Abstract
Purpose of review
Assessment of corneal biomechanics has been an unmet clinical need in ophthalmology for many years. Many researchers and clinicians have identified corneal biomechanics as source of variability in refractive procedures and one of the main factors in keratoconus. However, it has been difficult to accurately characterize corneal biomechanics in patients. The recent development of Brillouin light scattering microscopy heightens the promise of bringing biomechanics into the clinic. The aim of this review is to overview the progress and discuss prospective applications of this new technology.
Recent findings
Brillouin microscopy uses a low-power near-infrared laser beam to determine longitudinal modulus or mechanical compressibility of tissue by analyzing the return signal spectrum. Human clinical studies have demonstrated significant difference in the elastic properties of normal corneas versus corneas diagnosed with mild and severe keratoconus. Clinical data have also shown biomechanical changes after corneal cross-linking treatment of keratoconus patients. Brillouin measurements of the crystalline lens and sclera have also been demonstrated.
Summary
Brillouin microscopy is a promising technology under commercial development at present. The technique enables physicians to characterize the biomechanical properties of ocular tissues.
Keywords: biomechanics, Brillouin spectroscopy, cornea, keratoconus, refractive surgery
INTRODUCTION
Multiple techniques have emerged in recent years for measuring corneal biomechanics. The most common approach uses an air puff and analyzes corneal deformability against the external pressure using tonometry, topography, or optical coherence tomography (OCT). While providing clinically useful information, the output of these devices is influenced by intraocular pressure (IOP) and geometric properties of the cornea. Extracting corneal biomechanical data from such measurements relies on multiple assumptions and modeling. Another drawback of these techniques is that they do not provide spatial aspect of tissue strength as it may vary across stromal thickness, from center to periphery, and at the focal point of disease.
The study discusses a novel optical technique – Brillouin microscopy – that measures spectral shift of a probing laser beam as it scatters from a localized volume of tissue. Brillouin spectral shift has direct relationship to longitudinal modulus or mechanical compressibility of tissue. Using confocal imaging it can produce a full volumetric map of the elastic properties. We discuss latest clinical evaluation of the technique in human studies conducted under Institutional Review Board-approved protocols.
Box 1.
no caption available
BACKGROUND
As corneal refractive surgery had become widespread around the world, researchers and clinicians became more interested in biomechanics of the stroma [1]. Even with very precise modern-day excimer lasers, variability of tissue strength has been implicated in suboptimal outcomes of laser-assisted in situ keratomileusis (LASIK) and other procedures [2]. Significant differences in corneal strength have been noted between normal eyes, eyes after refractive surgery, and also eyes with keratoconus [3].
Today there are two instruments that provide some insight into corneal biomechanics: the ocular response analyzer (ORA) from Reichert and the Corvis ST from Oculus. Both are based on measurement of dynamic corneal response to a puff of air. The ORA device provides a corneal hysteresis measurement, which has been shown to correlate with known corneal pathologies such as keratoconus [4,5]. Although useful in some applications [6], corneal hysteresis values can be produced by various combinations of corneal thickness, rigidity, intraocular pressure, and hydration, and therefore do not truly represent corneal biomechanical properties. More recently available Corvis ST device provides more detailed information about corneal response by using high-speed Scheimpflug imaging. It provides a so called Corneal Biomechanical Index (CBI) which includes corneal thickness information. CBI has been shown to improve keratoconus detectability [7], and in combination with tomographic data in corneal ectasia detection [8▪]. Ongoing research may discover additional parameters [9]. Both ORA and Corvis ST devices have shown clinical usefulness, but do not provide direct measurement of corneal elasticity and do not allow three-dimensional mapping of these properties of stroma. The mapping of localized corneal properties has important clinical benefits. First, it allows localization of corneal weakening, such as in case of early keratoconus, which produces focal region of increased elasticity [10]. Second, it allows depth-dependent mapping of corneal strength important in refractive surgery [11] and monitoring effectiveness of corneal cross-linking treatments [12,13].
Some ex-vivo methods have been used to address this gap, but in-vivo imaging has been difficult. Ultrasound surface wave elastometry has been used for measurements in donor corneas [14], and recently in vivo[15]. OCT-based elastography has been attempted to measure variation in biomechanical strength between middle and posterior stroma [16]. Although promising, the methods require a complex finite element model of the cornea to deduce actual biomechanical moduli from available data. Other OCT elastography techniques that measure acoustic surface waves are under development in preclinical stages [17,18].
BRILLOUIN TECHNIQUE
The principle of Brillouin microscopy is illustrated in Fig. 1. Brillouin light scattering occurs by the interaction of light and intrinsic acoustic waves in tissues. The acoustic waves are naturally present in tissue. They originate from thermal fluctuations of molecules, which generate pressure fluctuations that propagate at the speed of sound (∼1620 m/s in corneal stroma). The acoustic waves modulate the refractive index periodically, and when light is reflected from this modulation, a Doppler frequency shift occurs. The frequency shift is proportional to the speed of sound (Brillouin frequency = 2 × acoustic speed/wavelength of light in the tissue). The acoustic speed is proportional to the square of longitudinal modulus. Longitudinal modulus is defined as the magnitude of hydrostatic pressure required to cause a fractional volume change and is approximately the inverse of the compressibility. Brillouin microscopy uses a low-power, focused laser beam and a high-resolution confocal spectrometer to measure the Brillouin frequency at the focus [19]. By scanning the beam, the spatial variation of longitudinal modulus of tissue is mapped.
FIGURE 1.
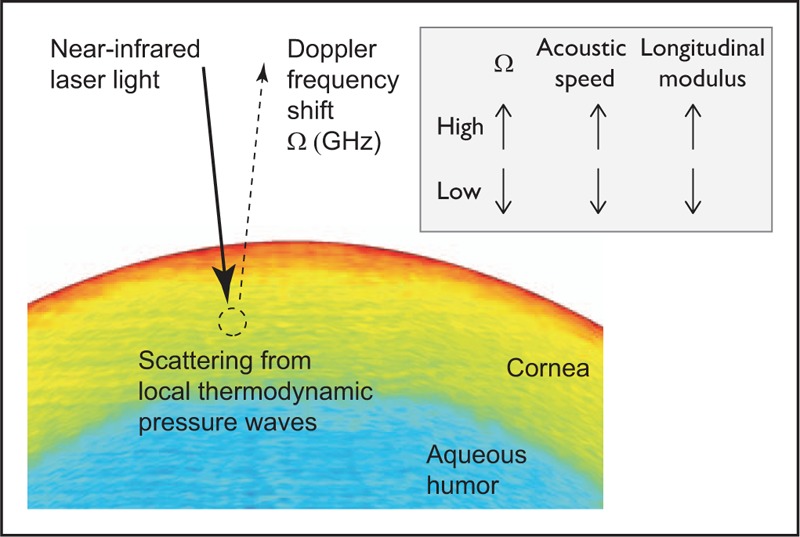
The principle of Brillouin microscopy. A low-power, narrowband NIR laser light is focused into the corneal tissue, and the Doppler Brillouin frequency shift of scattered light from the focus is analyzed by a confocal spectrometer. Spontaneous Brillouin scattering originates from thermodynamically induced pressure (acoustic) waves. The magnitude of Brillouin frequency is proportional to the acoustic propagation speed of tissue at the focus and provides a direct measurement of local longitudinal modulus of the tissue. NIR, near-infrared.
A compelling advantage of Brillouin microscopy is that it provides a direct readout of the local tissue property [20▪▪]. In the sense that the primary output of Brillouin microscopy is the acoustic speed information, this technique is analogous to high-frequency ultrasound microscopy [21]. However, as an all-optical technique, Brillouin microscopy does not require any physical contact, deposits no acoustic energy in the tissue, and provides microscopic three-dimensional resolution [22].
It should be noted that longitudinal modulus does not directly correlate with the elasticity or stiffness a physician can feel by touching the tissue. The latter mechanical property is better described in terms of shear (or Young's) modulus [23]. From the mechanical perspective, shear and longitudinal moduli are two independent elastic properties and have vastly different magnitudes in soft tissues. However, it is expected that during normal physiological changes or pathologic processes in vivo, these two moduli are more likely to change in the same direction. For example, the thickening (thinning) of collagen fibrils in cornea stroma would increase (decrease) both moduli. Nonetheless, caution is needed when relating longitudinal and shear moduli and interpreting Brillouin outputs.
CLINICAL APPLICATIONS
Ocular biomechanics plays significant role in diagnostic and treatment of eye diseases [24]. We overview the recent clinical results obtained with Brillouin microscopy and discuss several specific applications based on the biomechanical characterizations of the cornea, crystalline lens, and the sclera.
Baseline data of normal population
Currently available Brillouin microscopes use near-infrared (NIR) laser light at a wavelength of 780 nm [25▪▪]. At this wavelength, the Brillouin frequencies measured in normal human corneas fall in a tight range between 5.69 and 5.76 GHz, which correspond to longitudinal modulus of 2.74–2.81 GPa (Fig. 2a).
FIGURE 2.
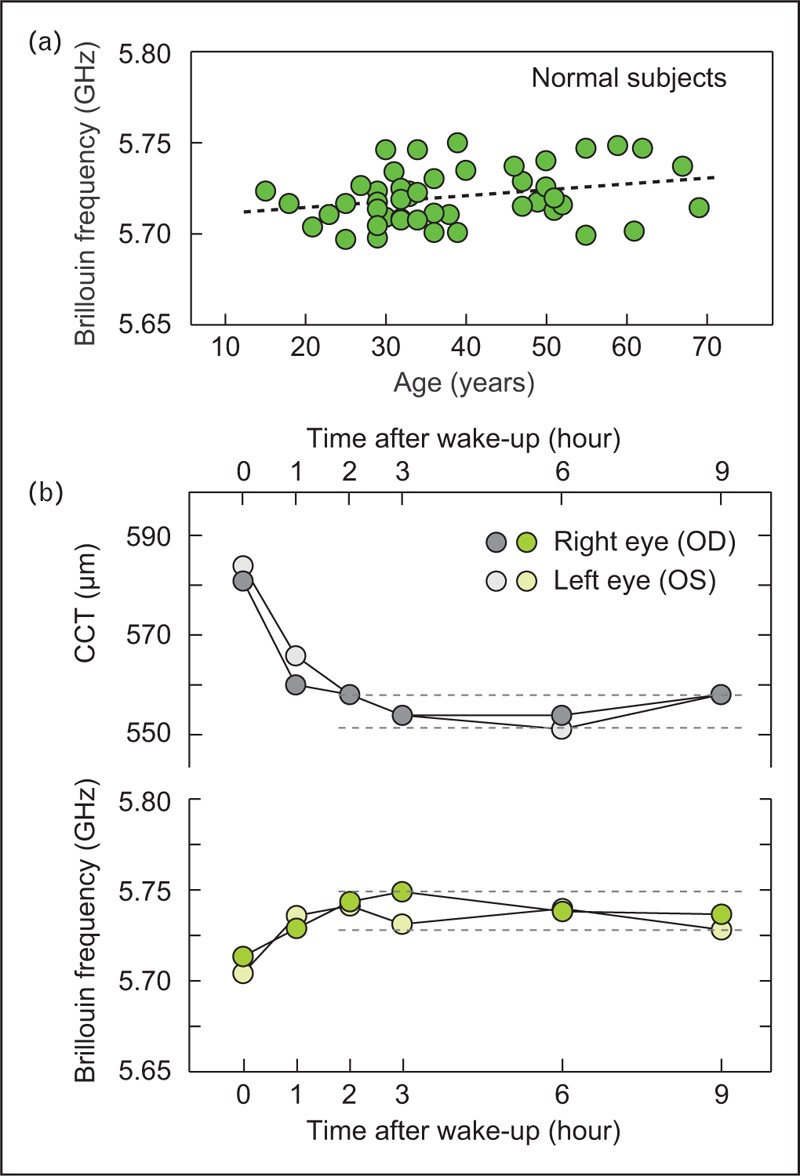
Brillouin measurements of normal population. (a) Central cornea Brillouin frequency values of normal individuals in an age range of 15–70. (b) Diurnal change of a healthy volunteer. Within 1 h after wake-up, the hydration level of corneal stroma is stabilized, so are the central corneal thickness (CCT) and Brillouin frequency. The magnitude of frequency variation during day time is less than the instrument's sensitivity of ±10 MHz.
Because the acoustic speed varies with water content in tissues [26], Brillouin frequency is sensitive to corneal hydration. This has been recently studied by measuring volunteers during daytime [27▪]. The time-lapse data showed the effects of hydration change in both corneal thickness and Brillouin frequency in the first 1 h after wake-up, but Brillouin frequency is stabilized after the transient period within instrument's sensitivity (Fig. 2b). The sensitivity of Brillouin frequency to hydration, not only the compressibility of collagen fibrils and extracellular matrix, must be considered in interpreting the underlying structural and molecular changes of tissue from the Brillouin frequency.
Diagnosis and screening for corneal diseases
Due to focal nature of keratoconus, identifying local variations in elastic modulus may allow earlier detection of the disease before it manifests itself morphologically. Early clinical data show statistically significant differences between Brillouin measurements in the cone region versus in other corneal loci [28]. Distinct biomechanical features that differentiate keratoconus from normal corneas have been identified, and they may serve as diagnostic metrics for keratoconus detection in clinical practice [25▪▪]. There are three clinical findings. First, the average Brillouin frequency of keratoconus eyes is significantly lower when compared to the normal population, and the difference increases with the severity of the disease; second, the reduction of Brillouin frequency is most pronounced in the cone region, whereas the surrounding corneal tissue have normal Brillouin frequencies (Fig. 3a); and third, mild keratoconus is most clearly distinguished by comparing the difference of Brillouin frequency between the left and right eyes (Fig. 3b). These results suggest that the biomechanical tissue properties are altered in keratoconic cones, and that the biomechanical bilateral asymmetry may serve as a diagnostic metric for detecting early-stage keratoconus and possibly identifying progressive keratoconus for early interventions. This expectation is supported by clinical experience that in many keratoconus patients, the severity of the disease is asymmetric [29].
FIGURE 3.
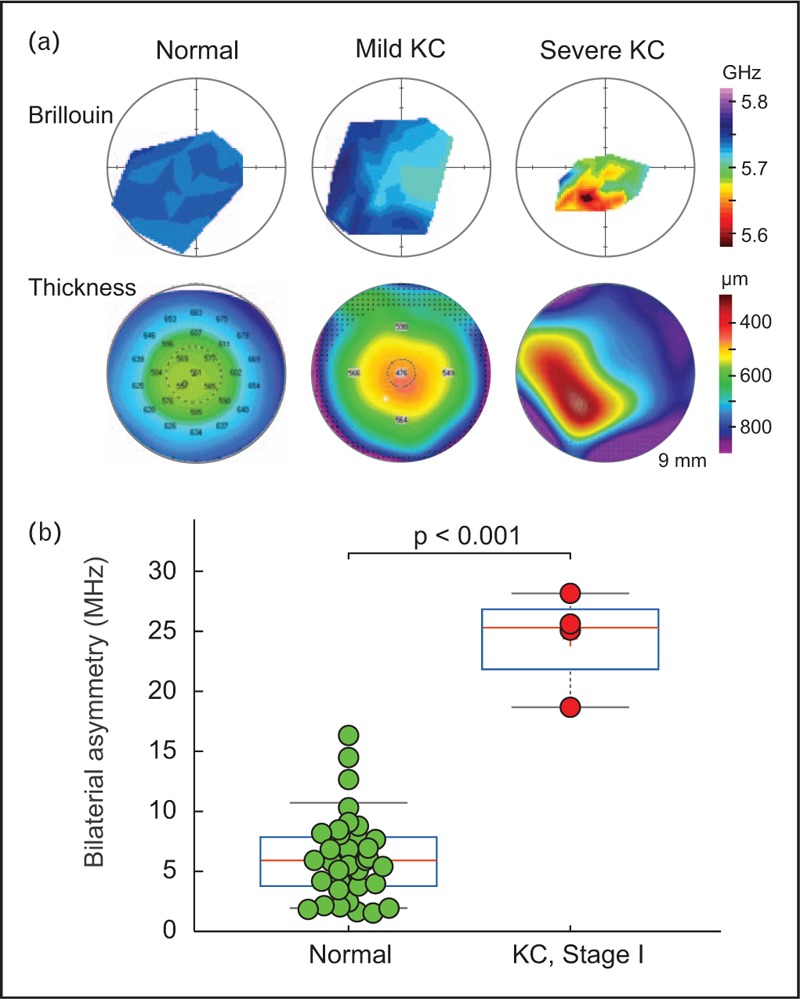
Brillouin measurement of keratoconus patients. (a) Brillouin maps and corresponding pachymetry maps by Pentacam (Oculus Gmbh) of patients diagnosed with mild KC (stage 1; middle) and severe KC (right), in comparison to a normal subject (left). (b) The difference in Brillouin frequency between left and right eyes. The bilateral asymmetry is significantly higher in early-stage keratoconus.
Fuchs’ dystrophy is associated the loss of endothelial cell function in water transport. The corneal thickness recovery in response to induced hydration control has been suggested as a test of endothelial function [30,31]. Brillouin microscopy may be used to measure abnormal hydration changes in patients with Fuchs’ dystrophy and help monitor the progression of the disease.
Refractive procedure evaluation
Corneal screening has been a standard procedure for evaluating the eligibility of candidates for refractive surgery. Having detailed biomechanical information about the stroma may improve this screening process to decrease the risk of surgical complications. Postrefractive surgery measurements may also provide useful information about each procedure and compare residual biomechanical stability of the eye between different surgical options, such as LASIK, photorefractive keratectomy (PRK), or small incision lenticule extraction procedure (SMILE) [32]. Such comparison has been limited to theoretical models [33] or nonposition-specific evaluations [34]. Having noninvasive, spatially-resolved biomechanical mapping can improve current evaluation methods.
Personalized treatment planning potential
Several theoretical models using finite element corneal mesh have predicted differential responses of ‘weak’ and ‘strong’ corneas to the same refractive procedure [35,36]. Peripheral disruption of collagen fibers produces an in-axis flattening effect responsible for the refractive effects of astigmatic keratotomy [37]. Ablation of tissue with LASIK or PRK procedure alters the corneal shape beyond the actual material that is removed from the stroma [38]. To properly account for differential response of the cornea to the refractive procedure, models must be loaded not just with geometric and morphological stromal properties, but also with its local elastic properties [39–41].
By measuring longitudinal modulus and hydrostatic tissue properties, Brillouin microscopy may enable clinicians to create patient-specific nomograms for refractive procedures reducing outcome variability and refractive outliers that may require secondary procedures (re-treatments). It has been shown that corneal hydration affects the excimer laser ablation rate in LASIK surgeries [42,43]. Biomechanical differences are thought to explain the variability in refractive outcomes following cataract and astigmatic keratotomy surgeries [44]. It may be possible to individually tailor ablation parameters or astigmatic keratotomy nomograms based on Brillouin measurements made prior to treatment.
Given the vastly different magnitudes and lack of a priori correlation between longitudinal and shear moduli, however, it is uncertain whether and how Brillouin data can be integrated into finite element modeling. Nevertheless, research effort is warranted to incorporate Brillouin data into patient-specific simulations of various refractive procedures to increase confidence in outcomes for both the patient and the surgeon.
Monitoring effectiveness of corneal cross-linking procedures
Corneal cross-linking (CXL) is one of the effective options to treat keratoconus and post-LASIK ectasia [45] by reinforcing the collagen and proteoglycans network in the corneal stroma. After ex-vivo studies [46,47▪,48], clinical Brillouin measurements of patients were conducted to evaluate biomechanical changes of corneal properties before and after CXL. The first clinical study on CXL patients has begun in collaboration with Dr Theo Seiler at IROC, Zurich, Switzerland, using a rack-mounted Brillouin system. Prior to the procedure, significant differences between keratoconus patients and controls were observed in the mean measurements of the Brillouin longitudinal modulus. After the procedure, the longitudinal moduli of the cross-linked corneas returned to the normal range [25▪▪]. Furthermore, measurement of a cohort of keratoconus patients who had received CXL at IROC in the past 4 years revealed a trend of increasing Brillouin frequencies after the treatment [49].
Measuring postoperative treatment penetration depth is difficult and imprecise with existing methods such as slit-lamp exam [12] or OCT [50]. Brillouin spectroscopy has the potential to more accurately demarcate treated zones. Having detailed preoperative assessment of corneal strength in keratoconus patient may also lead to improved treatment designs that are customized for a given individual [51,52] as opposed to a generic cross-linking protocol.
Characterization of the crystalline lens
In addition to corneal imaging, Brillouin microscopy can be used to evaluate the crystalline lens in vivo. Early studies have demonstrated feasibility of the approach in animal models [53,54]. Brillouin data showed remarkable correlation to Young's modulus obtained by other conventional mechanical techniques. Recently, human measurements have shown positional changes in Brillouin elastic modulus that are consistent with known lens anatomy (nucleus and cortex) as well as age-related changes in the central region of the lens associated with growth [55]. Figure 4 shows typical axial Brillouin lens profiles for two subjects. Such data can prove useful in characterizing the crystalline lens for age-related presbyopia and optimization of surgical or pharmacological procedures to reduce its symptoms.
FIGURE 4.
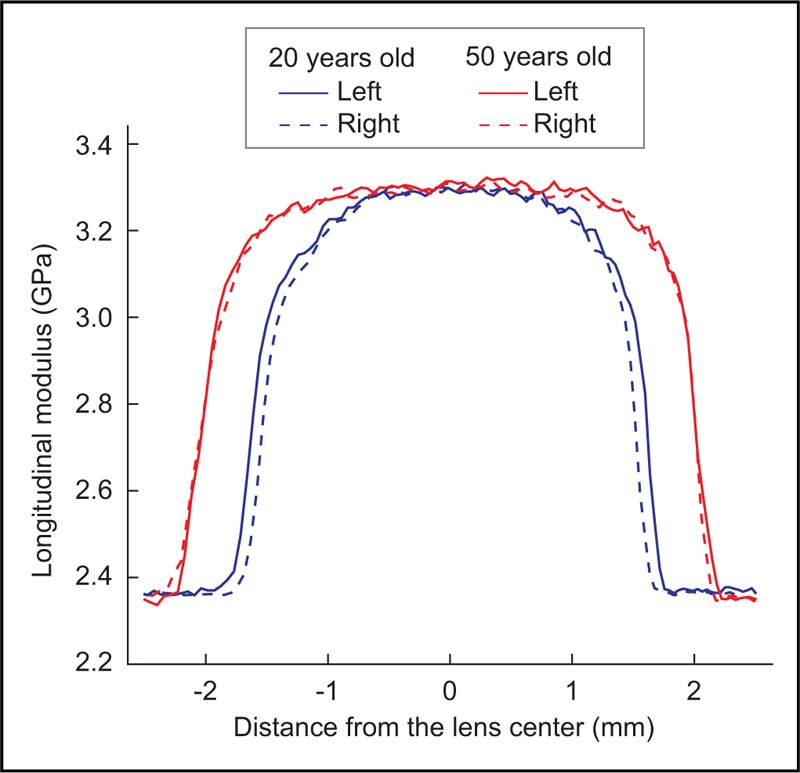
Brillouin axial profiles of the crystalline lens in two healthy volunteers with different ages.
Characterization of the sclera
Brillouin spectroscopy measurements of sclera are more challenging due to highly scattering properties of this tissue. However, an optical method for improving the resolution and signal for scleral measurements was developed and tested ex vivo[56]. Using additional optical filtering allows for imaging of sclera and conjunctiva of porcine eyes. Scleral measurements may help with detecting and monitoring of myopia progression due to scleral weakening and pave the way to early prevention and treatments such as scleral cross-linking [57].
CONCLUSION
Brillouin microscopy provides safe and noncontact method for measuring longitudinal elastic modulus of ocular tissues. It provides three-dimensional resolution of the imaged tissue revealing spatial variations in mechanical properties. Its clinical usefulness has already been demonstrated in corneal diagnostic and treatment monitoring and may eventually be a valuable tool for surgical planning of refractive and therapeutic procedures. Beyond corneal applications, crystalline lens imaging may help clinicians in assessing presbyopia and effectiveness of various treatments for restoring its elasticity; imaging of the sclera may determine predisposition to progressive myopia in patients and allow for earlier intervention before refractive error manifests itself.
Acknowledgements
We would like to thank Theo Seiler, Julian Stevens, Roberto Pineda, Dominik Beck, Lawrence Yoo, and Niaz Karim for valuable comments.
Financial support and sponsorship
The work was supported by National Institutes of Health (R01EY025454 and P41EB015903 to S.Y. and R41 EY028820 to D.C. and S.Y.)
Conflicts of interest
S.Y. has patents on related technologies. D.C. is a full-time executive employee of Intelon Optics, Inc.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Kotecha A. What biomechanical properties of the cornea are relevant for the clinician? Surv Ophthalmol 2007; 52:S109–S114. [DOI] [PubMed] [Google Scholar]
- 2.Roberts C. The cornea is not a piece of plastic. J Refract Surg 2000; 16:407–413. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz D, Pinero D, Shabayek M, et al. Corneal biomechanical properties in normal, postlaser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg 2007; 33:1371–1375. [DOI] [PubMed] [Google Scholar]
- 4.Touboul D, Roberts C, Kérautret J, et al. Correlation between corneal hysteresis intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg 2008; 34:616–622. [DOI] [PubMed] [Google Scholar]
- 5.Shah S, Laiquzzaman M, Bhojwani R, et al. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci 2007; 48:3026–3031. [DOI] [PubMed] [Google Scholar]
- 6.Kerautret J, Colin J, Touboul D, Roberts C. Biomechanical characteristics of the ectatic cornea. J Cataract Refract Surg 2008; 34:510–513. [DOI] [PubMed] [Google Scholar]
- 7.Vinciguerra R, Ambrósio R, Jr, Elsheikh A, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg 2016; 32:803–810. [DOI] [PubMed] [Google Scholar]
- 8▪.Ambrosio RJ, Jr, Lopes BT, Faria-Correia F, et al. Integration of Scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg 2017; 33:434–443. [DOI] [PubMed] [Google Scholar]; This study describes the latest progress in biomechanical analysis using air puff dynamic elastography.
- 9.Roberts CJ, Mahmoud AM, Bons JP, et al. Introduction of two novel stiffness parameters and interpretation of air puff-induced biomechanical deformation parameters with a dynamic Scheimpflug analyzer. J Refract Surg 2017; 33:266–273. [DOI] [PubMed] [Google Scholar]
- 10.Pahuja N, Kumar NR, Shroff R, et al. Differential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest Ophthalmol Vis Sci 2016; 57:5372–5382. [DOI] [PubMed] [Google Scholar]
- 11.Yang E, Roberts CJ, Mehta JS. A review of corneal biomechanics after LASIK and SMILE and the current methods of corneal biomechanical analysis. J Clin Exp Ophthalmol 2015; 6:507. [Google Scholar]
- 12.Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea 2006; 25:1057–1059. [DOI] [PubMed] [Google Scholar]
- 13.Gallhoefer NS, Spiess BM, Guscetti F, et al. Penetration depth of corneal cross-linking with riboflavin and UV-A (CXL) in horses and rabbits. Vet Ophthalmol 2016; 19:275–284. [DOI] [PubMed] [Google Scholar]
- 14.Dupps WJ, Netto MV, Herekar S, et al. Surface wave elastometry of the cornea in porcine and human donor eyes. J Refract Surg 2007; 23:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sit AJ, Lin SC, Kazemi A, et al. In vivo noninvasive measurement of Young's modulus of elasticity in human eyes: a feasibility study. J Glaucoma 2017; 26:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford MR, Dupps WJ, Rollins AM, et al. Method for optical coherence elastography of the cornea. J Biomed Optics 2011; 16:016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larin KV, Sampson DD. Optical coherence elastography: OCT at network in tissue biomechanics invited. Biomed Optics Express 2017; 8:1172–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy BF, Wijesinghe P, Sampson DD. The emergence of optical elastography in biomedicine. Nature Photon 2017; 11:215–221. [Google Scholar]
- 19.Scarcelli G, Yun SH. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nature Photon 2008; 2:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Hillen M. The Hubble telescope of the eye: the quest for truly noninvasive ocular biomechanical measurements. Ophthalmologist 2017; March:18–29. [Google Scholar]; This is a comprehensive article describing the clinical potential of biomechanics and Brillouin microscopy.
- 21.Miura K, Nasu H, Yamamoto S. Scanning acoustic microscopy for characterization of neoplastic and inflammatory lesions of lymph nodes. Sci Rep 2013; 3:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarcelli G, Pineda R, Yun SH. Brillouin optical microscopy for corneal biomechanics. Invest Ophthalmol Vis Sci 2012; 53:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 2005; 31:146–155. [DOI] [PubMed] [Google Scholar]
- 24.Girard MJ, Dupps WJ, Baskaran M, et al. Translating ocular biomechanics into clinical practice: current state and future prospects. Curr Eye Res 2015; 40:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪▪.Shao P, Eltony AM, Seiler TG, et al. Spatially-resolved Brillouin spectroscopy reveals biomechanical changes in early-stage ectatic corneal disease and post-crosslinking in vivo. Nature Commun 2018; arXiv:1802.01055. [Google Scholar]; This study reports the latest clinical data obtained from normal population, keratoconus patients, and patients post corneal cross-linking.
- 26.Silverman RH, Patel MS, Gal O, et al. Effect of corneal hydration on ultrasound velocity and backscatter. Ultrasound Med Biol 2009; 35:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Shao P, Ramier A, Tavakol B, et al. Effects of corneal hydration on Brillouin microscopy in vivo. Invest Ophthalmol Vis Sci 2018; 59: (in print). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the theoretical and experimental results on how Brillouin frequency depends on hydration and corneal thickness swelling.
- 28.Scarcelli G, Besner S, Pineda R, et al. In vivo biomechanical mapping of normal and keratoconus corneas. J Am Med Assoc Ophthalmol 2015; 133:480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naderan M, Rajabi MT, Zarrinbakhsh P. Intereye asymmetry in bilateral keratoconus, keratoconus suspect and normal eyes and its relationship with disease severity. Br J Ophthalmol 2017; 101:1475–1482. [DOI] [PubMed] [Google Scholar]
- 30.Mandell RB, Polse KA, Brand RJ, et al. Coneal hydration control in Fuchs dystrophy. Invest Ophthalmol Vis Sci 1989; 30:845–852. [PubMed] [Google Scholar]
- 31.Wacker K, McLaren JW, Kane KM, et al. Corneal hydration control in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 2016; 57:5060–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawson DG, Grossniklaus HE, McCarey BE, et al. Biomechanical and wound healing characteristics of corneas after excimer laser keratorefractive surgery: is there a difference between advanced surface ablation and sub-Bowman's keratomileusis? J Refract Surg 2008; 24:S90–S96. [DOI] [PubMed] [Google Scholar]
- 33.Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg 2013; 29:454–460. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen IB, Bak-Nielsen S, Vestergaard AH, et al. Corneal biomechanical properties after LASIK, ReLEx flex, and ReLEx smile by Scheimpflug-based dynamic tonometry. Graefes Arch Clin Exp Ophthalmol 2014; 252:1329–1335. [DOI] [PubMed] [Google Scholar]
- 35.Hanna KD, Jouve FE, Waring GO, 3rd, et al. Computer simulation of arcuate keratotomy for astigmatism. J Cataract Refract Surg 1992; 8:152–163. [PubMed] [Google Scholar]
- 36.Pinsky PM, Datye DV. Numerical modeling of radial, astigmatic, and hexagonal keratotomy. Refract Corneal Surg 1992; 8:164–172. [PubMed] [Google Scholar]
- 37.Dupps WJ, Jr, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg 2001; 17:658–669. [DOI] [PubMed] [Google Scholar]
- 38.Bryant MR, Frederiks DJ, Campos M, et al. Finite-element analysis of corneal topographic change after excimer laser laser phototherapeutic keratectomy. Invest Ophthalmol Vis Sci 1993; 34:804–1804. [Google Scholar]
- 39.Pandolfi A, Manganiello F. A model for the human cornea: constitutive formulation and numerical analysis. Biomech Model Mechanobiol 2006; 5:237–246. [DOI] [PubMed] [Google Scholar]
- 40.Roy AS, Dupps WJ. Effects of altered corneal stiffness on native and postoperative LASIK corneal biomechanical behavior: a whole-eye finite element analysis. J Refract Surg 2009; 25:875–887. [DOI] [PubMed] [Google Scholar]
- 41.Roy A, Dupps W. Patient-specific modeling of corneal refractive surgery outcomes and inverse estimation of elastic property changes. J Biomech Eng 2011; 133:11002. [DOI] [PubMed] [Google Scholar]
- 42.Dougherty PJ, Wellish KL, Maloney RK. Excimer-laser ablation rate and corneal hydration. Am J Ophthalmol 1994; 118:169–176. [DOI] [PubMed] [Google Scholar]
- 43.Kim WS, Jo JM. Corneal hydration affects ablation during laser in situ keratomileusis surgery. Cornea 2001; 20:394–397. [DOI] [PubMed] [Google Scholar]
- 44.Denoyer A, Ricaud X, Van Went C, et al. Influence of corneal biomechanical properties on surgically induced astigmatism in cataract surgery. J Cataract Refract Surg 2013; 39:1204–1210. [DOI] [PubMed] [Google Scholar]
- 45.Randleman JB, Khandelwal SS, Hafezi F. Corneal cross-linking. Surv Ophthalmol 2015; 60:509–523. [DOI] [PubMed] [Google Scholar]
- 46.Scarcelli G, Kling S, Quijano E, et al. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci 2013; 54:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Randleman JB, Su JP, Scarcelli G. Biomechanical changes after LASIK flap creation combined with rapid cross-linking measured with Brillouin microscopy. J Refract Surg 2017; 33:408–414. [DOI] [PubMed] [Google Scholar]; This study describes the application of Brillouin microscopy for evaluation of a LASIK CXL procedure.
- 48.Kwok SJJ, Kuznetsov IA, Kim M, et al. Selective two-photon collagen crosslinking in situ measured by Brillouin microscopy. Optica 2016; 3:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seiler T. In vivo Brillouin microscopy in keratoconus corneas. Refractive Surgery 2017. Am Acad Ophthalmol 2017; VI:61. [Google Scholar]
- 50.Yam JCS, Chan CWN, Cheng ACK. Corneal collagen cross-linking demarcation line depth assessed by Visante OCT after CXL for keratoconus and corneal ectasia. J Refract Surg 2012; 28:475–481. [DOI] [PubMed] [Google Scholar]
- 51.Roy AS, Dupps WJ., Jr Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Invest Ophthalmol Vis Sci 2011; 52:9174–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seiler TG, Fischinger I, Koller T, et al. Customized corneal cross-linking: one-year results. Am J Ophthalmol 2016; 166:14–21. [DOI] [PubMed] [Google Scholar]
- 53.Scarcelli G, Kim P, Yun SH. In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophys J 2011; 101:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reiß S, Burau G, Stachs O, et al. Spatially resolved Brillouin spectroscopy to determine the rheological properties of the eye lens. Biomed Opt Express 2011; 2:2144–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Besner S, Scarcelli G, Pineda R, et al. In vivo Brillouin analysis of the aging crystalline lens. Invest Ophthalmol Vis Sci 2016; 57:5093–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao P, Besner S, Zhang J, et al. Etalon filters for Brillouin microscopy of highly scattering tissues. Opt Express 2016; 24:22232–22238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwok SJJ, Kim M, Lin HH, et al. Flexible optical waveguides for uniform periscleral cross-linking. Invest Ophthalmol Vis Sci 2017; 58:2596–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]


