Abstract
Naturally occurring variations in maternal licking/grooming influence neural development and are transmitted from mother to female offspring. We found that the induction of maternal behavior in virgin females through constant exposure to pups (pup sensitization) was significantly shorter in the offspring of High compared with Low licking/grooming mothers, suggesting differences in maternal responsivity. In randomly selected females screened for individual differences in maternal responsivity and subsequently mated, there was a significant and negative correlation (r = −0.73) between the latency to exhibit maternal behavior in the pup sensitization paradigm and the frequency of pup licking/grooming during lactation. Females that were more maternally responsive to pups and that showed increased levels of pup licking/grooming also showed significantly higher oxytocin receptor levels in the medial preoptic area, the lateral septum, the central nucleus (n.) of the amygdala, the paraventricular n. of the hypothalamus, and the bed n. of the stria terminalis. Intracerebroventricular administration of an oxytocin receptor antagonist to mothers on postpartum day 3 completely eliminated the differences in pup licking/grooming, suggesting that differences in oxytocin receptor levels are functionally related to maternal behavior. Finally, estrogen treatment of virgin females significantly increased oxytocin receptor binding in the medial preoptic area and lateral septum of female offspring of High, but not Low, licking/grooming mothers. These findings suggest that maternal licking/grooming influences the development of estrogen sensitivity in brain regions that regulate maternal behavior, providing a potential mechanism for the intergenerational transmission of individual differences in maternal behavior.
Naturally occurring variations in maternal care influence the development of behavioral, endocrine, and cognitive stress responses in the rat (1–4). As adults, the offspring of mothers that show increased levels of pup licking/grooming and arched-back nursing (i.e., High LG-ABN mothers) exhibit more modest hypothalamic-pituitary-adrenal and behavioral responses to stress, and are more proficient in learning to navigate an escape path in the Morris water maze compared with the offspring of Low LG-ABN mothers. The results of cross-fostering studies suggest that the differences in maternal behavior are critical: On each of the measures mentioned above, the biological offspring of Low LG-ABN mothers reared by High LG-ABN dams resembled the normal offspring of High LG-ABN mothers (3, 4). Individual differences in maternal behavior show a comparable pattern of transmission across generations. Thus, the adult, female offspring of High LG-ABN mothers are, themselves, High LG-ABN mothers, whereas those of Low LG-ABN dams are Low LG-ABN mothers. The pattern is reversed with cross-fostering (3). These findings suggest that individual differences in stress reactivity and maternal care can be transmitted across generations through a behavioral mode of transmission linked to variations in maternal behavior.
One obvious question concerns the origin of these variations in maternal behavior. Female rats, unless they are in late pregnancy or lactating, generally show an aversion toward pups (5–9). The novelty of the pups is the source of aversion and is typical of the generally neophobic adult rat. The onset of maternal behavior clearly depends on decreasing the negative-withdrawal tendency associated with neophobia and increasing the positive-approach responses (6, 7, 9). A hormonal regimen that mimics the changes in estrogen and progesterone occurring in late pregnancy and parturition reduces the fear of novelty (10, 11) and facilitates the expression of maternal behavior in the rat (5, 12). In virgin females, habituation to the novelty, through continuous exposure to pups (i.e., the pup sensitization paradigm) ultimately results in the onset of maternal behavior even in the absence of hormonal priming (5, 8, 12). Interestingly, High LG-ABN mothers are significantly less fearful under conditions of novelty than are Low LG-ABN mothers (13).
In the natural condition, the relevant hormonal events occur in the latter phase of pregnancy and include an increase in estrogen levels, which is obligatory for the onset of maternal behavior (5, 12). Estrogen enhances the expression of maternal behavior (5, 7, 14, 15), and this effect is mediated, in part at least, by effects on central oxytocinergic systems (6, 16). Estrogen increases oxytocin receptor binding. Intracerebroventricular (ICV) administration of oxytocin rapidly stimulates maternal behavior in virgin rats (17, 18). The effect of oxytocin is abolished by ovariectomy and reinstated with estrogen treatment. Moreover, treatment with oxytocin-antisera or receptor antagonists blocks the effects of ovarian steroid treatments on maternal behavior (19–21). Oxytocin infusion directly into the medial preoptic area (MPOA) increases the expression of maternal behavior (22). Interestingly, chronic elevations in oxytocin are associated with a potent anxiolytic effect (23–25), and these effects are enhanced by estrogen (11). The estrogen-regulated increase in oxytocin activity in critical brain regions appears to produce a decreased level of fearfulness and increased attraction toward pup-related stimuli that are critical for the transition of the female rat to a high state of maternal responsivity (6, 7). Interestingly, lactating High LG-ABN mothers exhibit increased levels of oxytocin receptor binding in the MPOA, as well as in the lateral septum and the bed nucleus (n.) of the stria terminalis by comparison with lactating Low LG-ABN mothers (13), and these regions have been implicated in the expression of maternal behavior in the rat (7).
In the following studies, we examined the relationship among maternal responsivity in the pup sensitization paradigm, maternal behavior during lactation, and oxytocin receptor levels. The results suggest that differences in the maternal behavior of High and Low LG-ABN mothers are associated with differences in maternal responsivity to pups and that the mechanism for these differences involves alterations in estrogen-inducible oxytocin receptor levels in brain regions that mediate the expression of maternal behavior in the rat.
Materials and Methods
Animals.
The animals used were Long–Evans hooded rats (derived from animals obtained from Charles River Breeding Laboratories). Unless otherwise specified, the animals were born in our colony and, as adults, were housed in 46 cm × 18 cm × 30 cm Plexiglas cages that permitted a clear view of all activity within the cage. Food and water were provided ad libitum. The colony was maintained on a 12:12 light:dark schedule with lights on at 0800. The animals underwent routine cage maintenance beginning on day 12 of life, but were otherwise unmanipulated. All procedures were performed according to guidelines developed by the Canadian Council on Animal Care and protocols approved by the McGill University Animal Care Committee.
At the time of weaning on day 22 of life, the offspring were housed in same-sex, same-litter groups of two to three animals per cage until day 45 of life, and two animals per cage from this point until the time of testing, which occurred no earlier than 100 days of age. After mating and throughout lactation, adult females were housed singly. Likewise, except for mating, animals were housed singly after surgical interventions. All experiments were performed by individuals who were blind to the developmental history of the animals.
Maternal Behavior.
We examined maternal behavior in dams by using a version of the procedure developed by Myers et al. (26) and previously described (1). The behavior of each dam was observed for six 75-minute observation periods daily for the first 6 to 8 days postpartum, depending on the experiment. Observers were trained to a high level of interrater reliability (i.e., >0.90). Observations occurred at regular times each day with four periods during the light (0900, 1200, 1500, and 1800) and two periods during the dark (0600 and 2000) phases of the light:dark cycle. Within each 75-minute observation period, the behavior of each mother was scored every 3 min (25 observations/period × six periods per day = 150 observations/mother per day) for the following behaviors: mother off pups, mother carrying pup, mother licking and grooming any pup, or mother nursing pups in either an arched-back posture, a “blanket” posture in which the mother lays over the pups, or a passive posture in which the mother is lying either on her back or side while the pups nurse. A detailed description of these behaviors is provided in Myers et al. (26).
The frequency of maternal licking/grooming and arched-back nursing across a large number of mothers is normally distributed (A. Mar, F.C., C. Francis, and M.J.M., unpublished results). Hence, High and Low LG-ABN mothers represent two ends of a continuum, rather than distinct populations. To define these populations for the current studies, we observed the maternal behavior in cohorts of mothers, ranging 30–40 dams with their pups, and devised the group mean and standard deviation for each behavior over the first 8 days of life as previously described (1–4). For the cohorts used in these studies, the mean ± SEM percentage of licking/grooming across the first 8 days postpartum was 10.2 ± 2.1. High licking/grooming mothers were defined as females whose frequency scores for both licking/grooming and arched-back nursing were greater than 1 SD above the mean. Low licking/grooming mothers were defined as females whose frequency scores for both licking/grooming and arched-back nursing were greater than 1 SD below the mean.
Maternal Responsivity Testing.
We compared maternal responsivity in the adult, virgin female offspring of High and Low LG-ABN mothers by using the pup sensitization paradigm (see ref. 27). The choice of subjects was based on the finding that differences in maternal behavior are reliably transmitted across generations (3, 13). Four days before the start of behavioral testing, Plexiglas dividers were inserted into the home cage (30 × 45 cm) of individually housed adult female offspring of High or Low LG-ABN mothers. The dividers were 3.5 cm high and separated the cage into four equally sized quadrants. The dividers limited the movements of the pups to the quadrant. Animals were tested daily between 1000 and 1200. To initiate the test session, three recently fed, 3- to 6-day-old pups were placed in the three quadrants (one pup per quadrant) away from the nest site. The test animals were observed continuously for 1 h for pup retrieval of the young, crouching over the young in a nursing position, and pup licking/grooming. On test days 2–12, pups from the previous session were removed, and, 30 min later, a new set of recently fed pups of the same age were introduced into each test cage, thereby commencing another 1-h test session. Testing continued for 12 consecutive days or until a female displayed full maternal behavior on 2 consecutive days, whichever occurred first. Animals were scored as fully maternal if they retrieved all three test pups to the nest, grouped them in the nest, crouched over them, and lick/groomed within the 60-min test session. The latency of the animal to exhibit a maternal response was based on the test session in which the response was observed. For example, if an animal responded on test day 1, then the latency to exhibit that response is 0 days. Animals failing to respond within the 12-day test period were assigned a score of 11.
Oxytocin Receptor Autoradiography.
Slide-mounted sections were processed for receptor autoradiography by using [125I]d(CH2)5[Tyr(Me)2,Tyr-NH29]-OVT (New England Nuclear) as previously described (13, 28, 29). After a prewash in Tris⋅HCl (pH 7.4), slides were exposed to a 75-min incubation (at room temperature) of 60 pM [125I]OVT in Tris with MgCl (10 mM), BSA (0.1%), and bacitracin (0.05%). Nonspecific binding was defined in adjacent sections by adding 50 nM Thr4Gly7-OT to the incubation buffer. The final 35-min wash was performed at room temperature in 50 mM Tris (pH 7.4), 100 mM MgCl2 to reduce background. After air drying, the slides were exposed to BioMax MR film (Kodak) for 48 h. 125I autoradiographic standards (Amersham Pharmacia) were included in the cassette for quantification. The autoradiograms were analyzed by using an image-analysis system (MC1D-4, Imaging Research, St. Catherine's, ON, Canada). Three sections were analyzed bilaterally at each level. For each experiment, we ensured that the optical densities of all images were within the linear range of the film. For each rat, total and nonspecific binding were measured for each region, and the difference was taken to yield specific binding. Specific binding was greater than 90% of total binding. The statistical analysis was performed on the mean of these values for each animal by brain region according to the atlas of Swanson (30). Comparison was performed by applying two-way ANOVA (group × brain region).
ICV Cannulae Implantation and Oxytocin Antagonist (OTA) Infusion.
Previously confirmed High and Low LG-ABN mothers were anesthetized and implanted with stainless steel cannulae into the left lateral ventrical (1.5 mm posterior to bregma, 2.0 mm lateral to midline, and 3.0 mm ventral to the brain surface) as previously described (19–21). Guide cannulae were secured with dental cement and three stainless steel screws and kept patent with 30-gauge stainless steel stylets. After a 1-week recovery period, females were mated and allowed to give birth. Maternal observations were performed over the first 6 days after parturition. On day 3 postpartum, females were removed from their cages and infused with either an oxytocin receptor antagonist, [d(CH2)5,Tyr(Me)2,Thr4,Tyr-NH29]-OVT (Peninsula Laboratories), or saline at a rate of 1 μl/min. Infusion cannulae were left in place for an additional minute after infusion. After a 20-min recovery period, females were returned to their home cage. Observations of mother-pup interactions resumed 1 h after infusion.
Ovariectomy and E2 Replacement.
Animals were anesthetized and bilaterally ovariectomized. At the time of surgery, the animals were implanted s.c. with a single silastic capsule (Dow Corning; 0.0062 inner diameter (ID), 0.125 outer diameter (OD); 10 mm in length per 100 g body weight) containing 0, 3, 10, or 20 μg estradiol benzoate (β-estradiol 3 benzoate; Sigma) dissolved in peanut oil vehicle. Four days after ovariectomy, animals were killed by rapid decapitation. The 4-day treatment period was selected on the basis of previous studies examining the effects of estrogen on oxytocin receptor binding (e.g., refs. 6, 11, and 28). Brains were collected on ice and frozen for processing for in vitro receptor autoradiography (see above). Blood samples obtained at sacrifice were then assayed for estradiol levels by using an RIA kit obtained from ICN and [125I]estradiol as tracer.
Results
Maternal Responsivity.
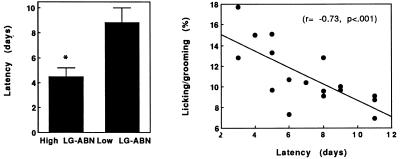
We first examined the latency to exhibit maternal behavior in the virgin female offspring of High and Low licking/grooming mothers by using the pup sensitization paradigm (Fig. 1 Left). The latency for the onset of full maternal behavior (retrieval of pups, crouching over pups, licking/grooming) was significantly (t = 3.2; df = 1,15; P < 0.01) shorter in the female offspring of High compared with Low LG-ABN mothers.
Figure 1.
(Left) Mean ± SEM latency (days) to exhibit maternal behavior in the virgin female offspring of High (n = 8) and Low (n = 8) licking/grooming mothers. *, P < 0.01. (Right) The percentage of the total number of observations across the first 6 days postpartum in which lactating mothers were observed to be engaged in pup licking/grooming as a function of the latency to exhibit full maternal responsivity in the pup sensitization paradigm in the same animals.
We then reasoned that, if naturally occurring variations in maternal care are associated with differences in maternal responsivity, then individual differences in the latency to express maternal behavior in the pup sensitization paradigm among randomly selected females should predict variations in maternal care during lactation. We tested this idea by obtaining virgin females from the local breeder (Charles River Breeding Laboratories Canada) at 70 days of age. The females were screened (by using the maternal responsivity test), mated, and allowed to give birth. The mean latency for the onset of maternal behavior across all females was 7.0 days, with a range from 3 to 11 days as is commonly reported in the literature (8). All females were mated, and observations of maternal behavior were then performed over the first 6 days postpartum. The results revealed a significant correlation (r = −0.73; P < 0.001; see Fig. 1 Right) between the latency to exhibit maternal behavior in the pup sensitization paradigm and pup licking/grooming during lactation; Virgin females that were more readily responsive to pups emerged as High licking/grooming mothers.
Oxytocin Receptor Binding.
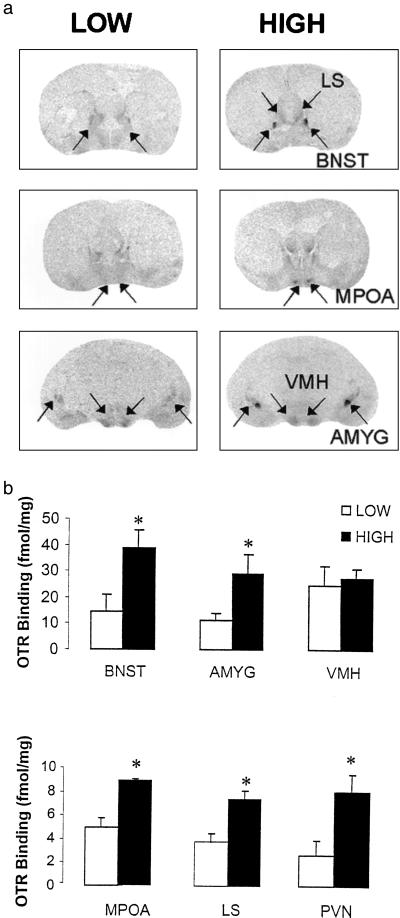
The lactating females from the previous study were killed on postpartum day 6 for measurement of oxytocin receptor binding. For the sake of comparisons, females were arbitrarily divided into High Responsivity (latency ≤ 6 days) and Low Responsivity (>8 days) females based on the results of the screening test. Not surprisingly, there was a significant differences in the frequency of pup licking/grooming between High and Low Responsivity groups (High = 13.6 ± 1.1; Low = 8.1 ± 0.8; P < 0.01), with no group differences in the percentage of total observations in which dams were observed to be in contact with pups (data not shown) The results of the receptor autoradiography (Fig. 2) revealed a significant Group effect across regions (F = 6.4; df = 1,5; P < 0.05). Post hoc analysis revealed significant group differences in oxytocin receptor binding in the MPOA (P < 0.03), the bed n. of the stria terminalis (P < 0.01), the central n. of the amygdala (P < 0.05), the lateral septum (P < 0.05) and the paraventricular n. of the hypothalamus (P < 0.01), but not in the ventromedial n. of the hypothalamus.
Figure 2.
(a) Representative autoradiographic images of [125I]OTA binding in various brain regions in females that were High (full maternal responsivity in <6 days) or Low (full maternal responsivity in >6 days) as determined in the pup sensitization paradigm. MPOA, medial preoptic area; LS, lateral septum; BNST, bed nucleus of the stria terminalis; AMYG, central nucleus of the amygdala; PVN, paraventricular nucleus; VMH, ventromedial nucleus of the hypothalamus. (b) Mean ± SEM levels of [125I]OTA binding (fmol/mg) in day 6 lactating females that were High (full maternal responsivity in <6 days) or Low (full maternal responsivity in >6 days; n = 6 per group).
Oxytocin Receptor Antagonist Treatment.
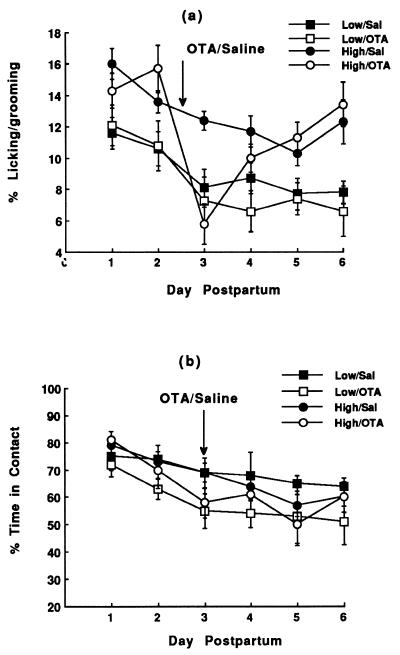
We then examined the functional link between differences in oxytocin receptor levels and maternal behavior by using ICV administration of an oxytocin receptor antagonist (Fig. 3). On each day of observation, there was a significant effect of Group, reflecting the increased frequency of licking/grooming in the High LG-ABN mothers. On day 3 (treatment day) there was no effect of Group, but a significant effect of Drug (F = 11.8; df = 1,26; P < 0.002) and more importantly a significant Group × Drug interaction effect (F = 3.8; df = 1,26; P < 0.05). The oxytocin receptor antagonist treatment on day 3 postpartum significantly (P < 0.05) reduced the observed frequency of maternal licking/grooming in High, but not Low, LG-ABN females (Fig. 3a). The net effect of the OTA treatment was to eliminate the group differences in licking/grooming. The frequency of pup licking/grooming in saline-control High and Low LG-ABN mothers differed significantly (P < 0.01) on day 3. By day 5 and onward, the frequency of pup licking/grooming was, again, significantly greater in High compared with Low LG-ABN mothers. There was no effect of OTA treatment on the percentage of total observations in which mothers were in physical contact with their pups (see Fig. 3b and ref. 20).
Figure 3.
Mean ± SEM percentage of total observations in which a mother was observed to be licking/grooming a pup (a) or to be in physical contact with pups (b) in High and Low licking/grooming mothers over the first 6 days postpartum. On day 3, all females received an intracerebroventricular infusion of either saline or the oxytocin receptor antagonist [d(CH2)5,Tyr(Me)2,Thr4,Tyr-NH29]-OVT. The frequency of pup licking/grooming in saline-treated High licking/grooming was significantly greater than that of Low licking/grooming mothers on each day of observation. In animals treated with the oxytocin receptor antagonist, differences in licking/grooming were significant only on days 1–2 and 5–6, but not on days 3–4 (n = 8 per group).
Estrogen Induction of Oxytocin Receptor Levels.
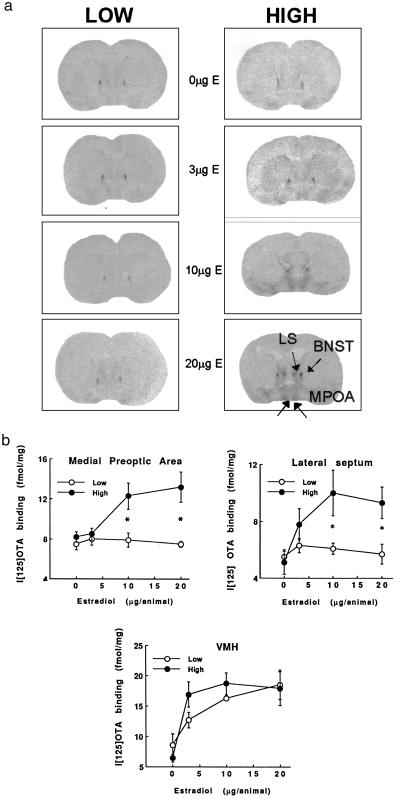
Because estrogen enhances oxytocin receptor levels, we examined the possibility of group differences in estrogen sensitivity. Adult, ovariectomized virgin female offspring of High and Low licking/grooming mothers were provided with estrogen replacement (0, 3, 10, or 20 μg) for 4 days and then killed for oxytocin receptor autoradiography. The circulating estradiol levels obtained with the 10- and 20-μg silastic implants were comparable to those reported in the later phase of gestation in pregnant rats (i.e., 60–90 pg/ml; refs. 12 and 32). In addition, there were no group differences (F = 0.2; df = 1,29; ns) in estradiol levels (3 μg, Low = 28.7 ± 3.3, High = 23.5 ± 3.0 pg/ml; 10 μg, Low = 53.1 ± 3.7, High = 54.3 ± 4.8 pg/ml; 20 μg, Low = 67.2 ± 8.2, High = 70.2 ± 2.4 pg/ml.
The results of the oxytocin receptor analysis (Fig. 4) revealed a significant Group (F = 11.3; df = 1,31; P < 0.01) and Group × Dose interaction effect (F = 3.7; df = 3,31; P < 0.03) for the MPOA. Similarly, in the lateral septum, there was a significant effect of Group (F = 11.3; df = 1,31; P < 0.01) and a Group × Drug interaction effect (F = 2.6; df = 3,31; P = .06). In each region, there was a significant and dose-related increase in oxytocin receptor binding in the female offspring of High, but not Low, LG-ABN mothers. Indeed, there was no effect of estrogen on oxytocin receptor binding in these brain regions at any dose. In the ventromedial n. of the hypothalamus, there was a significant effect of Drug (F = 10.7; df = 3,31; P < 0.0001), but not effect of Group or Group × Dose interaction.
Figure 4.
(a) Mean ± SEM levels of [125I]OTA binding (fmol/mg) in ovariectomized virgin female offspring of High or Low LG-ABN mothers as a function of estradiol replacement (n = 4–6 per group). *, P < 0.01. (b) Representative autoradiographic images of [125I]OTA binding in various brain regions in ovariectomized virgin female offspring of High or Low LG-ABN mothers as a function of estradiol replacement (see Fig. 2b for list of abbreviations).
Discussion
Virgin females rats initially avoid pups; however, most do become fully maternal after a period of continuous exposure (pup sensitization; refs. 5, 8, and 12). Habituation to the novelty of the pups appears to unmask maternal responsivity. Importantly, there is an impressive level of individual variation in the duration of pup exposure required for females to exhibit maternal behavior. Stern (8), for example, reported that 2 of 13 virgin females exhibited the full pattern of maternal behavior on the first day of testing. We wondered whether this variability in maternal responsivity corresponds to variations in specific forms of maternal behavior in lactating females, such as pup licking/grooming and arched-back nursing? Our approach was to examine the nulliparous, female offspring of High or Low LG-ABN mothers, because these animals reliably resemble their mothers on measures of maternal licking/grooming and arched-back nursing. In the pup sensitization paradigm, the latency to exhibit maternal behavior was significantly shorter in the virgin female offspring of High compared with Low LG-ABN mothers. Because these differences were apparent in virgin females exposed to pups drawn randomly from our breeding colony, it does not appear that the group differences in the maternal responsivity depend on the characteristics of the pups. Differences in maternal responsivity were also associated with variations in the maternal behavior. Thus, among a group of females obtained directly from a supplier, the latency to exhibit maternal behavior in the pup sensitization paradigm was strongly correlated with the frequency of pup licking/grooming during lactation.
The pup sensitization paradigm has been used to study the mechanisms that underlie the transition from a maternally nonresponsive to responsive state. Evidence for the validity of the model derives from studies showing that hormonal regimens that mimic the endocrine state of late pregnancy facilitate the expression of maternal behavior in virgin, nulliparous female rats in the pup sensitization paradigm (12, 31). Estrogen pretreatment, for example, reduces the period of pup exposure required for the onset of maternal behavior (5, 12). The effect of estrogen is, at least in part, mediated through an estrogen-induced increase in oxytocin receptor levels in brain regions thought to mediate the expression of maternal behavior, most notably the MPOA (22). We previously found increased oxytocin receptor levels in lactating High compared with Low LG-ABN mothers (13). These findings are consistent with the results of the present studies showing increased oxytocin receptor levels in various limbic brain regions, including the MPOA, in females characterized in the pup sensitization paradigm as High in maternal responsivity. Importantly, these females also emerge as High licking/grooming mothers (see Fig. 1b and ref. 2).
Enhanced maternal responsivity to pups was associated with increased oxytocin receptor levels and a greater frequency of pup licking/grooming. The results from the ICV infusion of the oxytocin receptor antagonist suggest that oxytocin does indeed regulate the expression of certain forms of maternal behavior, notably pup licking/grooming, for some period during the postpartum period. Administration of the oxytocin receptor antagonist on day 3 postpartum completely eliminated the difference in pup licking/grooming between High and Low LG-ABN mothers (Fig. 3a). There was a modest residual effect on day 4, which is consistent with the duration of action of the oxytocin receptor antagonist, [d(CH2)5,Tyr(Me)2,Thr4,Tyr-NH29]-OVT, used in this study. Thus, an ICV infusion of the same antagonist disrupted female sexual behavior for a period of ≈24 to 48 h after infusion (32). These findings suggest that the increased oxytocin receptor levels in the High LG-ABN female rats provide a mechanism for the increased levels of pup licking/grooming observed in the animals.
The results of earlier studies suggested that, whereas oxytocin was crucial for the initiation of maternal behavior, it had little influence of the expression of infant-directed behavior beyond the period immediately after parturition (6). Indeed, the maintenance of maternal behavior is thought to become hormonally independent over time (5). Such conclusions are based on studies showing that central infusion of an oxytocin receptor antagonist on day 6 of lactation has no effect on time in contact with pups and retrieval (20, 21). Our data do not conflict with this notion, because, in our study, as in earlier reports, there was no effect of the oxytocin receptor antagonist on time spent in contact with pups (see Fig. 3b). Interestingly, under normal conditions, High and Low LG-ABN mothers do not differ in time spent in contact with pups (1–3). Also note that in the present study there was no correlation between maternal responsivity in the pup sensitization paradigm and time in contact with pups during lactation (data not shown). However, the pronounced effect of the oxytocin receptor antagonist on pup licking/grooming in High LG-ABN mothers on day 3 of lactation suggests that not all aspects of the maintenance phase of maternal behavior in the rat are hormonally independent.
During lactation, High and Low LG-ABN mothers, as well as females High or Low in maternal responsivity, differ in oxytocin receptors in a number of brain regions, including the MPOA, the lateral septum, the bed n. of the stria terminalis, and the central n. of the amygdala (see ref. 13 and Fig. 2). Thus, each of these regions could potentially serve to mediate the relationship between differences in oxytocin receptor sensitivity and maternal behavior. The evidence for the importance of receptor differences at the level of the MPOA is certainly compelling. Infusions of either estrogen (14, 33) or oxytocin (22) directly into the MPOA facilitate the expression of maternal care. Moreover, lesions of the MPOA decrease pup licking/grooming (34). These findings suggest that the increased estrogen sensitivity in the MPOA of High LG-ABN mothers results in enhanced lactation-induced increases in oxytocin receptors in the MPOA and elevated tissue sensitivity to oxytocin, which drives pup licking/grooming.
The critical differences between High and Low LG-ABN mothers may ultimately lie in the rather dramatic, regionally specific differences in estrogen sensitivity. Estrogen produced a dose-related increased in oxytocin receptor levels in the MPOA and the lateral septum in the virgin female offspring of High, but not Low, LG-ABN mothers. Interestingly, we found no differences in oxytocin receptor levels in the ventromedial n. of the hypothalamus between High and Low LG-ABN mothers (13), or between the High and Low maternally responsive females in the current research (Fig. 2). Moreover, there was no difference in the effect of estrogen on oxytocin receptor binding in the ventromedial n. of the hypothalamus. These findings belie an impressive level of regional specificity in the effect of maternal care on estrogen sensitivity in the female offspring. Oxytocin receptor activation in the ventromedial n. of the hypothalamus has been linked to sexual, and not maternal, behavior (35). It is possible, therefore, that the specificity in estrogen sensitivity is associated with selective functional outcomes, influencing maternal, but not sexual, behavior. It is also interesting to note that, in the absence of estrogen, there were no group differences in oxytocin receptor levels. Indeed, we previously found that High and Low LG-ABN mothers differed in oxytocin receptor levels in the MPOA and the lateral septum only during lactation (13), which would further contribute to a functionally specific effect on maternal behavior.
The group differences in estrogen-inducible oxytocin receptor binding, even in the nonlactating, ovariectomized state, suggest the existence of stable differences in estrogen sensitivity in the female offspring of High and Low LG-ABN mothers. Although such differences could reflect altered levels of estrogen receptor expression, a number of endocrine systems, including the thyroid system (36), have been shown to have potent regulatory effects on estrogen sensitivity. Although the mechanism remains to be clarified, it is interesting to think that perhaps these findings represent an active process of “feminization” such that the behavior of the mother toward her female offspring sensitizes selected brain regions to the effects of estrogen in adulthood, and thus forms the basis for the transmission of individual differences in maternal behavior. However, an interesting question emerges in considering oxytocin receptor levels in the lactating females. Estradiol levels in lactating females are low. Thus, whereas variations in estrogen sensitivity might initiate differences in oxytocin receptor levels between High and Low LG-ABN mothers, it is not at all clear how such differences might relate to variations in oxytocin receptor levels observed on day 6 of lactation. What is interesting to consider here is the potential influence of the behavior on the neural mechanisms that mediate maternal behavior. Maternal licking/grooming is associated with increased activity in the mesolimbic dopamine system (8, 37). Dopamine has been found to activate estrogen receptors even in the absence of the cognate ligand (38, 39). Thus, it is possible that group differences in estrogen sensitivity serve to both initiate and maintain the group differences in oxytocin receptor levels. The relevant ligand for estrogen receptor activation during the peripartum period is estradiol itself, whereas in the later phase of gestation, it could, potentially, be that of behaviorally induced levels of dopamine. Regardless of the merits of this idea, we believe these findings provide an exciting opportunity to examine the molecular basis for the expression and transmission of individual differences in maternal behavior.
Acknowledgments
We gratefully acknowledge the very important and constructive comments of the reviewers, which contributed enormously to the revised version of this paper. This research was supported by grants from the National Institute for Mental Health and the Canadian Institutes for Health Research (to M.J.M.). F.C. holds a Graduate Fellowship and M.J.M. a Senior Scientist award from the Canadian Institutes for Health Research.
Abbreviations
- LG-ABN
licking and grooming-arched back nursing
- MPOA
medial preoptic area
- n.
nucleus
- ICV
intracerebroventricular
- OTA
oxytocin antagonist
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Liu D, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky P M, Meaney M J. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 2.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky P M, Meaney M J. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis D, Diorio J, Liu D, Meaney M J. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 4.Liu D, Diorio J, Day J C, Francis D D, Mar A, Meaney M J. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblatt J S. Acta Paediatr Suppl. 1994;397:3–8. doi: 10.1111/j.1651-2227.1994.tb13259.x. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen C A. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 7.Numan M, Sheehan T P. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- 8.Stern J M. Dev Psychobiol. 1997;31:19–37. doi: 10.1002/(sici)1098-2302(199707)31:1<19::aid-dev3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Fleming A S, O'Day D H, Kraemer G W. Neurosci Biobehav Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 10.Fleming A S, Cheung U, Myhal N, Kessler Z. Physiol Behav. 1989;46:449–453. doi: 10.1016/0031-9384(89)90019-x. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy M M. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 12.Bridges R S. Adv Study Behav. 1996;25:215–242. [Google Scholar]
- 13.Francis D, Champagne F, Meaney M J. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 14.Fahrbach S E, Pfaff D W. Horm Behav. 1986;20:354–363. doi: 10.1016/0018-506x(86)90043-7. [DOI] [PubMed] [Google Scholar]
- 15.Giordano A L, Siegel H I, Rosenblatt J S. Neuroendocinol. 1989;50:248–258. doi: 10.1159/000125230. [DOI] [PubMed] [Google Scholar]
- 16.Bale T L, Pedersen C A, Dorsa D M. Adv Exp Med Biol. 1995;395:269–280. [PubMed] [Google Scholar]
- 17.Pedersen C A, Prange A J., Jr Proc Natl Acad Sci USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen C A, Ascher J A, Monroe Y L, Prange A J., Jr Science. 1982;216:648–649. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen C A, Caldwell J D, Johnson M F, Fort S A, Prange A J., Jr Neuropeptides. 1985;6:175–182. doi: 10.1016/0143-4179(85)90108-8. [DOI] [PubMed] [Google Scholar]
- 20.Fahrbach S E, Morrell J I, Pfaff D W. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- 21.van Leengoed E, Kerker E, Swanson H H. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen C A, Caldwell J D, Walker C, Ayers G, Mason G A. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 23.Uvnas-Moberg K. Ann NY Acad Sci. 1997;807:146–163. doi: 10.1111/j.1749-6632.1997.tb51917.x. [DOI] [PubMed] [Google Scholar]
- 24.Windle R J, Shanks N, Lightman S L, Ingram C D. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 25.Neumann I D, Torner L, Wigger A. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 26.Myers M M, Brunelli S A, Shair H N, Squire J M, Hofer M A. Dev Psychobiol. 1989;22:55–67. doi: 10.1002/dev.420220105. [DOI] [PubMed] [Google Scholar]
- 27.Bridges R S, Ronsheim P M. Endocrinology. 1990;126:837–848. doi: 10.1210/endo-126-2-837. [DOI] [PubMed] [Google Scholar]
- 28.Insel T R. Neuroendocrinology. 1986;44:515–518. doi: 10.1159/000124694. [DOI] [PubMed] [Google Scholar]
- 29.Johnson A E, Coirini H, Ball G F, McEwen B S. Endocrinology. 1989;124:207–211. doi: 10.1210/endo-124-1-207. [DOI] [PubMed] [Google Scholar]
- 30.Swanson L. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 31.Bridges R S. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 32.Witt D M, Insel T R. Endocrinology. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- 33.Numan M, Rosenblatt J S, Komisaruk B R. J Comp Physiol Psychol. 1977;91:146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- 34.Lee A, Clancy S, Fleming A S. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy M M, Kleopoulos S P, Mobbs C V, Pfaff D W. Neuroendocrinology. 1994;59:432–440. doi: 10.1159/000126689. [DOI] [PubMed] [Google Scholar]
- 36.Dellovade T L, Chan J, Vennstrom B, Forrest F, Pfaff D W. Nat Neurosci. 2000;3:472–475. doi: 10.1038/74846. [DOI] [PubMed] [Google Scholar]
- 37.Hansen S, Bergvall A H, Nyidredi S. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 38.O'Malley B W, Schrader W T, Mani S K, Smith C, Weigel N L, Conneely O M, Clark J H. Rec Prog Horm Res. 1995;50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- 39.Gangolli E A, Conneely O M, O'Malley B W. J Ster Biochem Mol Biol. 1997;61:1–9. doi: 10.1016/s0960-0760(97)00003-4. [DOI] [PubMed] [Google Scholar]