Abstract
Exon skipping uses chemically modified antisense oligonucleotides to modulate RNA splicing. Therapeutically, exon skipping can bypass mutations and restore reading frame disruption by generating internally truncated, functional proteins to rescue the loss of native gene expression. Limb-girdle muscular dystrophy type 2C is caused by autosomal recessive mutations in the SGCG gene, which encodes the dystrophin-associated protein γ-sarcoglycan. The most common SGCG mutations disrupt the transcript reading frame abrogating γ-sarcoglycan protein expression. In order to treat most SGCG gene mutations, it is necessary to skip 4 exons in order to restore the SGCG transcript reading frame, creating an internally truncated protein referred to as Mini-Gamma. Using direct reprogramming of human cells with MyoD, myogenic cells were tested with 2 antisense oligonucleotide chemistries, 2’-O-methyl phosphorothioate oligonucleotides and vivo–phosphorodiamidate morpholino oligomers, to induce exon skipping. Treatment with vivo–phosphorodiamidate morpholino oligomers demonstrated efficient skipping of the targeted exons and corrected the mutant reading frame, resulting in the expression of a functional Mini-Gamma protein. Antisense-induced exon skipping of SGCG occurred in normal cells and those with multiple distinct SGCG mutations, including the most common 521ΔT mutation. These findings demonstrate a multiexon-skipping strategy applicable to the majority of limb-girdle muscular dystrophy 2C patients.
Keywords: Genetics, Muscle Biology
Keywords: Monogenic diseases, Neuromuscular disease, Skeletal muscle
Antisense oligonucleotides correct SGCG frameshift mutations in human cells derived from Limb Girdle Muscular Dystrophy 2C individuals harboring different SGCG mutations.
Introduction
Limb-girdle muscular dystrophy type 2C (LGMD 2C) is a debilitating muscle disease characterized by progressive muscle degeneration, loss of ambulation, and eventual deterioration of respiratory and cardiac function (1, 2). Autosomal recessive mutations in the γ-sarcoglycan gene (SGCG) cause LGMD 2C (3). The SGCG gene encodes a type II transmembrane protein that is a component of the sarcoglycan complex, which — in skeletal and cardiac muscle — is comprised of α-, β-, γ-, and δ-sarcoglycans (4, 5). The sarcoglycan complex is part of the larger dystrophin glycoprotein complex (DGC), which localizes to the muscle plasma membrane, stabilizes the sarcolemma, and connects the extracellular matrix to the intracellular cytoskeleton (6, 7). SGCG mutations commonly disrupt the reading frame and result in the absence of γ-sarcoglycan protein expression (3, 8, 9). There are currently no approved therapies for treating LGMD 2C, and the mainstay of medical treatment relies on supportive care (10).
Exon skipping uses chemically modified antisense oligonucleotides (AONs). The utility of exon skipping to correct reading frame disruption has been demonstrated for the treatment of Duchenne muscular dystrophy (DMD), which is caused by mutations in the dystrophin gene (11). The concept of exon skipping derived from observations that deletions in the dystrophin gene, which disrupt the transcript reading frame, cause the severe DMD, while in-frame deletions cause Becker muscular dystrophy (BMD), a milder form of disease with improved clinical outcomes (12, 13). Moreover, sporadic dystrophin-positive fibers, known as revertant fibers, have been observed in muscle biopsies from DMD patients and in mouse models of the disease (14, 15). Revertant fibers in dystrophin mutant muscle represent intrinsic bypass of mutations that correct the reading frame to restore expression of an internally deleted dystrophin protein (16). Exon skipping uses AONs to hinder sterically the pre-mRNA splicing of a target exon, blocking its incorporation into the mature mRNA transcript (17). For clinical benefit, the mRNAs produced after exon skipping are required to encode and produce an internally truncated protein with functional relevance.
AONs are short RNA or DNA oligomers that are the reverse complementary sequence to a target mRNA sequence (18). AON chemical modifications are designed to avoid nuclease degradation and enhance RNA binding (18). Further modifications are required for the purpose of splice modulation to avoid RNase-H degradation of the oligo-RNA hybrid, as the goal is transcript correction and not destruction (17). Multiple chemical modifications were developed for this purpose, with 2’-O-methyl phosphorothioate (2OMePS) AONs and phosphorodiamidate morpholino oligomers (PMOs) having been tested in human clinical trials for DMD (19–22). In 2016, the US Food and Drug Administration (FDA) granted accelerated approval of a PMO to treat DMD caused by specific mutations in the dystrophin gene, whose reading frame is corrected through the skipping of exon 51 (23). Eteplirsen represented an achievement in the fields of neuromuscular disease and molecular gene correction, and it was followed by the approval of nusinersen for treating spinal muscular atrophy (SMA) (23, 24). Progress toward clinical development of antisense-mediated splice modulating therapy has expanded beyond the treatment of DMD to include other disorders, such as Pompe disease, cystic fibrosis, cardiomyopathies, and laminopathies (25–29).
Recently, we explored the therapeutic potential of exon skipping to treat LGMD 2C by examining whether Mini-Gamma protein can successfully assume the functional roles of full-length γ-sarcoglycan (30). The SGCG gene is composed of 8 exons, with exons 2–8 encoding protein. The most common SGCG mutation is a single thymine deletion from a string of 5 thymines in SGCG exon 6, designated 521ΔT (3, 8). Reframing the 521ΔT transcript requires the skipping of exons 4, 5, 6, and 7, creating an internally truncated product encoded by exons 2, 3, and 8, termed Mini-Gamma. We demonstrated that transgenic expression of murine Mini-Gamma provided functional and pathological benefit to correct the loss of γ-sarcoglycan in both fly and mouse models of the disease (30). Here, we report the use of AON-mediated exon skipping to correct the reading frame of multiple SGCG mutations, including the 521ΔT mutation, in a human cell model of disease. To this end, multiexon-skipping AON cocktails were employed using either 2OMePS or vivo-PMO chemistries. The vivo-PMOs were more efficient than 2OMePS oligomers in generating the Mini-Gamma transcript. This approach targets any mutation occurring in SGCG exons 4–7, making this therapy applicable to the majority of LGMD 2C patients.
Results
An exon-skipping strategy to treat LGMD 2C.
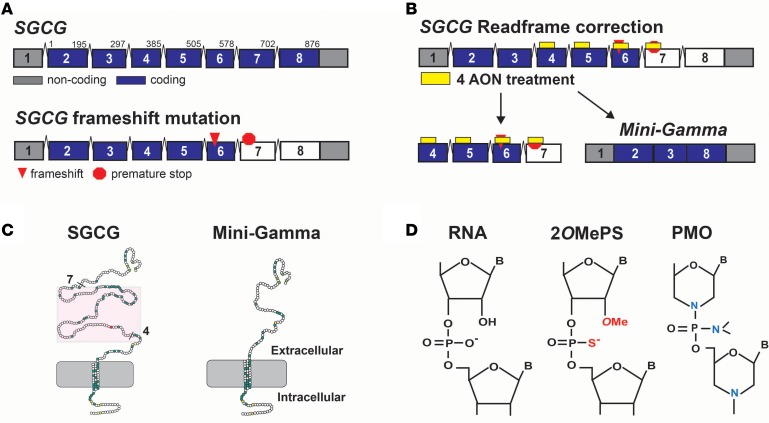
The 8 γ-sarcoglycan exons produce a type II transmembrane protein that is an essential component of the muscle plasma membrane. Homozygous SGCG mutations, which disrupt the reading frame, generate premature stop codons in the mRNA, preventing protein expression and causing LGMD 2C (Figure 1A). Exon skipping uses AONs to block pre-mRNA splicing sites, bypassing mutations and modifying the exon content of an mRNA. Maintaining the reading frame requires skipping of exons 4, 5, 6, and 7 to create an internally truncated transmembrane protein, termed Mini-Gamma, encoded by exons 2, 3, and 8 (Figure 1B). Mini-Gamma retains the essential intracellular, transmembrane, and extracellular SGCG domains required for functionality (Figure 1C), and transgenic expression of murine Mini-Gamma has been shown to rescue multiple features of muscular dystrophy in fly and mouse models of disease (30).
Figure 1. An exon-skipping strategy to treat LGMD 2C.
(A) The SGCG gene, which encodes γ-sarcoglycan, is comprised of 8 exons, and many SGCG mutations disrupt the transcript reading frame, causing Limb-girdle muscular dystrophy (LGMD) 2C. (B) Correction of the reading frame requires the skipping of SGCG exons 4, 5, 6, and 7 at the pre-mRNA level. Four antisense oligonucleotides (AON) were designed to exclude these exons from the mature mRNA transcript. The bypass of exons 4–7 generates an internally truncated product termed Mini-Gamma encoded by exons 2, 3, and 8. (C) Mini-Gamma protein lacks a portion of the extracellular domain encoded by exons 4–7 (red box) and retains the essential cytosolic, transmembrane, and extracellular domains required for functionality (30). (D) Exon skipping AONs are chemically modified to avoid nuclease degradation and destruction of the mRNA transcript. Shown are the chemical structures of 2 types of AONs used for exon skipping. 2’-O-methyl phosphorothioates (2OMePS) include a methyl group on the ribose ring and substitution of sulfur for oxygen to create a phosphothioate bond (red). Phosphorodiamidate morpholino oligomers (PMOs) incorporate morpholino rings rather than ribose rings and substitute nitrogen for oxygen to create a phosphorodiamidate bond (blue) (18). Vivo-PMOs are synthesized with a covalently linked 3′ octa-guanidine delivery moiety for enhanced cell delivery (31).
Chemical modifications of AONs are necessary to prevent nuclease attack and degradation of the transcript, while increasing their stability and interaction with the RNA target (17, 18). We evaluated 2 common AON chemical modifications for exon skipping for LGMD 2C: 2OMePS and PMO (Figure 1D). PMOs were synthesized as vivo-PMOs, which contained a covalently linked C-terminal octa-guanidine dendrimer delivery moiety that promotes cell membrane permeability (31). Overall, this exon-skipping strategy was designed to target SGCG missense, nonsense, or frame-shifting mutations in exons 4–7 that account for the majority of LGMD 2C disease–causing mutations.
A human cell–based model of LGMD 2C.
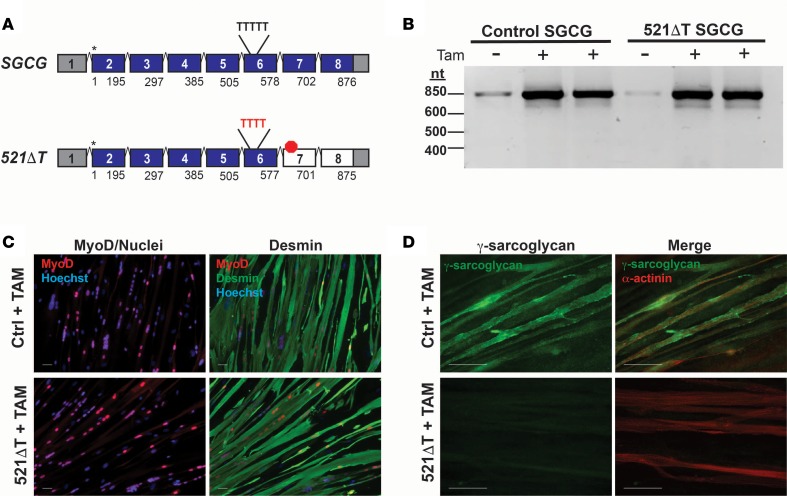
Fibroblasts were obtained from an individual with an autosomal recessive deletion of a single thymine in SGCG exon 6, a mutation designated 521ΔT, which disrupts the reading frame and results in the absence of γ-sarcoglycan protein (Figure 2A) (3). This is the most common mutation of the SGCG gene and accounts for almost half of all LGMD 2C patients (3, 8). We reprogrammed control and 521ΔT fibroblasts into the myogenic lineage using a tamoxifen-inducible MyoD (iMyoD) lentiviral system (32). To assess the possibility of reading frame correction via exon skipping, we first determined if the reprogrammed 521ΔT cells produce a stable mutant mRNA transcript. Analysis of PCR-amplified transcripts detected native and mutant SGCG expression in reprogrammed control and 521ΔT fibroblasts, respectively (Figure 2B) (See complete unedited blots in the supplemental material; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.99357DS1). Sequence analysis confirmed the 521ΔT deletion in the mutant transcript (Supplemental Figure 1, A and B). Each cell line was efficiently induced into the myogenic lineage, as demonstrated by the expression of desmin and the presence of elongated myotube-like structures (Figure 2C). The expression of the γ-sarcoglycan protein was observed in reprogrammed control cells and absent in the 521ΔT patient cells, commensurate with mutation status (Figure 2D).
Figure 2. SGCG mRNA and protein expression in fibroblasts reprogrammed into the myogenic lineage.
(A) Schematic of the SGCG pre-mRNA transcript. The most common LGMD 2C mutation is a single thymine (T) deletion from a string of 5Ts in SGCG exon 6. This mutation, designated 521ΔT, results in a transcript frame shift, which generates a premature stop codon (red octagon). Asterisks represent the transcription start sites, and the numbers denote the mRNA region encoded by each exon. Fibroblasts from a normal control subject (SGCG intact) and a LGMD 2C individual with the 521ΔT mutation were directly reprogrammed into the myogenic lineage using a tamoxifen (Tam) inducible MyoD lentiviral construct (iMyoD) (32). (B) RT-PCR analysis demonstrated expression of the SGCG mRNA in normal control and 521ΔT cells after tamoxifen induction and culture in differentiation media (5 μM 4OH-tamoxifen, 48 hours, 9 days differentiation). (C) Immunofluorescence microscopy (IFM) demonstrated the efficient transduction and myogenic reprogramming of both the control and 521ΔT cell lines (5 μM 4OH-tamoxifen, 48 hours, 12 days differentiation). Nuclear expression of MyoD (red) is observed in the iMyoD-transduced cells. Reprogrammed cells expressed the myogenic marker desmin (green) and formed multinucleated myotubes. Nuclei were labeled with Hoechst 3342 (blue). Scale bar: 50 μM. (D) γ-Sarcoglycan protein (green) was readily detected in the reprogrammed control cells, but not in reprogrammed 521ΔT cells, in accordance with their mutation status. Expression of α-actinin (red) indicated the myogenic reprogramming of each cell line. Scale bar: 50 μM.
2OMePS AON exon skipping of the 521ΔT transcript.
The 2OMePS AON chemical modification is among the most commonly used for exon-skipping splice manipulation and has efficiently corrected dystrophin frameshift mutations in cell models of DMD (33–35). For this LGMD 2C strategy, 2OMePS AONs targeting intraexonic regions in SGCG exons 4, 5, 6, and 7 (Table 1) were designed in accordance with previously described guidelines (36). Transfection of reprogrammed 521ΔT cells with individual 2OMePS AONs demonstrated skipping of individual exons 5 and 7, partial skipping of exon 4, and indeterminate skipping of exon 6 (Supplemental Figure 2, A and B). We also evaluated whether the normal SGCG sequence was targeted by AON-mediated exon skipping. Transfection of reprogrammed control cells with single 2OMePS elicited a similar skipping profile as 521ΔT SGCG cells (Supplemental Figure 2C), indicating that mutation in SGCG is not required for exon skipping.
Table 1. SGCG exon targets and AON sequences.
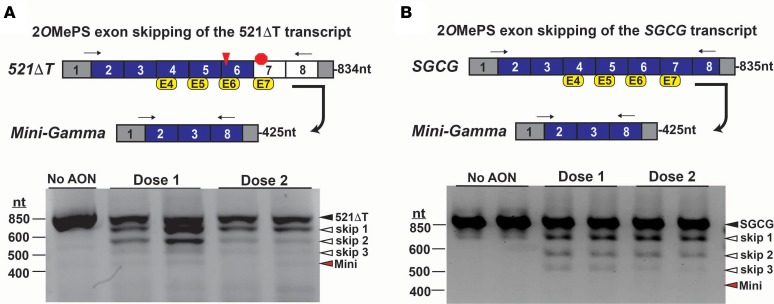
Cells were then treated with a 4-AON exon-skipping cocktail aimed at SGCG exons 4, 5, 6, and 7 (Supplemental Table 1). Analysis of PCR-amplified transcripts after 36 hours of treatment showed the expression of multiple intermediate products, which represented the skipping of 1, 2, and 3 exons (Figure 3A). Notably, there was minimal expression of the 4-exon–skipped Mini-Gamma transcript at the expected size (Figure 3A). Similar treatment of control cells produced intermediate transcripts with no evidence of Mini-Gamma encoding mRNA expression after 48 hours (Figure 3B). Collectively, these data indicate that reading frame correction of the 521ΔT SGCG frameshift mutation with the 2OMePS AON multiexon-skipping strategy was only modestly effective due to inefficient skipping of individual SGCG exons, specifically exon 6.
Figure 3. 2OMePS-mediated exon skipping of the SGCG transcript.
(A) Correction of the SGCG 521ΔT frameshift mutation (red triangle) requires a multi-AON exon-skipping strategy targeting exons 4, 5, 6, and 7 (yellow boxes) to generate the reading frame–corrected Mini-Gamma transcript encoded by exons 2, 3, and 8. Arrows denote the location of the RT-PCR primers, and the expected amplicon lengths are indicated. Myogenic cells were exposed to a multiexon–skipping cocktail of 2OMePS AONs targeting exons 4, 5, 6, and 7. RT-PCR analysis demonstrated the expression of the 521ΔT transcript (black arrowhead), along with PCR products representing the skipping of 1, 2, and 3 exons (white arrowheads). A PCR product representing the 4-AON–mediated generation of the Mini-Gamma was observed at the expected size (red arrowhead). (B) Treatment of reprogrammed normal control fibroblasts with 2OMePS AONs targeting exons 4, 5, 6, and 7 also induced exon skipping. RT-PCR analysis demonstrated the expression of the SGCG transcript (black arrowhead), along with PCR products representing the skipping of 1, 2, and 3 exons (white arrowheads). No PCR product representing the 4-AON–mediated generation of the Mini-Gamma was observed at the expected size (red arrowhead).
Vivo-PMO exon skipping of the 521ΔT transcript.
PMOs are another commonly used AON chemistry used for exon skipping. These molecules carry no net charge and appear nontoxic with minimal off-target interactions (37). In order to improve cellular uptake in vitro, PMOs were covalently conjugated to an octa-guanidine dendrimer used to generate vivo-PMOs. AONs using vivo-PMO chemistry were designed as described and targeted intraexonic regions in SGCG exons 4, 5, 6, and 7 (Table 1). Transfection of reprogrammed 521ΔT cells with single vivo-PMOs demonstrated dose-dependent skipping of individual SGCG exons 4, 5, 6, and 7 (Supplemental Figure 3A). Transfection of control cells with single vivo-PMOs demonstrated similar dose-dependent skipping of individual SGCG exons 4, 5, 6, and 7, which indicated the ability of vivo-PMOs to efficiently skip exons of the native SGCG transcript (Supplemental Figure 3B).
Additionally, there was evidence of double exon skipping after treatment with single vivo-PMOs targeting exons 4, 5, and 6 (Supplemental Figure 3, A and B), likely due to the dual skipping of individual exons 4, 5, and 6 coupled with the endogenous skipping of exon 7. Notably, single vivo-PMO treatments targeting exon 7 did not demonstrate double skipping (Supplemental Figure 3, A and B). Splice-site analysis of SGCG exon 7 revealed 2 potential regions of splice repression that were conserved between the human and mouse SGCG sequences (Supplemental Figure 3C).
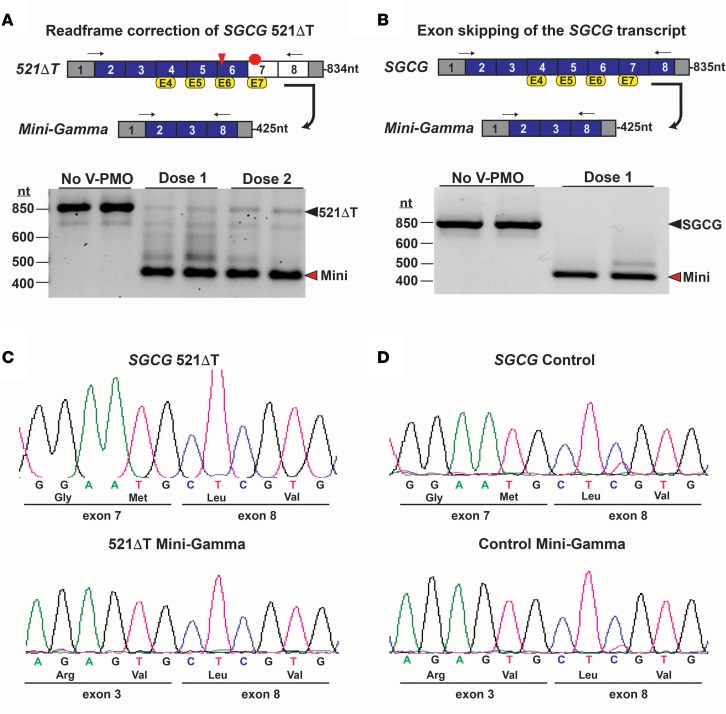
To correct the SGCG 521ΔT reading frame disruption and generate a Mini-Gamma transcript, reprogrammed cells were treated with a 4-AON cocktail of vivo-PMOs targeting SGCG exons 4, 5, 6, and 7 (Supplemental Table 1). Analysis of PCR-amplified transcripts after 36-hour treatment showed predominant expression of the reading frame–corrected, 4-exon–skipped Mini-Gamma transcript with minimal expression of intermediate products (Figure 4A). Treatment of control myogenic cells with the same vivo-PMO cocktail also elicited predominant expression of the Mini-Gamma transcript (Figure 4B). The internally truncated Mini-Gamma products were confirmed by sequence analysis (Figure 4, C and D). These results demonstrated efficient reading frame correction of the SGCG 521ΔT mutation with our multiexon-skipping strategy and the ability of vivo-PMOs to skip the native SGCG transcript. Furthermore, the data indicated improved efficiency of exon skipping with vivo-PMOs compared with 2OMePS AONs in the cell model.
Figure 4. Vivo-PMO–mediated exon skipping of the SGCG transcript.
(A) A multi-AON exon-skipping cocktail using vivo-PMO chemistry was employed to correct the SGCG 521ΔT reading frame in myogenic cells. RT-PCR analysis showed treatment with vivo-PMOs targeting exons 4, 5, 6, and 7 generated the Mini-Gamma reading frame–corrected transcript (red arrowhead) with minimal residual expression of the mutant 521ΔT transcript (black arrowhead). Arrows in schematic denote the location of the RT-PCR primers, and the expected amplicon lengths are indicated. (B) Normal control myogenic cells were treated with the same multiexon-skipping vivo-PMO cocktail. RT-PCR analysis showed expression of the Mini-Gamma transcript (red arrowhead), indicating that normal SGCG transcript could be skipped using vivo-PMOs. Expression of the SGCG transcript in untreated cells is indicated (black arrowhead). (C and D) Sequence analysis of PCR products confirmed the generation of the Mini-Gamma transcripts in each of the mutant cell lines, with the splicing of SGCG exons 3 and 8 demonstrating the removal of exons 4, 5, 6, and 7 from the mature transcripts.
Reading frame correction of multiple SGCG frameshift mutations.
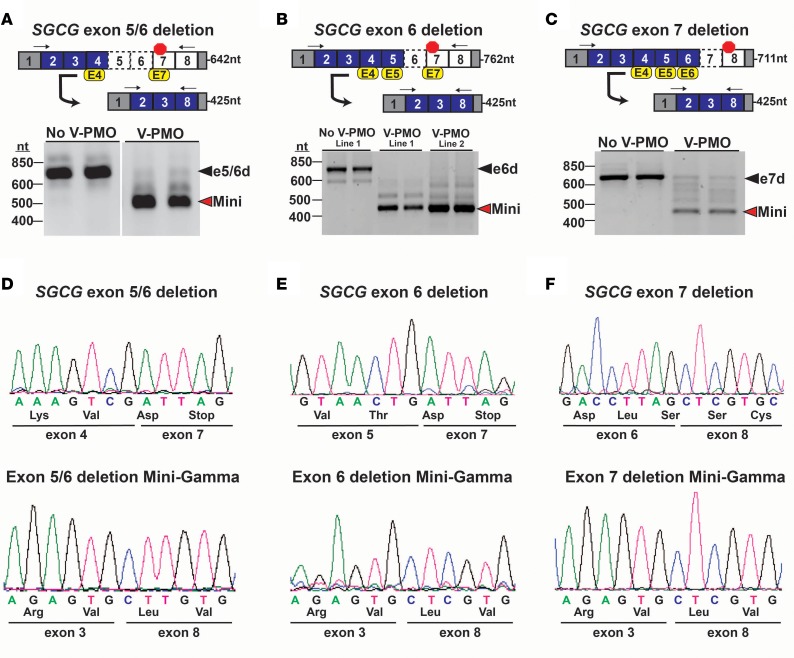
We next examined the ability of this exon-skipping strategy to correct unique SGCG frameshift mutations. Fibroblasts were obtained from 4 different LGMD 2C patients harboring 3 SGCG frameshift mutations. The first was from an individual with a large deletion of SGCG encompassing the entire SGCG gene and a second allele missing only exons 5 and 6 (ex5/6del). Two other cell lines were from 2 siblings with a large deletion of the entire SGCG gene on one allele and a deletion of only exon 6 (ex6del) on the second allele; the fourth was an individual with a homozygous deletion of SGCG exon 7 (ex7del) (Figure 5, A–C). Each cell line was reprogrammed into the myogenic lineage using the iMyoD system. To generate the reading frame–corrected Mini-Gamma transcripts for each mutation, the individual LGMD 2C myogenic cell cultures were treated with a mutation-specific cocktail of vivo-PMOs (Figure 5, A–C, and Supplemental Table 1). This mutation-specific approach limited the total AON exposure to that required for reading frame correction. Analysis of PCR-amplified transcripts after 36-hour treatment showed predominant expression of the internally truncated reading frame–corrected Mini-Gamma transcript in each of the LGMD 2C patient cell lines (Figure 5, A–C). Sequencing analysis confirmed the patient-specific mutations and the generation of Mini-Gamma after vivo-PMO treatment (Figure 5, D–F). These findings demonstrate the ability to correct multiple SGCG frameshift mutations with this exon-skipping strategy.
Figure 5. Reading frame correction of multiple SGCG frameshift mutations.
(A–C) Top: Mutation-specific multiexon-skipping strategies were designed to correct the SGCG reading frame of multiple unrelated LGMD 2C patients whose primary SGCG mutations include (A) the deletion of exons 5 and 6; (B) the deletion of exon 6; and (C) the deletion of exon 7. Myogenic cells were treated with vivo-PMO exon skipping cocktails. Arrows denote the location of the RT-PCR primers, and the expected amplicon lengths are indicated. (A–C) Bottom: RT-PCR analysis confirmed the mutant SGCG transcripts were expressed in the reprogrammed cells at the expected size, consistent with their exon deletion status (black arrowheads). Cells treated with the mutation-specific vivo-PMOs cocktails demonstrated predominate expression of the reading frame–corrected Mini-Gamma transcript (red arrowheads). (D–F) Sequence analysis of RT-PCR products: (D) a deletion of exons 5 and 6; (E) a deletion of exon 6; and (F) a deletion of exon 7. Sequence analysis also confirmed the generation of the Mini-Gamma transcript in each of the mutant cell lines, with the splicing of SGCG exons 3 and 8 demonstrating the removal of (D) exons 4 and 7; (E) exon 4, 5, and 7; and (F) exons 4, 5 and 6 from the mature transcripts.
Restoration of Mini-Gamma protein expression was demonstrated in the 521ΔT, ex5/6del, and ex6del mutations. Immunofluorescence microscopy (IFM) detected γ-sarcoglycan protein in reprogrammed LGMD 2C patient myotubes 3 days after treatment with reading frame–correcting vivo-PMOs (Figure 6, A, C, and E). Similarly conducted and acquired imaging did not detect γ-sarcoglycan protein expression in reprogrammed patient cells treated with a nontargeting control vivo-PMO. Quantitative image analysis confirmed the expression of Mini-Gamma in cells transfected with reading frame–correcting vivo-PMO cocktails as compared with cells treated with nontargeting control vivo-PMOs (Figure 6, B, D, and F). These findings demonstrate the ability to correct multiple SGCG frameshift mutations with this exon-skipping strategy, restoring Mini-Gamma expression at the protein level. Detection of Mini-Gamma protein by immunoblot was limited by the culture system, as reprogrammed control fibroblasts expressed only modest levels of even normal γ-sarcoglycan protein (Supplemental Figure 4A). Mini-Gamma protein expression was nonuniform across the culture system, with some myotubes expressing more Mini-Gamma protein (Figure 6, A–F). The ex5/6del line had the highest levels of Mini-Gamma expression, as seen by IFM (Figure 6, C and D). Correspondingly, Mini-Gamma was detected via immunoblot in reprogrammed ex5/6del cells after reading frame correction with vivo-PMOs (Supplemental Figure 4B).
Figure 6. Restoration of SGCG expression as Mini-Gamma protein.
Reprogrammed fibroblasts were treated for 3 days with either a nontargeting control vivo-PMO (nontarget) or a mutation-specific vivo-PMO exon skipping cocktail (Mini-Gamma). (A) Representative IFM images of reprogrammed 521ΔT cells that were treated as indicated. Treatment with reading frame–correcting vivo-PMOs resulted in the expression of the Mini-Gamma protein (green). Myotubes were labeled with α-actinin (red), and nuclei were labeled with Hoechst 3342 (blue). (B) Image analysis demonstrated a significant increase in Mini-Gamma fluorescence after treatment with vivo-PMOs that corrected the transcript reading frame (n = 6) as compared with nontargeting controls (n = 5). (C and D) Reprogrammed cells harboring a deletion of SGCG exons 5 and 6 (ex5/6del) were treated as indicated. IFM image analysis demonstrated a significant increase in Mini-Gamma fluorescence after treatment with vivo-PMOs that corrected the reading transcript reading frame (n = 4) as compared with nontargeting controls (n = 3). (E and F) Reprogrammed cells harboring a deletion of SGCG exons 6 (ex6del) were treated as indicated. IFM image analysis demonstrated a significant increase in Mini-Gamma fluorescence after treatment with vivo-PMOs that corrected the transcript reading frame (n = 5) as compared with nontargeting controls (n = 3). Data represent the mean γ-sarcoglycan fluorescence per α-actinin–positive area normalized to the mean of the untreated group. A minimum of 3 independent fields were analyzed for each sample. *P < 0.05 as determined by 2-tailed Student’s t test. Data represent the mean ± SEM. Scale bars: 50 μM.
The sarcoglycan complex includes α-, β-, δ-, and γ-sarcoglycans, with β-, δ-, and γ-sarcoglycans forming the core unit (4, 5). The absence of γ-sarcoglycan reduces the expression of β- and δ-sarcoglycan at the sarcolemma in intact muscle (21). To determine if Mini-Gamma stabilized the sarcoglycan complex, β- and δ-sarcoglycan expression levels were examined after treatment with reading frame–correcting vivo-PMOs. An increase of β- and δ-sarcoglycan protein expression was detected by IFM in those myotubes that expressed Mini-Gamma protein (Supplemental Figure 5, A–C, and Supplemental Figure 6, A–C).
Functional reading frame correction in SGCG reprogrammed cells.
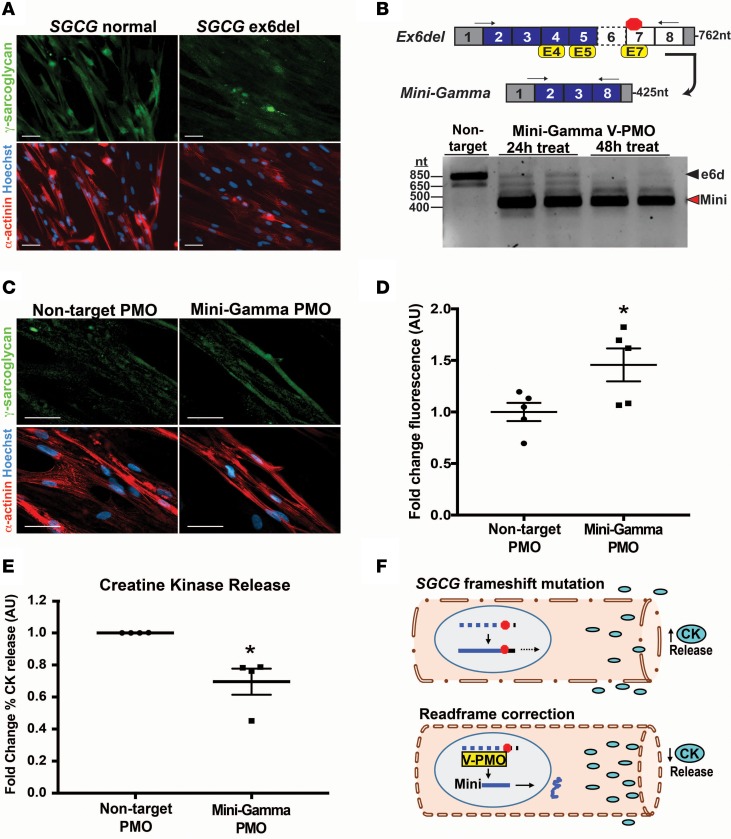
Previously, we showed the ability to directly reprogram urine-derived cells (UDC) into the myogenic lineage through the overexpression of MyoD (38). To further analyze this SGCG exon-skipping strategy, we obtained UDCs from an LGMD 2C patient harboring a frameshift mutation caused by the deletion of SGCG exon 6 (ex6del). Normal control and ex6del UDCs were reprogrammed into the myogenic lineage, seen as α-actinin expression and the appearance of elongated myotube-like structures (Figure 7A). γ-Sarcoglycan protein expression was detected in the reprogrammed normal control cells but not in LGMD 2C cells, commensurate with SGCG mutation status (Figure 7A). To generate the reading frame–corrected Mini-Gamma transcript, ex6del myogenic cells were treated with vivo-PMOs targeting exon 4, 5, and 7 (Supplemental Table 1). Analysis of PCR-amplified transcripts after 24 and 48 hours showed predominant expression of the internally truncated Mini-Gamma transcript (Figure 7B). IFM imaging detected Mini-Gamma protein expression in reprogrammed LGMD 2C mutant myotubes 3 days after treatment with vivo-PMO (Figure 7C). Protein expression was observed throughout the myotube, including at the plasma membrane (Supplemental Figure 7, A and B). Image analysis confirmed Mini-Gamma expression relative to reprogrammed cells transfected with nontargeting control vivo-PMOs (Figure 7D). The reprogrammed LGMD 2C patient cells were treated for 3 days with either reading frame–correcting vivo-PMOs or nontargeting control vivo-PMOs, then subjected to a hypo-osmotic shock to induce creatine kinase (CK) release. Treatment with vivo-PMOs to restore the reading frame resulted in a decrease in CK release in response to the hypo-osmotic shock, indicative of increased membrane integrity after the restoration of SGCG expression (Figure 7E). We tested reprogrammed 521ΔT, ex5/6del, and ex6del fibroblasts, using this same CK release assay. Treatment with reading frame–correcting vivo-PMOs resulted in a nonstatistically significant trend toward decreased CK release (Supplemental Figure 8, A–C). The trend toward increased CK release likely reflects that only a subset of the myotubes in culture expressed Mini-Gamma protein. However, taken together, these data demonstrate that exon skipping–mediated reading frame correction of human SGCG is feasible in human cell culture and protective against stress (Figure 7F).
Figure 7. SGCG reading frame correction in urine-derived cells (UDCs).
UDCs from a normal control subject and an LGMD 2C patient with a deletion of exon 6 (ex6del) were reprogrammed into a myogenic lineage. (A) γ-Sarcoglycan protein (green) was detected by IFM in the reprogrammed normal control myotubes but not in reprogrammed ex6del cells. α-Actinin, red; nuclei, blue. (B) RT-PCR analysis demonstrated reading frame–corrected Mini-Gamma transcript expression (red arrowhead). (C) Representative IFM images showed the restoration of γ-sarcoglycan protein expression in cells treated with Mini-Gamma vivo-PMOs. (D) Significant increase in γ-sarcoglycan protein fluorescence was observed after treatment with vivo-PMOs (n = 5) as compared with nontargeting control vivo-PMOs (n = 5). A minimum of 3 independent fields were analyzed for each sample. (E) To assess membrane stability in response to vivo-PMO treatment, reprogrammed cells were challenged with hypo-osmotic shock and membrane leak was monitored by release of creatine kinase (CK). Vivo-PMO treatment significantly decreased the relative amount of CK release consistent with increased membrane stability. Data represent the percent of CK released relative to the total CK from 4 independent experiments (n = 3–4, for each). Data are presented as the mean CK released in cells treated with exon-skipping vivo-PMOs relative to the mean in cells treated with a nontargeting vivo-PMO. (F) Model depicting the increased membrane stability that resulted from vivo-PMO–mediated reading frame correction of an SGCG frameshift mutation. *P < 0.05 as determined by 2-tailed Student’s t test. Data represent the mean ± SEM. Scale bars: 50 μM.
Discussion
Exon skipping for LGMD 2C.
Exon splice–modulating therapy has arrived in the clinic with the recent approval of AONs for the treatment of DMD and SMA (24, 39). The field of RNA-targeting therapeutics continues to expand with new splice-switching oligonucleotide therapies designed to treat multiple disorders (40). In the current study, we evaluated an AON-mediated exon-skipping strategy to treat LGMD 2C. This approach required the bypass of SGCG exons 4, 5, 6, and 7 to generate an internally truncated product. A multiexon-skipping cocktail of vivo-PMO oligomers successfully generated the reading frame–corrected Mini-Gamma transcript in myogenically reprogrammed cells derived from a patient with the most common SGCG 521ΔT mutation. Notably, this strategy is applicable to any mutation occurring in SGCG exons 4, 5, 6, and 7, and this approach was used to correct the reading frame of multiple distinct SGCG frameshift mutations. Successful correction of the reading frame resulted in the restoration of SGCG protein expression as Mini-Gamma. These results demonstrate that exon skipping is a viable therapeutic approach for the treatment of LGMD 2C caused by mutations occurring in SGCG exons 4–7, which encompasses the majority of known mutations.
An exon-skipping strategy must generate a functional internally truncated protein. We previously used transgenic expression of the internally truncated murine Mini-Gamma to demonstrate partial correction of many aspects of muscular dystrophy in a mouse model of LGMD 2C (30). In these studies, Mini-Gamma maintained proper interaction with components of the DGC, improved membrane integrity, and rescued the dystrophic phenotype in both fly and mouse models of the disease (30). Mini-Gamma retains the complete cytosolic and transmembrane domains. Importantly, Mini-Gamma retains the cysteine-rich C-terminal extracellular domain, which is known to play a role in sarcoglycan complex formation and plasma membrane targeting (9, 41–43). The high homology between the human and mouse SGCG genes supported that this strategy should work on human SGCG. AON-mediated expression of Mini-Gamma in human SGCG cells led to a reduction in the amount of CK released in response to hypo-osmotic shock, indicating a functional rescue of muscle membrane stability.
Vivo-PMOs appeared to have superior skipping compared with 2OMePS AONs.
Chemically modified AONs are used to promote compound stability and avoid RNase digestion. In this study, we observed minimal multi-exon skipping using 2OMePS AONs. This result was attributed primarily to the negligible skipping of exon 6 that was observed in both normal control and SGCG 521ΔT cells. SGCG exon 6 is only 73 nucleotides in length, and by m-fold is predicted to adopt a mainly closed secondary structure at the mRNA level (44). The sequence characteristics limited the number of potential AON candidates that could successfully target the exon. Altering the 2OMePS AON length or target sequence could result in improved single and multi-exon skipping of exon 6.
Several studies have suggested that PMOs have better exon-skipping efficacy compared with 2OMePS in the targeting of specific dystrophin exons, and the difference is thought to be sequence dependent (45–47). Unmodified PMOs were not assessed in the current study due to their difficulty of uptake in cultured cells (48). Instead, only vivo-PMOs were tested to enhance cell delivery. Improved molecular correction was observed using vivo-PMOs targeting the same exon sequences as the 2OMePS AONs. Single vivo-PMO treatment showed robust skipping of exon 6 in both the 521ΔT and normal control cells, and the multiexon-skipping cocktail targeting exons 4, 5, 6, and 7 generated predominate expression of the Mini-Gamma transcript with minimal expression of intermediate products.
The results also suggest that exon 7 has a high propensity toward skipping, even in the absence of AONs. Single vivo-PMO treatments targeting exons 4, 5, and 6 resulted in double–exon-skipping events, which each included exon 7. Splice-site analysis did not indicate any anomalies in the splice acceptor site for exon 7; however, there are 2 regions, one intronic and one exonic, that had canonical splice repressor motifs without an adjacent enhancer motif (49). Thus, there may be competition between the exon 7 and exon 8 splice acceptor sites. This could reduce the number and/or concentration of AONs needed for therapeutic effect, since only a low concentration of exon 7–directed sequences would be needed.
Challenges for in vivo translation.
Although multi-exon skipping was possible in a myogenic cell model, the delivery of multiple AONs in vivo is expected to face several hurdles. First, the previously published Sgcg mouse model is not molecularly suitable for testing exon skipping, as it was engineered to lack exon 2 (50). While studies in a canine and mouse models of DMD demonstrated the ability to skip multiple exons of the dystrophin gene using multi-AON cocktails (51, 52), sufficient dosing and potential side effects could limit the human translation of these observations. Advancements in oligonucleotide chemistries and strategies have sought to improve the efficacy and delivery of AON delivery. For example, the tri-cyclo AON modification demonstrated superior exon skipping efficiency as compared with 2OMePS AONs and PMOs in a mouse model of DMD, including access to the heart and brain (53). AONs have also been conjugated to ligands, nanoparticles, cell penetrating peptides, or covalently modified delivery moieties such as the octa-guanidine dendrimers (54). While toxicity concerns need to be addressed, these modifications represent continued progress toward the enhanced delivery of AONs at lower concentrations.
Methods
Cell culture.
Primary fibroblasts were isolated from skin biopsies taken from 5 LGMD 2C patients. Primary fibroblasts from a healthy control subject were obtained from the American Type Culture Collection (ATCC, CCD-1127Sk). Primary UDCs were isolated from the urine of deidentified healthy volunteers and an LGMD 2C patient harboring a deletion of SGCG exon 6, as previously described (38). Primary fibroblasts were cultured in proliferation media consisting of DMEM (Thermo Fisher Scientific, 11955-065) supplemented with 15% FBS (Thermo Fisher Scientific, 26140-079). Primary UDCs were cultured in UDC proliferation media as described (38).
Lentiviral constructs and transduction.
Primary fibroblasts were cotransduced with an hTert lentivirus containing a puromycin selection cassette (Applied Biological Materials [ABM], G200) and a iMyoD lentivirus as previously described (30). The 521ΔT fibroblast cell line was transduced with the single iMyoD lentivirus (MOI 200). The iMyoD lentiviral construct was provided by Jeffrey Chamberlain (University of Washington, Seattle, Washington, USA), and packaged by the Northwestern Skin Disease Research Center (Chicago, Illinois, USA).
Myogenic reprogramming of human fibroblasts.
Myogenic reprogramming of fibroblasts was performed as previously described with minor modifications (30). Briefly, iMyoD-transduced cells were seeded onto 24-well culture plates (Thermo Fisher Scientific, 353047) or coverslips (Thermo Fisher Scientific, 12-545-81) at a density of 30,000 cells/cm2 in proliferation media. When cells reached confluence, the media was replaced with proliferation media supplemented with 5 μM 4OH-tamoxifen (MilliporeSigma, H7904). After 48 hours, the media was replaced with differentiation media consisting of DMEM supplemented with 2.5% Normal Horse Serum (NHS, Thermo Fisher Scientific, 161050-122), 1% insulin-transferrin-selenium (MilliporeSigma, 41400-045), and 1 μM tamoxifen. After 3 days, the media was replaced with differentiation media without tamoxifen, and it was exchanged every 3 days.
IFM.
Prior to imaging, culture plates or coverslips were washed twice with DPBS (Thermo Fisher Scientific, 14190-144), fixed in ice cold methanol for 2 minutes, rinsed with DPBS, and blocked for 1 hour at 4oC in DPBS supplemented with 10% NHS and 0.1% triton (MilliporeSigma, X-100). Primary antibody incubations were performed in blocking buffer (DPBS supplemented with 5% NHS and 0.1% triton) at room temperature for 2 hours or overnight at 4oC. Secondary antibody incubations were performed in blocking buffer at room temperature for 1 hour. Nuclei were counterstained with Hoechast 3342 (Thermo Fisher Scientific, H3570) diluted 1:10,000 in DPBS. Coverslips were mounted with Vectashield mounting media (Vector Laboratories, H-1000). Imaging was performed using the Zeiss Axio Observer Z1 microscope using the 20× objective, or the Zeiss Axio Observer A1 microscope, using 40× or 63× objectives with ZEN 2 software (version 2011; Zeiss). Images were processed using FIJI (NIH) and Adobe Photoshop CC.
IFM antibodies.
Primary antibodies were as follows: rabbit polyclonal anti-MyoD (1:1,000, Santa Cruz Biotechnology Inc., sc-304), mouse monoclonal anti-desmin (1:1,000, MilliporeSigma, D1033), mouse monoclonal anti–α-actinin (1:1,000, MilliporeSigma, A7811), mouse monoclonal anti–β-sarcoglycan (1:250, Leica Biosystems, B-SARC-L-CE,clone BETASARC1/5B1), mouse monoclonal anti–δ-sarcoglycan (1:250, Leica Biosystems, D-SARC-CE, clone DELTASARC/12C1), and rabbit polyclonal anti–γ-sarcoglycan (1:1,000) (8). Secondary antibodies included Alexa Fluor 488 donkey anti-mouse (A21202), Alexa Fluor 594 donkey anti-mouse (A21203), Alexa Fluor 488 donkey anti-rabbit (A21206), and Alexa Fluor 594 donkey anti-rabbit (A21207) (all 1:1,000, Thermo Fisher Scientific).
2OMePS design and transfection.
2OMePS AON were synthesized as 25-mers by Integrated DNA Technologies (IDT) following sequence guidelines outlined in ref. 36. AON sequences and their exon targets are listed in Table 1. The 2OMePS AONs were transfected into fibroblasts in serum free media using Lipofectamine 3000 (Thermo Fisher Scientific) at a ratio of 2 μl:1 μg DNA. After 6 hours, the serum free media was replaced with differentiation media. For reading frame correction, 2OMePS AONs targeting exons 4, 5, 6, and 7 were cotransfected at the dosages listed in Supplemental Table 1. Single 2OMePS AONs or multiexon-skipping AON cocktails were transfected into fibroblasts on differentiation day 9. Cells were isolated for analysis after 36–48 hours.
Vivo-PMO design and transfection.
PMOs were designed according to described guidelines and synthesized as 25-mer vivo-PMOs by GeneTools. Specific AON sequences and their exon targets are listed in Table 1. A nontargeting vivo-PMO standard control was purchased from GeneTools (CCTCTTACCTCAGTTACAATTTATA). Vivo-PMO were resuspended in ultrapure water to a stock concentration of 0.5 mM. For cell culture application, vivo-PMO were diluted in serum free media and then directly added to cells cultured in differentiation media. For reading frame correction, vivo-PMOs were cotransfected as indicated in Supplemental Table 1. Single vivo-PMOs or multiexon-skipping AON cocktails were transfected into fibroblasts on differentiation day 9 or 10. Cells were isolated for analysis as indicated.
RNA analysis.
Prior to isolation, cells were washed once with DPBS. Total RNA was isolated in 1 ml of TRIzol (Thermo Fisher Scientific, 15596018) according to the manufacturer’s instructions and included the addition of glycogen (10 μg/ml) to the isopropanol before the RNA precipitation. The RNA was reverse-transcribed using qSCRIPT cDNA Supermix (Quanta Biosciences, 95048-025). To detect SGCG and Mini-Gamma, Taqman based reverse transcription PCR (RT-PCR) was used to amplify 50 ng of complementary DNA (cDNA) with the following primer set ex1/2Fwd 5′-TCTAAGATGGTGCGTGAGCAG-3′ and ex8R 5′-GCCACAGACAGGTACAGCTT-3′. PCR was performed by 35 cycles of 95°C (40 seconds), 57°C (30 seconds), and 72°C (60 seconds). The PCR products were separated on a 1.5% agarose gel (Denville Scientific Inc., CA3510-8) supplemented with 0.5 μg/ml ethidium bromide. Gels were imaged with either the UVP transluminator (BioSpectrum) or the FluorChem E (Protein Simple).
Sequence analysis.
RT-PCR was performed using the SGCG primer set as described. The PCR products were separated on a 1% low-melt agarose gel (Lonza, 50080). Individual PCR bands were excised from the gel and extracted using the Qiaquick Gel Extraction Kit (Qiagen, 28706). Sanger sequencing of the purified products was conducted by ACGT Inc. The sequences were analyzed using Lasergen 13 software from DNASTAR. Some isolated products were reamplified prior to sequencing. For these samples, 5–10 ng of the purified product was reamplified as described above. For this PCR, the number of cycles was reduced to 25 and the annealing temperature was increased by 3°C.
Splice-site analysis.
Human and murine γ-sarcoglycan genes were analyzed for exon splicing elements encoded in exons 7 and 8, along with 100 bp of the intronic region upstream of each exon. Splice-site analysis was conducted using the Human Splicing Finder software (www.umd.be/HSF3/) and the Ensembl transcript database (49, 55).
Mini-Gamma Image Analysis.
IFM was performed as described above. Imaging was performed using a Zeiss Axio Observer A1 microscope, using 40× or 63× objectives with ZEN 2 software (version 2011; Zeiss). Images were processed using FIJI (NIH) and Adobe Photoshop CC. Quantitation of fluorescence was performed from multiple fields per condition using FIJI (NIH) and is presented as mean γ-sarcoglycan fluorescence per α-actinin–positive area normalized to the mean of the nontargeted group.
Immunoblotting.
iMyoD-transduced cells were reprogrammed in 6-well culture plates and treated with vivo-PMOs as indicated. Total protein was isolated in radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, 89900) supplemented with a protease inhibitor cocktail (Roche Diagnostics, 11836170001). Briefly, culture media was removed, and cells were washed twice with ice-cold PBS. RIPA buffer was added to the cells, and they were collected using a cell scraper (Thermo Fisher Scientific, 08-100-241). Cell isolates were then sonicated using the Bioruptor 300 (Diagenode). Three 15-second pulses were performed at the medium setting. Cells were then centrifuged at 4°C for 10 minutes at 15,000 g, and the supernatant was collected. Protein samples were denatured using 4× NuPAGE LDS sample buffer supplemented with reducing agent (Thermo Fisher Scientific, NP0007 and NP0004). Proteins were resolved on precast 4-12% Bis-Tris gels (Thermo Fisher Scientific, NP0336), and then transferred to Immu-Blot PVDF membranes (Bio-Rad, 1620177). Membranes were blocked for 1 hour at room temperature with Starting Block T20 blocking buffer (Thermo Fisher Scientific, 37543), followed by incubation with primary antibody diluted in T20 blocking buffer for either 2 hours at room temperature or overnight at 4°C. After primary incubation, the membranes were washed 3 times, for 20 minutes each, at room temperature with TBS containing 0.1% Tween-20. The membranes were then incubated for 1 hour with a goat anti-mouse or goat anti–rabbit HRP secondary antibodies diluted in T20 blocking buffer, followed by 3 washes. Enhanced chemiluminescence was performed with the SuperSignal West Pico Plus (Thermo Fisher Scientific, 34580) and imaged using the FluorChem E (Protein Simple) imaging system. Primary antibodies were as follows: rabbit polyclonal anti–γ-sarcoglycan (1:1,000) (8); rabbit polyclonal anti–γ-sarcoglycan (1:1,000, Abnova; MaxPab H00006445-D01); and mouse monoclonal anti–γ-tubulin (1:1,000, MilliporeSigma, T5326/GTU-88). Secondary antibodies included HRP conjugated goat anti-mouse and goat anti-rabbit (Jackson ImmunoResearch; 115-035-003 and 111-035-144).
Mini-Gamma overexpression.
HEK293T cells were obtained from the ATCC (CCD-1127Sk) and cultured in DMEM supplemented with 10% FBS and 1× Pen/Strep, described above. Human Mini-Gamma was synthesized and cloned into the pcDNA3.1(+) vector by GenScript. Human Mini-Gamma was transfected into HEK293T cultured in Opti-MEM (Thermo Fisher Scientific, 31985062) using Lipofectamine 3000 according to the manufacturer’s instructions (Thermo Fisher Scientific, L3000015). Cells were cultured in the presence of transfection media and reagents for 6 hours; then, the media was replaced with fresh growth media. Total protein was isolated as described 3 days after transfection.
Reprogrammed UDCs.
Primary UDCs were transduced with the iMyoD lentivirus as previously described (38). Myogenic reprogramming of iMyoD-transduced urine cells was performed as previously described with minor modifications. Briefly, culture plates and coverslips were coated with 8 μg/cm2 and 24 μg/cm2 collagen I (MilliporeSigma, C3867), respectively. Cells were seeded at a density of 50,000 cells/cm2 in proliferation media and cultured until confluent. On the day of induction, cells were overlaid with Growth Factor Reduced Matrigel (Corning, 354230) and exposed to 2.5 μM tamoxifen in proliferation media. After 48 hours, the media was replaced with Hydrocortisone dexamethasone (HD) differentiation media supplemented with 1 μM tamoxifen. Half of the media was replenished every 3 days with HD differentiation media without tamoxifen. After 14 days, media was replaced with differentiation media (DMEM; 2.5% NHS; 1% insulin-transferrin-selenium), and half of the media was replenished every 3 days.
Vivo-PMO treatment of UDCs.
Vivo-PMO were diluted in serum free media and then directly added to urine cells cultured in differentiation media. For reading frame correction, vivo-PMOs were cotransfected as indicated in Supplemental Table 1. Multiexon-skipping AON cocktails or nontargeting control vivo-PMO were transfected into UDCs on differentiation day 25. Cells were isolated for analysis as indicated. RNA analysis, IFM, and image analysis were performed as described above.
Hypo-osmotic shock and CK release.
Reprogrammed fibroblasts and UDCs were challenged with hypo-osmotic shock through the replacement of culture media with deionized water (56, 57). Cells were cultured in 24-well plates and treated with vivo-PMO as indicated. Prior to treatment (3–12 hours), cells were washed with DPBS and differentiation media was replaced (500 μl). To induce hypo-osmotic shock, 350 μl of media was removed and replaced with 350 μl of deionized water. Cells were exposed to hypo-osmotic conditions for 20–30 minutes at 37°C. The supernatant, containing the released CK fraction, was collected and placed on ice. Following supernatant collection, deionized water was added to the remaining cell fraction and submitted to 3 cycles of freeze-thaw to rupture the cell membranes and release the remaining CK into solution. Prior to assay, each fraction was subject to centrifugation at 1,000 g for 10 minutes at 4°C. CK was measured using a kinetic assay, used according to manufacturers’ instructions (BioAssay System, ECPK-100). The cellular CK fraction and the released CK fraction represent the total CK. Released CK represented the percent of the CK in the supernatant fraction relative to the total CK.
Statistics.
Prism (GraphPad Software) was used for data analysis. A 2-tailed Student’s t test was used to compare results between 2 groups. P < 0.05 was considered significant.
Study approval.
Written informed consent was obtained from all human subjects. All work was approved by and conducted under the IRBs of the University of Chicago, Northwestern University, and the Biosciences Institute Ethics Committee on Human Subject at Universidade de São Paulo, which serve as the ethics boards for each institution. All studies were conducted in compliance with the Helsinki Declaration.
Author contributions
EJW performed the in vitro experiments. ARD analyzed the IFM data. EYK contributed to the isolation and culture of human cells. AHV contributed to the in silico analysis. LMDC contributed to the acquisition of patient samples and patient information. MZ, MV, and RCMP contributed to the acquisition, isolation, and culture of human cells. ARD, MJP, and QQG made critical contributions to the manuscript preparation. EMM directed the study. EJW and EMM designed the study, analyzed the data, and wrote the manuscript.
Supplementary Material
Acknowledgments
We thank the Kurt+Peter Foundation for facilitating these studies and the patients and families for participation. We thank Jeff Chamberlain for providing the inducible MyoD construct. We thank Jon Moulton, of GeneTools, for help in oligonucleotide design. This work was supported by NIH R01HL61322, NIH T32 HD009007, FAPESP-CEPID, and CNPq.
Version 1. 05/03/2018
Electronic publication
Footnotes
Conflict of interest: EMM and EJW are coinventors of a patent related to exon skipping (US patent 9,777,271).
Reference information: JCI Insight. 2018;3(9):e99357. https://doi.org/10.1172/jci.insight.99357.
Contributor Information
Andy H. Vo, Email: ahvo@uchicago.edu.
Mariz Vainzof, Email: mvainzof@usp.br.
Mayana Zatz, Email: mayazatz@usp.br.
References
- 1.Fayssoil A, et al. Natural History of Cardiac and Respiratory Involvement, Prognosis and Predictive Factors for Long-Term Survival in Adult Patients with Limb Girdle Muscular Dystrophies Type 2C and 2D. PLoS ONE. 2016;11(4):e0153095. doi: 10.1371/journal.pone.0153095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschner J, Lochmüller H. Sarcoglycanopathies. Handb Clin Neurol. 2011;101:41–46. doi: 10.1016/B978-0-08-045031-5.00003-7. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi S, et al. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science. 1995;270(5237):819–822. doi: 10.1126/science.270.5237.819. [DOI] [PubMed] [Google Scholar]
- 4.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-W. [DOI] [PubMed] [Google Scholar]
- 5.Ozawa E, Mizuno Y, Hagiwara Y, Sasaoka T, Yoshida M. Molecular and cell biology of the sarcoglycan complex. Muscle Nerve. 2005;32(5):563–576. doi: 10.1002/mus.20349. [DOI] [PubMed] [Google Scholar]
- 6.Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23(10):1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::AID-MUS2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122(4):809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNally EM, et al. Mild and severe muscular dystrophy caused by a single gamma-sarcoglycan mutation. Am J Hum Genet. 1996;59(5):1040–1047. [PMC free article] [PubMed] [Google Scholar]
- 9.McNally EM, et al. Mutations that disrupt the carboxyl-terminus of gamma-sarcoglycan cause muscular dystrophy. Hum Mol Genet. 1996;5(11):1841–1847. doi: 10.1093/hmg/5.11.1841. [DOI] [PubMed] [Google Scholar]
- 10.Straub V, Bushby K. Therapeutic possibilities in the autosomal recessive limb-girdle muscular dystrophies. Neurotherapeutics. 2008;5(4):619–626. doi: 10.1016/j.nurt.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 12.Koenig M, et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- 13.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 14.Fanin M, Danieli GA, Vitiello L, Senter L, Angelini C. Prevalence of dystrophin-positive fibers in 85 Duchenne muscular dystrophy patients. Neuromuscul Disord. 1992;2(1):41–45. doi: 10.1016/0960-8966(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman EP, Morgan JE, Watkins SC, Partridge TA. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990;99(1):9–25. doi: 10.1016/0022-510X(90)90195-S. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson LV. The “rescue” of dystrophin synthesis in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 1993;3(5-6):525–531. doi: 10.1016/0960-8966(93)90109-W. [DOI] [PubMed] [Google Scholar]
- 17.Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012;11(2):125–140. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JH, Lim S, Wong WS. Antisense oligonucleotides: from design to therapeutic application. Clin Exp Pharmacol Physiol. 2006;33(5-6):533–540. doi: 10.1111/j.1440-1681.2006.04403.x. [DOI] [PubMed] [Google Scholar]
- 19.Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378(9791):595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goemans NM, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364(16):1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 21.Mendell JR, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74(5):637–647. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 22.Voit T, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised, placebo-controlled phase 2 study. Lancet Neurol. 2014;13(10):987–996. doi: 10.1016/S1474-4422(14)70195-4. [DOI] [PubMed] [Google Scholar]
- 23.Aartsma-Rus A, Krieg AM. FDA Approves Eteplirsen for Duchenne Muscular Dystrophy: The Next Chapter in the Eteplirsen Saga. Nucleic Acid Ther. 2017;27(1):1–3. doi: 10.1089/nat.2016.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aartsma-Rus A. FDA Approval of Nusinersen for Spinal Muscular Atrophy Makes 2016 the Year of Splice Modulating Oligonucleotides. Nucleic Acid Ther. 2017;27(2):67–69. doi: 10.1089/nat.2017.0665. [DOI] [PubMed] [Google Scholar]
- 25.Clayton NP, et al. Antisense Oligonucleotide-mediated Suppression of Muscle Glycogen Synthase 1 Synthesis as an Approach for Substrate Reduction Therapy of Pompe Disease. Mol Ther Nucleic Acids. 2014;3:e206. doi: 10.1038/mtna.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gedicke-Hornung C, et al. Rescue of cardiomyopathy through U7snRNA-mediated exon skipping in Mybpc3-targeted knock-in mice. EMBO Mol Med. 2013;5(7):1128–1145. doi: 10.1002/emmm.201202168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gramlich M, et al. Antisense-mediated exon skipping: a therapeutic strategy for titin-based dilated cardiomyopathy. EMBO Mol Med. 2015;7(5):562–576. doi: 10.15252/emmm.201505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igreja S, Clarke LA, Botelho HM, Marques L, Amaral MD. Correction of a Cystic Fibrosis Splicing Mutation by Antisense Oligonucleotides. Hum Mutat. 2016;37(2):209–215. doi: 10.1002/humu.22931. [DOI] [PubMed] [Google Scholar]
- 29.Lee JM, et al. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J Clin Invest. 2016;126(4):1592–1602. doi: 10.1172/JCI85908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao QQ, et al. Reengineering a transmembrane protein to treat muscular dystrophy using exon skipping. J Clin Invest. 2015;125(11):4186–4195. doi: 10.1172/JCI82768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morcos PA, Li Y, Jiang S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques. 2008;45(6):613–618. doi: 10.2144/000113005. [DOI] [PubMed] [Google Scholar]
- 32.Kimura E, et al. Cell-lineage regulated myogenesis for dystrophin replacement: a novel therapeutic approach for treatment of muscular dystrophy. Hum Mol Genet. 2008;17(16):2507–2517. doi: 10.1093/hmg/ddn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunckley MG, Manoharan M, Villiet P, Eperon IC, Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7(7):1083–1090. doi: 10.1093/hmg/7.7.1083. [DOI] [PubMed] [Google Scholar]
- 34.van Deutekom JC, et al. Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum Mol Genet. 2001;10(15):1547–1554. doi: 10.1093/hmg/10.15.1547. [DOI] [PubMed] [Google Scholar]
- 35.Wilton SD, et al. Specific removal of the nonsense mutation from the mdx dystrophin mRNA using antisense oligonucleotides. Neuromuscul Disord. 1999;9(5):330–338. doi: 10.1016/S0960-8966(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 36.Aartsma-Rus A. Overview on AON design. Methods Mol Biol. 2012;867:117–129. doi: 10.1007/978-1-61779-767-5_8. [DOI] [PubMed] [Google Scholar]
- 37.Douglas AG, Wood MJ. Splicing therapy for neuromuscular disease. Mol Cell Neurosci. 2013;56:169–185. doi: 10.1016/j.mcn.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim EY, Page P, Dellefave-Castillo LM, McNally EM, Wyatt EJ. Direct reprogramming of urine-derived cells with inducible MyoD for modeling human muscle disease. Skelet Muscle. 2016;6:32. doi: 10.1186/s13395-016-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNally EM, Wyatt EJ. Mutation-Based Therapy for Duchenne Muscular Dystrophy: Antisense Treatment Arrives in the Clinic. Circulation. 2017;136(11):979–981. doi: 10.1161/CIRCULATIONAHA.117.028382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Havens MA, Hastings ML. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016;44(14):6549–6563. doi: 10.1093/nar/gkw533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan YM, Bönnemann CG, Lidov HG, Kunkel LM. Molecular organization of sarcoglycan complex in mouse myotubes in culture. J Cell Biol. 1998;143(7):2033–2044. doi: 10.1083/jcb.143.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, et al. Identification of functional domains in sarcoglycans essential for their interaction and plasma membrane targeting. Exp Cell Res. 2006;312(9):1610–1625. doi: 10.1016/j.yexcr.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Shi W, Chen Z, Schottenfeld J, Stahl RC, Kunkel LM, Chan YM. Specific assembly pathway of sarcoglycans is dependent on beta- and delta-sarcoglycan. Muscle Nerve. 2004;29(3):409–419. doi: 10.1002/mus.10566. [DOI] [PubMed] [Google Scholar]
- 44.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 2006;8(2):207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- 46.Heemskerk HA, et al. In vivo comparison of 2’-O-methyl phosphorothioate and morpholino antisense oligonucleotides for Duchenne muscular dystrophy exon skipping. J Gene Med. 2009;11(3):257–266. doi: 10.1002/jgm.1288. [DOI] [PubMed] [Google Scholar]
- 47.McClorey G, et al. Induced dystrophin exon skipping in human muscle explants. Neuromuscul Disord. 2006;16(9-10):583–590. doi: 10.1016/j.nmd.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Amantana A, Iversen PL. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr Opin Pharmacol. 2005;5(5):550–555. doi: 10.1016/j.coph.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hack AA, et al. Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J Cell Biol. 1998;142(5):1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aoki Y, et al. Bodywide skipping of exons 45-55 in dystrophic mdx52 mice by systemic antisense delivery. Proc Natl Acad Sci USA. 2012;109(34):13763–13768. doi: 10.1073/pnas.1204638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokota T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65(6):667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goyenvalle A, et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med. 2015;21(3):270–275. doi: 10.1038/nm.3765. [DOI] [PubMed] [Google Scholar]
- 54.Sardone V, Zhou H, Muntoni F, Ferlini A, Falzarano MS. Antisense Oligonucleotide-Based Therapy for Neuromuscular Disease. Molecules. 2017;22(4):E563. doi: 10.3390/molecules22040563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flicek P, et al. Ensembl 2013. Nucleic Acids Res. 2013;41(Database issue):D48–D55. doi: 10.1093/nar/gks1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha B, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144(3):402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang B, et al. Membrane blebbing as an assessment of functional rescue of dysferlin-deficient human myotubes via nonsense suppression. J Appl Physiol. 2010;109(3):901–905. doi: 10.1152/japplphysiol.01366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.