Abstract
Members of the N-methyl-d-aspartate (NMDA) class of glutamate receptors (NMDARs) are critical for development, synaptic transmission, learning and memory; they are targets of pathological disorders in the central nervous system. NMDARs are phosphorylated by both serine/threonine and tyrosine kinases. Here, we demonstrate that cyclin dependent kinase-5 (Cdk5) associates with and phosphorylates NR2A subunits at Ser-1232 in vitro and in intact cells. Moreover, we show that roscovitine, a selective Cdk5 inhibitor, blocks both long-term potentiation induction and NMDA-evoked currents in rat CA1 hippocampal neurons. These results suggest that Cdk5 plays a key role in synaptic transmission and plasticity through its up-regulation of NMDARs.
The N-methyl-d-aspartate (NMDA) class of glutamate receptors (NMDAR) are essential for learning, memory, and development in the central nervous system (1–5). NMDARs are multimeric complexes formed from both NMDA receptor subunit (NR1) and modulatory NR2 subunits (6–9). A single gene encodes the NR1 subunit. Eight possible alternative RNA-splice variants provide molecular diversity of NMDARs (10). NR2A-NR2D are encoded by four separate genes (11, 12). NMDARs consist of NR1/NR2 heteromeric complexes (13–15). The activation of NMDARs and the influx of Ca2+ into the postsynaptic cells through the receptor are important for the induction of long-term synaptic plasticity, including long-term potentiation (LTP; ref. 16).
NMDA-channel activity is dynamically modulated in both intracellular and extracellular sites (3). Phosphorylation sites have been identified on the NR1, NR2A, and NR2B subunits, but not on the NR2C-NR2D subunits. Protein kinase C phosphorylates Ser-890 and Ser-896, and protein kinase A phosphorylates Ser-897 within the C1 exon of the NR1 subunit (17–19). Calcium/calmodulin protein kinase II mediates phosphorylation of NR2B, but not NR2A (20). More recently, phosphorylation at tyrosine residues of NR2A and NR2B has been described as an important determinant for NMDAR functions (19–20). In particular, Fyn, a member of the Src family of nonreceptor protein tyrosine kinases, was shown to phosphorylate the NR2A (7, 21–23). However, no information is available concerning which kinases phosphorylate NR2A at serine/threonine sites. Cyclin-dependent kinase-5 (Cdk5) is a serine/threonine kinase that is activated by neuron-specific p35 and p39 proteins (24–27). It exists as a large, multimeric complex associated with cytoskeletal proteins in the neurons. Cdk5 has been shown to phosphorylate a wide variety of proteins, all of which have serine/threonine sites in (K/RT/SPXK)-type motifs (28, 29). A number of synaptic proteins have been identified as Cdk5 substrates (30–32). Cdk5 and p35, predominantly expressed in postmitotic neurons, play essential roles in neuronal migration, neurite outgrowth, and laminar configuration of the cerebral cortex (25, 27, 33). Cdk5 in association with p25, a truncated form of p35, hyperphosphorylates the microtubule-associated protein tau. This hyperphosphorylation is thought to disrupt the neuronal cytoskeleton and ultimately contributes to neurodegeneration in Alzheimer's disease (34).
Because of its virtue of phosphorylating so many diverse substrates, Cdk5 has been proposed to participate in synaptic transmission and plasticity by phosphorylating NMDAR subunits. Here, we show that Cdk5 directly phosphorylates NR2A on Ser-1232 both in vitro and in intact cells. This phosphorylation can be inhibited by roscovitine, a Cdk5-specific inhibitor. Moreover, we show that inhibition of the Cdk5 activity prevents induction of LTP in CA1 pyramidal cells of rats. These findings indicate that, through phosphorylation of the NR2A subunit, Cdk5 may play a key role in synaptic transmission.
Materials and Methods
Constructs.
The 920-bp wild-type and mutant NR2A fragments (C-terminal domain from amino acid 1159 to 1464) were generated by reverse transcription–PCR from rat brain RNA by using primers corresponding to the C-region of NR2A. Wild-type Cdk5, inactive mutant Cdk5 (K33T), and p35 in pcDNA3 were gifts from L.-H. Tsai (Harvard Medical School, Boston, MA). HA-tagged wild-type and mutant (S1232A) NR2A in pcDNA3, recombinant His-tagged wild-type and mutant (S1232A) NR2A C-terminal domain were generated by standard cloning methods. The putative phosphorylation site in NR2A was mutated by using the Quick-Change site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The mutation was verified by DNA sequencing.
Cell Culture.
The hippocampal region from E 16 Cdk5 wild-type (Cdk5+/+) and knockout mice (Cdk5−/−) embryos were dissected and minced well with scissors. The dissociated cells were collected by centrifugation and resuspended in a serum-free neurobasal medium supplemented with B-27 supplement and 0.5 mM l-glutamine (Life Technologies, Rockville, MD). Cells (25 × 103) were plated in 35-mm plastic dishes precoated with laminin (10 μg/ml; Life Technologies) and allowed to grow for 5 days. HEK293T cells were cultured in DMEM with 10% (vol/vol) FCS.
In Situ Hybridization.
Digoxigenin-labeled Cdk5 riboprobes were used for in situ hybridization. The digoxigenin probes were diluted to 1–2 ng/ml hybridization solution. Sections were incubated for 12–16 hr at 56°C with probe, then incubated in 0.002% RNase A (Roche Molecular Biochemicals) for 30 min. Sections then were incubated in sheep anti-digoxigenin primary antisera conjugated to alkaline phosphatase (1:1,000) overnight, and then incubated in a solution of nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate for 2–4 hr and dipped in 3% (vol/vol) parlodion (Life Technologies).
Phosphorylation Studies.
For in vivo phosphorylation studies, hippocampal neurons from E 16 Cdk5 wild-type (Cdk5+/+) and knockout mouse (Cdk5−/−) embryos were cultured for 5 days. Phosphate-free DMEM supplemented with 100 μCi/ml (1 Ci = 37 GBq) [32P]orthophosphoric acid was added, and the incubation continued for 3 hr. Lysates were collected, NR2A was immunoprecipitated (IP) with monoclonal anti-NR2A antibody (MAB5216; Chemicon), and 32P-incorporation was visualized by autoradiography after SDS/10–20% PAGE. NR2A levels in each lane were measured by Western blotting. For in vitro phosphorylation studies, histone H1 or recombinant NR2A purified from Escherichia coli were incubated either with wild-type or kinase-inactive Cdk5 immunoprecipitate from transfected HEK293T cells. Histone H1 or recombinant NR2A was incubated with [32P]γ-ATP (0.1 mM) in a buffer containing 50 mM Tris⋅HCl (pH 7.4), 1 mM EGTA, 1 mM dithiothretol, 5 mM MgCl2, 0.5 mM microcystin L R, and IP-Cdk5 for 30 min at room temperature. In experiments examining in vitro phosphorylation of wild-type and mutant NR2A, glutathione S-transferase (GST)-Cdk5 and GST-p35 fusion proteins (synthesized by using pGEX-4T-2 vector; Amersham Pharmacia) (N. D. Amin and H.C.P., unpublished work) were incubated with wild-type or S1232A NR2A (2 μg) under the same conditions as described above. Proteins were resolved by SDS/PAGE. 32P-incorporation and protein levels were determined by autoradiography and Coomassie staining, respectively.
Hippocampal Slice Preparation and Electrophysiology.
Male Sprague–Dawley rats (200–250 g) were decapitated. The brains were removed and rapidly cooled to 4°C in a modified artificial cerebrospinal fluid (CSF) containing 124 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4 26 mM NaHPO4, 1.25 mM NaH2PO4, and 10 mM glucose, and then gassed in a 95% O2/5% CO2 atmosphere. Hippocampi were sliced (400 μm), and kept in an interface chamber in the CSF. For LTP experiments, CA1 responses were recorded at 30–31°C with glass micropipettes filled with 3 M potassium acetate, pH 7.25 (tip resistance 60–120 MΩ) from the cell body layer of the CA1 region. One bipolar stimulating electrode (Teflon-insulated PtIr wire of 25 μm in diameter) was positioned in the stratum radiatum of the CA1 subfield to stimulate the neurons in the Schaffer collateral pathway. Two trains of tetanic stimulation at the same-set intensity (100 Hz, 100 μs for 1 s each at 10-s intervals) were administered to induce LTP. Signals were amplified with an AxoClamp-2B amplifier, digitized, stored, and analyzed with DIGIDATA 1200 and P-CLAMP 6 software (Axon Instruments, Foster City, CA). A discontinuous, single-electrode voltage-clamp mode was used for voltage-clamp experiments (tip resistance 60–70 MΩ). The sampling rate was 3.0–5.0 kHz. Electrode capacitance was optimized during discontinuous current-clamp mode before and after cell penetration to neutralize capacitance and reduce overshoot/undershoot errors. Gain was routinely set at 6–8 nA⋅mV−1. The effective voltage clamp was continuously monitored during the course of the experiment. Unstable and unsettled voltage-clamp recordings were discarded. Test stimulation intensity (30–50 μA, 50 μs, single pulse) was set to elicit an excitatory postsynaptic potential (EPSP) that was about 40% amplitude of the threshold for evoking action potentials. All of the neurons recorded for this study had a resting membrane potential ranging from −71 mV to −78 mV. The holding potential for voltage-clamp recordings was −75 mV.
Results
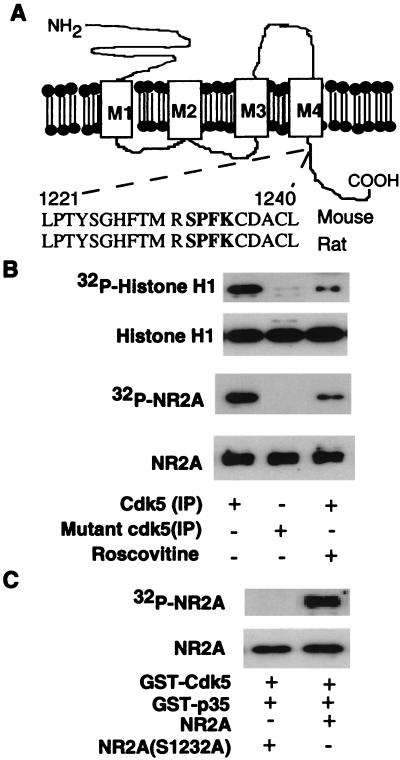
The consensus phosphorylation sequence of Cdk5 is XR/KS/TPXK/R. One such putative Cdk5 phosphorylation motif (RSPFK) is present in the NR2A subunit (Ser-1232; see Fig. 2A) but not in other NR2 subunits (NR2B-D). To find out whether NR2A is an in vivo Cdk5 substrate, we first investigated the distribution of Cdk5 in rat brain to see whether it colocalized with NR2A. The highest levels of Cdk5 were detected in the cerebral cortex and hippocampal (Fig. 1 A–D) regions expressing high levels of NR2A (35, 36). Cdk5 is detected in both pre- and postsynaptic sites in the cerebral cortex by immunogold labeling (Fig. 1E). These ultrastructural studies add support to light microscopic and biochemical findings (described later), indicating a presence of Cdk5 in synaptic regions. To determine whether Cdk5 is present in the PSD fraction of the brain, we followed the association of Cdk5 protein during biochemical purification of the PSD. By Western blot analysis, we observed that Cdk5 protein is in whole-cell extracts of the cerebral cortex, in a synaptosomal fraction, and in purified PSD (Fig. 1F).
Figure 2.
Phosphorylation of NR2A by Cdk5 in vitro. (A) The Cdk5 phosphorylation motif RSPFK is indicated in Mouse and Rat (NR2A). (B) HEK293T cells were transfected with Cdk5/p35 or mutant Cdk5 (K33T)/p35, and lysates were IP by using anti-Cdk5 antibody (C-8). The cells were incubated in a kinase assay system in vitro with histone H1 (Top and Top Middle) or recombinant NR2A (Bottom Middle and Bottom) as substrates. [32P]-incorporation into substrates is indicated in Top and Bottom Middle, and the amount of substrate stained by Coomassie stain is indicated in Top Middle and Bottom. (C) Recombinant wild-type NR2A and mutant NR2A (S1232A) were purified, and equal amounts were incubated with GST-Cdk5 and GST-p35 (see Materials and Methods). (Top) The incorporation of [32P] into NR2A. (Middle) The amount of NR2A substrate by Coomassie blue staining of the gel.
Figure 1.
Cdk5 expression and association with NR2A in the central nervous system. (A–D) Rat coronal brain sections were analyzed by in situ hybridization with a digoxigenin-labeled Cdk5 probe. High levels of Cdk5 mRNA expression were observed in the cerebral cortex (Ctx) (A), hippocampal CA3 (B), dentate gyrus (DG) (C), and CA1 region (D). (E) Postembedding immunogold EM labeling of Cdk5 in a representative section from adult rat cerebral cortex showing Cdk5 labeling over presynaptic (Upper) and postsynaptic (Lower) regions. (F) Immunoblot analysis of Cdk5 in rat brain homogenate synaptosomal and postsynaptic density (PSD) fractions. Whole-brain extract (lane 1, 50 μg), synaptosomes (lane 2, 10 μg), and PSD fraction (lane 3, 5 μg) were separated by SDS/PAGE and subjected to immunoblotting with anti-Cdk5, anti-NR2A, and anti-PSD-95 antibodies. (G) Rat brain extracts were solubilized with 1% deoxycholate, and the resulting detergent extract (Input) was used to immunoprecipitate the proteins indicated. The immunoprecipitates then were immunoblotted for Cdk5, NR2A, or control (preimmune IgG) antibodies. The input lane was loaded with 20% of the amount of extract used for IP. (H–J) Colocalization of Cdk5 with NR2A in hippocampal neurons. Rat hippocampal neurons were stained with Cdk5 polyclonal and monoclonal NR2A antibodies; Cdk5 and NR2A staining was visualized with a rhodamine-coupled secondary and FITC-coupled secondary antibody, respectively.
We also asked whether Cdk5 is associated with NR2A by coimmunoprecipitation. Cell extracts from the cerebral cortex were solubilized with 1% deoxycholate and IP with Cdk5, NR2A, PSD-95 (NR2A is known to associate with PSD-95; ref. 37), or control (preimmune IgG) antibodies. Cdk5, NR2A, and PSD-95 were all co-IP by antibodies specific for these proteins, but not by control antibody (preimmune IgG; Fig. 1G). Because antibodies to Cdk5 brought down a significant amount of both PSD-95 and NR2A, a ternary complex containing Cdk5/NR2A/PSD-95 is likely to exist. To examine whether Cdk5 also is colocalized specifically with NR2A, we performed double-labeled immunofluorescence staining of cultured hippocampal neurons. Cdk5 colocalizes with NR2A in hippocampal neurons (Fig. 1 H–J).
To test whether NR2A is an in vitro substrate for Cdk5 phosphorylation, HEK293T cells were cotransfected with wild-type or the kinase-inactive mutant Cdk5 (K33T; ref. 38), and the status of phosphorylation of the recombinant NR2A was assessed by immunoprecipitation of the wild-type and mutant (K33T)-Cdk5. Cells transfected with active Cdk5/p35 phosphorylated both histone H1 and recombinant NR2A (Fig. 2B), whereas the immunoprecipitate from cells transfected with mutant (K33T) or treated with roscovitine, a Cdk5-specific inhibitor (39), did not phosphorylate either substrate (Fig. 2B). The expression levels of the wild type and the mutant Cdk5 K33T were the same in these cells (data not shown).
Because Ser-1232 of NR2A is the ideal consensus sequence (RSPFK) of Cdk5 (Fig. 2A), Ser-1232 was mutated to Ala (S1232A). The ability of Cdk5 to phosphorylate wild-type and mutant HA-NR2A (C-region, S1232A) was examined by an in vitro kinase assay with GST-Cdk5/GST-p35. Cdk5 phosphorylated wild-type NR2A but not the mutant S1232A (Fig. 2C), which supports Ser-1232 as the phosphorylation site of Cdk5.
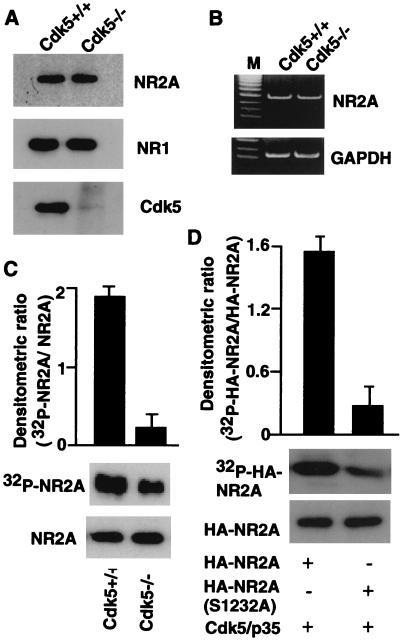
To establish further that Cdk5 activity is necessary for phosphorylation of NR2A, we first compared expression of NR2A in cultured neurons from Cdk5 knockout (Cdk5−/−) and wild-type (Cdk5+/+) mice by reverse transcription–PCR and Western blot analysis. Cdk5−/− and wild-type mice exhibited similar levels of NR2A mRNA and protein expression (Fig. 3 A and B). We then compared the phosphorylation state of NR2A by using cultured hippocampal neurons from Cdk5−/− and wild-type mice. Cells were metabolically labeled with [32P]orthophosphate. The levels of phosphorylated NR2A were measured by immunoprecipitation with anti-NR2A antibody and autoradiography, and its phosphorylation state and protein levels were quantified. Neurons from wild-type mice exhibited greater than 2-fold phosphorylation of NR2A, compared with neurons from Cdk5−/− mice (Fig. 3C). To demonstrate further that Ser-1232 in NR2A is phosphorylated by Cdk5, the HEK293T cells were cotransfected with wild-type HA-NR2A (C region, S1232), or mutant HA-NR2A (S1232A) were cotransfected with wild-type Cdk5/p35 and then metabolically labeled with [32P]orthophosphate. The levels of phosphorylated HA-NR2A were measured by immunoprecipitation with anti-HA-tagged antibody, and its phosphorylation state and protein levels were quantified. Coexpression of wild-type HA-NR2A and Cdk5/p35 resulted in enhancement of HA-NR2A phosphorylation compared with coexpression of mutant HA-NR2A and Cdk5/p35 (Fig. 3D).
Figure 3.
Comparison of NR2A phosphorylation with Cdk5 knockout mice and wild-type mice. (A and B) NR2A expression in wild-type and Cdk5−/− mice was analyzed by Western blotting (A) and reverse transcription–PCR analysis (B). (C) The extracts from 32P-labeled cultured hippocampal neurons from wild-type and Cdk5−/− mice were processed for immunoprecipitation by using anti-NR2A antibody and subjected to autoradiography (Middle) or Western blotting (Bottom). Histogram (n = 3) reflects the relative amount of labeled NR2A to the amount of immunoreactive NR2A. (D) 32P-labeled wild type or Ser mutant (S1232A) of HA-NR2A was IP with anti-HA-tagged antibody from transfected HEK293T cells and subjected to autoradiography (Top Middle) or Western blotting (Bottom Middle). The histogram shows the relative amount of labeled HA-NR2A to the amount of immunoreactive HA-NR2A.
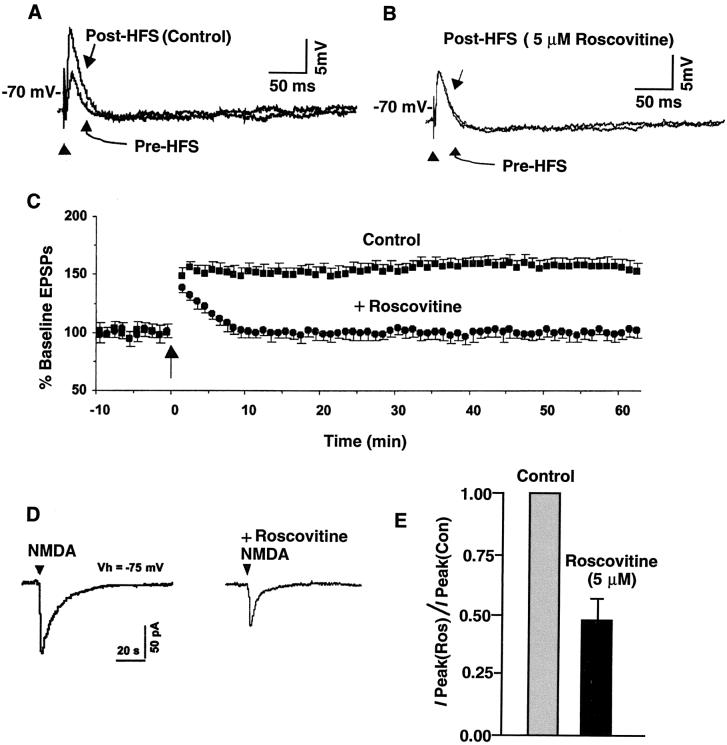
LTP is an activity-dependent strengthening of synaptic efficacy that is considered to be a basic mode of learning and memory (40). In area CA1 of the hippocampus, either one or multiple trains of high-frequency stimulation are used to induce the most commonly studied forms of LTP (41). This form requires postsynaptic Ca2+ influx and depends on NMDAR activation (42–44). Therefore, we investigated whether Cdk5 is involved in NMDAR-dependent LTP induction. Stimulation of the Schaffer collateral inputs to CA1 neurons evoked EPSP. It is well established that the LTP at these synapses depends on the activation of the postsynaptic NMDARs (10, 22). To determine whether Cdk5 affects LTP induction, we made use of roscovitine, a specific inhibitor of Cdk5 (39), to examine its effects on the rat hippocampal CA1 long-term synaptic potentiation. Tetanic trains induced EPSPs and LTP in the absence of the roscovitine. For instance, 40 min after tetanization, the EPSPs increased (156.9 ± 7.2% of baseline EPSP; n = 6) significantly (P < 0.05) from their control values (Fig. 4 A and C). In contrast, in the presence of extracellular roscovitine (5 μM), tetanic stimulation induced only a brief posttetanic potentiation of EPSPs (Fig. 4 B and C). LTP, however, was not induced. Thus, 40 min after the tetanization (100.9 ± 5.2% of baseline ESPS; n = 6; Fig. 4C), the EPSPs did not differ significantly (P > 0.05, paired t test) from the value before the tetanization in the presence of roscovitine (5 μM).
Figure 4.
Inhibition of Cdk5 activity in hippocampal CA1 neurons results in reduced LTP- and NMDA-evoked currents. (A–C) Tetanic trains induced LTP in the absence, but not in the presence, of 5 μM roscovitine (20 min preincubation). Representative responses in A and B were recorded before and 40 min after high-frequency stimulation. The data points in C are means ± SEM. (D) The inward current evoked by the local application of NMDA was reduced by roscovitine [compare Right with Left (control)]. (E) Normalized peak currents (I Peak) in the presence of roscovitine (5 μM) compared with those in the absence of roscovitine during the application of NMDA. The responses to NMDA decreased in the presence of roscovitine (n = 6), compared with neurons in the absence of roscovitine (n = 6).
The blockade of LTP by roscovitine did not seem to involve an action at non-NMDARs. The EPSPs were largely eliminated (98.5 ± 4.2% of baseline value; n = 7; P < 0.05) by 100 μM extracellular 6-cyano-7-nitroquinoxaline-2, 3-dione, a selective non-NMDAR antagonist (data not shown). Roscovitine (5 μM), when applied alone, had no detectable effects on the EPSPs (n = 6) but significantly reduced the inward current induced by local application of NMDA (110.5 ± 6.8 pA to 60.3 ± 4.2 pA; n = 5; P < 0.05) (Fig. 4 D and E). These responses were blocked by 50 μM dextrorotatory and levorotatory (DL)-2-amino-5-phosphovaleric acid, a selective NMDAR antagonist (data not shown).
Discussion
The evidence presented here identifies Cdk5 as a potential regulator of NMDA receptor, NR2A. Cdk5 phosphorylation of NR2A at Ser-1232 affects NMDAR activity, as indicated by the regulation of NMDA-induced LTP in CA1 neurons. These effects were inhibited by roscovitine, a specific Cdk5 inhibitor. These results indicate that the observed changes of NMDAR activity in neurons are caused by, at least in part, NR2A-subunit phosphorylation by Cdk5, which, in turn, up-regulates NMDAR activity.
Cdk5−/− mice exhibit a unique phenotype with perinatal mortality and associated disruption of cerebral cortical layering, cerebellar foliation, and degeneration of neurons in the brainstem and the spinal cord (24, 45). Transgenic knockout mice that express Cdk5 only in the p35-expressing brain regions in endogenous Cdk5-null mice reversed the phenotype observed in Cdk5-null mice (46). These Cdk5 transgenic animals were viable and fertile. These studies indicate that neuronal Cdk5 activity is critical for embryonic development and survival. The Cdk5/p35 complex recently has been shown to phosphorylate Munc-18 and amphiphysin, which in turn affect neuronal exocytosis and neurite outgrowth (30, 32, 47–48), and dopamine and cAMP-regulated phosphoprotein of Mr 32,000 (DARPP-32), a bifunctional signal-transduction molecule that controls the activity of protein phosphatase 1 and protein kinase A through the phosphorylation of Thr-75 by Cdk5. Phosphorylation of DARPP-32 by Cdk5/p35 has been implicated in the regulation of dopamine signaling (49). In a recent study, Cdk5 was shown to phosphorylate the regulatory subunit of cGMP-phosphodiesterase in a GTP-dependent manner in photoreceptor outer segment membrane (50). These studies indicate that Cdk5 plays an important role in synaptic transmission.
Adult mice lacking NR2A show defective LTP and impaired spatial memory (51), indicating that NR2A is an important modulator during LTP. Several reports have related biochemical events underlying the induction of LTP in hippocampal CA1 neurons with the signaling cascades initiated by Ca2+ influx through NMDARs (40, 41, 52). Our results demonstrate that roscovitine, a specific Cdk5 inhibitor, reduced LTP induction and significantly inhibited the inward current induced by the local application of NMDA in CA1 pyramidal neurons. Cdk5 also has been shown to phosphorylate Munc-18, which interacts with syntaxin 1a and, thereby, modulates the level of vesicle soluble N-ethylmaleimide-sensitive factor attachment protein receptor association with syntaxin 1a in the regulation of exocytosis (26). It has been shown that LTP is accompanied by the increased phosphorylation of NR2 subunits of NMDARs (51). The present study provides evidence that Cdk5 can phosphorylate the NR2A subunit of the NMDAR, and that this phosphorylation affects the NMDA-regulated synaptic activity. Thus, Cdk5 seems to exert an important role in synaptic transmission.
Acknowledgments
We thank Drs. Philip Grant, Robert Gould, Pamela Voulalas, Kiyoshi Kusano, Mary Jo Danton and Mrs. Devee Schoenberg for critically reading this manuscript. We also thank Dr. Carolyn Smith, National Institute of Neurological Disorders and Stroke Light Microscopy Facility; for her assistance in confocal microscopy.
Abbreviations
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptors
- LTP
long-term potentiation
- Cdk
cyclin dependent kinase
- IP
immunoprecipitated
- GST
glutathione S-transferase
- EPSP
excitatory postsynaptic potential
- PSD
postsynaptic density
Footnotes
See commentary on page 12323.
References
- 1.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Nature (London) 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 2.Ryan T A, Ziv N E, Smith S J. Neuron. 1996;17:125–134. doi: 10.1016/s0896-6273(00)80286-x. [DOI] [PubMed] [Google Scholar]
- 3.McBain C J, Mayer M L. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 4.Dingledine R, Borges K, Bowie D, Traynelis S F. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 5.Schwarzschild M A, Cole R L, Meyers M A, Hyman S E. J Neurochem. 1999;72:2248–2255. doi: 10.1046/j.1471-4159.1999.0722248.x. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi S. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 8.Westbrook G L. Curr Opin Neurobiol. 1994;4:337–346. doi: 10.1016/0959-4388(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 9.Mori H, Mishina M. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- 10.Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Biochem Biophys Res Commun. 1992;185:826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- 11.Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Nature (London) 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- 12.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg P H. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 13.Behe P, Stern P, Wyllie D J, Nassar M, Schoepfer R, Colquhoun D. Proc R Soc London Ser B. 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- 14.Blahos J, Wenthold R J. J Biol Chem. 1996;271:15669–15674. doi: 10.1074/jbc.271.26.15669. [DOI] [PubMed] [Google Scholar]
- 15.Sheng M, Cummings J, Roldan L A, Jan Y N, Jan L Y. Nature (London) 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 16.Zukin R S, Bennett M V. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]
- 17.Tingley W G, Roche K W, Thompson A K, Huganir R L. Nature (London) 1993;364:70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- 18.Tingley W G, Ehlers M D, Kameyama K, Doherty C, Ptak J B, Riley C T, Huganir R L. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 19.Leonard A S, Hell J W. J Biol Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- 20.Omkumar R V, Kiely M J, Rosenstein A J, Min K T, Kennedy M B. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- 21.Kohr G, Seeburg P H. J Physiol (London) 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y T, Salter M W. Nature (London) 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 23.Yu X M, Askalan R, Keil G J, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 24.Ohshima T, Ward M, Huh C G, Longenecker G, Veeranna, Pant H C, Brady R O, Martin L J, Kulkarni A B. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai L H, Delalle I, Caviness V S, Jr, Chae T, Harlow E. Nature (London) 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 26.Chae T, Kwon Y T, Bronson R, Dikkes P, Li E, Tsai L H. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 27.Humbert S, Dhavan R, Tsai L-H. J Cell Sci. 2000;113:975–983. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- 28.Shetty K T, Link W T, Pant H C. Proc Natl Acad Sci USA. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant P, Sharma P, Pant H C. Eur J Biochem. 2001;268:1534–1546. [PubMed] [Google Scholar]
- 30.Shuang R, Zhang L, Fletcher A, Groblewski G E, Pevsner J, Stuenkel E L. J Biol Chem. 1998;273:4957–4966. doi: 10.1074/jbc.273.9.4957. [DOI] [PubMed] [Google Scholar]
- 31.Matsubara M, Kusubata M, Ishiguro K, Uchida T, Titani K, Taniguchi H. J Biol Chem. 1996;271:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- 32.Rosales J L, Nodwell M J, Johnston R N, Lee K Y. J Cell Biochem. 2000;78:151–159. doi: 10.1002/(sici)1097-4644(20000701)78:1<151::aid-jcb14>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Lew J, Huang Q Q, Qi Z, Winkfein R J, Aebersold R, Hunt T, Wang J H. Nature (London) 1994;371:423–425. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 34.Patrick G N, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai L H. Nature (London) 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 35.Jin D H, Jung Y W, Ko B H, Moon I S. Mol Cells. 1997;7:749–754. [PubMed] [Google Scholar]
- 36.Jin D H, Jung Y W, Ham S H, Ko B H, Moon I S. Mol Cells. 1997;7:64–71. [PubMed] [Google Scholar]
- 37.Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. Proc Natl Acad Sci USA. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolic M, Dudek H, Kwon Y T, Ramos Y F, Tsai L-H. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 39.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 40.Bliss T V, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 41.Malenka R C, Lancaster B, Zucker R S. Neuron. 1992;9:121–128. doi: 10.1016/0896-6273(92)90227-5. [DOI] [PubMed] [Google Scholar]
- 42.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Nature (London) 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 43.Collingridge G L, Kehl S J, McLennan H. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collingridge G L, Singer W. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- 45.Ohshima T, Gilmore E C, Longenecker G, Jacobowitz D M, Brady R O, Herrup K, Kulkarni A B. J Neurosci. 1999;19:6017–6026. doi: 10.1523/JNEUROSCI.19-14-06017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka K, Veeranna, Ohshima T, Rajan P, Amin N D, Cho A, Sreenath T, Pant H C, Brady R O, Kulkarn A B. J Neurosci. 2001;21:550–558. doi: 10.1523/JNEUROSCI.21-02-00550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fletcher A I, Shuang R, Giovannucci D R, Zhang L, Bittner M A, Stuenkel E L. J Biol Chem. 1999;274:4027–4035. doi: 10.1074/jbc.274.7.4027. [DOI] [PubMed] [Google Scholar]
- 48.Floyd S R, Porro E B, Slepnev V I, Ochoa G C, Tsai L H, De Camilli P. J Biol Chem. 2001;276:8104–8110. doi: 10.1074/jbc.M008932200. [DOI] [PubMed] [Google Scholar]
- 49.Bibb J A, Snyder G L, Nishi A, Yan Z, Meijer L, Fienberg A A, Tsai L H, Kwon Y T, Girault J A, Czernik A J, et al. Nature (London) 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 50.Matsuura I, Bondarenko V A, Maeda T, Kachi S, Yamazaki M, Usukura J, Hayashi F, Yamazaki A. J Biol Chem. 2000;275:32950–32957. doi: 10.1074/jbc.M000702200. [DOI] [PubMed] [Google Scholar]
- 51.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Nature (London) 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 52.Lisman J E. Trends Neurosci. 1994;17:406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]