Herpes simplex virus type 2 seroprevalence among male blood donors from different Middle East and North Africa nationalities was in the range of few percentage points.
Supplemental digital content is available in the text.
Abstract
Background
There are limited data on herpes simplex virus type 2 (HSV-2) seroprevalence in the Middle East and North Africa (MENA). We examined country- and age-specific HSV-2 seroprevalence among select MENA populations residing in Qatar.
Methods
Sera were collected from male blood donors attending Hamad Medical Corporation between June 2013 and June 2016. Specimens were screened for anti-HSV-2 IgG antibodies following a 2-test algorithm: HerpeSelect 2 ELISA was used to identify HSV-2–positive specimens, and Euroline-WB was used to confirm positive and equivocal specimens for final HSV-2 status. Trends and associations with HSV-2 seropositivity were assessed.
Results
Of the 2077 tested sera, 61 were found and confirmed positive. The proportion of those confirmed positive increased steadily with HerpeSelect 2 ELISA index value, ranging from 16.3% for index values of 1.101 to 1.999 to 92.9% for index values of 4 or greater. Nationality-specific seroprevalence was 6.0% (95% confidence interval [CI], 4.1%–8.8%) in Qataris, 5.3% (95% CI, 2.5%–11.1%) in Iranians, 4.2% (95% CI, 1.8%–9.5%) in Lebanese, 3.1% (95% CI, 1.2%–7.7%) in Sudanese, 3.0% (95% CI, 1.4%–6.4%) in Palestinians, 2.2% (95% CI, 1.1%–4.3%) in Egyptians, 2.0% (95% CI, 1.0%–5.0%) in Syrians, 1.0% (95% CI, 0.3%–3.6%) in Jordanians, 0.7% (95% CI, 0.1%–3.7%) in Yemenis, and 0.5% (95% CI, 0.1%–2.8%) in Pakistanis. There was evidence for higher seroprevalence in older age groups.
Conclusions
The seroprevalence of HSV-2 was in the range of few percentage points. There were no major differences in seroprevalence by nationality. These findings add to our understanding of HSV-2 epidemiology in MENA and indicate unmet needs for sexual health and control of sexually transmitted infections.
Herpes simplex virus type 2 (HSV-2) is a lifelong infection and a prevalent sexually transmitted infection (STI).1,2 It was estimated that, for 2012, there were more than 400 million prevalent HSV-2 infections worldwide with an annual incidence of nearly 20 million infections.3 Herpes simplex virus type 2 is a cause of a range of diseases,4 most notably genital ulcer disease, where HSV-2 is a leading, if not the leading, cause of genital ulcer disease in developed and developing countries.4,5 Evidence suggests an epidemiologic synergy between HSV-2 infection and HIV infection.6,7
An intriguing aspect of HSV-2 epidemiology is that HSV-2 antibody prevalence (seroprevalence) could be used as a “summary collective measure” of sexual risk behavior and HIV epidemic potential.8 A recent mathematical modeling study demonstrated that HSV-2 seroprevalence in a population is a reflection of key statistics of sexual network structure.9 The mean and variance of the number of sexual partners, as well as concurrency and clustering coefficient, were the strongest predictors of HSV-2 seroprevalence.9 A systematic review and meta-analyses of global HSV-2 and HIV data,10 and mathematical modeling9 identified a strong and statistically significant association, with a Spearman rank correlation of approximately 0.7, between HSV-2 seroprevalence and HIV seroprevalence across populations. These findings highlight how HSV-2 seroprevalence can identify populations and/or sexual networks at risk for future HIV expansion.
Despite the growth in STI research in the Middle East and North Africa (MENA) recently,11,12 our knowledge of HSV-2 epidemiology remains limited. A systematic review of HSV-2 seroprevalence data in MENA identified only a small number of studies.8 Most studies used also diagnostic tests of questionable validity and with cross-reactivity with the highly prevalent HSV-1 antibodies.8,13 With only few acceptable-quality studies in MENA, we aimed in the present study to provide measures of the nationality-specific and age-specific HSV-2 seroprevalence in select MENA populations residing in Qatar. This is the first time that HSV-2 seroprevalence measures are reported for several nationalities. We used a 2-test algorithm to screen specimens for HSV-2 antibodies, to avoid key limitations in existing literature.8
Qatar is a MENA country with a resident population of 2.2 million in 2014, of which only 12% are Qataris.14 Qatar provided an opportune setting for our study, as most of the population are short-term expatriate residents.14 These expatriates came to Qatar in recent years for contractual employment with the rapid economic expansion.14 With a fraction of these expatriates being MENA expatriates14 and with the existing availability of a sample of specimens from male blood donors,15–18 we aimed to assess HSV-2 seroprevalence in male blood donors from 10 MENA nationalities.
MATERIALS AND METHODS
Study Design and Participants
This was an opportunistic cross-sectional study on volunteer male blood donors attending the donation center at Hamad Medical Corporation, the primary provider of health care in Qatar, between June 2013 and June 2016. Blood donation in Qatar is a common and accessible practice, and individuals from diverse socioeconomic strata participate in donation campaigns. A total of 5973 anonymized blood sera specimens were originally obtained for other studies.15–18 All specimens were anonymously collected and unidentified at the donation center and subsequently provided to study investigators for testing.
Collected basic demographic data included only nationality, age, and sex. A total of 4525 specimens satisfied the eligibility criteria (male sex and MENA nationals residing in Qatar) and served as the original sampling cohort. There were too few specimens (only 88) to extend the study to female residents. The anonymized specimen collection was approved by Hamad Medical Corporation ethics board, and this study was also approved by the ethics boards and research committees at Qatar University and Weill Cornell Medicine—Qatar.
We powered final sample sizes of the study for 5% significance level. To estimate a 2% age-specific HSV-2 seroprevalence with a 4% precision level, we calculated a sample size of 50 for each 5-year age group in each nationality. To estimate a 2% nationality-specific HSV-2 seroprevalence with a 2% precision level, we calculated a sample size of 200 for each nationality. We based the 2% seroprevalence on observed seroprevalence in Saudi Arabia,19 a neighboring country.
We used a similar sampling strategy to that of a previous study on HSV-1 seroprevalence.13 We selected a random sample of 50 subjects per age group for estimating the age-specific HSV-2 seroprevalence for each of Egypt, the Fertile Crescent (merged sample for Iraq, Jordan, Lebanon, Palestine, and Syria), and Qatar. The neighboring countries of Iraq, Jordan, Lebanon, Palestine, and Syria were merged into one sample “Fertile Crescent” because of insufficient sample size for any country individually, and because of socioeconomic and sociocultural similarity. For estimating the nationality-specific seroprevalence, we selected a random sample of 200 subjects for each of Jordan, Pakistan, Palestine, and Syria, whereas all available specimens (<200) for Iran, Lebanon, Sudan, and Yemen were included.
Biological Specimen Laboratory Analysis
In light of known limitations and recommendations in conducting HSV-2 serology,20–22 we used a 2-test algorithm for identifying positive specimens, using 2 of the most commonly used commercial assays.23,24 The first stage included screening for the presence of anti-HSV-2 IgG antibodies using an enzyme-linked immunosorbent assay (ELISA), and the second stage included confirmation of HSV-2 IgG seropositivity using a Western blot (WB) assay for both positive and equivocal specimens.
For screening, we aliquoted 50 μL of sera from existing sera specimens and tested for the presence of anti–HSV-2 IgG using HerpeSelect 2 ELISA kits (Cat. No. EL0910G-5; Focus Diagnostics, Cypress, CA).25 This kit was approved for laboratory diagnosis of anti–HSV-2 IgG by the US Food and Drug Administration.25 We interpreted the analysis and results according to the manufacturer's instructions: sera with optical density index values (cutoff) less than 0.90 were considered negative, values greater than 1.10 were considered positive, and values ranging between 0.90 and 1.10 were considered equivocal.25 Equivocal specimens were retested by using HerpeSelect 2 ELISA kits for final ELISA results, and if remained equivocal, they were considered equivocal by ELISA testing.
For confirmation of seropositivity, we retested all positive and equivocal specimens for the presence of anti–HSV-2 IgG using Euroline-WB assays (Cat. No. DY 2531-2401-1G; Euroimmun Laboratory, Luebeck, Germany).26 Analysis and results were interpreted according to the manufacturer's instructions.
The final results for the equivocal specimens by Euroline-WB and for the specimens with no sufficient serum for Euroline-WB confirmatory testing were determined, as informed by previous work,20,21 based on the HerpeSelect 2 ELISA index value (positive if index value ≥3 and negative if index value <3).
Data Analysis
We analyzed the data using SPSS version 24. We categorized age into 8 brackets: ≤24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, and ≥55 years. To assess the age-specific seroprevalence of the study population for Egypt, Fertile Crescent, and Qatar, we cross-tabulated age and HSV-2 serostatus. We assessed trends using the Cochran-Armitage test. To estimate crude and adjusted associations of age and nationality with HSV-2 serostatus, we conducted logistic regression to determine the odds ratios.
RESULTS
We tested a total of 2077 specimens for HSV-2 IgG antibodies using HerpeSelect 2 ELISA kits. The median age was 37 years. HerpeSelect 2 ELISA testing identified 1955 sera as negative, 116 as positive, and 6 as equivocal (Fig. 1). We performed confirmatory testing for HSV-2 antibodies using Euroline-WB assays for 117 specimens. Fifty-eight specimens were confirmed positive for HSV-2 antibodies and 3 were equivocal.
Figure 1.
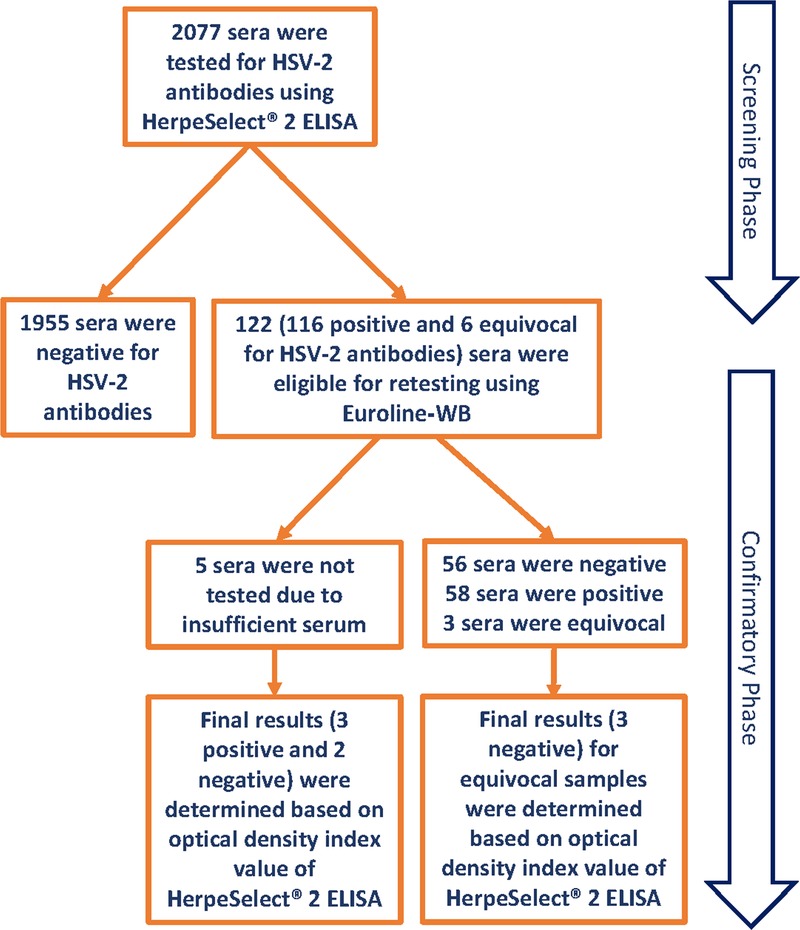
Overview of the serological testing for anti-HSV-2 IgG antibodies in its 2 stages of screening and confirmation.
There was not enough serum for confirmatory testing for 5 specimens identified as positive using HerpeSelect 2 ELISA (Fig. 1). Final results for these specimens, as well as for the 3 equivocal specimens by Euroline-WB, were determined based on the HerpeSelect 2 ELISA index value (positive if index value ≥3 and negative if index value <3). The final results for these 8 specimens were 5 negative and 3 positive. Accordingly, final results of the serology testing for the 2077 specimens identified 61 sera as positive and 2016 as negative.
Figure 2 shows the proportion of HSV-2 HerpeSelect 2 ELISA positive results confirmed positive by Euroline-WB assays, by index value. Of the 117 HerpeSelect 2 ELISA (111 positive and 6 equivocal) specimens retested (Fig. 1), 49.6% were confirmed positive by Euroline-WB (Fig. 2). The proportion of specimens confirmed positive increased steadily with index value and ranged from a low of 16.3% for index value of 1.101 to 1.999 to a high of 92.9% for index value of 4 or greater. The trend of higher positive concordance with higher index value was statistically significant (P value for trend < 0.001). Of the 6 equivocal specimens, 5 were confirmed negative by Euroline-WB assays and 1 remained equivocal.
Figure 2.
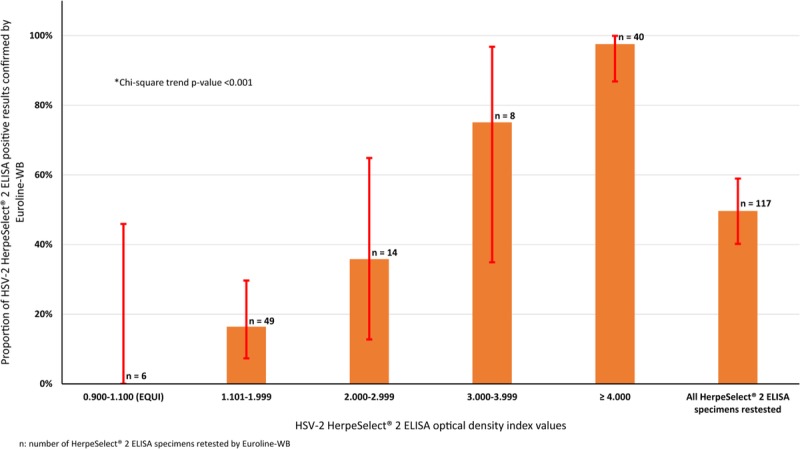
Proportion of HSV-2 HerpeSelect 2 ELISA positive results confirmed positive by the Euroline-WB assay, by optical density index value.
We estimated the nationality-specific HSV-2 seroprevalence for 10 populations (Fig. 3A and Table S1 of Supplementary Content, http://links.lww.com/OLQ/A234). Seroprevalence ranged between 0.5% (95% confidence interval [CI], 0.1%–2.8%) for Pakistanis and 6.0% (95% CI, 4.1%–8.8%) for Qataris. Seroprevalence was 5.3% (95% CI, 2.5%–11.1%) in Iranians, 4.2% (95% CI, 1.8%–9.5%) in Lebanese, 3.1% (95% CI, 1.2%–7.7%) in Sudanese, 3.0% (95% CI, 1.4%–6.4%) in Palestinians, 2.2% (95% CI, 1.1%–4.3%) in Egyptians, 2.0% (95% CI, 1.0%–5.0%) in Syrians, 1.0% (95% CI, 0.3%–3.6%) in Jordanians, and 0.7% (95% CI, 0.1%–3.7%) in Yemenis.
Figure 3.
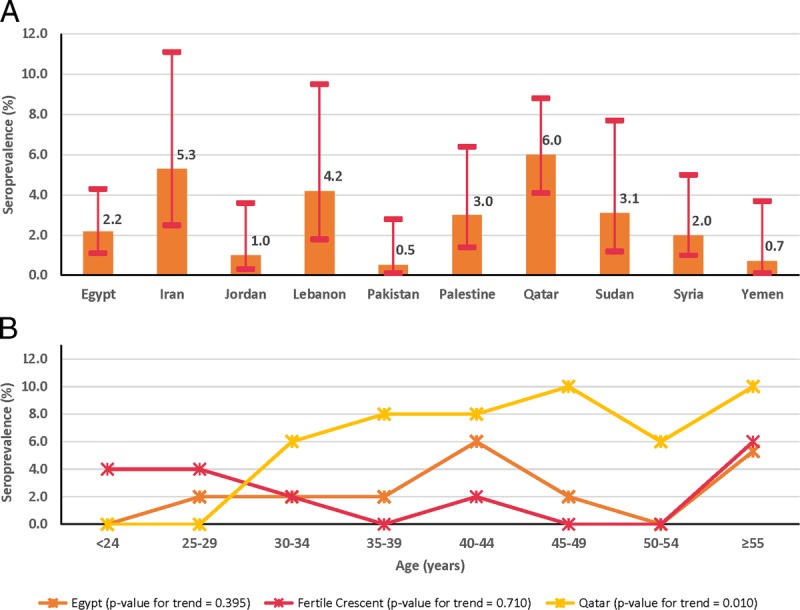
Estimates of HSV-2 nationality-specific (A) and age-specific (B) seroprevalence among male blood donors currently residing in Qatar, but from different MENA countries.
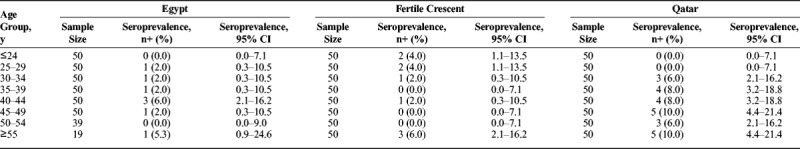
Table 1 and Figure 3B show the estimated age-specific HSV-2 seroprevalence for Egyptians, Fertile Crescent nationals, and Qataris. Seroprevalence tended to grow with age, but the trend was statistically significant only for Qataris (P value for trend = 0.010). Highest seroprevalence was in the older age groups.
TABLE 1.
Estimates of HSV-2 Age-Specific Seroprevalence Among Male Blood Donors Currently Residing in Qatar, But From Egypt, the Fertile Crescent (Including Iraq, Jordan, Lebanon, Palestine, and Syria), and Qatar
There were only few significant associations between age or nationality and HSV-2 infection in univariable analyses (Table S2 of Supplementary Content, http://links.lww.com/OLQ/A234). For example, compared with participants 24 years or younger, the crude odd ratios (ORs) for seropositivity were significantly higher in those aged 40 to 44 years (OR, 4.93; 95% CI, 1.10–22.06) and in those 55 years or older (OR, 7.97; 95% CI, 1.74–36.45). All associations remained significant in the multivariable analysis.
DISCUSSION
This is, to our knowledge, the largest study of HSV-2 seroprevalence in MENA. We reported the nationality-specific and age-specific seroprevalence for several national populations for the first time in the literature, and we used a 2-test algorithm using quality testing kits and confirmatory testing to avoid limitations in existing studies.8 We found that HSV-2 seroprevalence is in the range of few percentage points—on the low side compared with global levels.1,2,8,27 By world region, seroprevalence seems broadly in the range of 10% to 30% in Asia, 5% to 25% in Europe, 20% to 40% in Latin America, 15% to 25% in North America, and 10% to 70% in sub-Saharan Africa.1,2,8,27
The seroprevalence of HSV-2 tended to grow with age, as expected reflecting cumulative risk of exposure.1 Although there was some evidence for variation in seroprevalence by nationality, seroprevalence was overall similar. This is to be contrasted with HSV-1, which showed large variations in seroprevalence, by age and nationality, in this very same blood donor sample.13
Observed HSV-2 seroprevalence was comparable to that found in other MENA general population studies that used reasonable-quality diagnostics.8,19 Seroprevalence, however, was substantially less than that found in studies that used poor-quality diagnostics known for cross-reactivity with the highly prevalent HSV-1 antibodies.8 This affirms the need to insure use of quality type-specific diagnostics, to avoid generating clinically or epidemiologically noninterpretable, or even misleading results.
Only half of the HerpeSelect 2 ELISA positive results were confirmed positive by Euroline-WB. The proportion of those confirmed positive increased steadily with index value and was greater than 50% only for index values of greater than 3. These findings concur with earlier studies that used HerpeSelect 2 ELISA.20,21 Given the variation in test performance by population,21,22 our study provides useful data for the performance of this commonly used assay in MENA populations. They also testify to the importance of a 2-test algorithm,21 or the use of a different cutoff index value in interpreting results.21
Because HSV-2 seroprevalence can be seen as a proxy “biomarker” of sexual risk behavior in a population,9 our results suggest a lower risk behavior than that in most other regions. This finding is supported by studies of self-reported sexual behavior,27,28 notwithstanding the many limitations of such self-reported data.8 Importantly, this finding is supported by the generally lower levels of other STIs in MENA.27,28 Despite this, our study demonstrates that there are sexual networks where STIs are propagating. There is also likely an HSV-2 disease burden that needs to be addressed in a context of limited programs for sexual health and STIs.
There was overall a weak association between HSV-2 seroprevalence and age. This pattern remains to be explained, but we speculate that this may relate to statistical power with the small number of positives. Alternatively, the force of infection may be low in MENA resulting in a small prevalence and a more diffused seroprevalence age distribution.29 Another explanation is that successive birth cohorts may have had a variable experience in being exposed to HSV-2 in different eras, possibly because of variation in risk behavior over time.27
This study has limitations. The sample consisted of male blood donors with no population-based and probability-based sampling. Depending on the main outcome, nationality- versus age-specific seroprevalence, different sample selection methodologies were used, which may affect estimated seroprevalence. Although blood donation is a common practice in Qatar, blood donors are more likely to be a healthy population with possibly lower levels of sexual risk behavior and STIs. The seroprevalence HSV-2 is often 2-fold higher among women than among men,1–3,30 but our sample was exclusively from men. These considerations suggest that our estimated seroprevalence is possibly underestimating actual HSV-2 seroprevalence in the population at large.
Because all recruited individuals were residing in Qatar, we cannot be certain as to how representative these individuals are of citizens in their home countries. However, expatriates in Qatar are not permanent immigrants, most often stay for few years, and are predominantly recent residents. Because they have spent most of their lifetime in their home countries, their seroprevalence is probably representative of the exposure risk in their home countries, rather than in Qatar.
Very basic sociodemographic variables were collected, restricting our ability to assess relevant individual-level associations such as with sexual behavior. Collecting sexual behavior data is challenging in the MENA context—supporting the value of using HSV-2 (among other STIs) as proxy biomarkers of sexual risk behavior.8,9
Because of logistical considerations (regulations of shipping specimens and cost of conducting the University of Washington [UW] WB), we did not use the criterion standard UW-WB assay for serology testing. However, to minimize issues with diagnosis, we used a 2-test algorithm for identifying positive specimens, using 2 of the most commonly used commercial assays, including a WB assay that resembles the UW-WB assay.23,24 Moreover, used assays used different biological formats, and their performance has already been investigated independently with reference to UW-WB.20–24 Having said so, with the relative paucity of data on the Euroline-WB assay, it is of value to examine further the performance of this assay relative to the UW-WB.
In conclusion, this study fills a gap in HSV-2 regional and nationality-specific seroprevalence data. Seroprevalence in men was in the range of few percentage points—on the low side of the global range. Seroprevalence increased with age, but no major differences by nationality were identified. Performance of HerpeSelect 2 ELISA in MENA populations demonstrated similarities to its performance elsewhere, stressing the need for careful interpretation of the results by index value and the utility of a 2-test algorithm for diagnosis. These findings add to our understanding of STI epidemiology and sexual behavior in MENA. Despite the rather low HSV-2 seroprevalence, they also highlight the need for programs to tackle STIs and address broader sexual health needs.
Supplementary Material
Footnotes
Acknowledgments: The authors gratefully acknowledge the administrative support of Ms Adona Canlas. They are also grateful to Dr Asmaa Al-Marwani, Ms Maria Samatti, and Ms Sana Abohasera for their work on blood specimen collection. The authors are further grateful for support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine—Qatar.
Conflict of Interest and Sources of Funding: The authors have no conflicts of interest to disclose.
Contributors: L.J.A., G.K.N., and S.R.D. designed the study and developed the research methodology. G.K.N. provided the specimens and led the laboratory component of this study including testing of all specimens. L.I.M., R.S.A., M.Y.A., and E.S.A. conducted laboratory work on the specimens and contributed to data management. S.R.D. conducted the data analysis and interpretation of the results, and wrote the initial draft of the article. L.J.A. conceived the study and led the data analysis, interpretation of the results, and drafting of the article. All authors contributed to the interpretation of the results and drafting and revision of the article.
Funding: Testing kits were provided through pilot funding by the Biomedical Research Program at Weill Cornell Medicine—Qatar. G.K.N. acknowledges support by Qatar University internal grant No. QUST-CHS-SPR-15/16-7. L.J.A. and S.R.D. acknowledge study conception and design support through NPRP grant number 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation), and G.K.N. acknowledges support from the Qatar National Research Fund UREP grant number UREP18-001-3-001. The findings achieved herein are solely the responsibility of the authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J Infect Dis 2002; 186(Suppl 1):S3–S28. [DOI] [PubMed] [Google Scholar]
- 2.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes 2004; 11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 3.Looker KJ, Magaret AS, Turner KM, et al. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes KK. Sexually Transmitted Diseases. 4th ed New York: McGraw-Hill Medical, 2008. [Google Scholar]
- 5.Morse SA. Etiology of genital ulcer disease and its relationship to HIV infection. Sex Transm Dis 1999; 26:63–65. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008; 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis 2017; 17:1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Raddad LJ, Schiffer JT, Ashley R, et al. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics 2010; 2:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omori R, Abu-Raddad LJ. Sexual network drivers of HIV and herpes simplex virus type 2 transmission. AIDS 2017; 31:1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouymjian SP, Heijnen M, Chaabna K, et al. Population-level ecological association between HSV-2 prevalence and HIV prevalence: systematic review and meta-analyses. 2017. Submitted for publication. [Google Scholar]
- 11.Saba HF, Kouyoumjian SP, Mumtaz GR, et al. Characterising the progress in HIV/AIDS research in the Middle East and North Africa. Sex Transm Infect 2013; 89(Suppl 3):iii5–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Raddad LJ, Ghanem KG, Feizzadeh A, et al. HIV and other sexually transmitted infection research in the Middle East and North Africa: Promising progress? Sex Transm Infect 2013; 89(Suppl 3):iii1–iii4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasrallah GK, Dargham SR, Mohammed LI, et al. Estimating seroprevalence of herpes simplex virus type 1 among different Middle East and North African male populations residing in Qatar. J Med Virol 2018; 90:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Development Planning and Statistics. Qatar's Fourth National Human Development Report: Realising Qatar National Vision 2030. The Right to Development. Available at: http://www.gsdp.gov.qa/portal/page/portal/gsdp_en/knowledge_center/Tab2/NHDR4%20Complete%20Report%20English%20LowResolution%2028May2015.pdf). Ministry of Development Planning and Statistics, 2015.
- 15.AbuOdeh R, Al-Mawlawi N, Al-Qahtani AA, et al. Detection and genotyping of torque teno virus (TTV) in healthy blood donors and patients infected with HBV or HCV in Qatar. J Med Virol 2015; 87:1184–1191. [DOI] [PubMed] [Google Scholar]
- 16.AbuOdeh RO, Al-Absi E, Ali NH, et al. Detection and phylogenetic analysis of human pegivirus (GBV-C) among blood donors and patients infected with hepatitis B virus (HBV) in Qatar. J Med Virol 2015; 87:2074–2081. [DOI] [PubMed] [Google Scholar]
- 17.Al-Qahtani AA, Alabsi ES, AbuOdeh R, et al. Prevalence of anelloviruses (TTV, TTMDV, and TTMV) in healthy blood donors and in patients infected with HBV or HCV in Qatar. Virol J 2016; 13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasrallah GK, Al Absi ES, Ghandour R, et al. Seroprevalence of hepatitis E virus among blood donors in Qatar (2013–2016). Transfusion 2017; 57:1801–1807. [DOI] [PubMed] [Google Scholar]
- 19.Memish ZA, Almasri M, Chentoufi AA, et al. Seroprevalence of herpes simplex virus type 1 and type 2 and coinfection with HIV and syphilis: The first national seroprevalence survey in Saudi Arabia. Sex Transm Dis 2015; 42:526–532. [DOI] [PubMed] [Google Scholar]
- 20.Mujugira A, Morrow RA, Celum C, et al. Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect 2011; 87:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delany-Moretlwe S, Jentsch U, Weiss H, et al. Comparison of focus HerpesSelect and Kalon HSV-2 gG2 ELISA serological assays to detect herpes simplex virus type 2 antibodies in a South African population. Sex Transm Infect 2010; 86:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashley-Morrow R, Nollkamper J, Robinson NJ, et al. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect 2004; 10:530–536. [DOI] [PubMed] [Google Scholar]
- 23.Neal JD, Tobian AA, Laeyendecker O, et al. Performance of the Euroline Western blot assay in the detection of herpes simplex virus type 2 antibody in Uganda, China and the USA. Int J STD AIDS 2011; 22:342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingappa J, Nakku-Joloba E, Magaret A, et al. Sensitivity and specificity of herpes simplex virus-2 serological assays among HIV-infected and uninfected urban Ugandans. Int J STD AIDS 2010; 21:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Focus Diagnostics. HerpeSelect 1 ELISA IgG (English). 2011.
- 26.Euroimmun. Anti-HSV-1/HSV-2-gG2 Euroline-WB (IgG/IgM). 2011.
- 27.Abu-Raddad LJ, Akala FA, Semini I. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa: Time for strategic action. Middle East and North Africa HIV/AIDS Epidemiology Synthesis Project. World Bank/UNAIDS/WHO Publication. Washington, DC: The World Bank Press, 2010. [Google Scholar]
- 28.Abu-Raddad LJ, Hilmi N, Mumtaz G, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS 2010; 24(Suppl 2):S5–S23. [DOI] [PubMed] [Google Scholar]
- 29.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford University Press, 1991. [Google Scholar]
- 30.Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis 2014; 209:325–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


