Abstract
Transcripts of human endogenous retrovirus K are expressed in most breast cancers (BCs). Yellow fever vaccine 17D (YFV) expresses a protein with a closely homologous epitope. Cross-reactive immunity could hypothetically inhibit BC growth at least in women aged around 50 years at diagnosis, in whom the prognosis of BC was found to be better than that in women younger or older. A cohort of 12 804 women who received YFV in the Veneto Region, Italy, was divided into two subcohorts according to age at vaccination and followed up through the Veneto Tumor Registry. The time since vaccination until cancer incidence was categorized (≤1.9; 2–3.9; 4–5.9; 6–7.9; 8–10.9; ≥11 years) and, using the lowest class as a reference, the incidence rate ratio for BC with a 95% confidence interval and P-value was estimated by Poisson regression in each time since vaccination class, adjusting for age and calendar period. In 3140 women vaccinated at 40–54 years of age, YFV administration resulted in a protective effect of long duration slowly fading over time with a U-shaped pattern of response. Overall, BC risk was reduced by about 50% (incidence rate ratio=0.46; 95% confidence interval=0.26–0.83; P=0.009) 2 years after vaccination. Cross-reactive antigens could not be the mechanism because no protection was observed in women vaccinated before 40 or after 54 years of age. BC cells in a microscopic stage of disease can be destroyed or severely damaged by YFV if BC is not very aggressive. To prove that treatment is truly effective, a placebo-controlled double-blind trial should be conducted.
Keywords: breast cancer, cancer vaccine, epidemiology, human endogenous retrovirus K, primary prevention, yellow fever vaccine 17D
Introduction
In previous studies, it has been shown that vaccination with bacille Calmette–Guérin (BCG) vaccine and/or smallpox vaccine and/or a history of certain serious infections reduced the risk of melanoma by about 50% (Kölmel et al., 1999; Pfahlberg et al., 2002; Krone et al., 2003). Moreover, previous vaccinations with vaccinia and/or BCG improved survival of patients with melanoma (Kölmel et al., 2005). A transcript of the human endogenous retrovirus K (HERV-K) was found to be frequently expressed in melanomas and therefore termed HERV-K-MEL (Schiavetti et al., 2002). A BLAST (Basic Local Alignment Search Tool) search showed that vaccines and infectious agents protecting against melanoma expressed epitopes homologous to HERV-K-MEL (Krone et al., 2005; Krone et al., 2014). One possible explanation for melanoma protection was therefore a cross-reactive immunity against the common antigenic epitope. Additional considerations on the possible underlying immunology have been published previously (Krone et al., 2005; Krone and Grange, 2010; Cegolon et al., 2013).
Yellow fever vaccine 17D (YFV) expresses a protein with a closely homologous epitope (Krone et al., 2005). In a cohort study carried out in 28 306 individuals vaccinated with YFV in Italy (Mastrangelo et al., 2009) and in a case–control study carried out on 7010 military of the US armed forces vaccinated with YFV (Hodges-Vazquez et al., 2012), the risk estimates for melanoma suggested a protective effect, but did not reach the level of statistical significance.
There is evidence for HERV-K activation in several solid tumors including breast cancer (BC) (Downey et al., 2015). Therefore, an immune response against HERV-K-MEL could destroy or control BC cells.
Multiple clinical, pathological, and molecular analyses support the theory that BC is a heterogeneous disease rather than one biologic entity with a common etiology (Kravchenko et al., 2011). Age at diagnosis as a prognostic indicator in BC has been considered in several publications. Large epidemiological studies based on tumor registries (Adami et al., 1986; Høst and Lund, 1986; Holli and Isola, 1997; Sant et al., 1998) and clinical studies (LeMarchand et al., 1984; Jayasinghe et al., 2005) have shown a better prognosis in women aged around 50 years at diagnosis compared with those younger or older. Other studies have shown early age at diagnosis as an adverse factor affecting prognosis even in the contemporary era of systemic therapy and BC subtyping (Anders et al., 2008; Arvold et al., 2011; Colzani et al., 2011; Vicini et al., 2013; Kong et al., 2014). Various underlying biological mechanisms have been suggested. An alternative explanation for the effect of age on survival may be that, on average, tumors from age groups with favorable survival are biologically less aggressive than tumors from age groups with lower survival (de la Rochefordiere et al., 1993).
The heterogeneous nature of BC suggests a stratified rather than a unified approach to BC research, prevention, and treatment (Anderson et al., 2006). Therefore, to identify a preventive approach against BC, we carried out an epidemiological study in a cohort of women vaccinated against yellow fever, examining BC risk in women vaccinated at 40–54 years of age and, separately, in women vaccinated before 40 or after 54 years of age. No previous research has been carried out on this topic; therefore, the present study is an exploratory one that could help to gain a better understanding of the problem and form the basis for a more definitive conclusive investigation.
Methods
Study design
The design was a longitudinal retrospective treatment-outcome study in which a cohort of vaccinees against yellow fever was retrospectively followed up across a cancer registry to assess cancer incidence. The study was carried out in a region (Veneto, Italy) that is in large part covered by the Veneto Tumor Registry (VTR). Rather than considering a fixed study size, we decided to recruit all individuals vaccinated in Local Health Units (LHUs) covered by the VTR. Under Italian law, YFV may only be administered in authorized centers and the personal data of vaccinees must be recorded. The participating centers (and period of recruitment) were the Regional Office of Air and Maritime Health (1985–2000); centers of Verona (1983–2000), Padua (1991–1993), Vicenza (1998–2000), Venice (1998–2000), Treviso (1998–2000), and Bassano (1999–2000). Three centers (Adria, Belluno, Dolo) were excluded because they were active only after 1999; one center (Negrar) refused to cooperate.
Eligibility was established when a vaccination report was available in the archive of the participating centers for a YFV recipient. The original number of eligible participants was reduced according to the following exclusion criteria.
Age younger than 18 years at vaccination (data not inputted).
Receiving booster doses of YFV.
Residence outside the Veneto Region.
Residence in LHUs not covered by the VTR.
YFV after the diagnosis of cancer.
Male sex.
The follow-up was performed through record linkage with the VTR data from 1987 (start year of the VTR) to 31 December 2005. VTR also provided dates and causes of death in patients who had died before 31 December 2005.
The University of Padua Ethics Committee and the Italian National Authority for Protection of Sensitive Data approved the study protocol.
Variables
In the present exposure-only study, where participants were vaccinated once, only the time–effect relationship could be assessed. This was done in relation to the years elapsed from vaccination to cancer diagnosis (time since vaccination, TSV). TSV was categorized into six classes (≤1.9; 2–3.9; 4–5.9; 6–7.9; 8–10.9; ≥11 years); the class intervals were chosen in such a manner as to distribute the mass of person-years into groups of (utmost possible) similar weight. The obvious confounder ‘age’ was divided into 5-year classes: ≤34; 35–39; 40–44; 45–49; 50–54; 55–59; 60–64; 65–69: 70–74; and ≥75 years. The period of observation was divided into classes of 5 calendar years (1987–1991; 1992–1996; 1997–2001; 2002–2005). There were no missing data for any variable.
Statistical analysis
The number of person-years was calculated by taking as entry the date of vaccination or date of VTR coverage, whichever was the latest. The exit date was 31 December 2005 (31 December 1999 for Padua), date of incidence, death, or loss to follow-up, whichever was the earliest.
The outcome was BC incidence. Therefore, using the lowest class as a reference, the incidence rate ratio (IRR) with 95% confidence interval (CI) and P-values were estimated in each TSV class with different models of Poisson regression: univariable regression (unadjusted analysis) and multivariable regression with age and calendar period used as factorial variables (adjusted analysis). The relationship between predictors and outcome was analyzed separately in subcohort 1 (women vaccinated from 40 to 54 years of age) and subcohort 2 (women vaccinated before the age of 40 or after the age of 54 years).
Stata 13 (Stata Corporation, College Station, Texas, USA) was used for statistical analysis.
Results
Participants
The process of selection of cohort members is shown as a flow diagram in Fig. 1. After excluding 1467 individuals who received more than one vaccination (exclusion criterion no. 2 of the above list), the database was linked to the regional registry of residents; at this stage, the 18 852 individuals who did not link (residency outside the region) were excluded (exclusion criterion no. 3). The remaining 70 343 individuals were linked with cancer data of the VTR to obtain cancer incidence; then, 42 037 were excluded because they lived outside the LHUs covered by the VTR (exclusion criterion no. 4) or were vaccinated after cancer diagnosis (exclusion criterion no. 5). The number of participants reached 28 306. When the VTR updated its archive, we repeated the record linkage. At this stage, 476 participants could not be traced in the regional registry of residents, most probably because they were no longer resident in Veneto. Excluding participants lost to follow-up, the updated cohort comprised 27 830 participants, 15 026 men (omitted, exclusion criterion no. 6) and 12 804 women, the final cohort. The latter was divided into subcohort 1, which included 3140 women vaccinated at 40 to 54 years of age, and subcohort 2, which included 9664 women vaccinated before the age of 40 or after the age of 54 years.
Fig. 1.
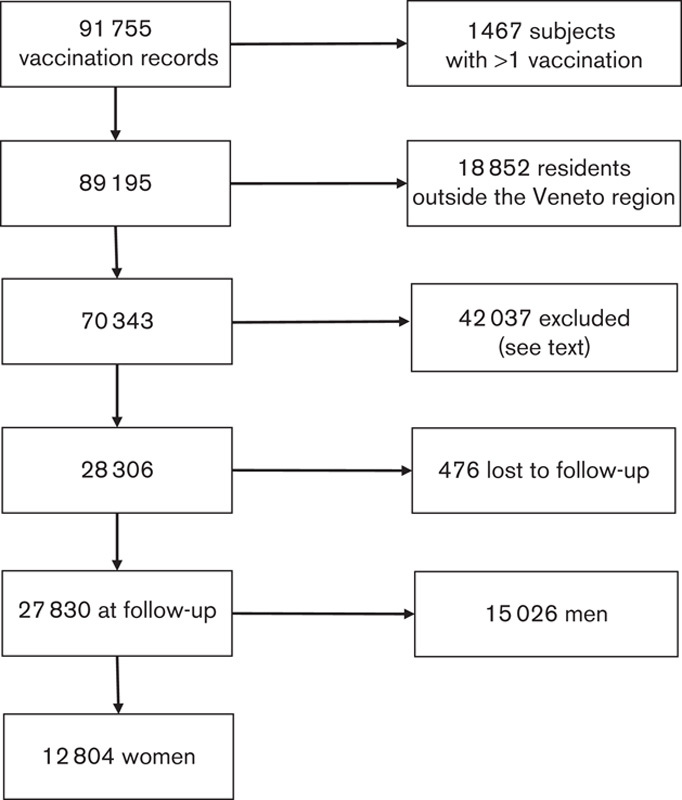
Cohort members: flow diagram of the selection process.
Descriptive data
The number of person-years was 110 664.1 (mean=8.64) in the entire cohort, 27 493.4 (mean=8.76) in subcohort 1 and 83 170.7 (mean=8.61) in subcohort 2.
Person-years were concentrated in the classes 45–54 years in subcohort 1, whereas they were distributed in all age classes in subcohort 2. In subcohorts 2 and 1, respectively, the percentage of person-years was 67 and 0% for age classes under 40 years and 13 and 39% for age classes above 54 years. Therefore, subcohort 2 was younger than subcohort 1.
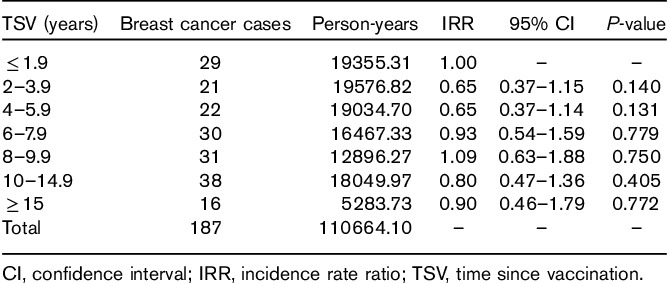
The joint distribution of person-years by age and TSV is shown in Table 1. Across the classes of TSV, the number of person-years ranged from 4400.0 to 4847.6 in subcohort 1 and from 12 391.6 to 14 723.2 in subcohort 2. No different subdivision of TSV produced smaller variations in person-years among TSV classes. Despite the quite constant number of person-years, the composition by age of TSV classes varies because age increases with increasing TSV. As higher TSV classes include older populations, the risk of BC is expected to increase with TSV.
Table 1.
Breast cancer cases, person-years, incidence rate ratio with 95% confidence interval, and P-value in the classes of time since vaccination against yellow fever in the overall cohort of 12 804 women
Outcome data
Among 517 incident tumors of all sites identified in the entire cohort with the record linkage, we found 187 BC cases (89 and 98 in subcohorts 1 and 2, respectively).
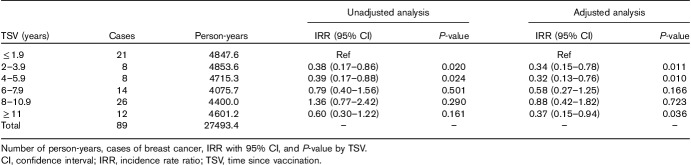
Table 2 shows cases of BC, number of person-years, and IRR with 95% CI and P-value by TSV classes in 3140 women vaccinated between 40 and 54 years of age (subcohort 1). Adjustment for age and calendar periods decreased IRR, particularly in the last three classes of TSV. Compared with the reference category (TSV≤1.9 years), IRR was significantly lower in classes ‘2–3.9’ and ‘4–5.9 years’ of TSV (unadjusted and adjusted analysis) and in the last TSV class (adjusted analysis). When all other TSV categories as a whole (68 BC cases; 22 645.8 person-years) were contrasted with the reference class (21 cases; 4847.6 person-years), the age-adjusted and calendar period-adjusted IRR became 0.46 (95% CI=0.26–0.83; P=0.009) and the prevented fraction (1−IRR) increased to 54% (=1−0.46). In the absence of a protective effect, there should have been 148 BC cases (=68/0.46); the number of cases of BC prevented would be, therefore, 80 (=148−68).
Table 2.
Unadjusted and adjusted analysis of Poisson regression in subcohort 1 (3140 women vaccinated from 40 to 54 years of age)
Figure 2 shows that in subcohort 1, YFV resulted in a protective effect of long duration slowly fading over time with a U-shaped pattern of response, as is the case for most vaccines.
Fig. 2.
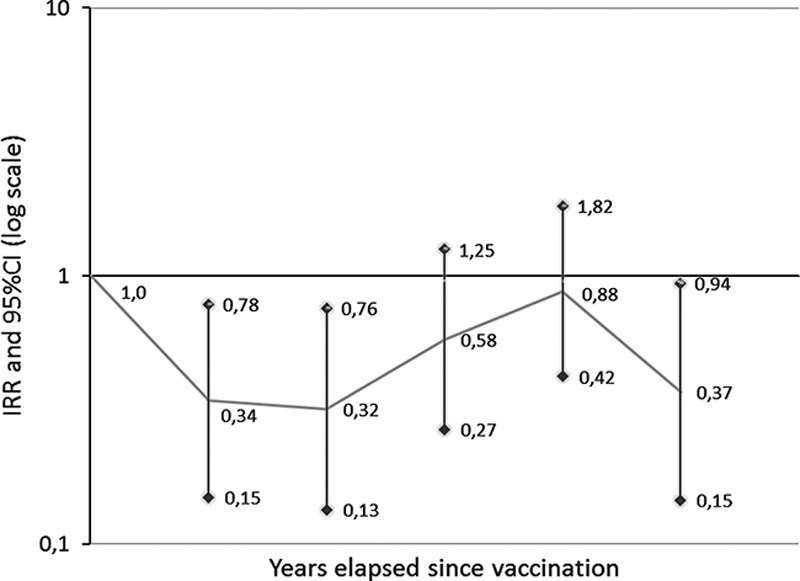
Breast cancer risk in subcohort 1 by years elapsed since vaccination: incidence rate ratio (IRR) and 95% confidence intervals (CIs).
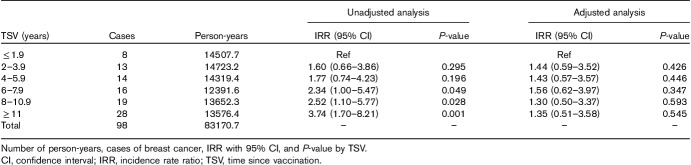
Table 3 shows cases of BC, number of person-years, and IRR with 95% CI and P-value by TSV classes in 9664 women vaccinated before the age of 40 or after the age of 54 years (subcohort 2). The increasing trend of IRRs with increasing TSV years in unadjusted Poisson regression disappeared at adjusted analysis, suggesting confounding by age (in particular) and calendar period.
Table 3.
Unadjusted and adjusted analysis of Poisson regression in subcohort 2 (9664 women vaccinated before the age of 40 or after the age of 54 years)
In the beginning, women vaccinated before 40 years and those vaccinated after 54 years of age were analyzed separately; as the trend of IRRs across TSV was similar (data not shown), these participants were combined in subcohort 2.
Discussion
Key results
The live-attenuated YFV 17D, administered as a single injection to 3140 healthy women aged between 40 and 54 years, resulted in a protective effect of long duration slowly fading over time with a U-shaped pattern of response. Overall, BC risk was reduced by about 50% two years after vaccination. The estimated number of BC cases prevented would have been 80. As 27.6 euros is the unit cost of the YFV, the total cost of vaccination was 86 664 (=27.6×3140) euros in subcohort 1 and the cost per case prevented would have been ∼1083 (=86 664/80) euros. The latter represents a very small fraction of the cost of treating a single case of BC.
Study limitations
In Italy, vaccination against yellow fever at an authorized health service is free of charge, does not require a prescription from a doctor, and an official certificate of vaccination is issued that is compulsory for travel to and between several countries. Any alternative is highly unlikely because it involves obtaining a prescription from a doctor and purchasing the vaccine without the release of a valid certificate. It can therefore be concluded that the cohort is complete. YFV is only recommended for those traveling to hot countries where yellow fever is endemic, and individuals who travel to these countries tend to belong to a higher than average social class of the general population. The reference group (the TSV class of <1.9 years) was a subset of the same study population; the class intervals were chosen in such a manner as to minimize the variation of person-years among TSV classes. Therefore, the method of collection of exposed and unexposed individuals could not have introduced a selection bias.
Assessment of exposure was based on a vaccination report and cancer diagnosis was found in a tumor registry. Although it was determined retrospectively, information was accurate and an information bias can be excluded.
In observational studies, the lack of randomization leads to the potential problem of confounding. Secular changes of BC incidence have been reported in the USA over the past 70 years (Toriola and Colditz, 2013); to account for the distorting effects of this potential confounder (as well as age), we used a multivariate analysis technique. It might be that another causal factor is both a protection factor for the disease and a factor associated with the exposure of interest. Potential confounders were, for example, BCG and smallpox vaccinations that, according to a BLAST search (Krone et al., 2014), express epitopes homologous to HERV-K-MEL. No information was available on these because the BCG vaccine is not required for travel abroad and vaccinia vaccination was halted in Italy in 1981. As the cohort members (with the exception of 15 individuals) were born before 1981, almost all had been vaccinated against smallpox. Yet, a protective effect of YFV was detected only in the women of subcohort 1.
The lack of information on HERV-K activation status in BC cases (and study participants at large) could be another limitation. If protection was because of a cross-immune reaction between YFV products and transcripts of HERV-K, we should presume that only women of subcohort 1 were carriers of HERV-K. This seems unlikely, however, in view of the evidence that among 59 patients with BC, HERV-K-ENV was expressed in 62% of women aged 60 years or younger and in 64% of women above 60 years of age (P=0.866) (Zhao et al., 2011).
No information was available on hormone receptor status, genotype-based subtypes, and histologic grade. The triple-negative BCs – defined by the absence of the estrogen receptor, progesterone receptor, and receptor 2 of the human epidermal growth factor – are most commonly diagnosed in women younger than 40 years of age (Bauer et al., 2007; Anders et al., 2008), and have a more aggressive clinical course than non-triple-negative BCs (higher relapse and distant recurrence rate; and shorter post recurrence and overall survival) (Haffty et al., 2006; Dent et al., 2007). Breast tumors characterized by poor survival would be numerous in subcohort 2 because here 67% of person-years were found in age classes younger than 40 years.
Interpretation of results
BC cells in a preclinical microscopic stage of disease can be destroyed or severely damaged by YFV 17D through an unpredicted immunologic/inflammatory ‘bustle’ if BC is not very aggressive.
Future perspectives
The present observational study provided an idea of what works in the real world, but clearly needs confirmatory investigations. There are no similar studies for comparison of these encouraging results. Rather than (or in parallel with) other observational studies, a placebo-controlled double-blind trial is required to prove that treatment is truly effective. Our study showed that about 6500 women should be enrolled (3250 in each group of treatment and control) to detect a significant 65% relative reduction in invasive BC. Eligible women should be 40–54 years of age, have a Gail 5-year risk score (percent chance of invasive BC within 5 years) more than 1.66%, and some previous atypical findings. The annual incidence of invasive BCs would be measured, along with toxic effects. Given that the protective effect begins 2 years after vaccination, a short follow-up would help to reduce difficulties and costs of the investigation. A significant relative reduction in the annual incidence of invasive BC for YFV 17D compared with the placebo will mark the conclusion of epidemiologic investigations and the beginning of laboratory evaluation (search for mechanisms). Despite its efficacy (YFV 17D is one of the most successful vaccines ever developed in humans) and widespread use (in >600 million individuals), the mechanisms by which YFV stimulates protective immunity remain poorly understood (Ravindran et al., 2014). Irrespective of their common origin, vaccinology and immunology have evolved such different trajectories that immunologists remain largely ignorant of the mechanisms of action of successful vaccines, and vaccinologists have until recently shown little interest in the intricacies of immune regulation (Pulendran et al., 2010). YFV 17D is a live-attenuated vaccine. To date, it is known that viral replication peaks at days 5–7 and is undetectable by 14 days. The vaccine induces neutralizing antibodies (IgM persisting up to 18 months and IgG that can persist for up to 40 years), CD4+ T-cell response (of a mixed T-helper 1 and T-helper 2 profile), and CD8+ T-cell responses (Pulendran, 2009). In addition, YFV was found to induce a significant modulation of about 600 genes in whole-blood cells (Gaucher et al., 2008). However, numerous potential mechanisms may explain the favorable association of infection with carcinogenesis: cross-reactive antigens, suppression of inflammation, promotion of antitumor immunity, induction of preimmunity, alteration of the tumor microenvironment, production of low-level ‘danger’ signals, removal of carcinogens, and inhibition of angiogenesis (Oikonomopoulou et al., 2013). Extensive work is thus needed to dissect the complex molecular and cellular pathways involved in BC protection elicited by YFV. This work could be carried out after (and only if justified by) the clinical results. This ‘clinics to laboratory’ path (called reverse pharmacology) reverses the conventional paradigm ‘laboratory to clinics’ – namely testing compounds in vitro and then in animals before evaluating them in humans – reducing costs, time, and toxicity (Patwardhan et al., 2008; Willcox et al., 2011).
Unfortunately, despite decades of promising preclinical and clinical research, vaccines against human BC remain an unfulfilled promise (Lollini et al., 2013). Except for vaccines against viruses that are associated with specific cancers, a reliable, safe, easy to use, and reasonably priced vaccine that can treat solid tumors or prevent their metastasis is not available (Gao et al., 2012). It will probably take decades to develop an agent that will match YFV 17D in its ability to reduce the incidence of BC for 6–8 years after a single dose administered to healthy women aged 40–54 years.
The occurrence of adverse effects is of particular concern for vaccines because they are supplied to healthy individuals on the scale of millions. YFV-associated neurologic or viscerotropic diseases are rare serious adverse events of the live-attenuated virus vaccine. A systematic search of adverse events associated with YFV was carried out in nine electronic bibliographic databases and reference lists of included papers. The review identified nine studies of adverse events in infants and children, eight studies of adverse events in pregnant women, nine studies of adverse events in human immunodeficiency virus-positive patients, five studies of adverse events in individuals 60 years of age and older, and one study of adverse events in individuals taking immunosuppressive medications. Two case studies of maternal–neonate transmission resulted in serious adverse events. The five passive surveillance databases identified very small numbers of cases of YFV-associated viscerotropic disease, YFV-associated neurotropic disease, and anaphylaxis in individuals 60 years or older (Thomas et al., 2012). Other data suggested a higher than expected number of deaths from YFV-associated viscerotropic disease among women 19–34 years of age without known immunodeficiency (Seligman, 2011). Furthermore, a mass vaccination campaign (2007–2010) was launched in sub-Saharan Africa (Benin, Burkina Faso, Cameroon, Guinea, Liberia, Mali, Senegal, Sierra Leone, and Togo) after an outbreak of yellow fever. Out of 38 million doses of YFV, cases of neurotropic disease, viscerotropic disease, and hypersensitivity reactions induced by YFV were 6, 5, and 11, respectively (attack rates per 100 000 individuals vaccinated were 0.016, 0.013, and 0.029), according to the Global Advisory Committee on Vaccine Safety (No authors listed, 2013). The indication of YFV as a BC vaccine even now excludes most of the vulnerable groups reported in the literature; nonetheless, attention should be paid to pregnancy and immune-suppression status. All risks could be minimized by using an inactivated vaccine that was recently found to induce neutralizing antibodies against the yellow fever virus (Monath et al., 2011). However, it will be necessary to determine whether the inactivated YFV would have a comparable protective effect.
Conclusion
A single administration of YFV to healthy women aged 40–54 years reduced BC risk by about 50% 2 years after vaccination. The translation of this observational research into a new regimen of BC prevention requires a randomized-controlled clinical trial.
Acknowledgements
Professor G. Mastrangelo received funding from Ministry of Education, University and Research (share funds ex60%).
Conflicts of interest
There are no conflicts of interest.
References
- Adami HO, Malker B, Holmberg L, Persson I, Stone B. (1986). The relation between survival and age at diagnosis in breast cancer. N Engl J Med 315:559–563. [DOI] [PubMed] [Google Scholar]
- Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. (2008). Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324–3330. [DOI] [PubMed] [Google Scholar]
- Anderson WF, Pfeiffer RM, Dores GM, Sherman ME. (2006). Comparison of age distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev 15:1899–1905. [DOI] [PubMed] [Google Scholar]
- Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, et al. (2011). Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol 29:3885–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. (2007). Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer 109:1721–1728. [DOI] [PubMed] [Google Scholar]
- Cegolon L, Salata C, Weiderpass E, Vineis P, Palù G, Mastrangelo G. (2013). Human endogenous retroviruses and cancer prevention: evidence and prospects. BMC Cancer 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, Hall PF, Czene K. (2011). Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol 29:4014–4021. [DOI] [PubMed] [Google Scholar]
- De la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, et al. (1993). Age as prognostic factor in premenopausal breast carcinoma. Lancet 341:1039–1043. [DOI] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. (2007). Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434. [DOI] [PubMed] [Google Scholar]
- Downey RF, Sullivan FJ, Wang-Johanning F, Ambs S, Giles FJ, Glynn SA. (2015). Human endogenous retrovirus K and cancer: innocent bystander or tumorigenic accomplice? Int J Cancer 137:1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Whitaker-Dowling P, Griffin JA, Bergman I. (2012). Treatment with targeted vesicular stomatitis virus generates therapeutic multifunctional anti-tumor memory CD4 T cells. Cancer Gene Ther 19:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, et al. (2008). Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 205:3119–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. (2006). Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24:5652–5657. [DOI] [PubMed] [Google Scholar]
- Hodges-Vazquez M, Wilson JP, Hughes H, Garman P. (2012). The yellow fever 17D vaccine and risk of malignant melanoma in the United States military. Vaccine 30:4476–4479. [DOI] [PubMed] [Google Scholar]
- Holli K, Isola J. (1997). Effect of age on the survival of breast cancer patients. Eur J Cancer 33:425–428. [DOI] [PubMed] [Google Scholar]
- Høst H, Lund E. (1986). Age as a prognostic factor in breast cancer. Cancer 57:2217–2221. [DOI] [PubMed] [Google Scholar]
- Jayasinghe UW, Taylor R, Boyages J. (2005). Is age at diagnosis an independent prognostic factor for survival following breast cancer? ANZ J Surg 75:762–767. [DOI] [PubMed] [Google Scholar]
- Kölmel KF, Pfahlberg A, Mastrangelo G, Niin M, Botev IN, Seebacher C, et al. (1999). Infections and melanoma risk: results of a multicentre EORTC case–control study. European Organization for Research and Treatment of Cancer. Melanoma Res 9:511–519. [PubMed] [Google Scholar]
- Kölmel KF, Grange JM, Krone B, Mastrangelo G, Rossi CR, Henz BM, et al. (2005). Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European Organization for Research and Treatment of Cancer cohort study on 542 patients. Eur J Cancer 41:118–125. [DOI] [PubMed] [Google Scholar]
- Kong I, Narod SA, Taylor C, Paszat L, Saskin R, Nofech-Moses S, et al. (2014). Age at diagnosis predicts local recurrence in women treated with breast-conserving surgery and postoperative radiation therapy for ductal carcinoma in situ: a population-based outcomes analysis. Curr Oncol 21:e96–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko J, Akushevich I, Seewaldt VL, Abernethy AP, Lyerly HK. (2011). Breast cancer as heterogeneous disease: contributing factors and carcinogenesis mechanisms. Breast Cancer Res Treat 128:483–493. [DOI] [PubMed] [Google Scholar]
- Krone B, Grange JM. (2010). Melanoma, Darwinian medicine and the inner world. J Cancer Res Clin Oncol 136:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone B, Kölmel KF, Grange JM, Mastrangelo G, Henz BM, Botev IN, et al. (2003). Impact of vaccinations and infectious diseases on the risk of melanoma – evaluation of an EORTC case–control study. Eur J Cancer 39:2372–2378. [DOI] [PubMed] [Google Scholar]
- Krone B, Kölmel KF, Henz BM, Grange JM. (2005). Protection against melanoma by vaccination with Bacille Calmette–Guerin (BCG) and/or vaccinia: an epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur J Cancer 41:104–117. [DOI] [PubMed] [Google Scholar]
- Krone B, Kölmel KF, Grange JM. (2014). The biography of the immune system and the control of cancer: from St Peregrine to contemporary vaccination strategies. BMC Cancer 14:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMarchand L, Kolonel LN, Nomura AM. (1984). Relationship of ethnicity and other prognostic factors to breast cancer survival patterns in Hawaii. J Natl Cancer Inst 73:1259–1265. [PubMed] [Google Scholar]
- Lollini PL, Cavallo F, de Giovanni C, Nanni P. (2013). Preclinical vaccines against mammary carcinoma. Expert Rev Vaccines 12:1449–1463. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G, Krone B, Fadda E, Buja A, Grange JM, Rausa G, et al. (2009). Does yellow fever 17D vaccine protect against melanoma? Vaccine 27:588–591. [DOI] [PubMed] [Google Scholar]
- Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, et al. (2011). An inactivated cell-culture vaccine against yellow fever. N Engl J Med 364:1326–1333. [DOI] [PubMed] [Google Scholar]
- [No authors listed] (2013). Global Advisory Committee on Vaccine Safety 12–13 June 2013. Wkly Epidemiol Rec 88:301–312.23909011 [Google Scholar]
- Oikonomopoulou K, Brinc D, Kyriacou K, Diamandis EP. (2013). Infection and cancer: revaluation of the hygiene hypothesis. Clin Cancer Res 19:2834–2841. [DOI] [PubMed] [Google Scholar]
- Patwardhan B, Vaidya ADB, Chorghade M, Joshi SP. (2008). Reverse pharmacology and systems approaches for drug discovery and development. Curr Bioact Compd 4:201–212. [Google Scholar]
- Pfahlberg A, Kölmel KF, Grange JM, Mastrangelo G, Krone B, Botev IN, et al. (2002). Inverse association between melanoma and previous vaccinations against tuberculosis and smallpox: results of the FEBIM study. J Invest Dermatol 119:570–575. [DOI] [PubMed] [Google Scholar]
- Pulendran B. (2009). Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol 9:741–747. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Li S, Nakaya HI. (2010). Systems vaccinology. Immunity 33:516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, et al. (2014). Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 343:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant M, Capocaccia R, Verdecchia A, Estève J, Gatta G, Micheli A, et al. (1998). Survival of women with breast cancer in Europe: variation with age, year of diagnosis and country. The EUROCARE Working Group. Int J Cancer 77:679–683. [DOI] [PubMed] [Google Scholar]
- Schiavetti F, Thonnard J, Colau D, Boon T, Coulie PG. (2002). A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer Res 62:5510–5516. [PubMed] [Google Scholar]
- Seligman SJ. (2011). Yellow fever virus vaccine-associated deaths in young women. Emerg Infect Dis 17:1891–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. (2012). The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: systematic review. Am J Trop Med Hyg 86:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriola AT, Colditz GA. (2013). Trends in breast cancer incidence and mortality in the United States: implications for prevention. Breast Cancer Res Treat 138:665–673. [DOI] [PubMed] [Google Scholar]
- Vicini FA, Shaitelman S, Wilkinson JB, Shah C, Ye H, Kestin LL, et al. (2013). Long-term impact of young age at diagnosis on treatment outcome and patterns of failure in patients with ductal carcinoma in situ treated with breast-conserving therapy. Breast J 19:365–373. [DOI] [PubMed] [Google Scholar]
- Willcox ML, Graz B, Falquet J, Diakite C, Giani S, Diallo D. (2011). A ‘reverse pharmacology’ approach for developing an anti-malarial phytomedicine. Malar J 10 (Suppl 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Rycaj K, Geng S, Li M, Plummer JB, Yin B, et al. (2011). Expression of human endogenous retrovirus type K envelope protein is a novel candidate prognostic marker for human breast cancer. Genes Cancer 2:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]