Abstract
Aims
Vascular ageing is characterized by arterial stiffening, dilation, and arterial wall thickening. We investigated the extent to which these changes are related and their heritability during 5 year follow-up in the Twins UK cohort.
Methods and results
Carotid-femoral pulse wave velocity (PWVcf), carotid diameter, carotid distensibility, and carotid intima-media thickness (IMT) were measured in 762 female twins (mean age 57.9 ± 8.6 years) at two time-points over an average follow-up of 4.9 ± 1.5 years. Magnetic resonance imaging (MRI) was performed in a sub-sample of 38 women to measure aortic pulse wave velocity (PWVaorta), diameter, and wall thickness. Heritability of changes in arterial wall properties was estimated using structural equation modelling. Annual increases in PWVcf, carotid diameter, distensibility, and IMT were 0.139 m/s, 0.028 mm, −0.4 kPa−1, and 0.011 mm per year, respectively. In regression analysis, predictors of progression in PWVcf included age, mean arterial pressure (MAP), and heart rate (HR) at baseline, and progression in MAP, HR, and body mass index (BMI). Predictors of progression in IMT included progression in MAP, BMI, and triglyceride levels. Progression of PWV and distensibility correlated with progression in carotid diameter but not with IMT. Heritability of progression of PWVcf, diameter, and IMT was 55%, 21%, and 8%, respectively. In a sub-sample of women that underwent MRI, aortic wall thickness increased by 0.19 mm/year, but aortic wall thickening was not correlated with an increase in lumen diameter or PWVaorta.
Conclusion
Arterial stiffening, as measured by PWVcf, and dilation are heritable but independent of arterial wall thickening. Genetic and cardiovascular risk factors contribute differently to progression of PWV and IMT.
Keywords: Aortic stiffness, Wall thickness, Lumen diameter, Imaging
Introduction
Vascular aging of large arteries plays a major role in contributing to cardiovascular morbidity and mortality.1 Structural changes include an increase in wall thickness and lumen diameter.2–8 Intima-media thickening (IMT) is widely attributed to sub-clinical atherosclerosis and is independently associated with future cardiovascular events.9 The most marked functional change in large arteries with age is an increase in stiffness i.e. ‘stiffening’. Arterial stiffness, assessed using pulse wave velocity (PWV), is also a strong independent predictor of cardiovascular events1 and an important determinant of the age-related increase in systolic blood pressure and pulse pressure,1 components of blood pressure most closely associated with cardiovascular risk in middle-aged to older subjects.
The aetiology of arterial stiffening and wall thickening may differ with degenerative/calcific processes predominating in the former and atherosclerotic processes in the latter, and the extent to which age-related changes in these properties are related is uncertain. Existing cross-sectional data have reported inconsistent findings for the direction of association between progression of PWV and IMT.2,10–12 This may be due to the region over which arterial wall thickness, diameter, and stiffness are measured. Wall thickness and diameter are commonly measured in accessible superficial arteries such as the carotid artery using ultrasound techniques. In contrast, PWV is commonly measured between the carotid and femoral arteries including the majority of the aorta (carotid-femoral PWV, PWVcf). In addition, cross-sectional studies have limited power to detect direction of causality and are susceptible to reverse causality bias. Thus, the aim of this study was to investigate the relation between longitudinal changes in PWVcf, carotid diameter, and IMT over a 5 year follow-up period in the Twins UK cohort. In a sub-sample, wall thickness and PWV were measured over the aorta using magnetic resonance imaging (MRI). Using the Twins UK cohort, we were able to determine the extent to which arterial stiffening and carotid IMT may be due to genetic factors or exposure to environmental risk factors.
Methods
Subjects
Subjects were 762 asymptomatic female twins recruited from the Twins UK cohort. All subjects underwent an ultrasound exam of the left carotid artery to determine IMT and lumen diameter and measurement of PWVcf at baseline (between 2006 and 2012) and ∼5 years later (between 2009 and 2015). A sub-sample of 38 subjects additionally underwent an MRI scan at baseline (between 2010 and 2011) and ∼4 years later (between 2013 and 2015) to determine aortic PWV (PWVaorta), aortic wall thickness, and aortic lumen diameter. The study was approved by St Thomas’ Hospital Research Ethics Committee, and written informed consent was obtained from all subjects.
Clinical characteristics
Clinical characteristics recorded at baseline and at follow-up included height and weight, smoking status, menopausal status, medication, and comorbidities including presence of diabetes mellitus. Brachial blood pressure was measured with the participants in a supine position according to British and Irish Hypertension Society Guidelines using a validated automated oscillometric device (Omron, 705 IT, Omron Health Care, Japan). Measurements were taken after at least 5 min of rest supine and an average of three measurements was used. Fasting serum total cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol), triglycerides, and creatinine were measured in 634 participants and fasting glucose in 448 participants.
Arterial structure and function
All measurements were made in a single centre by a small team of observers who undertook a training programme to verify adequate reproducibility. The majority of the carotid measurements were undertaken by a single observer. Carotid-femoral pulse wave velocity was measured by ECG-referenced sequential tonometry of the carotid and femoral arteries using the SphygmoCor system (AtCor Medical, Australia), as previously described.13 The intra-observer coefficient of variation (CV) for repeated of measurements of carotid-femoral PWV was <5%; inter-observer (CV) was <8%. Carotid IMT was measured using high resolution ultrasound with the participant supine (Siemens CV70, Siemens, Erlangen, Germany and GE Health Care Vivid 7 Dimension, Wauwatosa, WI, USA with a 13-MHz and 12-MHz vascular probe, respectively). Intima-media thickness was measured during diastole in the anterior and posterior wall 1 cm proximal to the bifurcation over a length of 1–2 cm using automated software (Carotid analyser 5, Medical Imaging Applications, Coralville, IA, USA). This technique provides highly reproducible measurements with previously reported within subject SD < 0.02 mm and CV < 3%.14 In the present study, the intra-observer CV was <4%. Average measurements for the anterior and posterior wall were used for analysis. Carotid diameter and distensibility were obtained with the same software using automated wall tracking. Diameter was measured as the distance between the anterior and posterior wall during diastole. Carotid distensibility was measured as the relative change in carotid lumen area (ΔA/A) over the cardiac cycle for a given change in central aortic pulse pressure (cPP), as measured by the SphygmoCor system15,16: Distensibility = (ΔA/A)/cPP.
Magnetic resonance imaging
Magnetic resonance imaging was performed using a 1.5-T Achieva MR scanner (Philips Healthcare, Best, The Netherlands) with a five-element cardiac phased-array receiver coil. An oblique-sagittal single-slice segmented gradient-echo scout image was initially performed to identify the aorta as previously described.17 For measurement of PWV, contrast magnetic resonance was performed at the ascending aorta and proximal to the aortic bifurcation. One directional through-plane non-segmented velocity-encoding was applied perpendicular to the aorta at these two levels using free breathing with retrospective ECG gating.17 Flow velocity data were acquired at 4–7 ms intervals, and two signal averages were used to generate aortic flow-time data over the cardiac cycle. Aortic flow-time curves at the two levels were visualized using software developed in house in MATLAB (Release 2009 b, version 7.9; Mathworks, Natick, MA, USA). An intersecting tangent algorithm was applied to the curves to identify the ‘foot’ of the pulse wave at each level from which the ECG R-wave to pulse transit time for each level was determined and PWVaorta calculated by dividing the distance (measured as described below) between flow measures by the difference in pulse transit time.17
For measurement of distance between the two flow levels, aortic wall thickness and lumen diameter, the aorta was visualized by obtaining 66 transverse slices (no slice gaps) spanning from the aortic arch to the aortic bifurcation using zoom imaging (free-breathing, double inversion, black-blood, two-dimensional proton density weighted, and turbo-spin-echo sequence) as previously described.18 To maximize signal-to-noise, the cardiac coil was centred about the thoracic and abdominal aorta, respectively. Other imaging parameters included: pixel bandwidth 416 Hz, repetition time of 2 heart beats, shortest trigger delay (∼500 ms), inversion time ∼500 ms, echo time of 5.0 ms, 60 ms acquisition window, 12 lines per heartbeat, field of view 220 × 67, acquired matrix size 224 × 208 (acquired resolution 0.98 × 1.06 mm), slice thickness 5 mm, and partial Fourier imaging factor = 0.75.18 Thoracic imaging was respiratory-gated using an 8 mm navigator window, and compensation for respiratory motion in the abdominal aorta was achieved using two signal averages. The distance between the flow measures in the ascending thoracic aorta and abdominal aorta was determined from zoom imaging18 by placing a curved line along the centre of the aorta using a centreline plot and multiplanar curved reformat (View Forum; Philips Healthcare). For each cross-sectional slice, a circular region of interest was drawn around the inner and outer aortic wall using Osirix Medical Imaging software (Geneva, Switzerland; www.osirix-viewer.com) and the area for both recorded and averaged along the length of the aorta (ascending aorta to bifurcation). Average aortic lumen and outer adventitial diameters were calculated from the inner and outer areas assuming a circular cross-section and average aortic wall thickness calculated as the difference between inner and outer diameters divided by two. For regional analysis, the aorta was divided into the thoracic aorta (including the ascending aorta, aortic arch, and thoracic aorta above the level of the diaphragm) and abdominal aorta (including the aorta below the diaphragm and just above the aortic bifurcation). Average lumen diameter and wall thickness was calculated for each of these regions. Additional flow measurement at the level of the diaphragm was performed to calculate thoracic PWV (from ascending aorta to diaphragm) and abdominal PWV (from diaphragm to aortic bifurcation) as described above.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA). Subject characteristics are presented as means ± SD unless otherwise stated. Comparisons between measures at baseline and follow-up were made using Student’s paired t-test and McNemar’s χ2 test. Generalized estimating equations (GEE) were used to examine correlations of annual progression (taken as the difference between the first and second visits divided by time between visits) in PWVcf, carotid diameter, distensibility, and IMT (and corresponding MRI measures from the aorta), to conventional risk factors. Covariates included were age, height, weight, mean arterial pressure (MAP), heart rate (HR), total cholesterol, HDL-cholesterol, triglycerides, fasting glucose, antihypertensive treatment, lipid-lowering treatment, and type 2 diabetes mellitus treatment. Values of these variables at baseline and annual progression were included in the analysis. The contribution of risk factors to the progression of arterial measures was first assessed in univariate and then multivariate analysis adjusted for baseline measurements. Generalized estimating equations were also used to examine the inter-relationship of vascular measures and their progression. Pulsatile components of blood pressure were not included in the analysis because they are largely determined by PWV. Heritability was assessed in the total cohort for PWVcf, IMT, and carotid lumen diameter at baseline and for progression in these parameters using the classic twin model. Variance of a phenotype was attributed to additive genetic component, shared environmental, and unique environmental component. These components were estimated using structural equation modelling with corresponding confidence intervals (CIs) (Mx software, University of Virginia).10
Results
Subject characteristics at baseline and follow-up are shown in Table 1. The sample had a prevalence of hypertension similar to that in the Health Survey for England (HSE) 2009,19 the median year for baseline measurements in the present study, for women aged 55–65 (18% vs. 21%, for the present sampled compared to HSE) but lower body mass index (BMI) (26.0 vs. 28.2 kg/m2) and lower rate of current smoking (7.3 vs. 16.2%) compared to HSE. The average duration of follow-up was 4.9 ± 1.5 years. There was a significant increase, over the follow-up period in blood pressure, total cholesterol, HDL-cholesterol, antihypertensive therapy, and lipid-lowering therapy. The number of smokers decreased from 7% to 5%.
Table 1.
Subject characteristics at baseline and follow-up in the total cohort (n = 762)
| Baseline | Follow-up | P-value | |
|---|---|---|---|
| Age (years) | 57.9 ± 8.6 | 62.8 ± 8.6 | – |
| Height (cm) | 161.8 ± 6.2 | 160.9 ± 6.5 | <0.0001 |
| Weight (kg) | 68.3 ± 12.2 | 68.4 ± 13.0 | 0.454 |
| Current smoker (n [%]) | 56 [7.3] | 37 [4.9] | 0.002 |
| Type 2 diabetes (n [%]) | 7 [0.9] | 13 [1.7] | 0.07 |
| Antihypertensive therapy (n [%]) | 139 [18.2] | 189 [24.8] | <0.0001 |
| Lipid-lowering therapy (n [%]) | 91 [11.9] | 141 [18.5] | <0.0001 |
| Systolic blood pressure (mmHg) | 124.1 ± 16.2 | 129.5 ± 16.4 | <0.0001 |
| Diastolic blood pressure (mmHg) | 72.7 ± 8.5 | 75.7 ± 8.4 | <0.0001 |
| Mean arterial pressure (mmHg) | 91.9 ± 11.2 | 96.1 ± 11.0 | <0.0001 |
| Total cholesterol (mmol/L) | 5.66 ± 1.1 | 5.80 ± 1.1 | <0.05 |
| HDL-cholesterol (mmol/L) | 1.92 ± 0.5 | 2.04 ± 0.6 | <0.0001 |
| Triglycerides (mmol/L) | 1.01 ± 0.5 | 1.03 ± 0.5 | 0.543 |
| Glucose (mmol/L) | 5.08 ± 0.7 | 4.96 ± 0.9 | <0.0001 |
| Carotid intima-media thickness (mm) | 0.67 ± 0.13 | 0.72 ± 0.12 | <0.0001 |
| Carotid lumen diameter (mm) | 6.6 ± 0.7 | 6.8 ± 0.7 | <0.0001 |
| Carotid distensibility (0.4 kPa−1 × 10−3) | 26.6 ± 11.7 | 24.5 ± 11.0 | <0.0001 |
| Carotid-femoral pulse wave velocity (m/s) | 9.1 ± 1.8 | 9.8 ± 2.0 | <0.0001 |
Values represent means ± standard deviation; or number [percentage].
HDL, high-density lipoprotein.
Progression of arterial properties and relation to vascular risk factors
Over the follow-up period, PWVcf increased by 7.9% (annual increase of 0.139 m/s/year), carotid lumen diameter increased by 3% (annual increase of 0.028 mm/year), carotid distensibility decreased by 7.9% (annual decrease of 0.4 kPa−1 × 10−3/year), and carotid IMT increased by 7.4% (annual increase of 0.011 mm/year). The relation of progression in PWVcf, carotid distensibility, and IMT to cardiovascular risk factors (including values at baseline and progression over the follow-up period) in univariate and multivariable analysis is shown in Table 2. In multivariable analysis, progression of PWVcf was associated with age, MAP, and HR at baseline, and progression from baseline in MAP, HR, and BMI. There was also an association with added treatment for hypercholesterolaemia and smoking status over the follow-up period (Table 2). Progression in carotid distensibility was negatively associated with age and with MAP at baseline and progression in MAP. Progression of IMT was associated with progression in BMI and triglyceride levels and treatment for hypercholesterolaemia and negatively with progression in treatment for diabetes and hypertension.
Table 2.
Univariate and multivariable generalized estimating equations: relation of annual progression of pulse wave velocity, carotid distensibility, intima media, and thickness to traditional cardiovascular risk factors in the total cohort
| Univariate analysis |
Multivariate analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variable | ΔPWV |
ΔCD |
ΔIMT |
ΔPWV |
ΔCD |
ΔIMT |
||||||
| Beta | P-value | Beta | P-value | Beta | P-value | Beta | P-value | Beta | P-value | Beta | P-value | |
| Baseline measures | ||||||||||||
| Age (years) | 0.32 | <0.0001 | −0.37 | <0.0001 | 0.15 | <0.01 | 0.27 | <0.0001 | −0.24 | <0.0001 | ||
| MAP (mmHg) | −0.08 | 0.08 | −0.10 | <0.05 | 0.00 | 0.97 | 0.13 | <0.05 | −0.24 | <0.0001 | ||
| HR (b.p.m.) | −0.01 | 0.82 | −0.14 | <0.0001 | 0.00 | 0.90 | 0.12 | <0.01 | ||||
| BMI | 0.01 | 0.12 | −0.02 | <0.001 | 0.00 | 0.62 | ||||||
| HT treatment (y/n) | 0.18 | 0.08 | −0.32 | <0.0001 | −0.03 | 0.08 | −0.21 | <0.05 | −0.30 | <0.05 | ||
| T2DM (y/n) | 0.09 | 0.88 | 0.17 | 0.62 | 0.10 | <0.0001 | ||||||
| LL treatment (y/n) | 0.22 | 0.07 | −0.30 | <0.01 | −0.01 | 0.96 | ||||||
| Current smoker (y/n) | 0.16 | 0.27 | 0.08 | 0.53 | 0.14 | 0.20 | −0.32 | <0.05 | ||||
| TC (mmol/L) | 0.02 | 0.70 | 0.03 | 0.52 | 0.02 | 0.47 | −0.08 | <0.05 | ||||
| HDL (mmol/L) | −0.01 | 0.72 | 0.5 | 0.14 | 0.00 | 0.95 | ||||||
| TG (mmol/L) | 0.05 | 0.16 | −0.11 | <0.01 | 0.01 | 0.77 | ||||||
| Glucose (mmol/L) | 0.07 | 0.08 | −0.04 | 0.19 | 0.03 | 0.31 | ||||||
| Follow-up measures | ||||||||||||
| ΔMAP (mmHg) | 0.40 | <0.0001 | −0.30 | <0.0001 | 0.02 | 0.79 | 0.32 | <0.0001 | −0.28 | <0.0001 | ||
| ΔHR (b.p.m.) | 0.12 | <0.05 | 0.05 | 0.40 | −0.05 | 0.55 | 0.29 | <0.0001 | ||||
| ΔBMI | 0.13 | <0.01 | −0.09 | 0.11 | 0.01 | 0.99 | 0.12 | <0.05 | 0.21 | <0.0001 | ||
| ΔHT treatment (y/n) | −0.13 | <0.01 | −0.05 | 0.16 | −0.03 | 0.51 | −0.13 | <0.05 | ||||
| ΔT2DM (y/n) | 0.04 | 0.07 | 0.05 | 0.14 | −0.02 | 0.56 | −0.15 | <0.01 | ||||
| ΔLL treatment (y/n) | −0.01 | 0.90 | −0.03 | 0.49 | 0.02 | 0.57 | 0.10 | <0.05 | 0.13 | <0.01 | ||
| Δcurrent smoking (y/n) | −0.15 | <0.01 | 0.06 | 0.22 | −0.04 | 0.40 | −0.09 | <0.01 | ||||
| ΔTC (mmol/L) | 0.05 | 0.25 | −0.01 | 0.72 | 0.06 | 0.27 | ||||||
| ΔHDL (mmol/L) | 0.02 | 0.59 | −0.01 | 0.91 | 0.06 | 0.17 | ||||||
| ΔTG (mmol/L) | 0.13 | <0.05 | 0.01 | 0.83 | 0.06 | 0.30 | 0.10 | 0.07 | ||||
| ΔGlucose (mmol/L) | 0.07 | <0.05 | −0.04 | 0.27 | 0.04 | 0.28 | ||||||
Univariate analysis included was performed using the enter method. The association with annual progression in dependent variables was adjusted for baseline values. Multivariable analysis included all variables considered in univariate analysis using backward method. All analyses were adjusted for time between visits.
ΔBMI, annual progression in body mass index; ΔCD, annual progression in carotid distensibility; ΔHDL, annual progression in high-density lipoprotein; ΔHR, annual progression in heart rate; ΔHT, annual progression in hypertensive treatment; ΔIMT, annual progression in intima-media thickness; ΔLL treatment, annual progression in lipid-lowering treatment; ΔMAP, annual progression in mean arterial pressure; ΔPWV, annual progression in PWV; ΔT2DM, annual progression in type 2 diabetes mellitus; ΔTC, annual progression total cholesterol; ΔTG, annual progression in triglycerides; BMI, body mass index; HDL, high-density lipoprotein; HR, heart rate; HT, hypertensive treatment; LL treatment, lipid-lowering treatment; MAP, mean arterial pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides. Bold font indicates a P-value greater than 0.05.
Correlation of arterial stiffening, dilation, and thickening
At baseline, PWVcf correlated positively with IMT and carotid lumen diameter (r = 0.37 and r = 0.34 respectively, each P < 0.0001). These correlations were less marked but still significant when adjusted for age (r = 0.14 and r = 0.21, for correlations of PWVcf with IMT and carotid lumen diameter respectively, each P < 0.0001). Carotid distensibility correlated negatively with IMT and carotid lumen diameter (r = −0.35and r = −0.38 respectively, each P < 0.0001; r = −0.11 and r = 0.26, each P < 0.01, when adjusted for age). There was no significant correlation between progression in PWVcf and that of carotid IMT (r = 0.03, P = 0.36). There was, however, a significant correlation of PWVcf with progression in carotid diameter (r = 0.14, P < 0.0001). Similarly, there was no significant correlation between progression in carotid distensibility and that of carotid IMT (r = −0.04, P = 0.33), but there was a correlation with progression in carotid diameter (r = −0.41, P < 0.0001), so that local stiffness (as the inverse of distensibility) showed the same relation to carotid IMT and diameter as did PWVcf. Progression in carotid diameter was significantly associated with that of IMT (r = 0.17, P < 0.001). Multivariate regression analysis also showed that progression of PWVcf did not significantly correlate with baseline or progression in carotid IMT. This lack of correlation was observed despite a wide distribution of progression of IMT. Thus, when comparing subjects in the upper to lower quartiles of progression of IMT for which there was a greater than three-fold difference, there was no significant difference in progression of PWVcf (0.21 and 0.20 m/s per year for the lower and upper tertiles respectively with 95% CIs for difference between these values of −0.05 to 0.07 m/s per year, Figure 1). Progression in PWVcf remained significantly positively correlated with baseline and progression in carotid lumen diameter on multivariate analysis (P < 0.0001, Table 3). Corresponding relationships between progression of carotid distensibility, diameter, and IMT were similar to those observed on univariate analysis (Table 3).
Figure 1.
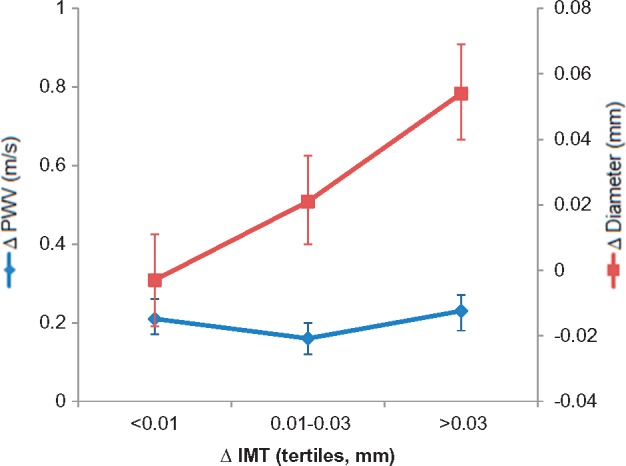
Annual progression in pulse wave velocity and carotid diameter (with corresponding 95% confidence intervals) with tertiles of progression in intima-media thickness.
Table 3.
Predictors of progression in carotid-femoral pulse wave velocity and carotid distensibility by generalized estimating equation analysis in the total cohort
| Δ cfPWV |
Δ carotid distensibility |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||||
| Variable | Beta | 95% CI | P-value | Beta | 95% CI | P-value | Beta | 95% CI | P-value | Beta | 95% CI | P-value |
| Carotid IMT | 0.03 | −0.07 to 0.13 | 0.59 | −0.02 | −0.12 to 0.08 | 0.68 | −0.11 | −0.20 to −0.16 | <0.05 | −0.06 | −0.16 to 0.04 | 0.22 |
| ΔCarotid IMT | – | – | – | 0.03 | −0.06 to 0.11 | 0.51 | – | – | – | −0.05 | −0.18 to 0.08 | 0.43 |
| Carotid diameter | 0.07 | −0.03 to 0.16 | 0.16 | 0.18 | 0.09 to 0.26 | <0.0001 | 0.08 | −0.01 to 0.18 | 0.08 | −0.02 | −0.12 to 0.08 | 0.68 |
| ΔCarotid diameter | – | – | – | 0.17 | 0.10 to 0.25 | <0.0001 | – | – | – | −0.18 | −0.38 to 0.02 | 0.07 |
| cfPWV | −0.41 | −0.53 to −0.28 | <0.0001 | −0.43 | −0.53 to −0.32 | <0.0001 | 0.04 | −0.04 to 0.12 | 0.30 | 0.09 | 0.10 to 0.18 | <0.05 |
| ΔcfPWV | – | – | – | – | – | – | – | – | – | 0.09 | −0.03 to 0.20 | 0.16 |
Model 1 included dependant variables at baseline (enter method). Model 2 included dependant variables both a baseline and progression (backward method). Adjusted for age, mean arterial pressure, heart rate, and time between visit and baseline parameter.
95% CI, 95% confidence intervals; cfPWV, carotid-femoral pulse wave velocity; IMT, intima-media thickness.
Aortic stiffening, dilation and thickening as assessed by magnetic resonance imaging
Characteristics of participants that underwent MRI are shown in Table 4. The average duration of follow-up was 3.6 ± 1.2 years. The number of women on treatment for hyperlipidaemia increased from 32% to 42% and low-density lipoprotein (LDL)-cholesterol decreased. There was no significant change in antihypertensive treatment or in blood pressure between the two visits. Over the follow-up period, the most marked structural change was an increase in aortic wall thickness which increased by 0.74 mm or 47% (P < 0.0001, Table 4) with no significant change in aortic lumen diameter (P = 0.69). There was no significant change in PWVaorta between the two visits. Thus despite the 47% increase in aortic wall thickness over the follow-up period, the mean increase in PWVaorta was −0.01% and the upper 95% CI for an increase in PWVaorta was 7.5%. Progression of PWVaorta was significantly positively correlated with progression in aortic lumen diameter (P < 0.01, Table 5) but not with wall thickness (Table 5).
Table 4.
Subject characteristics at baseline and follow-up in the subgroup that had an magnetic resonance imaging scan (n = 38)
| Visit 1 | Visit 2 | P-value | |
|---|---|---|---|
| Age (years) | 65 ± 6 | 69 ± 6 | – |
| Height (cm) | 161.3 ± 5 | 160.8 ± 6 | <0.05 |
| Weight (kg) | 64 ± 9 | 63 ± 7 | 0.078 |
| Antihypertensive therapy (n [%]) | 11 [29] | 11 [29] | 1 |
| Lipid-lowering therapy (n [%]) | 12 [32] | 16 [42] | <0.0001 |
| Systolic blood pressure (mmHg) | 132 ± 17 | 134 ± 19 | 0.365 |
| Diastolic blood pressure (mmHg) | 75 ± 10 | 75 ± 11 | 0.804 |
| Mean arterial pressure (mmHg) | 97 ± 13 | 97 ± 13 | 0.671 |
| Total cholesterol (mmol/L) | 5.65 ± 1.0 | 5.56 ± 1.0 | 0.638 |
| HDL-cholesterol (mmol/L) | 2.17 ± 0.6 | 2.20 ± 0.7 | 0.576 |
| Triglycerides (mmol/L) | 1.16 ± 0.7 | 1.09 ± 0.5 | 0.243 |
| Creatinine (μmol/L) | 71.5 ± 10 | 74.7 ± 8.8 | <0.01 |
| Mean wall thickness (cm) | 0.16 ± 0.02 | 0.23 ± 0.05 | <0.0001 |
| Mean inner wall diameter (cm) | 2.02 ± 0.14 | 2.03 ± 0.14 | 0.69 |
| Ratio wall thickness to inner diameter | 0.08 ± 0.01 | 0.11 ± 0.02 | <0.0001 |
| Mean outer wall diameter (cm) | 2.34 ± 0.15 | 2.49 ± 0.21 | <0.0001 |
| PWVaorta (m/s) | 7.74 ± 1.7 | 7.70 ± 1.5 | 0.85 |
Values represent means ± standard deviation; or number [percentage].
HDL, high-density lipoprotein; PWVaorta, aortic pulse wave velocity.
Table 5.
Predictors of progression in aortic pulse wave velocity by generalized estimating equations in the subgroup that had an magnetic resonance imaging scan (n = 38)
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Variable | Beta | 95% CI | P-value | Beta | 95% CI | P-value |
| PWVaorta | -0.65 | -1.02 to −0.28 | 0.001 | −0.70 | -1.01 to −0.40 | <0.0001 |
| Wall thickness | −0.13 | −0.37 to 0.12 | 0.31 | −0.11 | −0.36 to 0.13 | 0.36 |
| ΔWall thickness | – | – | – | 0.04 | −0.29 to 0.37 | 0.81 |
| Diameter | 0.01 | −0.13 to 0.16 | 0.86 | 0.01 | −0.29 to 0.29 | 0.99 |
| ΔDiameter | – | – | – | 0.37 | 0.10 to 0.64 | <0.01 |
Model 1 included dependant variables at baseline. Model 2 included dependant variables both a baseline and progression. Adjusted for age, MAP, HR, time between visit, and baseline parameter.
95% CI, 95% confidence interval; PWVaorta, aortic pulse wave velocity. Bold font indicates a P-value greater than 0.05.
Regional differences in aortic stiffening, dilation and thickening as assessed by magnetic resonance imaging
Wall thickness was higher in the thoracic compared abdominal aorta although this did not reach statistical significance (0.34 ± 0.07 cm vs. 0.32 ± 0.05 cm, respectively, P = 0.20). Aortic pulse wave velocity was not significantly different between the thoracic and abdominal aorta (7.88 ± 1.76 m/s vs. 8.23 ± 3.79 m/s for thoracic and abdominal aorta, respectively, P = 0.58). Over the follow-up period increase in wall thickness was significantly higher in the abdominal compared to thoracic aorta (wall thickness increased by 0.83 ± 0.66 mm in the abdominal aorta compared to 0.59 ± 0.60 mm in the thoracic aorta, P ≤ 0.01). In contrast, there was no significant regional difference in progression of diameter or PWVaorta.
Heritability of arterial properties and their progression
Heritability of cfPWV, diameter, and distensibility and IMT at baseline was estimated to be 38% (95% CI 16–63%), 42% (16–69%), 65% (39–72%) and 34% (9–50%), respectively. After adjustment for age, heritability estimates were 52% (23–70%), 47% (18–69%), 37% (20–50%), and 49% (17–63). Heritability of annual progression of cfPWV, diameter, distensibility, and IMT between the two visits (adjusted for baseline values) was estimated to be 58% (32–66%), 20% (0–52%), 44% (7–57%), and 7% (0–36%), respectively. After further adjustment for age, heritability estimates were 55% (31–64%), 21% (0–53%), 44% (14–57%), and 8% (0–36%), respectively.
Discussion
Although there are many cross-sectional data on the relationship of arterial stiffness to age20 there are few longitudinal data on progression of stiffness. The arterial stiffening we observed with PWVcf increasing by 0.14 m/s per year is within the range reported by Benetos et al.21 of annual increases of 0.08 and 0.15 m/s in 296 normotensive and 187 hypertensive subjects respectively over a 6 year follow-up. Lack of a significant increase in PWVaorta in the MRI subgroup may have been due to the relatively small sample size in this subgroup with 95% CIs for increase in PWVaorta of –0.48 to 0.58 m/s. Annual increases in carotid IMT, thoracic, and abdominal wall thickness of 0.01 mm/year, 0.15 mm/year, and 0.22 mm/year, respectively were consistent with previous studies where increase in carotid and aortic walls of between 0.0045 and 0.32 mm/year have been reported.3–5 In the Multi-Ethnic Study of Atherosclerosis, annual increase in aortic wall thickness was comparable to the present findings at 0.32 ± 0.46 mm/year over 10 year follow-up where measurement of wall thickness was confined to a single point in the thoracic aorta.2 Carotid artery diameter increased by 0.04 mm/year consistent with previous findings.6–8
To our knowledge, this is the first study to investigate the relation between longitudinal change in aortic stiffness, wall thickness, and diameter. Consistent with previous cross-sectional studies, we found a modest correlation between unadjusted PWV and measures of arterial wall thickness at baseline.2,12,22 However, a major finding of the present study was that there was no association between longitudinal progression of PWVcf and carotid IMT, nor between carotid distensibility and carotid IMT, nor between PWVaorta and aortic wall thickness as measured by MRI. Thus the results were consistent whether assessed locally (carotid distensibility vs. carotid IMT), regionally (aortic PWV vs. aortic wall thickening) or loco-regionally (carotid-femoral PWV vs. carotid IMT).
Previous cross-sectional studies have reported a differential association of PWVcf and IMT to traditional cardiovascular risk factors, with PWV being mainly associated with MAP and HR and IMT relating more closely to smoking and blood lipids.23–25 Our findings confirm a strong association between progression in PWVcf and longitudinal exposure to MAP and HR. Association of progression in PWVcf with baseline MAP is consistent with MAP being a determinant of PWV, rather than vice versa.26 However, we cannot exclude a bi-directional association of PWV with MAP. In addition to a differential relationship of arterial stiffening and thickening to conventional risk factors, heritability analysis demonstrated that stiffening is moderately heritably whereas thickening relates more to environmental factors.
Taken together, the disassociation of arterial stiffening and thickening, their differential relationship to classical risk factors and differing heritability points to a differing aetiology underlying these processes. Arterial thickening has generally been attributed to atherosclerosis, although it may also be influenced by smooth muscle hypertrophy in response to hypertension.27 From the ultrasound and MRI zoom imaging of the carotid artery and aorta, respectively, we are unable to distinguish whether changes in wall thickness result from atherosclerosis or vascular smooth muscle hypertrophy. However, the most marked increase in wall thickness was in the abdominal aorta, a site more predisposed to atherosclerosis than the thoracic aorta.7 Furthermore, in the MRI subgroup, there was no significant change in central blood pressure over the 4 year follow-up. This suggests that aortic wall thickening likely results from development of atherosclerosis rather than smooth muscle cell hypertrophy. In contrast to atherosclerosis, the process of arterial stiffening is thought to relate to degenerative change in the elastin component of the arterial wall accompanied by calcification.28 The association of progression of PWV with MAP and HR in the present study is consistent with degeneration of the arterial wall being influenced by mechanical stresses, as has been previously suggested.29 However, the relatively high heritability of arterial stiffening suggests a genetic predisposition to such mechanical factors and/or other mechanisms of degeneration.
Arterial dilation associated with ageing has been attributed to a common degenerative process driving both dilation and stiffening and the association of stiffening with dilation (again consistent whether assessed locally, regionally, or loco-regionally) that we observed in the present study would be consistent with this. Furthermore, the correlation between intrinsic stiffening of the artery and dilation may be more marked than between PWV and dilation, since dilation may offset an increase in PWV caused by intrinsic stiffening of the wall.17 However, we also found a significant correlation between dilation and thickening suggesting that dilation is driven by both degenerative and atherosclerotic processes. Local activation of matrix metalloproteinases by atherosclerotic plaque could be one mechanism implicated in this process.30 Arterial dilation may represent a compensatory process to preserve lumen diameter in the face of atherosclerotic thickening of the wall.31
Limitations
We recognize several limitations in the present study. The study is limited to female participant of the Twins UK cohort. However, this cohort has been shown to be comparable to women in the general population32 and observed prevalence of hypertension was similar to the latest Health Survey data for England, but we did observe lower rates for current smoking and BMI. Although regarded as the most meaningful measure of arterial stiffness, predictive of subsequent events, PWV is a functional measurement, influenced not only by the intrinsic stiffness of the arterial wall but by arterial lumen diameter and the thickness of the load-bearing part of the wall of the artery.33 Thus conclusions relating to progression of PWV are not necessarily applicable to intrinsic stiffening of the arterial wall as measured, for example, by progression in Young’s Elastic modulus. Progression/regression of carotid IMT does not necessarily relate to clinical events34 and the dissociation between progression of IMT and PWV that we observed would argue against progression of these measures being equally valuable predictors of cardiovascular events. Thus, the study should be interpreted in purely pathophysiological terms. Finally, these findings are limited to a 5 year follow-up period, with mean age at baseline 58 years. Relationships between arterial stiffening, dilation, and thickening at younger or older ages or during longer follow-up may differ.
In conclusion, in women from the Twins UK cohort, arterial stiffening was associated with arterial dilation but not with carotid IMT and, unlike carotid IMT was heritable. These findings suggest that the aetiology of arterial stiffening differs from atherosclerosis, and that there is a genetic predisposition to degenerative process linked to arterial stiffening around middle age.
Funding
British Heart Foundation Centre for Research Excellence Career Development Fellowship and a British Heart Foundation Special Project Grant SP/12/4/29573; Twins UK is funded by the Wellcome Trust, Medical Research Council, European Union, the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London.
Conflict of interest: none declared.
Footnotes
See page 2289 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy231)
References
- 1. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 2. Liu CY, Chen D, Bluemke DA, Wu CO, Teixido-Tura G, Chugh A, Vasu S, Lima JA, Hundley WG.. Evolution of aortic wall thickness and stiffness with atherosclerosis: long-term follow up from the multi-ethnic study of atherosclerosis. Hypertension 2015;65:1015–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stein JH, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, Chen W, Berenson GS.. Distribution and cross-sectional age-related increases of carotid artery intima-media thickness in young adults: the Bogalusa Heart Study. Stroke 2004;35:2782–2787. [DOI] [PubMed] [Google Scholar]
- 4. Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, Timaran CH.. Sex, race, and age distributions of mean aortic wall thickness in a multiethnic population-based sample. J Vasc Surg 2011;53:950–957. [DOI] [PubMed] [Google Scholar]
- 5. Li AE, Kamel I, Rando F, Anderson M, Kumbasar B, Lima JA, Bluemke DA.. Using MRI to assess aortic wall thickness in the multiethnic study of atherosclerosis: distribution by race, sex, and age. Am J Roentgenol 2004;182:593–597. [DOI] [PubMed] [Google Scholar]
- 6. Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB.. The relationship of age with regional aortic stiffness and diameter. JACC Cardiovasc Imaging 2010;3:1247–1255. [DOI] [PubMed] [Google Scholar]
- 7. O’Rourke MF, Nichols WW.. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 2005; 45:652–658. [DOI] [PubMed] [Google Scholar]
- 8. Redheuil A, Yu WC, Mousseaux E, Harouni AA, Kachenoura N, Wu CO, Bluemke D, Lima JA.. Age-related changes in aortic arch geometry: relationship with proximal aortic function and left ventricular mass and remodeling. J Am Coll Cardiol 2011;58:1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB Sr.. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med 2011;365:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cecelja M, Jiang B, Bevan L, Frost ML, Spector TD, Chowienczyk PJ.. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women. A twin study. J Am Coll Cardiol 2011;57:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zureik M, Temmar M, Adamopoulos C, Bureau JM, Courbon D, Thomas F, Bean K, Touboul PJ, Ducimetiere P, Benetos A.. Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens 2002;20:85–93. [DOI] [PubMed] [Google Scholar]
- 12. Taniwaki H, Kawagishi T, Emoto M, Shoji T, Kanda H, Maekawa K, Nishizawa Y, Morii H.. Correlation between the intima-media thickness of the carotid artery and aortic pulse-wave velocity in patients with type 2 diabetes. Vessel wall properties in type 2 diabetes. Diabetes Care 1999;22:1851–1857. [DOI] [PubMed] [Google Scholar]
- 13. Cecelja M, Jiang B, McNeill K, Kato B, Ritter J, Spector T, Chowienczyk P.. Increased wave reflection rather than central arterial stiffness is the main determinant of raised pulse pressure in women and relates to mismatch in arterial dimensions: a twin study. J Am Coll Cardiol 2009;54:695–703. [DOI] [PubMed] [Google Scholar]
- 14. Davis PH, Dawson JD, Riley WA, Lauer RM.. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation 2001;104:2815–2819. [DOI] [PubMed] [Google Scholar]
- 15. Cecelja M, Jiang B, Spector TD, Chowienczyk P.. Progression of central pulse pressure over 1 decade of aging and its reversal by nitroglycerin. A Twin Study. J Am Coll Cardiol 2012;59:475–483. [DOI] [PubMed] [Google Scholar]
- 16. Sinha MD, Keehn L, Milne L, Sofocleous P, Chowienczyk PJ.. Decreased arterial elasticity in children with nondialysis chronic kidney disease is related to blood pressure and not to glomerular filtration rate. Hypertension 2015;66:809–815. [DOI] [PubMed] [Google Scholar]
- 17. Abbas A, Cecelja M, Hussain T, Greil G, Modarai B, Waltham M, Chowienczyk PJ, Smith A.. Thoracic but not abdominal phase contrast magnetic resonance-derived aortic pulse wave velocity is elevated in patients with abdominal aortic aneurysm. J Hypertens 2015;33:1032–1038. [DOI] [PubMed] [Google Scholar]
- 18. Hussain T, Clough RE, Cecelja M, Makowski M, Peel S, Chowienczyk P, Schaeffter T, Greil G, Botnar R.. Zoom imaging for rapid aortic vessel wall imaging and cardiovascular risk assessment. J Magn Reson Imaging 2011;34:279–285. [DOI] [PubMed] [Google Scholar]
- 19. Mindell J, Biddulph JP, Hirani V, Stamatakis E, Craig R, Nunn S, Shelton N.. Cohort profile: the Health Survey for England. Int J Epidemiol 2012;41:1585–1593. [DOI] [PubMed] [Google Scholar]
- 20. Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L.. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002;105:1202–1207. [DOI] [PubMed] [Google Scholar]
- 22. Brandts A, Westenberg JJ, van Elderen SG, Kroft LJ, Roes SD, Tamsma JT, van der Geest RJ, Lamb HJ, Doornbos J, Putter H, Stuber M, de Roos A.. Site-specific coupling between vascular wall thickness and function: an observational MRI study of vessel wall thickening and stiffening in hypertension. Invest Radiol 2013;48:86–91. [DOI] [PubMed] [Google Scholar]
- 23. Cecelja M, Chowienczyk P.. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009;54:1328–1336. [DOI] [PubMed] [Google Scholar]
- 24. Takahara M, Katakami N, Osonoi T, Saitou M, Sakamoto F, Matsuoka TA, Shimomura I.. Different impacts of cardiovascular risk factors on arterial stiffness versus arterial wall thickness in japanese patients with type 2 diabetes mellitus. J Atheroscler Thromb 2015;22:971–980. [DOI] [PubMed] [Google Scholar]
- 25. Rosvall M, Persson M, Ostling G, Nilsson PM, Melander O, Hedblad B, Engstrom G.. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: the Malmo Diet and Cancer Study. Atherosclerosis 2015;239:615–621. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 2014;64:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhailidis DP, Naka KK, Protogerou AD, Rizzoni D, Schmidt-Trucksäss A, Van Bortel L, Weber T, Yamashina A, Zimlichman R, Boutouyrie P, Cockcroft J, O’Rourke M, Park JB, Schillaci G, Sillesen H, Townsend RR.. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015;241:507–532. [DOI] [PubMed] [Google Scholar]
- 28. Cecelja M, Chowienczyk P.. Arterial stiffening: cause and prevention. Hypertension 2010;56:29–30. [DOI] [PubMed] [Google Scholar]
- 29. McEniery CM, Spratt M, Munnery M, Yarnell J, Lowe GD, Rumley A, Gallacher J, Ben-Shlomo Y, Cockcroft JR, Wilkinson IB.. An analysis of prospective risk factors for aortic stiffness in men: 20-year follow-up from the Caerphilly prospective study. Hypertension 2010;56:36–43. [DOI] [PubMed] [Google Scholar]
- 30. Yasmin McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB.. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 2005;25:372. [DOI] [PubMed] [Google Scholar]
- 31. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ.. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371–1375. [DOI] [PubMed] [Google Scholar]
- 32. Andrew T, Hart DJ, Snieder H, de Lange M, Spector TD, MacGregor AJ.. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res 2001;4:464–477. [DOI] [PubMed] [Google Scholar]
- 33. O’Rourke MF, O’Brien C, Edelman ER.. Arterial stiffening in perspective: advances in physical and physiological science over centuries. Am J Hypertens 2016;29:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Costanzo P, Perrone-Filardi P, Vassallo E, Paolillo S, Cesarano P, Brevetti G, Chiariello M.. Does carotid intima-media thickness regression predict reduction of cardiovascular events? A meta-analysis of 41 randomized trials. J Am Coll Cardiol 2010;56:2006–2020. [DOI] [PubMed] [Google Scholar]


