Abstract
We report the first case of cerebral Fonsecaea monophora infection in a German woman without immunodeficiency or travel history. F. monophora infection is a rare differential diagnosis of cerebral tumors; it was previously considered a tropical fungus. This case adds to the scarce reports in nontropical regions.
Keywords: cerebral, melanized, Fonsecaea monophora, immunocompetent, Europe
CASE
A 78-year-old German woman presented with subacute onset of right lower extremity paresis and facial nerve palsy. She denied preexisting headache, nausea, vomiting, fatigue, anorexia, fever, chills, cough, dyspnea, skin lesions, or visual complaints. Her medical history was significant for chronic obstructive pulmonary disease, presumably related to her work history as a baker. Her medications included inhaled budesonide/formoterol and fenoterol, but no systemic steroids. She denied travel outside Germany or previous exposure to or injury from exotic plants or wooden objects.
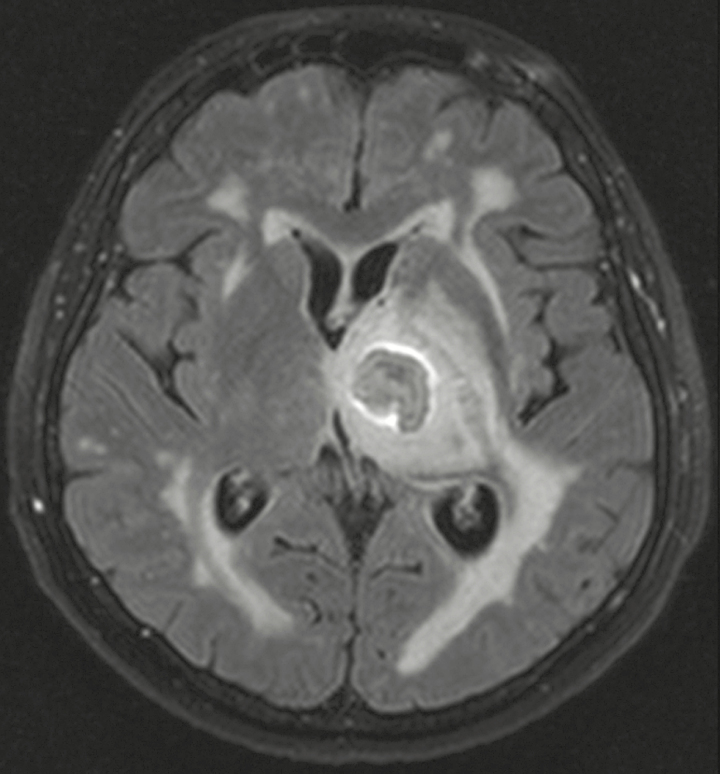
Relevant physical findings on admission were a right lower extremity weakness and mild facial nerve palsy. Vital signs were within normal limits. Pertinent laboratory findings were a white blood cell count of 10.5/µL (normal range, 3.8–9.8/µL), C-reactive protein, creatinine, and liver function tests within normal limits, and negative test for HIV serology. Brain magnetic resonance imaging (MRI) showed an 18 × 13-mm left thalamic mass with surrounding edema, with a 4-mm central area of restricted diffusion on diffusion-weighted imaging and a strong peripheral ring enhancement after intravenous contrast administration (Figure 1). Intravenous (IV) dexamethasone was started.
Figure 2.
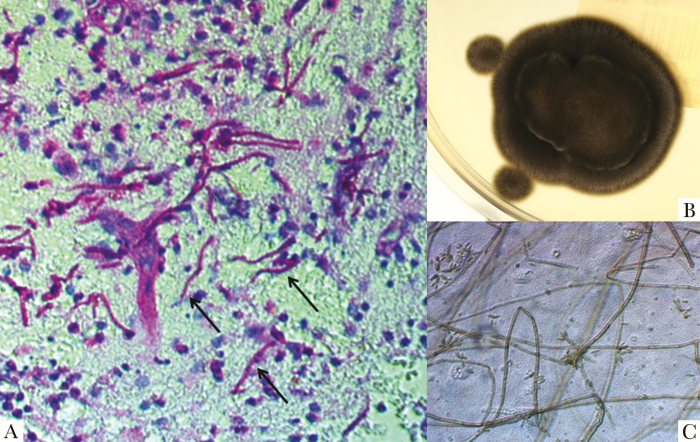
A, Numerous purple-stained hyphae (black arrows) on Periodic acid Schiff (PAS) stain of brain tissue (magnification x40). B, Black velvet-like colonies of Fonsecaea monophora on Sabouraud Glucose Agar after 12 days of incubation at 25°C. C, Micromophology of colony fragments on lactophenol cotton blue stain (LCBS) with septate hyphae, conidiophores, and many solitary conidias (magnification x400).
Stereotactic biopsy of the thalamic mass while on dexamethasone treatment revealed branching septated hyphae morphologically suggestive of aspergillus without evidence of malignancy on histopathology (Figure 2A). No microbiologic evaluation was performed at that time. Serum cryptococcus and aspergillus antigen were negative. Antifungals were not started. Cerebrospinal fluid analysis showed: 1 cell/µL, protein 1364 mg/L, glucose 2.94 mmol/L, cryptococcal antigen negative, bacterial, fungal, and mycobacterial culture negative, and negative blood culture and mycobacterial blood culture. Thoracic computed tomography scan was not suggestive of fungal disease. Ear, nose, throat, and ophthalmologic exams were unremarkable.
Figure 1.
Fluid attenuated inversion recovery (FLAIR) magnetic resonance image in the axial plane showing the left thalamic mass with FLAIR hyperintense perifocal edema.
Follow-up MRI 1 week after admission showed progressive enlargement of the ring-enhancing mass (24 × 20 mm) and the perifocal edema, prompting radical open excision of the mass. Histopathologic evaluation showed again many septated branching hyphae with chronic and acute inflammation and necrosis. A brain tissue culture was performed that showed growth of a filamentous fungus (Figure 2B and C). Pan-fungal internal transcribed spacer-based polymerase chain reaction (rDNA) revealed Fonsecaea monophora. Final species confirmation as F. monophora and in vitro susceptibility testing were performed by the National Reference Laboratory for Cryptococcosis, Scedosporiosis, and Imported Systemic Mycoses (Robert Koch Institute, Berlin, Germany). Susceptibility to voriconazole was demonstrated (minimal inhibitory concentration 0.125 µg/mL).
Voriconazole 5 mg/kg bodyweight twice daily IV was then initiated, and it was well tolerated. The patient’s neurological and functional capacity had been improving; therefore no follow-up MRI was obtained. Unfortunately, the patient died 4 months postsurgery due to pneumonia.
DISCUSSION
Fonsecaea spp. is a melanized tropical fungus. There are, however, sporadic reports in temperate climate zones. Classically, Fonsecaea spp. causes chromoblastomycosis [1, 2]. Cerebral infection is rare and frequently lethal.
The fungus is found on tropical plants and wooden objects. The way of transmission can rarely be elicited. Transmission via traumatic inoculation, the pulmonary route, and per continuitatem after sinus infection has been speculated. Long latency periods of up to years between exposure and infection have been reported [1, 2]. We describe the first cerebral F. monophora infection in Germany; it is the second in Europe [3]. Nine more cases have been published outside temperate Europe (MEDLINE via PUBMED, English language) [4–7], one-third were presumably acquired in nonendemic regions [3, 6, 7]. In these cases, the pathogenesis is unknown. F. monophora seems to have a unique neurotropic affinity. Although most fungi cause opportunistic infections in immunosuppressed hosts, F. monophora appears to affect immunocompetent hosts alike [1]. Of the 11 cases reported so far, 8 (including our case) occurred in immunocompetent patients and 3 in immunodeficient patients (AIDS, solid organ transplant) [6, 7].
Cerebral fungal infection may appear as a tumor-like mass lesion. The surprising diagnosis is often made by histopathology reporting fungal elements. Microbiologic evaluation is prudent for pathogen identification.
Neuroimaging findings in fungal infections are nonspecific. Fungi with hyphal growth, like Fonsecaea, tend to cause intraaxial, parenchymal lesions, varying from cerebritis to abscesses. On MRI, a fungal abscess may have a “target-like appearance,” as in our patient (Figure 1); the annular enhancement is believed to correspond with granulation tissue, the central nonenhancing nodule in the abscess cavity with hyphae [8].
Unfortunately, cerebral infection by melanized fungi has been associated with high mortality. Due to the paucity of the disease, the optimal treatment strategy remains unknown.
This is the first report of F. monophora cerebral abscess in Germany. Our report adds to the scarce literature and highlights the consideration of F. monophora as a rare differential diagnosis of a cerebral tumor also in temperate climate zones and immunocompetent hosts.
Acknowledgments
Financial support. No funding was received for this study.
We thank Dr. Volker Rickerts of the National Reference Laboratory for Cryptococcosis, Scedosporiosis, and Imported Systemic Mycoses (Robert Koch Institute, Berlin, Germany) for confirmatory ITS sequencing and susceptibility testing. We thank Dr. Matthias Meinhardt of the Institute of Pathology, University Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany, for providing the histopathological stain.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev 2010; 23:884–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng S, Tsui CK, Gerrits van den Ende AH, et al. . Global spread of human chromoblastomycosis is driven by recombinant Cladophialophora carrionii and predominantly clonal Fonsecaea species. PLoS Negl Trop Dis 2015; 9:e0004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Surash S, Tyagi A, De Hoog GS, et al. . Cerebral phaeohyphomycosis caused by Fonsecaea monophora. Med Mycol 2005; 43:465–72. [DOI] [PubMed] [Google Scholar]
- 4. Saberi H, Kashfi A, Hamidi S, et al. . Cerebral phaeohyphomycosis masquerading as a parafalcian mass: case report. Surg Neurol 2003; 60:354–9; discussion 359. [DOI] [PubMed] [Google Scholar]
- 5. Doymaz MZ, Seyithanoglu MF, Hakyemez İ, et al. . A case of cerebral phaeohyphomycosis caused by Fonsecaea monophora, a neurotropic dematiaceous fungus, and a review of the literature. Mycoses 2015; 58:187–92. [DOI] [PubMed] [Google Scholar]
- 6. Bagla P, Loeffelholz M, Blanton LS. Cerebral phaeohyphomycosis by Fonsecaea monophora: report in a patient with AIDS and a ring enhancing lesion. Med Mycol Case Rep 2016; 12:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takei H, Goodman JC, Powell SZ. Cerebral phaeohyphomycosis caused by ladophialophora bantiana and Fonsecaea monophora: report of three cases. Clin Neuropathol 2007; 26:21–7. [DOI] [PubMed] [Google Scholar]
- 8. Luthra G, Parihar A, Nath K, et al. . Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. Am J Neuroradiol 2007; 28:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]