Abstract
BACKGROUND:
Most previous reports to analyze risk factors for peritoneal recurrence in patients with colon cancer have been observational studies of a population-based cohort.
OBJECTIVE:
This study aimed to determine the risk factors for peritoneal recurrence in patients with stage II to III colon cancer who underwent curative resection.
DESIGN:
This was a pooled analysis using a combined database obtained from 3 large phase III randomized trials (N = 3714).
SETTINGS:
Individual patient data were collected from the Japanese Foundation for Multidisciplinary Treatment of Cancer clinical trials 7, 15, and 33, which evaluated the benefits of postoperative 5-fluorouracil–based adjuvant therapies in patients with locally advanced colorectal cancer.
PATIENTS:
We included patients who had stage II to III colon cancer and underwent curative resection with over D2 lymph node dissection.
MAIN OUTCOME MEASURES:
Main outcomes measured were risk factors for peritoneal recurrence without other organ metastasis after curative surgery.
RESULTS:
Peritoneal recurrence occurred in 2.3% (86/3714) of all patients undergoing curative resection. Mean duration from operation to peritoneal recurrence was 17.0 ± 10.3 months. Of these patients with peritoneal recurrence, 29 patients (34%) had recurrence in ≥1 other organ. Multivariate analysis showed that age (≥60 y: HR = 0.531; p = 0.0182), pathological T4 (HR = 3.802; p < 0.0001), lymph node involvement (HR = 3.491; p = 0.0002), and lymphadenectomy (D2: HR = 1.801; p = 0.0356) were independent predictors of peritoneal recurrence. The overall survival was lower in patients who developed peritoneal recurrence than in those with other recurrence (HR = 1.594; p = 0.002).
LIMITATIONS:
The regimens of adjuvant chemotherapy were limited to oral 5-fluorouracil.
CONCLUSIONS:
Our findings clarified the risk factors for peritoneal recurrence in patients who underwent curative resection for colon cancer. See Video Abstract at http://links.lww.com/DCR/A609.
Keywords: Colon cancer, Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy, Metachronous peritoneal carcinomatosis, Peritoneal recurrence
Approximately 14.1-million new cancer cases and 8.2-million cancer deaths occurred worldwide in 2012. Colorectal cancer is the third most common cancer (1.4-million cases, 9.7% of total) and the fourth most frequent cause of cancer death (694,000 deaths, 8.5%).1 In Japan, colorectal cancer is a major cause of death, accounting for the largest number of cancer deaths among women and the third largest number among men. Although surgical resection is the only curative treatment for colorectal cancer, postoperative recurrences often occur in patients who have undergone resection. The typical sites of local recurrence are divided into 4 groups: the anastomotic site, mesentery or nodal basin, retroperitoneum, and peritoneum.2
Peritoneal carcinomatosis (PC) is a rare pattern of recurrence after potentially curative resection, accounting for 3% to 6% of local recurrences, as reported in population-based cohort studies.3–5 Although several investigators have reported that complete cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) contributes to improved overall survival (OS) of patients with PC,6,7 the standard therapy for recurrent PC has not been established, and curative therapy is difficult to achieve, in contrast to liver metastases.
Most previous reports that analyzed risk factors for PC have been observational studies of a population-based cohort, examining patients who underwent noncurative resection for stage IV cancers, such as synchronous PC, including ovarian metastases, in addition to stages I to III. Population-based cohort studies are impeded by unspecified indications for treatment, heterogeneous surgery and chemotherapy, heterogeneous examination schedule, incomplete follow-up, and poor data reliability. Because recurrent PC after surgery for colon cancer is rare, inappropriate target population selection and unreliable clinical data would decrease the reliability of the results. For these reasons, we conducted our pooled analysis using a combined database of individual patient data obtained from 3 large phase III randomized trials to evaluate adjuvant chemotherapy for stages I to III to examine the risk factors for recurrent PC after curative surgery for stage II to III colon cancer.
PATIENTS AND METHODS
Patients
Individual patient data from the Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC) clinical trials 7, 15, and 33 were pooled for this analysis.8
JFMC Studies 7, 15, and 33
In JFMC trials 7 and 15, patients with locally advanced colorectal cancer were randomly allocated either to adjuvant chemotherapy with oral 5-fluorouracil (FU) or surgery alone.9 In the Japanese Classification of Colorectal Carcinoma, regional lymph nodes are classified into 3 groups according to their position: D1 (pericolic) nodes are situated close to the bowel wall, D2 (intermediate) nodes lie along the feeding arteries, and D3 (main) nodes are located at the origin of the feeding artery.10 In these trials, curative resection with over D2 lymph node dissection was performed. The study design was very similar for both trials. The main inclusion criteria were as follows: macroscopic Dukes B (invasion through the bowel wall penetrating the muscle layer but not involving lymph nodes) or Dukes C (involvement of lymph nodes) based on intraoperative judgment, age <75 years, and no severe complications. Between 1986 and 1990, the total of number of patients enrolled for JFMC 7 and 15 were 3394 and 2315. The adjuvant chemotherapy was a 1-year administration of oral 5-FUs (JFMC 7: 200 mg/d or 300 mg/d; JFMC 15: 300 mg/d). In the JFMC 33 trial, patients with advanced colorectal cancer were allocated to adjuvant tegafur plus leucovorin after surgery for 6 or 18 months.11 The main inclusion criteria were as follows: stage IIB (T4, N0, or M0) or stage III according to the TNM classification, 6th edition; age 20–75 years; an Eastern Cooperative Oncology Group performance status of 0 or 1; no previous chemotherapy or radiotherapy; and an ability to start postoperative adjuvant chemotherapy within 6 weeks after surgery. Between 2005 and 2007, 1071 patients were enrolled at 233 hospitals in Japan.
In the present pooled analysis, we included patients who had stage II to III colon cancer and underwent curative resection with over D2 lymph node dissection. In total, 3714 patients were examined (Fig. 1). Thus, we excluded all patients with rectal cancer, stage I colon cancer, or stage IV colon cancer with synchronous PC; ovarian metastasis; positive cytology; or other organ metastasis. The key findings of each trial have been published in peer-reviewed journals.
FIGURE 1.
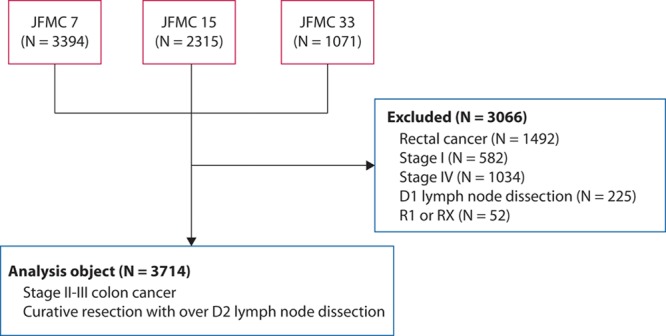
Flow diagram. We included patients who had stage II to III colon cancer and underwent curative resection with over D2 lymph node dissection. In total, 3714 patients were included in the present study.
Follow-up
After completion of the treatment protocol, patients were followed up, according to a schedule defined by each clinical trial protocol until recurrence or death, for 5 years after surgery. Briefly, recurrence was assessed based on CT scans that were carried out every 4 months during the first 2 years after surgery and once every 6 months from the third year onward. The peritoneal recurrence-free survival was determined from the date of surgery to the date of peritoneal recurrence for each patient. Recurrences attributed to other causes, death, or survival without recurrence were treated by censoring. OS was determined from the date of surgery to the date of death from any cause. All follow-ups were censored at 5 years from the surgery date. This study was conducted in accordance with ethical principles of the Declaration of Helsinki and approved by the institutional review board of JFMC.
Statistical Analysis
Baseline clinical and pathological variables were expressed as mean ± SD for continuous variables or numbers and proportions for categorical variables. For the primary analysis of peritoneal recurrence-free survival, we applied competing risk analysis, classifying all other recurrences with/without peritoneal recurrence or death as competing events, by the method of Fine and Gray.12 In multivariable analysis, prognostic factors for only peritoneal recurrence were selected using backward variable elimination with the exclusion criterion of p > 0.20. The Kaplan–Meier curve of the OS by recurrence sites (PC/not PC) was depicted using log-rank testing. In all of the analyses, statistical significances were defined as p < 0.05. All of the analyses were performed using SPSS version 19 software (IBM Corp, Armonk, NY) and SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
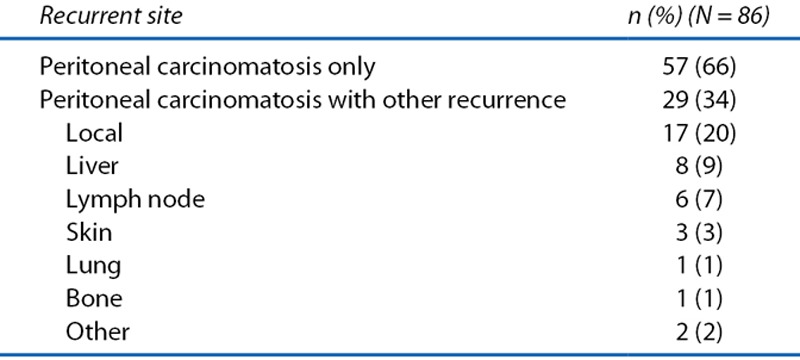
The clinicopathological parameters of the patients in the pooled analysis are shown in Table 1. The present cohort was characterized by the following: sigmoid colon was the major location, tumor depth was pT3 in most patients, lymph node involvement was observed in half, and 5-FU–based adjuvant chemotherapy was administered in ≈30%. Recurrent PC occurred in 2.3% of all patients (86/3714). Of the 86 patients with recurrent PC, 57 patients had PC alone, whereas 29 patients had ≥1 other organ recurrence in addition to PC (Table 2). The patients with recurrent PC and other organ metastasis had PC with liver (8%), lymph nodes (7%), skin (3%), and lung (1%).
TABLE 1.
Patient clinicopathological parameters
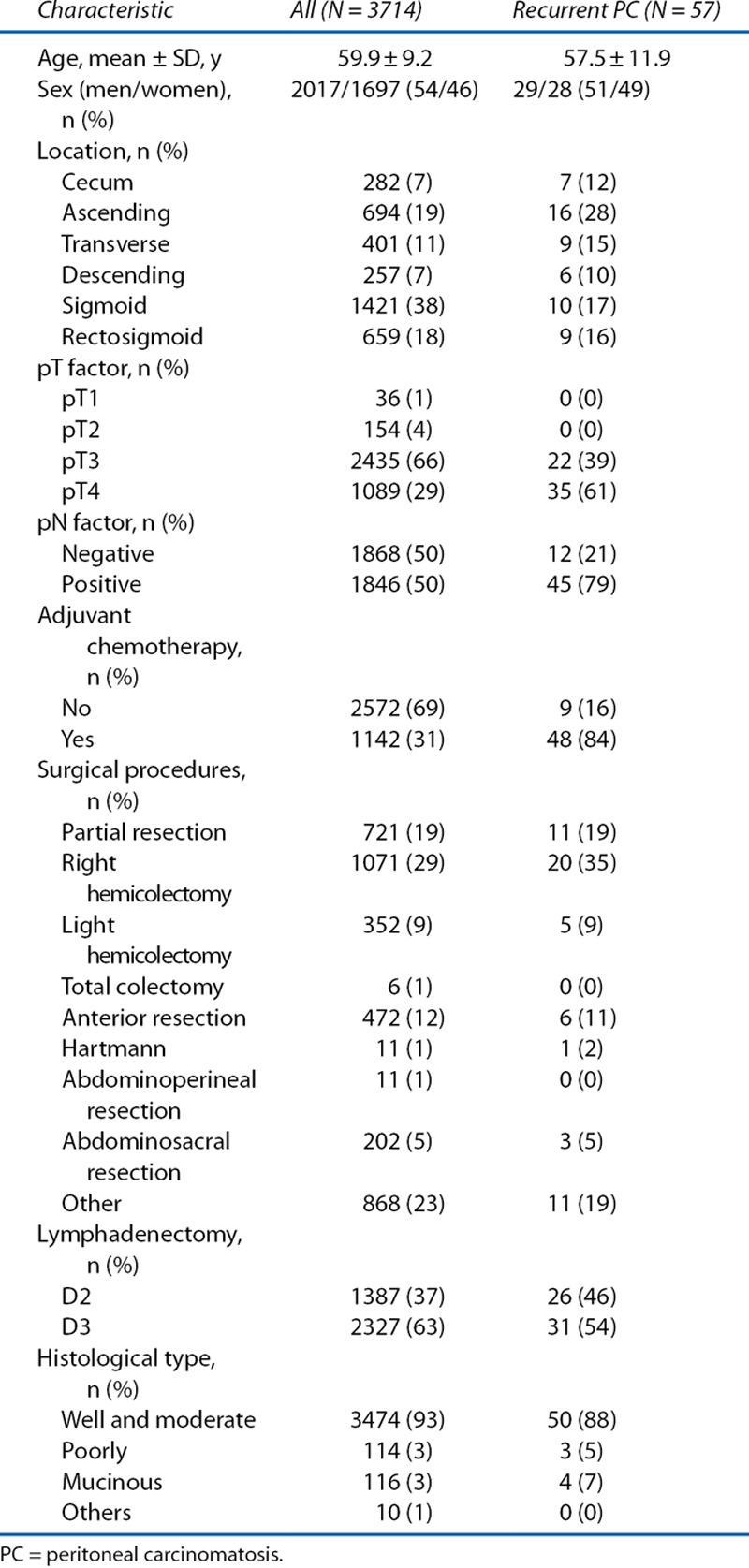
TABLE 2.
Recurrent site in patients with peritoneal carcinomatosis
Age, pT, lymph node involvement, histological type, and lymphadenectomy were found to influence recurrent PC. Finally, in multivariate analysis, age (≥60 y; HR = 0.531; p = 0.0182), pathological T4 (HR = 3.802; p < 0.0001), lymph node involvement (HR = 3.491; p = 0.0002), and lymphadenectomy (D2: HR = 1.801; p = 0.0356) were independent predictors of recurrent PC (Table 3). Patients receiving oral 5-FU–based adjuvant chemotherapy were not at reduced risk of recurrent PC. The OS was lower in patients with recurrent PC than in those with recurrence other than PC (HR = 1.594 (95% CI, 1.177–2.161); p = 0.002; Fig. 2).
TABLE 3.
Multivariate Cox Regression analysis for recurrent peritoneal carcinomatosis
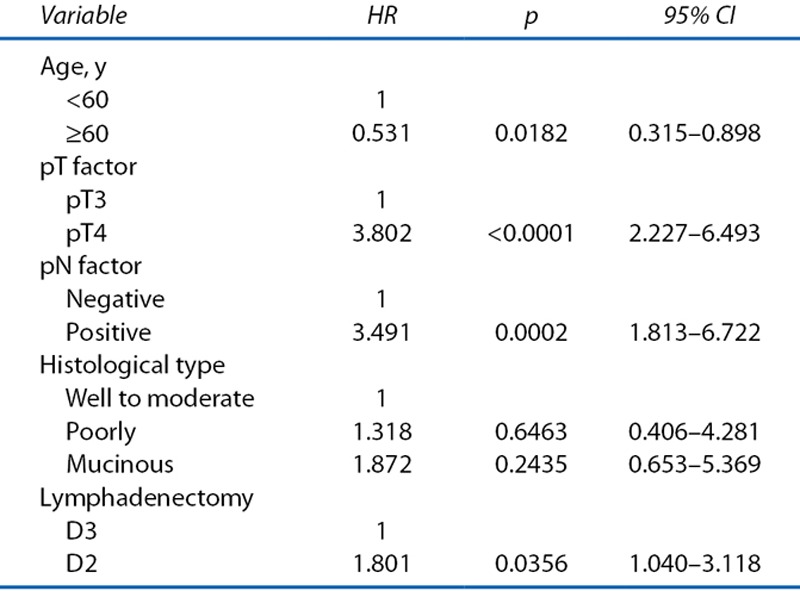
FIGURE 2.
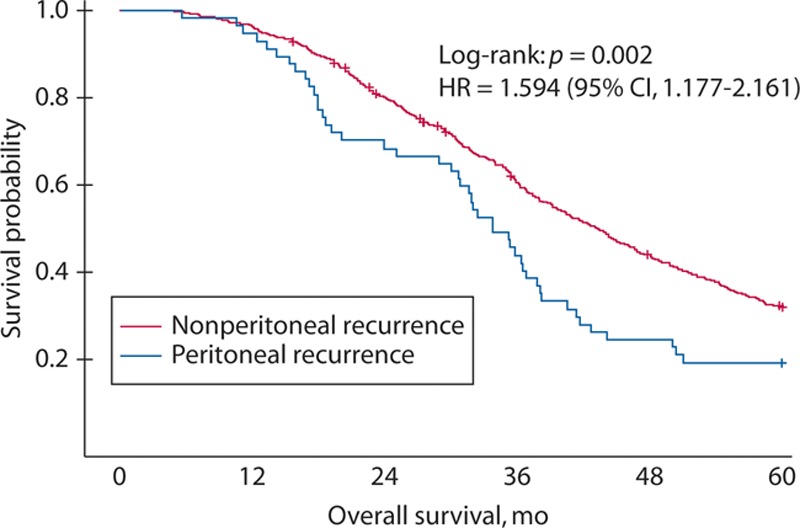
The Kaplan–Meier graph of overall survival (OS). The OS is lower among patients with recurrent peritoneal carcinomatosis than among those with other recurrence (p = 0.002).
DISCUSSION
The present study is the first to examine pooled individual patient data from 3 large phase III studies to identify risk factors for recurrent PC for stage II to III colon cancer. The study found that, in multivariate analysis, younger age, pT4, lymph node involvement, and D2 lymphadenectomy were associated with recurrent PC in patients who underwent curative resection for colon cancer. These results are useful to identify optimal subgroups for high risk of recurrent PC.
The planned second-look surgery followed by HIPEC is a useful diagnostic and therapeutic strategy for recurrent PC. Because second-look surgery is invasive, the indication of second-look surgery should be carefully selected. In the previously mentioned systematic review, the patients with T4 cancer were categorized as “low-risk for PC.13” In this review, however, the risk of recurrent PC in patients with T4 cancer was only relatively lower than those in patients with very high risk, for example, synchronous PC or ovarian metastases at primary surgery. Meanwhile, several reports showed that recurrent PC occurred frequently in 17% to 33% (HR, 1.93–9.98) of patients with T4 colorectal cancer.3–5 In our present study, HR for recurrent PC increased as pT factor progressed, and the HR of patients with pT4 tumors was high. Therefore, pT factor was one of the leading risk factors for recurrent PC, regardless of the histology of the primary tumor. Our result supported the planned second look (followed by cytoreductive surgery and HIPEC if positive and resectable PC) for patients with T4 cancer.
With respect to the lymph node involvement, the population-based cohort studies also reported that both T and N parameters,3,4 or the pathological stage of TNM classification,5 were risk factors for recurrent PC in patients with colorectal cancer after resection of primary tumors. Our results showed that both lymph node metastasis and D2 lymphadenectomy had a negative impact on recurrent PC. We calculated that ≈200 D3 lymphadenectomies were necessary to prevent 1 case of recurrent PC in all stage I to III patients on the basis of the 5-year PC recurrence proportions, 1.5% and 2.1% for D3 and D2 lymphadenectomy, estimated by Kaplan–Meier method, and 60 D3 lymphadenectomies per 1 case of recurrent PC in node-positive patients on the basis of the 5-year PC recurrence proportions, 2.3% and 3.8% for D3 and D2 lymphadenectomy. Although the contribution of D3 lymphadenectomy to prevent PC is not so high, the risk of D3 lymphadenectomy is almost similar to that of D2.14 Considering the risk and benefit of D3 lymphadenectomy, we recommended D3 lymphadenectomy for lymph node–positive patients.
We think that free cancer cells or tiny nodules that are disseminated from the primary colon cancer or extracapsular invasive lesion will enter the free peritoneal space. pT4 or lymph node–involved cancer has a higher incidence of dissemination than T2/T3 or lymph node–negative cancer. Sugarbaker7 reported a concept concerning the fact that local recurrence and PC have the same natural history. That was, at low density, these cancer cells will implant at a distance from the primary malignancy and develop into peritoneal metastases. Moreover, at higher density within the resection site, these free cancer cells or tiny nodules will implant and grow within the colon or rectal resection site.
With regard to histology of the tumor, several clinical and postmortem studies have already suggested that colorectal mucinous adenocarcinoma seems to metastasize more frequently to the peritoneum compared with other types of adenocarcinoma.15–17 Although the detailed mechanisms of peritoneal metastasis from mucinous colorectal adenocarcinoma have not been clarified, the production of mucus under pressure might allow cancer cells to separate tissue planes in the bowel wall and more frequently gain access to the peritoneal cavity.18 However, our study suggests that a mucinous adenocarcinoma subtype is not an independent risk factor for recurrent PC, and it might show no significant differences in recurrent PC because of a small proportion (3%) of mucinous adenocarcinoma in our cohort.
In our study, there was a significant proportion of patients with PC who had ≥1 other site involved with disease recurrence. As in previous reports, van Gestel et al19 indicated that, of the 197 patients with recurrent PC in their population-based study, the peritoneum was the only affected site in 81 patients (41%), whereas 62 patients (32%) had metastatic spread to the peritoneum and 1 other organ, and 54 patients (27%) presented with PC and metastases in ≥2 other organs. Furthermore, the patients with synchronous PC and 1 other organ metastasis had PC with liver (18%), lung (5%), lymph nodes (4%), and others (5%). As compared with our study, there was little difference regarding synchronous distant metastases at the time of PC recurrence. A large number of patients with recurrent PC had another organ metastasis, therefore we should give careful attention to the possibility of multiple organ metastases at the time of PC recurrence.
We found the incidence of recurrent PC to be 2.3% of all patients, which was lower than those in previous population-based cohort studies.3–5 Because the JFMC studies were designed to compare the effects of adjuvant chemotherapy with surgery alone for patients with colorectal cancer who underwent curative resection, all of the patients had undergone complete resection. Patients at high risk of recurrent PC, such as those with synchronous PC, ovarian metastasis, positive cytology, or tumor perforation, had not been considered eligible. In fact, recurrent PC was confirmed to be a relatively rare pattern of first recurrence in this pooled analysis using large-scale randomized controlled trial data. There were several reasons for a discrepancy of the incidence of recurrent PC between previous reports and our study. One of the factors affecting recurrent PC was tumor perforation. In our study, there was no perforated tumor patient compared with a considerable part of those in previous studies (2.0%–4.2%).4,5 Moreover, a relatively high rate of tumor obstruction (6.5%)5 and emergency operation (12.2%)4 would also have a negative effect for recurrent PC. The other reason was a positive resection margin. The patients with R1 resection were virtually absent in our cohort; however, those of previous studies were present at 3.0% to 10.5%.3,4
The present study had a major limitation regarding an adjuvant chemotherapy regimen. The regimens of adjuvant chemotherapy were limited to oral 5-FUs in JFMC trials. Because these trials were conducted in the 1980s to the 2000s, the currently preferred standard regimens, for example, oxaliplatin, leucovorin, and bolus and infusional fluorouracil or oxaliplatin and capecitabine, were not performed as adjuvant chemotherapies. Therefore, there would need to be additional evaluation of the appropriate adjuvant chemotherapy regimen to prevent recurrent PC.
CONCLUSION
Our findings clarified the risk factors for recurrent PC in patients with stage II to III colon cancer who underwent curative resection. These results will be useful to identify optimal subgroups for high risk of recurrent PC.
ACKNOWLEDGMENTS
This study was designed and organized by the Japanese Foundation for Multidisciplinary Treatment of Cancer Manuscript Writing Committee.
Footnotes
Funding/Support: This study was supported by Japanese Foundation for Multidisciplinary Treatment of Cancer and, in part, by the Epidemiological and Clinical Research Information Network.
Financial Disclosure: None reported.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.. [DOI] [PubMed] [Google Scholar]
- 2.Galandiuk S, Wieand HS, Moertel CG, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27–32.. [PubMed] [Google Scholar]
- 3.van Gestel YR, Thomassen I, Lemmens VE, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40:963–969.. [DOI] [PubMed] [Google Scholar]
- 4.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A.Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699–705.. [DOI] [PubMed] [Google Scholar]
- 5.Quere P, Facy O, Manfredi S, et al. Epidemiology, management, and survival of peritoneal carcinomatosis from colorectal cancer: a population-based study. Dis Colon Rectum. 2015;58:743–752.. [DOI] [PubMed] [Google Scholar]
- 6.Elias D, Honoré C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254:289–293.. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker PH.Update on the prevention of local recurrence and peritoneal metastases in patients with colorectal cancer. World J Gastroenterol. 2014;20:9286–9291.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayanagi S, Oba K, Hamada C, et al. Risk factors associated with recurrence by peritoneal dissemination or paraaortic lymph node metastasis after curative surgery in patients with colorectal cancer. Ann Cancer Res Ther. 2016;24:58–59.. [Google Scholar]
- 9.Hamada C, Sakamoto J, Satoh T, et al. Does 1 year adjuvant chemotherapy with oral 5-FUs in colon cancer reduce the peak of recurrence in 1 year and provide long-term OS benefit? Jpn J Clin Oncol. 2011;41:299–302.. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma. 2nd English ed. 2009Tokyo, Japan: Kanehara & Co, Ltd; [Google Scholar]
- 11.Sadahiro S, Tsuchiya T, Sasaki K, et al. Randomized phase III trial of treatment duration for oral uracil and tegafur plus leucovorin as adjuvant chemotherapy for patients with stage IIB/III colon cancer: final results of JFMC33-0502. Ann Oncol. 2015;26:2274–2280.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ.A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.. [Google Scholar]
- 13.Honoré C, Goéré D, Souadka A, Dumont F, Elias D.Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20:183–192.. [DOI] [PubMed] [Google Scholar]
- 14.Emmanuel A, Haji A.Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? A review of the literature. Int J Colorectal Dis. 2016;31:797–804.. [DOI] [PubMed] [Google Scholar]
- 15.Mekenkamp LJ, Heesterbeek KJ, Koopman M, et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer. 2012;48:501–509.. [DOI] [PubMed] [Google Scholar]
- 16.Catalano V, Loupakis F, Graziano F, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pande R, Sunga A, Levea C, et al. Significance of signet-ring cells in patients with colorectal cancer. Dis Colon Rectum. 2008;51:50–55.. [DOI] [PubMed] [Google Scholar]
- 18.Sugarbaker PH.Mucinous colorectal carcinoma. J Surg Oncol. 2001;77:282–283.. [DOI] [PubMed] [Google Scholar]
- 19.van Gestel YR, de Hingh IH, van Herk-Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014;38:448–454.. [DOI] [PubMed] [Google Scholar]


