV-1 R5 envelope-pseudotyped viruses (HIV-1ADA and HIV-1YU-2), with an average IC50 value of 0.69 nM (Fig. 3A).
Figure 3.
SCH-C inhibits HIV-1 entry and replication in vitro. (A) SCH-C inhibits the entry of Env-pseudotyped HIV-1 into U87-CD4-CCR5 cells. SCH-C at the concentrations indicated was added to the cells immediately before infection with HIV-1 pseudotypes containing the envelope glycoproteins of the ADA (⧫) or YU-2 (●) isolates. The extent of infection was determined by measuring luciferase expression 3 days after infection and is plotted as a percentage of that occurring in the absence of inhibitor. (B) SCH-C has no effect on HIV-1 entry via CXCR4. U87-CD4-CCR5 cells (Left) or U87-CD4-CXCR4 cells (Right) were infected with a dual-tropic primary virus, HIV-1 P17 (solid bars) or HIV-1 2073cl.3 (hatched bars), in the presence or absence of SCH-C (1 μM) or AMD3100 (1 μM) as indicated. The extent of infection was determined by measuring p24 antigen production and is plotted as a percentage of that occurring in the absence of inhibitor.
The specificity of SCH-C for the CCR5 coreceptor was then tested by using U87-CD4-CCR5 cells or U87-CD4 cells expressing CXCR4 (U87-CD4-CXCR4). The cells were treated with 1 μM SCH-C or the CXCR4-specific inhibitor, AMD3100, and then infected with the dual-tropic (R5X4) primary isolates, HIV-1 P17 or HIV-1 2073cl.3. SCH-C efficiently inhibited the replication of both viruses in the cells expressing CCR5 but had no effect on viral replication in the CXCR4-expressing cells (Fig. 3B). The converse was observed with AMD3100. Thus, the antiviral activity of SCH-C requires cell-surface CCR5 expression, as expected.
The breadth and potency of SCH-C was also assessed in replication assays using PBMC cultures and a panel containing 21 genetically diverse, primary, R5 HIV-1 isolates. The average IC50 and IC90 values derived from multiple experiments using cells from at least two different donors are summarized in Table 1. SCH-C inhibited the replication of most isolates with geometric mean IC50 values ranging from 0.40 to 8.9 nM and IC90 values from 3 to 78 nM. This potency range is similar to that observed with other antiretroviral agents (e.g., AZT, indinavir) in this assay system (data not shown).
Table 1.
SCH-C inhibits infection of PBMC by genetically diverse, primary, R5 HIV-1 isolates
| Virus | Clade/origin | # expts.* | IC50 (nM)†
|
IC90 (nM)
|
||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||
| ASJM 108 | B/USA | n = 5 | 0.76 | 0.40–1.6 | 9.2 | 6.0–17 |
| ASM 80 | B/USA | n = 6 | 0.40 | 0.17–0.95 | 3.0 | 0.76–13 |
| ASM 57 | B/USA | n = 10 | 7.8 | 3.5–18 | 72 | 38–170 |
| 301657 | B/USA | n = 8 | 1.9 | 0.78–5.1 | 25 | 9.8–64 |
| 302056 | B/USA | n = 6 | 0.61 | 0.29–1.4 | 6.1 | 3.0–14 |
| 92US715 | B/USA | n = 6 | 0.41 | 0.1–1.7 | 8.5 | 3.9–18 |
| US91005 | B/USA | n = 6 | 0.71 | 0.22–2.3 | 8.3 | 3.9–18 |
| Ba-L | B/USA | n = 6 | 1.7 | 0.51–5.9 | 19 | 5.8–52 |
| YU-2 | B/USA | n = 7 | 0.46 | 0.14–1.5 | 9.3 | 2.5–35 |
| QZ4589 | B/Trinidad | n = 10 | 2.1 | 1.0–4.4 | 37 | 13–98 |
| ADA-M | B/USA | n = 10 | 3.2 | 1.6–6.6 | 44 | 22–91 |
| JR-FL | B/USA | n = 17 | 1.2 | 0.58–2.5 | 14 | 6.3–29 |
| Ba-L (Gladstone) | B/USA | n = 7 | 8.9 | 3.2–25 | 70 | 25–204 |
| JV1083 | G/Nigeria | n = 11 | 7.1 | 7.7–20 | 78 | 36–170 |
| G3 | G/Nigeria | n = 4 | 3.9 | 0.98–15 | 61 | 10–360 |
| RU570 | G/Russia | n = 3 | 200 | 150–250 | >1000 | ND |
| 93MW959 | C/Malawi | n = 2 | 0.64 | 0.46–0.91 | 7.8 | 4.7–13 |
| 93MW960 | C/Malawi | n = 2 | 0.68 | 0.65–0.74 | 15 | 4.2–58 |
| 92UG035 | A/D/Uganda | n = 2 | 1.4 | 0.43–4.3 | 14 | 6.9–30 |
| 93UG082 | D/Uganda | n = 3 | 0.71 | 0.1–3.2 | 8.2 | 0.8–79 |
| BCF01 | 0/Cameroon | n = 3 | 1.2 | 0.1–30 | 16 | 0.5–490 |
| Overall average | 1.8 | 1.3–2.4 | 20 | 15–26 | ||
n = number of experiments for which IC values determined.
Geometric mean IC values (nM) calculated for multiple experiments; range values represent 95% confidence intervals based on SEM for all isolates except RU570, for which an IC90 value was not determined.
Only one of the 21 test isolates (RU570; subtype G) was resistant to SCH-C; its IC50 value was ≈200 nM, and its IC90 value was >1,000 nM. The relative resistance of this isolate to SCH-C may be due to its use of an alternate coreceptor, such as CCR3, or alternatively to a naturally occurring variation in its envelope glycoprotein that allows it to interact with CCR5 despite the presence of SCH-C.
Antiviral Activity of SCH-C in SCID-hu Thy/Liv Mice.
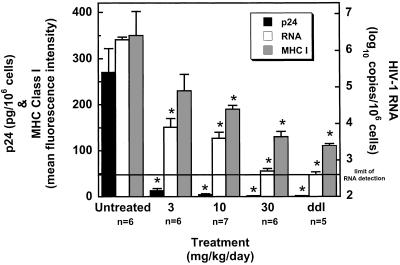
The in vivo antiviral potency of SCH-C was investigated in the SCID-hu Thy/Liv mouse model of HIV-1 infection (34, 35). Severe combined immunodeficient (SCID) mice were implanted under the left kidney capsule with human fetal thymus and liver from a single donor 20 weeks before inoculation with HIV-1. SCH-C was administered by twice daily oral gavage at 3, 10, and 30 mg/kg per day, beginning 1 day before direct inoculation of each implant with 2,000 TCID50 of the R5 isolate HIV-1 Ba-L. Another group of mice received 50 mg/kg per day of ddI by twice-daily i.p. administration, also beginning 1 day before virus inoculation. Mice were dosed twice daily until the implants were collected 28 days after inoculation for determination of cell-associated p24, viral RNA, and the level of MHC class I expression on immature (CD4+ CD8+) thymocytes.
SCH-C had potent and dose-dependent antiviral activity in this SCID-hu model (Fig. 4). No p24 antigen was detected in the implants of the seven mice treated with 30 mg/kg per day of SCH-C, and viral RNA was reduced by 3.6 log10 compared with untreated mice. Because HIV infection can up-regulate MHC class I expression on immature thymocytes in this model system (38), we measured HLA-ABC levels with the mAb W6/32 on implant cells from treated and untreated mice by FACS analysis. As shown, MHC class I expression on implant cells from SCH-C-treated mice was reduced in a dose-dependent manner compared with cells from untreated mice, which confirms further the inhibition of viral replication by SCH-C.
Figure 4.
SCH-C inhibits HIV-1 replication in vivo. SCID-hu Thy/Liv mice were treated by twice-daily oral gavage with SCH-C at the indicated doses or i.p. with ddI at 50 mg/kg per day. Mice were treated 1 day before inoculation of Thy/Liv implants with 2,000 TCID50 of HIV-1 Ba-L by direct injection, and dosing was continued until implant collection 28 days after inoculation. Antiviral efficacy was assessed by determining cell-associated p24, viral RNA, and MHC class I expression on immature CD4+ CD8+ thymocytes. Asterisks indicate P < 0.050 compared with untreated mice by the Mann–Whitney u test.
Pharmacokinetic Studies in Rats and Monkeys.
In exploratory pharmacokinetic studies, an amorphous tartrate salt form of [3H]SCH-C, formulated in 20% hydroxypropyl-β-cyclodextrin for i.v. studies and 0.4% methylcellulose for oral studies, was administered to fasted male Sprague–Dawley rats (10 mg/kg) and cynomolgus monkeys (2 mg/kg). Plasma samples were collected periodically up to 24 h after administration for quantitation of the parent compound by a liquid chromatography and mass spectrometry assay. The profile of total radioactivity across the time course was also determined and is expressed as the AUC. In these studies, SCH-C was found to be well absorbed and highly bioavailable in both species based on AUC determinations (Table 2). The maximal plasma concentrations (Cmax) of SCH-C after oral administration were 2.5 μM in the rat and 0.76 μM in the monkey. The compound half-life (t1/2) and clearance rate (CL) after i.v. administration were 5.4 h and 12.8 ml/min per kg for the rat and 6.0 h and 4.28 ml/min per kg for the monkey (Table 2). More important, plasma levels exceeding the mean in vitro IC90 inhibitory concentrations can be obtained and sustained at least 12–24 h after oral administration. In addition, metabolic studies demonstrated that SCH-C did not show significant inhibition or induction of the major P450-metabolizing enzymes (data not shown).
Table 2.
Pharmacokinetic parameters of SCH-C in rats and monkeys
| Pharmacokinetic parameters | Rat 10 mg/kg IV/PO | Monkey 2 mg/kg IV/PO |
|---|---|---|
| Absorption (%) | 57 | 80 |
| Bioavailability (%) | 63 | 52 |
| IV Half-life (h) | 5.4 | 6.0 |
| IV CL (ml/min/kg) | 12.8 | 4.28 |
| Vd (ss) (liter/kg) | 5.09 | 1.89 |
| Oral Cmax (μM) | 2.5 | 0.76 |
| Tmax (h) | 0.5 | 2.0 |
| Oral AUC(0–24 h) (h⋅μM) | 15 | 6.9 |
Discussion
The compound we have described here, SCH-C, is a potent and specific small molecule inhibitor of HIV-1 infection, both in vitro and, in a murine model system, in vivo. SCH-C is clearly a CCR5 receptor antagonist, a feature that is important for its planned use in humans. SCH-C also has favorable pharmacokinetic profiles in animals, including good oral bioavailability and absorption. As such, SCH-C has properties that are entirely suitable for its advancement into the clinical stages of a drug-development program.
Most primary, R5 HIV-1 isolates tested in this study were sensitive to SCH-C, with IC50 values typically in the low nM range. This potency is comparable with or greater than that of TAK-779, a small molecule inhibitor of CCR5 that has previously been described (29). However, unlike TAK-779, SCH-C is orally bioavailable and has no cross-reactivity with CCR2 or other chemokine receptors.
SCH-C was a potent inhibitor of a broad panel of diverse HIV-1 isolates from different genetic subtypes and geographic regions with the exception of one virus, the Russian subtype G strain, RU570, that was insensitive to this compound. This insensitivity may reflect an unusual coreceptor usage profile of this virus or an atypical interaction between its envelope glycoproteins and CCR5 that causes the binding event to be unaffected by the inhibitor. Further studies will be required to determine the basis of resistance of this isolate to SCH-C.
The in vitro potency of SCH-C against HIV-1 replication was an encouraging finding, but a further validation of its antiviral activity required use of an animal infection model. SCH-C is specific for human CCR5 and does not cross-react with high affinity with the macaque CCR5 receptor (M. Endres, personal communication), so it was not feasible to evaluate its in vivo efficacy in a simian immunodeficiency virus or chimeric simian human virus (SHIV) monkey infection model. We therefore used the SCID-hu Thy/Liv mouse model because this has previously proven to be useful for the evaluation of experimental new therapies (35, 36). In this model system, SCH-C had robust antiviral activity against a pathogenic R5 virus, HIV-1 Ba-L.
Finally, pharmacokinetic evaluation of SCH-C demonstrated that this compound is orally bioavailable and well absorbed in both monkeys and rats. Based on the animal pharmacokinetic data, a dosing paradigm model predicts that this compound may be effectively dosed in a once- or twice-daily regimen. Preliminary data from our laboratories and others (M. Hirsch, personal communication) indicate that SCH-C acts synergistically with other antiretroviral agents in vitro, suggesting that inhibitors of viral entry could be used in conjunction with existing therapies to suppress HIV-1 replication.
In summary, SCH-C is an orally bioavailable small molecule antagonist of the CCR5 coreceptor with in vivo activity against HIV-1. We believe from these preclinical studies that small molecule antagonists of chemokine receptors can be developed as a new class of potent antiretroviral drugs.
Acknowledgments
We are grateful to Michael Murray, Elizabeth Smith, Stuart Cox, Robert Bryant, Robert Watkins, Alan Bass, and Elizabeth Maxwell for their intellectual and technical contributions to this work. We also thank Xiping Zhang and Lisa Broske for their assistance in the pharmacokinetic evaluation of SCH-C and Waldemar Gonsiorek for his assistance with the statistical analysis of the saturation binding data. The Department of Microbiology and Immunology at the Weill Medical College gratefully acknowledges the support of the William Randolph Hearst Foundation. Research conducted in J.P.M.'s lab was funded by National Institutes of Health Grant RO1 AI41420. J.P.M. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation, a Stavros S. Niarchos Scholar, and a consultant to Schering Plough Research Institute.
Abbreviations
- PBMC
peripheral blood mononuclear cells
- TCID50
tissue culture 50% infective dose
- CHO
Chinese hamster ovary
- GTPγS
guanosine 5′-[γ-thio]triphosphate
- AUC
area under the curve
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Palella F J, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D The HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Lucas G M, Chiasson R E, Moore R D. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 3.Yerly S, Kaiser L, Race E, Bru J P, Clavel F, Perrin L. Lancet. 1999;354:729–733. doi: 10.1016/S0140-6736(98)12262-6. [DOI] [PubMed] [Google Scholar]
- 4.Furtado M R, Callaway D S, Phair J P, Kunstman K J, Stanton J L, Macken C A, Perelson A S, Wolinsky S M. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 5.Michael N L, Moore J P. Nat Med. 1999;5:740–742. doi: 10.1038/10462. [DOI] [PubMed] [Google Scholar]
- 6.Moore J P, Stevenson M. Nat Rev Mol Cell Biol. 2000;1:40–49. doi: 10.1038/35036060. [DOI] [PubMed] [Google Scholar]
- 7.Doms R W, Moore J P. J Cell Biol. 2000;151:F9–F14. doi: 10.1083/jcb.151.2.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C H, Matthews T J, McDanal C B, Bolognesi D P, Greenberg M L. J Virol. 1995;69:3771–3777. doi: 10.1128/jvi.69.6.3771-3777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilby J M, Hopkins S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldredge L, Hunter E, Lambert D, Bolognesi D, et al. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 10.Pilcher C D, Eron J J, Jr, Ngo L, Dusek A, Sista P, Gleavy J, Brooks D, Venetta T, DiMassimo E, Hopkins S. AIDS. 1999;13:2171–2173. doi: 10.1097/00002030-199910220-00024. [DOI] [PubMed] [Google Scholar]
- 11.De Clercq E. Drugs. 1999;2:321–331. doi: 10.2165/00126839-199902050-00010. [DOI] [PubMed] [Google Scholar]
- 12.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 13.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 14.Deng H K, Liu R, Ellmeier W, Choe H, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 16.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1159. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M, E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–378. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 18.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. Nature (London) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 19.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien S J, Moore J P. Immunol Rev. 2000;177:99–111. doi: 10.1034/j.1600-065x.2000.17710.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Kurihara T, Ryseck R P, Yang Y, Ryan C, Loy J, Warr G, Bravo R. J Immunol. 1998;160:4018–4029. [PubMed] [Google Scholar]
- 22.Sato N, Kuziel W A, Melby P C, Reddick R L, Kostecki V, Zhao W, Maeda N, Ahuja S K, Ahuja S S. J Immunol. 1999;163:5519–5525. [PubMed] [Google Scholar]
- 23.Huffnagle G B, McNeil L K, McDonald R A, Murphy J W, Toews G B, Maeda N, Kuziel W A. J Immunol. 1999;163:4642–4646. [PubMed] [Google Scholar]
- 24.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 26.Auraro S, Menten P, Struyf S, Proost P, Van Damme J, De Clercq E, Schols D. J Virol. 2001;75:4402–4406. doi: 10.1128/JVI.75.9.4402-4406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, et al. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson W C, Rabut G E, Nagashima K A, Tran D N, Anselma D J, Monard S P, Segal J P, Thompsoon D A, Kajumo F, Guo Y, et al. J Virol. 1999;73:4145–4155. doi: 10.1128/jvi.73.5.4145-4155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Olonogi K, Ogawa Y, et al. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dragic T, Trkola A, Thompson D A D, Cormier E G, Kajumo F A, Maxwell E, Lin S W, Ying W, Smith S O, Sakmar T P, et al. Proc Natl Acad Sci USA. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. . (First Published April 25, 2000; 10.1073/pnas.090576697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fawzi A B, Zhang H, Weig B, Hawes B, Graziano M P. Eur J Pharmacol. 1997;336:233–242. doi: 10.1016/s0014-2999(97)01227-2. [DOI] [PubMed] [Google Scholar]
- 32.Connor R I, Sheridan K E, Lai C, Zhang L, Ho D D. J Virol. 1996;70:5206–5311. doi: 10.1128/jvi.70.8.5306-5311.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trkola A, Ketas T, KewalRamani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCune J M. Semin Immunol. 1996;8:187–196. doi: 10.1006/smim.1996.0024. [DOI] [PubMed] [Google Scholar]
- 35.Rabin L. Antimicrob Agents Chemother. 1996;40:755–762. doi: 10.1128/aac.40.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoddart C A, Moreno M E, Lindquist-Stepps V D, Bare C, Bogan M R, Gobbi A, Buckheit R W, Jr, Bedard J, Rando R F, McCune J M. Antimicrob Agents Chemother. 2000;44:783–786. doi: 10.1128/aac.44.3.783-786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palani, A., Shapiro, S., Clader, J. W., Greenlee, W. J., Cox, K., Strizki, J., Endres, M. & Baroudy, B. M. (August 31, 2001) J. Med. Chem., 10.1021/jm015526o. [DOI] [PubMed]
- 38.Kovalev G, Duus K, Wang L, Lee R, Bonyhadi M, Ho D, McCune J M, Kaneshima H, Su L. J Immunol. 1999;162:7555–7562. [PMC free article] [PubMed] [Google Scholar]