Abstract
Group 3 pulmonary hypertension (PH) is a common complication of advanced chronic lung disease. Our hypothesis was that group 3 PH is associated with a more severe baseline presentation and a more severe prognosis compared to group 1 pulmonary arterial hypertension (PAH), chronic thromboembolic PH (group 4), and group 5 PH. We retrospectively analyzed consecutive incident PH patients in a single center between January 2006 and November 2014. Data were acquired from a prospective database. Clinical, functional, and hemodynamic characteristics, as well as survival, were compared between the four groups of precapillary PH. A total of 363 patients were analyzed; 164 patients (45.2%) belonged to group 1 PAH, 109 (30%) to group 3 PH, 65 (17.9%) to group 4 PH, and 25 (6.9%) to group 5 PH. Group 3 patients were predominantly male and were more frequently in New York Heart Association (NYHA) class III/IV. Patients with group 3 and 4 PH were older, had significantly lower 6-min walking distance (6MWD), higher mean pulmonary arterial pressure, higher pulmonary vascular resistance (PVR), and lower cardiac index (CI) than PAH patients. Group 3 and 5 patients had significantly lower total lung capacity (TLC), forced vital capacity (FVC), and FEV1; group 3 patients had the lowest carbon monoxide transfer coefficient values. PH therapy was used in 90.9% of group 3 patients. Univariate analysis of prognostic factors in the overall population showed that age, male gender, NYHA class, groups 3 and 4 PH (vs. PAH), 6MWD, FVC, TLC, carbon monoxide transfer coefficient (KCO), PVR, CI, and venous oxygen saturation were significantly associated with greater mortality. Multivariate analysis showed that age, PH group 4, 6MWD, and KCO but no longer PH group 3 were significantly associated with mortality. Patients with group 3 PH are older, have more severe baseline presentation and lower survival rates than PAH patients in univariate analysis, that seemed to be related to older age.
Keywords: emphysema, interstitial lung disease, pulmonary hypertension
Introduction
Pulmonary hypertension (PH) is a disease characterized by increased pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), progressively leading to right heart failure and death. PH is classified into five groups, each of them characterized by specific pathophysiological features.1 Group 3 PH is due to obstructive or restrictive lung diseases and/or hypoxemia.2 It is a common complication of severe chronic obstructive pulmonary disease (COPD),3,4 interstitial lung disease (ILD),5–7 and combined pulmonary fibrosis and emphysema (CPFE). When present, PH is associated with decreased exercise capacity and survival rates.8 In contrast with group 1 pulmonary arterial hypertension (PAH),9–11 specific treatments have not shown major beneficial effects in group 3 PH12–14 and are therefore not recommended.1 However, in specialized centers, treatment may be discussed on an individual basis in patients with severe PH or right heart failure. We hypothesized that group 3 PH has a more severe initial presentation of the disease and a worse prognosis than the other PH groups, especially group 1. For that purpose, we described and compared clinical, hemodynamic, and therapeutic characteristics of patients with precapillary PH diagnosed in our expert center.
Materials and methods
Study population
This monocentric study was conducted in the pneumology department of the Louis Pradel Hospital, Hospices Civils de Lyon (Lyon), France. This department is designated as a PH expert center as well as a national reference center for rare pulmonary diseases. We performed a retrospective analysis of data acquired in a prospective database. Incident patients were included if a diagnosis of precapillary PH was established using right heart catheterization (RHC) with a mean PAP (mPAP) ≥ 25 mmHg and a pulmonary capillary wedge pressure (PCWP) ≤ 15 mmHg. Pulmonary vascular resistance (PVR) > 3 Wood Units (WU) was required for the diagnosis of PAH. Patients with post-capillary PH (PCWP > 15 mmHg) were excluded. Inhaled nitric oxide (NO) was used for vasoreactivity testing. Pulmonary vascular pressures were measured at the end of expiration at RHC. All patients underwent echocardiography, ventilation/perfusion lung scan, pulmonary function tests (PFT), overnight oximetry, thoracic computed tomography (CT), a 6-min walk test (6MWT), and screening for HIV and autoimmune antibodies. COPD was defined as forced expiratory volume in 1 s to forced capacity ratio (FEV1/FVC) < 0.7. Diagnosis of emphysema was performed on CT scan and/or PFT showing hyperinflation (total lung capacity > 120% predicted). Diagnosis of idiopathic pulmonary fibrosis (IPF) and other ILD was established by multidisciplinary discussion after a thorough analysis of clinical, radiological, histological (when available), and biological findings (screening for connective tissue disease) according to international guidelines.15,16 Obesity-hypoventilation syndrome was defined as the combined presence of obesity (body mass index > 30 kg/m2) with daytime arterial hypercapnia (PaCO2 > 45 mmHg) in the absence of other causes of hypoventilation. CPFE was defined radiologically by the presence of centrilobular and/or paraseptal emphysema in the upper lobes and pulmonary fibrosis in the lower lobes. Differential diagnosis between group 1 PAH and group 3 PH was made according to the criteria proposed in the fifth World PH symposium.8 Criteria favoring group 1 PAH included normal or mildly impaired PFT (FEV1 > 60% predicted for COPD ; FVC > 70% for ILD), absence or modest airway or parenchymal abnormalities on CT scan, and features of exhausted circulatory reserve on cardiopulmonary exercise testing (when available). The study started on 1 January 2006; data were censored on the date of death or lung transplantation, or on 1 November 2014. In accordance with the World Health Organization definition, patients were considered elderly when they were aged > 65 years.
The database was anonymous and complied with the restrictive requirements of the CNIL (Comission nationale de l’informatique et des libertés, the French organization that regulates the collection, storage, and use of personal data) and the CCTIRS (Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé, the French advisory committee on health research data processing). Written consent was obtained from all patients. The study was approved by the CEPRO (Comité d’évaluation des protocoles de recherche observationnels, the Institutional Review Board of the French learned society for respiratory medicine, Société de Pneumologie de Langue Française).
Statistical analysis
Statistical analysis was performed with SPSS Statistics (International Business Machines, New York, NY, USA). Quantitative variables were expressed as median (minimum–maximum value) and were compared using Mann–Whitney and Kruskal–Wallis tests. Qualitative variables were compared using the chi-squared test and Fisher’s exact test when necessary. Survival status was estimated using the Kaplan–Meier method. The Log-rank test was used to compare survival distribution. Cox regression model was used to analyze factors associated with mortality. The proportional hazards assumption was verified by creating a Cox model with time-by-covariate interactions for each variable (introducing products between the variables and a linear function of time) and testing for their significance. A graphical method was also used to analyze the log-minus-log of the survival function versus the survival time plots (the stratum-specific log-minus-log plots exhibiting constant differences being parallel). Parameters associated with survival in univariate analysis were used as covariates in the Cox proportional model. Post-hoc analysis between group 1 and other PH groups were performed. A P value < 0.05 was considered statistically significant.
Results
Overall population
A total of 363 patients met inclusion criteria and were included in the study. Their baseline clinical and functional characteristics are detailed in Table 1. Median age was 69 years (age range = 14–89 years) and 62% (n = 225) of the study population was aged > 65 years. One-, three-, and five-year overall survival rates were 84.8%, 70.8%, and 61.3%, respectively.
Table 1.
Baseline clinical and functional characteristics of patients with PH.
| Overall | Group 1 | Group 3 | Group 4 | Group 5 | P * | |
|---|---|---|---|---|---|---|
| n (%) | 363 (100) | 164 (45.2) | 109 (30) | 65 (17.9) | 25 (6.9) | – |
| Age (years)† | 69 (14–89) | 65 (14–87) | 72 (44–89) | 76 (46–86) | 54 (27–97) | <0.001 |
| Age > 65 (n (%)) | 225 (62) | 84 (51.2) | 82 (75.2) | 53 (81.5) | 6 (24) | <0.001 |
| Female/Male ratio | 0.81 | 1.4 | 0.14 | 1.5 | 1.27 | <0.001 |
| Co-morbidities (n (%)) | ||||||
| HFpEF | 10 (3) | 3 (1.8) | 5 (4.5) | 1 (1.5) | 1 (5.2) | 0.06 |
| CHD | 64 (21) | 25 (15) | 29 (26.6) | 9 (13.8) | 1 (4.0) | 0.15 |
| Diabetes | 71 (23) | 33 (20.3) | 25 (22.9) | 10 (15.4) | 3 (12) | 0.32 |
| Hypertension | 159 (51) | 73 (44.3) | 52 (47.9) | 26 (40) | 8 (31.7) | 0.23 |
| COPD | 70 (23) | 20 (12) | 40 (36.7) | 6 (9.2) | 4 (16) | <0.001 |
| Liver cirrhosis | 23 (8) | 15 (9) | 2 (1.8) | 4 (6.2) | 3 (10.4) | 0.07 |
| Solid tumor | 43 (14) | 16 (9.8) | 14 (12.8) | 9 (13.8) | 4 (15.6) | 0.95 |
| Renal failure | 49 (16) | 22 (13.5) | 18 (16.5) | 6 (9.2) | 3 (12) | 0.57 |
| Charlson index | 4 (0–10) | 3 (0–10) | 4 (0–8) | 3 (0–8) | 3 (0–7) | 0.02 |
| NYHA class (n (%)) | <0.001 | |||||
| I | 11 (3) | 8 (4.9) | 0 (0) | 2 (3.1) | 1 (4) | |
| II | 134 (36.9) | 79 (48.2) | 19 (17.4) | 28 (43.1) | 8 (32) | |
| III | 189 (52.1) | 67 (40.8) | 75 (68.8) | 33 (50.7) | 14 (56) | |
| IV | 29 (8) | 10 (6.1) | 15 (13.8) | 2 (3.1) | 2 (8) | |
| 6MWD (m)† | 271 (0–640) | 320 (0–640) | 222 (0–511) | 271 (0–600) | 348 (0–500) | <0.001 |
| Follow-up time† (years) | 3.4 (2.8) | 3.5 (2.7) | 2.3 (2.6) | 3.8 (2.2) | 5 (3.8) | <0.001 |
| PFT† | ||||||
| FVC (%) | 89 (34–128) | 91 (66–123) | 78 (34–125) | 100 (55–128) | 75 (38–109) | <0.001 |
| TLC (%) | 86 (34–124) | 88 (69–121) | 78 (34–125) | 91 (58–109) | 75 (38–109) | 0.02 |
| FEV1 (%) | 76 (18–111) | 81 (66–111) | 62 (25–108) | 87 (33–111) | 56 (18–102) | <0.001 |
| KCO (%) | 60 (7–109) | 64 (15–107) | 35 (7–108) | 71 (12–108) | 52 (37–109) | <0.001 |
| PaO2 (mmHg)† | 60 (30–93) | 63 (34–93) | 52 (30–83) | 60 (34–82) | 62 (32–87) | <0.001 |
| LTO (n (%)) | 200 (55) | 70 (42.6) | 96 (88) | 26 (40) | 11 (44) | <0.001 |
| Deaths (n (%)) | 129 (35.5) | 50 (30.5) | 63 (57.8) | 11 (16.9) | 5 (20) | <0.001 |
Comparison of four PH groups.
Values are expressed as median with minimal–maximal value or interquartile range.
HfpEF, heart failure with preserved ejection fraction; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease, 6MWD: 6-min walking distance; PFT, pulmonary function test; FVC, forced vital capacity; TLC, total lung capacity; FEV1, forced expiratory volume in 1 s; KCO, carbon monoxide transfer coefficient; PaO2, partial pressure in arterial oxygen; LTO, long-term oxygen therapy.
PAH
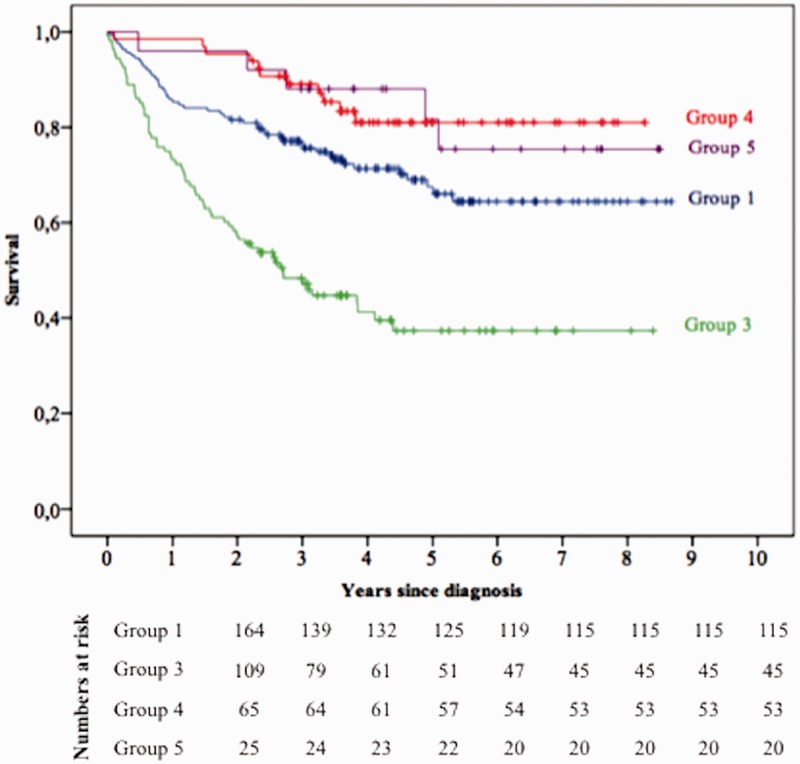
In total, 164 patients (45.2%) belonged to group 1 PH (Table 1). Idiopathic PAH (IPAH) was the most common cause of group 1 PH (40.9%), followed by connective tissue disease (CTD) (26.2%), portopulmonary hypertension (PPH) (13.4%), drug-induced PAH (8.6%), congenital heart disease (CHD) (6.1%), HIV (2.4%), and heritable PAH (2.4%) (Table 2). Nine patients in group 1 PH (5.5%) were considered responders to acute vasoreactivity testing and were treated with high-dose calcium channel blockers (CCB) (Table 3). Five of these nine patients (3%) were long-term responders, i.e. their New York Heart Association (NYHA) class changed to class I or II after one year of treatment. One-, three-, and five-year survival rates for PAH patients were 85.3%, 75.7%, and 66%, respectively (Fig. 1). The five-year survival rate was 70.8% for IPAH, 55.5% for CTD-PAH, 64.6% for drug-induced PAH, 50.6% for PPH, 90% for CHD-PAH, and 100% for HIV-PAH.
Table 2.
Etiologies of PH in groups 1, 3, and 5.
| PH group | Etiology | n (%) |
|---|---|---|
| Group 1 | IPAH | 67 (40.9) |
| CTD | 43 (26.2) | |
| PoPH | 22 (13.4) | |
| Anorexigen | 14 (8.6) | |
| CHD | 10 (6.1) | |
| HIV | 4 (2.4) | |
| Heritable | 4 (2.4) | |
| Group 3 | COPD | 40 (36.7) |
| CPFE | 37 (33.9) | |
| IPF | 14 (12.8) | |
| Non-IPF ILD | 12 (11) | |
| Other | 6 (5.6) | |
| Group 5 | Sarcoidosis | 10 (40) |
| Hematological disorders | 5 (20) | |
| LAM | 4 (16) | |
| LCH | 3 (12) | |
| Chronic renal failure | 2 (8) | |
| Splenectomy | 1 (4) |
IPAH, idiopathic pulmonary arterial hypertension; CTD, connective tissue disease; PoPH, portopulmonary hypertension; CHD, congenital heart disease; HIV, human immunodeficiency virus; COPD, chronic obstructive pulmonary disease; CPFE, combined pulmonary fibrosis and emphysema; IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; LAM, lymphangioleiomyomatosis; LCH, Langerhans cell histiocytosis.
Table 3.
Treatment initiated in the first three months following PH diagnosis.
| Overall | Group 1 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| Treatment strategy (n (%)) | |||||
| No treatment | 25 (6.9) | 12 (7.3) | 10 (9.1) | 2 (3.1) | 4 (16) |
| Monotherapy | 297 (81.8) | 134 (81.7) | 92 (84.5) | 49 (21.5) | 19 (76) |
| Combination therapy | 41 (11.3) | 18 (11) | 7 (6.4) | 14 (75.4) | 2 (8) |
| Therapeutic class (n (%)) | |||||
| Prostanoids | 13 (3.6) | 7 (4.3) | 5 (4.6) | 1 (1.5) | 0 (0) |
| ERA | 148 (40.8) | 67 (40.9) | 38 (34.9) | 32 (49.3) | 11 (44) |
| PDE-5i | 124 (34.2) | 51 (31.1) | 49 (44.9) | 16 (24.6) | 8 (32) |
| CCB | 12 (3.3) | 9 (5.5) | 0 (0) | 0 (0) | 0 (0) |
| PDE-5i + ERA | 33 (9.1) | 14 (8.5) | 3 (2.8) | 14 (21.5) | 2 (8) |
| Prostanoids + PDE-5i | 2 (0.5) | 1 (0.6) | 1 (0.9) | 0 (0) | 0 (0) |
| Prostanoids + ERA | 6 (1.6) | 3 (1.8) | 3 (2.8) | 0 (0) | 0 (0) |
ERA, endothelin receptor antagonist; PDE-5i, phosphodiesterase 5 inhibitor; CCB, calcium channel blocker.
Fig. 1.
Survival of patients according to PH group.
Group 3 PH
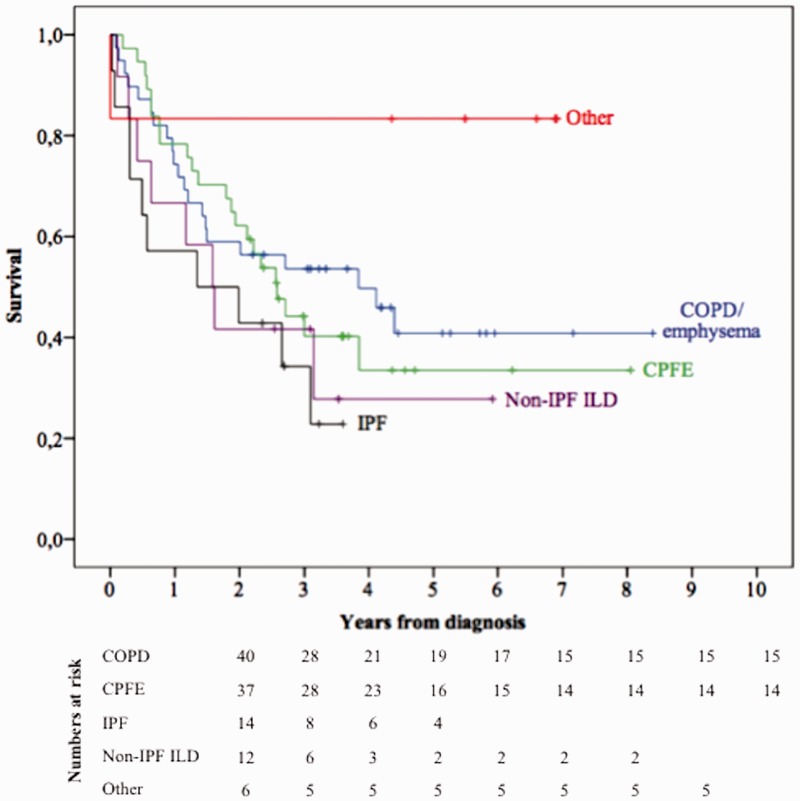
A total of 109 patients (30%) belonged to group 3 PH (Table 1). COPD/emphysema was the most common cause (36.7%), followed by combined pulmonary fibrosis and emphysema (CPFE) (33.9%), IPF (12.8%), non-IPF ILD (11%), diffuse bronchiectasis and other causes of chronic lung disease such as tuberculosis sequelae and obesity hypoventilation syndrome (Table 2). Pulmonary function tests for group 3 patients are detailed in Table 4. Ninety-four patients (86.2%) had severe PH as defined by mPAP > 35 mmHg alone, or mPAP ≥ 25 mmHg in the presence of a low cardiac output (cardiac index [CI] < 2.5 L/min/m2). In the first three months following PH diagnosis, monotherapy was initiated in 92 patients (84.5%) and combination therapy was initiated in seven patients (6.4%) (Table 3). Following initial diagnosis, phosphodiesterase-5 inhibitors (PDE-5i) were prescribed in 44.9% of group 3 patients, endothelin receptor antagonists (ERA) in 34.9%, and prostanoids in 4.6% of patients (Table 3). One-, three-, and five-year survival rates for group 3 PH patients were 72.2%, 47.1%, and 37.3%, respectively (Fig. 1). The three-year survival rate was 51.1% for COPD/emphysema, 40.2% for CPFE, 22.9% for IPF, 30% for non IPF-ILD, and 100% for the remaining causes combined (Fig. 2).
Table 4.
Pulmonary function tests in patients with group 3 PH.
| COPD | CPFE | IPF | Non-IPF ILD | Other | |
|---|---|---|---|---|---|
| FVC (%) | 90 (45–125) | 87 (47–124) | 48 (34–77) | 59 (36–94) | 63 (46–74) |
| TLC (%) | 93 (67–124) | 80 (34–115) | 50 (34–83) | 72 (37–94) | 58 (38–78) |
| FEV1 (%) | 61 (25–69) | 78 (43–108) | 54 (37–90) | 59 (33–103) | 75 (50–77) |
| KCO (%) | 35 (12–87) | 31 (7–126) | 56 (24–67) | 62 (20–96) | 106 (104–108) |
Values are expressed as median (min–max).
COPD, chronic obstructive pulmonary disease; CPFE, combined pulmonary fibrosis and emphysema; IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease.
Fig. 2.
Survival of patients with group 3 PH. COPD, chronic obstructive pulmonary disease; CPFE, combined pulmonary fibrosis and emphysema; IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; other, bronchiectasis, tuberculosis sequelae, and obesity-hypoventilation syndrome.
Group 4 PH
Chronic thromboembolic pulmonary hypertension (CTEPH) was diagnosed in 65 patients (17.9%) based on ventilation/perfusion lung scan (Table 1). Ten patients (15.3%) underwent surgical thromboendarteriectomy. One-, three-, and five-year survival rates were 98.5%, 89%, and 81%, respectively (Fig. 1). There was no significant difference in the five-year survival rates between patients who underwent surgical thromboendarteriectomy (87.5%) and patients who were treated medically (79.8%) (P = 0.54).
Group 5 PH
Twenty-five patients (6.9%) belonged to group 5 PH (Table 1). Sarcoidosis was the most common cause (40%), followed by hematologic disorders (20%), lymphangioleiomyomatosis (LAM) (16%), pulmonary Langerhans cell histiocytosis (PLCH) (12%), splenectomy (8%), and chronic renal failure (4%) (Table 2). One-, three-, and five-year survival rates were 96%, 88%, and 81.7%, respectively (Fig. 1). The three-year survival rate for sarcoidosis with PH was 70%, and 100% for all the other diseases.
Comparison between PH groups
Comparison of clinical and functional baseline characteristics (Table 1) showed that patients with group 3 or 4 PH were significantly older (P < 0.001) and had lower 6-min walking distance (6MWD) (P < 0.001) than patients with group 1 and 5 PH. Group 3 patients had a significantly higher Charlson Comorbidity Index. Gender distribution was significantly different between groups (P < 0.001) and group 3 patients were predominantly male. NYHA class was significantly different between groups (P < 0.001) and group 3 patients were the most numerous to initially be class III or IV. Patients with group 3 or 5 PH had lower FVC (P < 0.001), TLC (P = 0.002), and FEV1 values (P < 0.001). Group 3 patients had the lowest carbon monoxide transfer coefficient (KCO) values (P < 0.001), PaO2 values (P < 0.001), and received long-term oxygen therapy more frequently (P < 0.001).
Comparison of hemodynamic baseline characteristics (Table 5) showed that patients with group 3 or 4 PH had higher mPAP (P = 0.01) and PVR (P = 0.001) values than patients with group 1 and 5 PH; they also had lower venous oxygen saturation (SvO2) (P < 0.001) and CI (P = 0.001) values than patients with group 1 and 5 PH.
Table 5.
Baseline echocardiographic and hemodynamic characteristics of PH patients.
| Overall | Group 1 | Group 3 | Group 4 | Group 5 | P * | |
|---|---|---|---|---|---|---|
| Pericardial effusion (n (%)) | 57 (15.7) | 26 (15.8) | 18 (16.5) | 8 (12.3) | 5 (20) | 0.82 |
| mPAP† (mmHg) | 40 (25–83) | 38 (25–83) | 41 (26–72) | 42 (26–68) | 35 (26–63) | 0.01 |
| PCWP† (mmHg) | 9 (1–15) | 9 (1–15) | 10 (2–15) | 8 (1–15) | 11 (3–15) | 0.13 |
| PVR† (WU) | 9.1 (3.3–45.5) | 8.5 (3.3–28.5) | 9.7 (3.9–23.1) | 10.3 (4.5–45.5) | 7.4 (3.9–17.9) | 0.001 |
| CI† (L/min/m2) | 2.4 (0.8–5.6) | 2.6 (1.1–5.2) | 2.4 (1.1–4) | 2.2 (0.8–3.7) | 3 (1.7–5.6) | 0.001 |
| RAP† (mmHg) | 6 (0–27) | 6 (0–27) | 6 (1–21) | 5 (0–21) | 6 (0–16) | 0.54 |
| SvO2† (%) | 64 (25–94) | 66 (33–93) | 61 (40–93) | 61 (25–94) | 65 (34–89) | <0.001 |
Comparison of four PH groups.
Values are expressed as median (min–max).
mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; CI, cardiac index; RAP, right atrial pressure; SvO2, venous oxygen saturation.
Overall survival was significantly different between the four groups (P < 0.001) (Fig. 1). Univariate analysis of prognostic factors in the overall population showed that age, male gender, NYHA class, groups 3 and 4 PH (versus PAH), 6MWD, FVC, TLC, KCO, PVR, CI, and SvO2 were significantly associated with greater mortality (Table 6). When these factors were used as covariates in the Cox proportional model, multivariate analysis showed that age, PH group 4, 6MWD, and KCO but no longer PH group 3 were significantly associated with mortality (Table 6). Among patients with group 3 PH, multivariate analysis showed that age and TLC were significantly associated with mortality (Table 7).
Table 6.
Factors associated with mortality in univariate and multivariate analysis in the overall PH cohort.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Age (years) | 0.001 | 1.82 (1.28–2.59) | 0.006 | 1.03 (1.01–1.05) |
| NYHA class | <0.001 | 2.05 (1.54–2.73) | 0.26 | 0.8 (0.52–1.2) |
| Male gender | 0.003 | 1.74 (1.21–2.51) | 0.10 | 1.6 (0.9–2.8) |
| Group 3 vs. PAH | <0.001 | 2.50 (1.71–3.64) | 0.76 | 0.90 (0.48–1.7) |
| Group 4 vs. PAH | 0.04 | 0.51 (0.26–0.98) | 0.002 | 0.18 (0.06–0.53) |
| Group 5 vs. PAH | 0.22 | 0.56 (0.22–1.41) | 0.09 | 0.28 (0.06–1.2) |
| 6MWD (m)* | <0.001 | 0.996 (0.995–0.997) | <0.001 | 0.99 (0.995–0.998) |
| FVC (%)* | 0.015 | 0.99 (0.981–0.998) | 0.87 | 1.002 (0.98–1.02) |
| TLC (%)* | 0.004 | 0.985 (0.975–0.995) | 0.13 | 0.98 (0.96–1.005) |
| FEV1 (%)* | 0.06 | 0.992 (0.984–1) | – | |
| KCO (%)* | <0.001 | 0.983 (0.975–0.992) | 0.004 | 0.98 (0.97–0.99) |
| mPAP (mmHg)* | 0.8 | 1 (0.986–1.12) | – | |
| PVR (WU)* | 0.01 | 1.03 (1.008–1.06) | 0.70 | 1.01 (0.95–1.07) |
| CI (L/min/m2)* | 0.01 | 0.61 (0.46–0.81) | 0.56 | 0.87 (0.55–1.38) |
| SvO2 (%)* | <0.001 | 0.96 (0.94–0.98) | 0.13 | 0.97 (0.95–1.006) |
Per 1 unit increment.
PAH, pulmonary arterial hypertension.
Table 7.
Factors associated with mortality in univariate and multivariate analysis of the group 3 PH population.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Age | 0.003 | 1.04 (1.01–1.07) | 0.01 | 1.04 (1.01–1.08) |
| NYHA class | 0.12 | 1.46 (0.90–2.36) | – | |
| Male gender | 0.22 | 0.64 (0.31–1.30) | – | |
| PH etiology | 0.42 | 0.97 (0.92–1.03) | – | |
| 6MWD (m)* | <0.001 | 0.996 (0.994–0.998) | 0.19 | 0.998 (0.996–1.001) |
| FVC (%)* | 0.49 | 0.99 (0.98–1.01) | – | |
| TLC (%)* | 0.01 | 0.984 (0.971–0.997) | 0.005 | 0.97 (0.96–0.99) |
| FEV1 (%)* | 0.40 | 1.005 (0.99–1.01) | – | |
| KCO (%)* | 0.33 | 0.99 (0.98–1.01) | – | |
| mPAP (mmHg)* | 0.51 | 1.009 (0.98–1.03) | – | |
| PVR (WU)* | 0.006 | 1.08 (1.02–1.15) | 0.23 | 1.08 (0.94–1.24) |
| CI (L/min/m2)* | 0.06 | 0.66 (0.42–1.03) | – | |
| SvO2 (%)* | 0.004 | 0.95 (0.92–0.98) | 0.13 | 0.96 (0.92–1.01) |
Per 1 unit increment.
Discussion
This study describes the spectrum of patients in a PH expert center in France over an eight-year period. It is one of the rare studies comparing clinical, functional, hemodynamic, and survival characteristics between PH groups. It shows that group 3 PH, when associated with lung diseases, has distinctive features which are themselves associated with a more severe prognosis.
Compared to the other PH groups, group 3 patients are older, have a higher co-morbidity index, and have more severe functional and hemodynamic impairments. Their survival is significantly poorer, even though PAH therapy was used in the majority of cases. These findings may be explained by the combined burdens of the parenchymal disease on one hand and of the pulmonary vascular disease on the other hand. They are also consistent with results of randomized controlled trials that showed the lack of a significant effect of PAH drugs in improving symptoms or mortality in group 3 PH.17–22 Among group 3 patients, survival was poorer in patients with IPF, non-IPF ILD, and CPFE. In our study, one-year and three-year survival rates of CPFE patients are higher than what we reported in a previous study;7 nevertheless, these survival rates remain low. Our results are concordant with previous recent studies. One study compared the phenotypic characteristics and outcomes of 118 patients with lung disease and severe PH with 74 IPAH patients.12 Lung disease patients were older, more hypoxemic, had lower gas transfer, worse NYHA functional class, and lower 6MWD than IPAH patients. PH therapy in severe PH lung disease did not lead to improvement in 6MWD or functional class. A second study derived from the COMPERA registry compared 151 patients with PH associated to idiopathic ILD and 798 patients with IPAH.13 Survival rates were significantly worse in PH-ILD than in IPAH. PH therapy was associated with short-term functional improvement in some of these patients but whether this treatment affects survival was not clear. Another study derived from the ASPIRE registry compared 42 patients with COPD and mild PH to 59 patients with COPD and severe PH.14 Compassionate treatment with targeted therapies in 43 patients with severe PH was not associated with a survival benefit, although improvement in functional class and/or fall in PVR > 20% identified patients with improved survival.
The high prevalence of group 3 patients in our study may be due to a referral bias, our center being designated as a PH expert center as well as a national reference center for rare pulmonary diseases. Moreover, patients with chronic lung disease referred to our center are likely to have much more severe hemodynamics and symptoms than patients who are not referred by their respiratory physician because they have milder PH. In our study, 86.2% of group 3 patients had severe PH. This bias may hinder us from applying our results to a broader cohort of lung disease patients. This finding also explains the high rate of PH therapy in group 3 patients in our cohort, especially when distinction between group 1 PH and severe PH associated with lung disease—previously designated as “out of proportion PH”—was difficult. However, when no significant clinical or hemodynamic effect of PH therapy was found in these patients, treatment was subsequently discontinued. Over the years, the performance of RHC in patients with chronic lung disease has become restrained with respect to the lack of significant beneficial effect of PH therapy in this population. Presently, RHC is performed according to current guidelines, in patients with normal or mildly impaired lung function, normal or modest airway, or parenchymal abnormalities and features of exhausted circulatory reserve (8).
Another finding of our study is the high median age of the overall PH population and of the PAH population. This result is corroborated by the reported increase in patient age at the time of diagnosis23–25 from a mean age of 36 years in the NIH registry three decades ago,26 to a mean age of 50 years in the French27 and REVEAL registries,28 and up to a median age of 68 years in the COMPERA study.29
More than half of PAH patients in our study are aged over 65 years and are thus considered elderly. Epidemiological studies have shown a global increase in the proportion of the elderly population in France.30 Therefore, an expansion in the number of elderly patients diagnosed with PH is expected. This category of patients may have different characteristics than PAH patients described in previous cohorts. Older patients generally have a higher co-morbidity index31 and a higher percentage of left heart disease. Another particular aspect which might mislead PH diagnosis is the high prevalence of heart failure with preserved ejection fraction in the elderly population.32,33 The safety of invasive diagnostic procedures, as well as the efficacy and safety of PAH therapies in the elderly population, are unknown because most randomized controlled trials involve younger patients.
A surprising finding in our study was the low rate of group 4 patients who underwent surgical thromboendarteriectomy (15.3%). In the European registry, 37% of patients were considered inoperable.34 Although no clear explanation for this low rate was found, it could be explained by the patients’ refusal of surgery (done in a single center in France) or to the lack of referral of CTEPH patients to the National Reference Center for PAH for the operability evaluation (severe co-morbidities, a priori inaccessible peripheral disease). This might also be explained by the higher age of group 4 patients in our study (median age = 76 years) in comparison to the CHEST-1 trial (mean age = 59 years)35 and the European CTEPH registry (median age = 63 years).34 The higher age of group 4 patients may imply a larger delay in the diagnosis of CTPEH in this category of patients. The low percentage of surgical thromboendarteriectomy in our cohort may have affected survival analysis in group 4 patients. However, there was no difference in survival between patients who underwent surgery or not.
Our study has limitations. Retrospective analysis is prone to selection and information bias, although data were acquired prospectively. Twelve percent of PAH patients and 9.2% of group 4 patients had COPD. However, the majority of these patients (94.8%) had mild obstructive disease with FEV1 > 60% and no significant radiological abnormalities; hence, they were subsequently not classified as group 3 patients. Complete hemodynamic measures were missing for eight patients (0.02%). However, survival data were obtained for all patients and no other data concerning the baseline characteristics were missing. Another limitation of our study is its monocentric design, which may hinder the extrapolation of results. However, our study shows similar one-year survival rates for PAH patients in comparison to other registries (85% vs. 79–84%).
In conclusion, the four groups of precapillary PH have different clinical, functional, and hemodynamic presentations. Group 3 PH is characterized by the worst prognosis, partially due to the higher proportion of elderly people. Underlying respiratory diseases are certainly involved in the poor survival rates of group 3 patients. Our findings confirm that PH therapy is not beneficial in group 3 patients and should therefore not be used in this context. The epidemiology of PAH is progressively changing: it affects more and more elderly patients. The high prevalence of elderly patients in PAH requires a comprehensive study of this population, in order to better evaluate the appropriateness of invasive diagnostic procedures and the suitability of available therapies.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–41. [DOI] [PubMed] [Google Scholar]
- 3.Chaouat A, Bugnet A-S, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. [DOI] [PubMed] [Google Scholar]
- 4.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012; 31: 373–380. [DOI] [PubMed] [Google Scholar]
- 5.Lettieri CJ, Nathan SD, Barnett SD, et al. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006; 129: 746–752. [DOI] [PubMed] [Google Scholar]
- 6.Nathan SD, Shlobin OA, Ahmad S, et al. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration 2008; 76: 288–294. [DOI] [PubMed] [Google Scholar]
- 7.Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J 2010; 35: 105–111. [DOI] [PubMed] [Google Scholar]
- 8.Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–116. [DOI] [PubMed] [Google Scholar]
- 9.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780–788. [DOI] [PubMed] [Google Scholar]
- 10.Sitbon O, Humbert M, Jaïs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111: 3105–3111. [DOI] [PubMed] [Google Scholar]
- 11.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 12.Brewis MJ, Church AC, Johnson MK, et al. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J 2015; 46: 1378–1389. [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Behr J, Held M, et al. Pulmonary hypertension in patients with chronic fibrosing idiopathic interstitial pneumonias. PloS One 2015; 10: e0141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013; 41: 1292–1301. [DOI] [PubMed] [Google Scholar]
- 15.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolz D, Rasch H, Linka A, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008; 32: 619–628. [DOI] [PubMed] [Google Scholar]
- 18.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010; 181: 270–278. [DOI] [PubMed] [Google Scholar]
- 19.Zisman DA, Schwarz M, Anstrom KJ, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 2010; 363: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lederer DJ, Bartels MN, Schluger NW, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD 2012; 9: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42: 982–992. [DOI] [PubMed] [Google Scholar]
- 22.Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2014; 2: 293–300. [DOI] [PubMed] [Google Scholar]
- 23.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 2011; 139: 128–137. [DOI] [PubMed] [Google Scholar]
- 24.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. [DOI] [PubMed] [Google Scholar]
- 25.Hoeper MM, Simon R, Gibbs J. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014; 23: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987; 107: 216–223. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 28.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 29.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013; 168: 871–880. [DOI] [PubMed] [Google Scholar]
- 30.Béland D, Viriot Durandal J-P. Aging in france: population trends, policy issues, and research institutions. The Gerontologist 2013; 53: 191–197. [DOI] [PubMed] [Google Scholar]
- 31.Shimony A, Fox BD, Afilalo J, et al. Pulmonary arterial hypertension in the elderly-clinical characteristics and long-term survival. Lung 2012; 190: 645–649. [DOI] [PubMed] [Google Scholar]
- 32.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 33.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007; 50: 768–777. [DOI] [PubMed] [Google Scholar]
- 34.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 35.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]