Abstract
Although lung cancer remains the leading cause of death from cancer worldwide, the advent of immunotherapy is changing the survival of patients affected by non-small cell lung cancer (NSCLC). A multitude of clinical trials are evaluating different immune checkpoints inhibitors in this new field of thoracic oncology.
At the beginning of the immunotherapy era, nivolumab, pembrolizumab and atezolizumab showed high efficacy in patients with advanced NSCLC in second-line setting, receiving approvals for clinical practice. Nivolumab and atezolizumab are approved independently from programmed death lig and 1 (PD-L1) expression, while pembrolizumab is currently approved only for patients with PD-L1 expression ≥1%.
The role of PD-L1 expression acquired more interest considering first-line clinical trials, in which the role of immunotherapy as monotherapy was confirmed only for pembrolizumab in patients with PD-L1 expression ≥50%. These data were analysed in this paper, focusing on the implications in clinical practice and how to use them to an accurate clinical benefit of patients with advanced NSCLC.
We report a review based on a MEDLINE/PubMed, searched for randomised phase 2/3 trials evaluating immune checkpoint inhibitors and NSCLC, that moved to an approval from Food and Drug Administration (FDA) and European Medicine Agency (EMA). The evidence discussed in this manuscript and the final therapeutic algorithm, coming out from an International Experts Panel Meeting of the Italian Association of Thoracic Oncology.
Keywords: NSCLC, immunotherapy, nivolumab, pembrolizumab, atezolizumab, algorithm
Introduction
Lung cancer is the leading cause of cancer-related death worldwide. In the last 20 years, the survival has been modestly improved by standard chemotherapy, but there can be toxicity, and long-term benefit is rare. The identification of predictive biomarkers of response to tyrosine kinase inhibitors, such as Epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) or c-ros proto-oncogene 1 (ROS1), have led to a significant improvement in survival and response, but are only effective in about of only 20% or patients with non-squamous histology.1–4 More recently, the development of novel immune checkpoint inhibitors have showed to restore patient’s immune response to cancer cells and improve survival in some patients with non-small cell lung cancer (NSCLC).5–8
Nowadays, monoclonal antibodies against PD-1 (nivolumab and pembrolizumab) and PD-L1 (atezolizumab) showed to be effective in squamous and non-squamous NSCLC when compared with standard chemotherapy in second line and first line for patients with high expression of PD-L1. The role of PD-L1 expression is debating considering drug, setting and cut-off of evaluation.9–11
Looking to the future, combination treatment appear to be very promising, with the potential to overcome the role of PD-L1 as a mandatory predictive biomarkers of response.12 13 In this review, we built a methodological algorithm, considering previous approved treatment and new clinical options, which takes into account all the results of randomised clinical trials.
Immune checkpoint inhibitors in second-line setting
Nivolumab in squamous NSCLC (CheckMate 017)
The CheckMate 017, phase 3 trial, was designed to evaluate the efficacy and safety of nivolumab compared with standard docetaxel in patients with stage IIIB/IV NSCLC in second line, after a first-line platinum-based chemotherapy. Overall, 272 patients were randomised to receive respectively nivolumab (n=135) at a dose of 3 mg/kg every 2 weeks or docetaxel (n=137) at a dose of 75 mg/m2 every 3 weeks. Overall survival (OS) was the primary endpoint; overall responsive rate (ORR), progression-free survival (PFS), safety profile and outcomes according to PD-L1 expression were the secondary endpoints. PD-L1 expression was evaluated retrospectively on pretreatment (archival or recent) tissue by immunohistochemistry, using Dako clone 28–8 rabbit monoclonal antibody, considering three cut-off levels of PD-L1 expression of ≥1%, ≥5% or ≥10%.14 OS was significantly improved in favour of nivolumab (median 9.2 vs 6.0 months, respectively; HR=0.59; p<0.001) with a 1-year survival rate of 42% (95% CI 34% to 50%) compared with 24% (95% CI 17% to 31%). Moreover, the results confirmed the superiority of nivolumab for all predefined endpoints, including PFS (3.5 vs 2.8 months, respectively; HR 0.62; p<0.001) and ORR (20% vs 9%, respectively; p=0.008). PD-L1 expression, evaluated on 83% of study population, was not prognostic or predictive in patients with squamous cell lung cancer in the second-line setting. Based on these results, nivolumab was approved by FDA and EMA for the treatment of advanced squamous NSCLC after progression on first-line platinum therapy.
Nivolumab in non-squamous NSCLC (CheckMate 057 trial)
The efficacy and safety of nivolumab in advanced or metastatic non-squamous NSCLC was evaluated in the twin phase 3 trial named CheckMate 057. In this study, 582 patients that progressed to front-line platinum-based doublet chemotherapy were randomly assigned to receive 1:1 nivolumab at a dose of 3 mg/kg every 2 weeks (292 patients) or docetaxel at a dose of 75 mg/m2 every 3 weeks (290 patients) until disease progression. Treatment with nivolumab showed a significant improvement in favour of nivolumab for median OS (12.2 vs 9.4 months, HR 0.73, 95% CI 0.59 to 0.89; p=0.0015), 1-year OS rate of (51% vs 39%) median duration of response (17.2 vs 5.6 months) and ORR (19% vs 12%, p=0.0246). There were no significant differences in PFS (table 1).15 PD-L1 expression analysis was assessable in 78% (455/582) of overall study population and in 63% (123) of patients treated with nivolumab. Subgroups analysis of different PD-L1 expression levels confirm an increased median OS of 17.2, 18.2 and 19.4 months, respectively, for ≥1%, ≥5% or ≥10% patient groups. In patients with low or PD-L1 groups, no difference in survival was reported between nivolumab and docetaxel arms. Based on these results, FDA and EMA approved nivolumab in second-line setting for non-squamous population independently from PD-L1 analysis.
Table 1.
Activity of immune checkpoint inhibitor in second-line non-small cell lung cancer (NSCLC)
| Squamous NSCLC | ||||||
| Clinical trial | CheckMate 01714 | KEYNOTE-01011 | OAK16 | |||
| Drug | Nivolumab | Docetaxel | Pembolizumab* 2–10 mg/kg |
Docetaxel | Atezolizumab | Docetaxel |
| No. of patients | 135 | 137 | 156 | 66 | 112 | 110 |
| m OS | 9.2 | 6.0 | NA | NA | 8.9 | 7.7 |
| HR | 0.62 (0.48–0.81) | 0.74 (0.50–1.09) | 0.73 (0.54–0.98) | |||
| 1-year overall survival (OS) (%) | 42 | 24 | 43/52** | 35 | 55§ | 41§ |
| 2-year OS (%) | 23 | 15 | 30.1a/ 37.5b§ | 14.5 | 31§ | 21§ |
| 3-year OS (%) | 16 | 6 | ||||
| Non-squamous NSCLC | ||||||
| Clinical trial | CheckMate 05715 | KEYNOTE-01011 | OAK16 | |||
| Drug | Nivolumab | Docetaxel | Pembolizumab* 2–10 mg/kg |
Docetaxel | Atezolizumab | Docetaxel |
| No. of patients | 287 | 268 | 444 | 240 | 313 | 315 |
| m OS | 12.2 | 9.5 | NA | NA | 15.6 | 11.2 |
| HR | 0.75 (0.63–0.91) | 0.63 (0.50–0.79) | 0.73 (0.60–0.89) | |||
| 1-year OS (%) | 51 | 39 | 43/52§ | 35§ | 55§ | 41§ |
| 2-year OS (%) | 29 | 13 | 30.1a/37.5b § | 14.5 | 31§ | 21§ |
| 3-year OS (%) | 18 | 9 | ||||
m OS, median overall survival.
Nivolumab safety profile
Nivolumab evaluated in both phase 3 trials resulted to be better tolerated compared with docetaxel, with approximately 70% of overall adverse events (AEs), but only 10% grade 3 or 4 AEs. Fatigue and decreased appetite were the most common adverse events (≥20%). Immune-related AEs were reported in 9% in both of squamous and non-squamous clinical trials.14 15
Pembrolizumab in squamous and non-squamous NSCLC (KEYNOTE-010 trial)
Pembrolizumab was evaluated in KEYNOTE-010 phase 3 randomised clinical trial. Only pretreated patients with NSCLC and PD-L1 expression on at least 1% were enrolled and randomised (1:1:1) to receive pembrolizumab 2 mg/kg (n=346), pembrolizumab 10 mg/kg (n=346) or standard docetaxel 75 mg/m2 (n=343).11 PD-L1 expression was prospectively evaluated by immunohistochemistry with Dako PD-L1 IHC clone 22C3, considering two cut-offs ≥1% and ≥50% of tumour cells (TCs). Primary endpoints were OS and PFS both in overall study population and in subgroup of patients with PD-L1 expression on at least 50% of TCs.
Considering overall study population with PD-L1 ≥1% of TCs, patients treated with both doses of pembrolizumab achieved an improvement in OS: 10.4 months (95% CI 9.4 to 11.9) for pembrolizumab 2 mg/kg dose, 12.7 months (95% CI 10.0 to 17.3) for pembrolizumab 10 mg/kg dose and 8.5 moths for docetaxel (95% CI 7.5 to 9.8). Differences was highly statistical significant: pembrolizumab 2 mg/kg versus docetaxel (HR 0.71, 95% CI 0.58 to 0.88; p=0.0008) and pembrolizumab 10 mg/kg versus docetaxel (HR 0.61, 95% CI 0.49 to 0.75; p<0.0001). PFS resulted not significantly improved with both doses of pembrolizumab compared with docetaxel (3.9 vs 4.0 vs 4.0 months) in all patients treated.
In the subgroup of patients with PD-L1 ≥50% of TCs (n=346), median OS was 14.9, 17.3 and 8.2, respectively, for pembrolizumab 2 mg/kg group, pembrolizumab 10 mg/kg group and the docetaxel group.
The improvement in favour of pembrolizumab was statistically significant for both doses compared with docetaxel: pembrolizumab 2 mg/kg (HR 0.54, 95% CI 0.38 to 0.77; p=0.0002) and pembrolizumab 10 mg/kg (HR 0.50, 95% CI 0.36 to 0.70; p<0.0001).
Differing from the result of the overall population, PFS in patients with high expression of PD-L1 was significantly longer with pembrolizumab that with standard chemotherapy (5.0 vs 5.2 vs 4.1 months), respectively, for pembrolizumab 2 mg/kg dose, 10 mg/kg dose and docetaxel.
Statistically significant (p<0.0001) improvement in ORR was achieved in each group of patients with highly PD-L1 expression treated with pembrolizumab: 30%/29% for pembrolizumab 2 mg/kg and 10 mg/kg doses, compared with 8% of docetaxel.
Results about long-term survival showed 1-year survival rate of 52/43% and 58/53%, respectively, for patients receiving pembrolizumab 2 mg/10 mg in overall population and PD-L1 ≥50% groups, compared with 35/38% of docetaxel arm.
Safety profile confirmed a low rate of treatment-related adverse events (TRAEs) G3-G5 of pembrolizumab, compared with docetaxel. Immuno-related toxicities occurred in 20% of patients treated with each dose of pembrolizumab.
Based on the results of the KEYNOTE-010, pembrolizumab was approved for the treatment of second-line treatment by FDA and EMA, exclusively for patients with PD-L1 ≥1% of tumours, at the dose of 2 mg/kg only for patients.
Atezolizumab in squamous and non-squamous (OAK trial)
Atezolizumab is an anti-PD-L1 immune checkpoint inhibitor that was evaluated in a randomised phase 3 trial (OAK) compared with standard docetaxel. In this trial, 1225 patients were enrolled with squamous or non-squamous NSCLC previously treated with a front-line platinum-based chemotherapy, with stage IIIB or IV.16 Prospectively, evaluation of PD-L1 expression was assessed on archival or fresh tissue, with the VENTANA SP142 PD-L1 immunohistochemistry assay.
Differing from the analysis performed with Dako 28–8 for nivolumab and Dako 22C3 for pembrolizumab, with SP142 antibody, the expression of PD-L1 was evaluated on TCs or tumour-infiltrating immune cells (ICs) with different cut-off, identified in the POPLAR phase 2 trial:
TC0/IC0: PD-L1 expression on less than 1% of TCs or tumour-infiltrating ICs.
TC1/2/3 or IC1/2/3: PD-L1 expression on 1% or more of TCs or tumour-infiltrating ICs.
TC2/3 or IC2/3: PD-L1 expression on 5% or more of TCs or tumour-infiltrating ICs.
TC3: PD-L1 expression on 50% or more of TCs.
IC3: PD-L1 expression on 10% or more of tumour-infiltrating ICs.
Patients were stratified by PD-L1 expression. Coprimary endpoints were OS in overall study population and PD-L1 expression different population TC1/2/3 or IC1/2/3. Patients were randomised to receive standard docetaxel (n=578) or atezolizumab 1200 mg flat dose every 3 weeks.
OS resulted improved in intent-to-treat (ITT) population and all the PD-L1 expression subpopulations in favour of atezolizumab:
ITT population: 13.8 versus 9.6 months (HR 0.73; 95% CI 0.62 to 0.87); p=0.0003.
TC0-IC0 group: 12.6 versus 8.9 months (HR 0.75; 95% CI 0.59 to 0.96), p<0.0001.
TC 1/2/3 or IC 1/2/3 group: 15.7 versus 10.3 months (HR 0.74; 95% CI 0.58 to 0.93); p=0.0102.
TC 2/3 ore IC 2/3 group: 16.3 versus 10.08 months (HR 0.67; 95% CI 0.49 to 0.90); p=0.0080.
TC3 or IC3 group: 20.5 versus 8.9 months (HR 0.41; 95% CI 0.27 to 0.64), p<0.0001.
The OS improvement was higher in non-squamous histology of 15.6 versus 11.2 months (HR 0.73; 95% CI 0.60 to 0.89; p=0.0015) than in then squamous histology group 8.9 versus 7.7 months (HR 0.73; 95% CI 0.54 to 0.98; p=0.0383). Survival resulted independently from response achieved, both in squamous and non-squamous. No difference was noted in terms of PFS in the ITT population (4.0 vs 2.8 months, HR 0.95; 95% CI 0.82 to 1.10).
As for nivolumab and pembrolizumab, atezolizumab treatment resulted as well tolerated with 15% of TRAEs, grade ¾ and a low rate of immune-mediated toxicities.
Atezolizumab received FDA approval and positive evaluation by EMA for the treatment of patients with NSCLC in second-line setting.
Immune checkpoint inhibitors in first-line setting
Pembrolizumab (KEYNOTE-024)
Pembrolizumab was also evaluated in as a front-line therapy in KEYNOTE-024 randomised phase 3 clinical trial. In this study, untreated patients with advanced NSCLC, negative for ALK and EGFR mutations, with PDL1 ≥50% staining, were randomly assigned to received, in a 1:1 ratio, pembrolizumab in a flat dose of 200 mg every 3 weeks or a standard of care (SOC) platinum-based chemotherapy for 4–6 cycles. Continuous maintenance was allowed as an investigator option. ECOG PS2 and untreated brain metastases were considered as exclusion criteria. PFS was the primary endpoint. OS, ORR and safety profile were secondary endpoints. In SOC group, crossover to pembrolizumab was allowed at the time of disease progression.17
A total of 1934 patients were screened for the enrolment and 1653 were tested for PD-L1. Of these, 500 (30.2%) had a PD-L1 ≥50%. A total of 305 patients with high PD-L1 expression met the inclusion criteria and were randomised to receive selected treatment (n=151 in the chemotherapy arm; n=151 in the pembrolizumab arm). Median PFS was 10.4 versus 6.0 months (HR=0.50; 95% CI 0.37 to 0.68, p<0.001) in favour of pembrolizumab arm. Objective response rate (ORR), assessed according RECIST criteria, was 44.8% (95% CI 36.8 to 53.0) versus 27.8% (95% CI 20.8 to 35.7), respectively, for pembrolizumab and chemotherapy group (p=0.0011). The median duration of response was 6.3 months with platinum-base chemotherapy and not reached in the pembrolizumab arm.
Data about OS, presented at World Congress on Lung Cancer (WCLC) International Association for the Study of lung Cancer (IASLC) 2017 in Yokohama, showed median OS 30.0 versus 14 months in favour of pembrolizumab treatment (HR=0.63; 95% CI 0.47 to 0.86, p=0.002).
After disease progression, about 50% of patients who received chemotherapy had first-line crossed over to pembrolizumab.
The improvement of outcomes was observed in all subgroups of overall study population in favour of pembrolizumab. PFS was improved independently from multiple clinical variables: age, sex, ECOG Performance Status, smoking habit and brain metastases. Patients with squamous features histology showed a greatest benefit in terms of ORR and PFS (HR=0.35; 95% CI 0.17 to 0.71).
Safety profile was different considering the two arms of treatment favouring pembrolizumab. Discontinuation rate was 7.1% and 10.7%, respectively, for pembrolizumab and chemotherapy. Control arm with platinum-based chemotherapy was characterised by bone marrow toxicities (anaemia 44%, neutropaenia 28.7%), nausea (43.3%) and vomiting (20%), overlapping the safety results of multiple clinical trials. Patients treated with pembrolizumab showed as most common TRAEs: diarrhoea (14.3%), fatigue (10.4%) and fever (10.4%). Immuno-mediated adverse of any grade occurred in 29.2% of patients treated with pembrolizumab. Of these, 9.7% was of grades 3–5.
Based on these results, pembrolizumab was approved by FDA and EMA as a new ad front line for patients with PD-L1 expression on at least 50% of TCs.
Nivolumab (CheckMate 026)
Nivolumab in first line was evaluated in the CheckMate 026, phase 3 clinical trial, compared with platinum-based chemotherapy. Only patients with PD-L1 expression ≥1% evaluated with Dako 28.8 antibody were randomised in 1:1 ratio to receive nivolumab at a dose of 3 mg/kg or the investigator’s choice of platinum-based chemotherapy, customised for histology. Continuous maintenance was allowed.18 Randomisation and primary analysis were stratified according to PD-L1 expression level (<5% vs ≥5%).
PFS among patients with PD-L1 was the primary endpoint. Secondary endpoints includes: PFS in overall study population (PD-L1 ≥1%) and OS in PD-L1 ≥5%.
Globally, 541 patients with PD-L1 ≥1% were randomised. Of these, 423 (78.2%) showed PD-L1 ≥5%. In this subgroup, the PFS was 4.2 versus 5.9 (HR=1.15; 95% CI 0.91 to 1.45, p=0.25) months in favour of chemotherapy, and median OS was 14.4 versus 13.2 (HR=1.02; 95% CI 0.80 to 1.30), respectively, for chemotherapy and nivolumab. Neither of these differences was statistically significant. Also, in the subgroup of patients with PD-L1 ≥50% (n=214), PFS was not statistically significant between the two treatments (HR 1.07; 95% CI 0.77 to 1.49). In this group, ORR was 39% for chemotherapy, and 34% for nivolumab. As in the previous trials for nivolumab, atezolizumab and pembrolizumab, safety profile favoured nivolumab treatment with 18% of TRAEs of grades 3–4, compared with 51% of standard platinum chemotherapy.
Chemotherapy with or without pembrolizumab (KEYNOTE-021)
Combination treatment of carboplatin-pemetrexed with pembrolizumab was evaluated in a cohort of a randomised phase 2 trial. In this trial, patients with metastatic NSCLC, negative for EGFR and ALK, were randomised to receive chemotherapy with or without pembrolizumab in a flat dose (200 mg total). Randomisation was stratified according to different PD-L1 expression (<1% vs 1% vs 1%–49% vs ≥50%). Objective response rate according to RECIST 1.1 was the primary endpoint. Secondary endpoints include PFS, OS, safety profile and correlation between PDL1 and efficacy results. A total of 123 patients were randomised (n=63 in the control arm; n=60 in the combo arm).19
Results confirmed high ORR in favour of the combo treatment 55% versus 29% (p=0.0016). Median PFS was 13.0 and 8.9, respectively, for chemotherapy plus pembrolizumab and chemotherapy alone (HR=0.53; 95% CI 0.31 to 0.91, p=0.0102). No differences about OS and 1-year OS (75% vs 72%) rate were noted between the two groups.
Among patients treated with chemotherapy plus pembrolizumab, the results in terms of response showed no significant differences according to the different PD-L1 expression level: ORR was 57% in PD-L1 <1 group: 54% for PD-L1 ≥1%; 26% for PD-L1 1%–49%; and 80% for PD-L1 ≥50% group.
Safety analysis showed that TREAs occurred in 93% of patients treated with combo versus 90% of chemotherapy alone. The FDA granted accelerated approved the combination of carboplatin-pemetrexed plus pembrolizumab as first-line setting, independently from PD-L1 expression. An ongoing phase 3 trial (KEYNOTE-189) will evaluate if this preliminary signal of benefit is confirmed and if an OS advantage is achieved.
The updated algorithm for the treatment of advanced or metastatic NSCLC
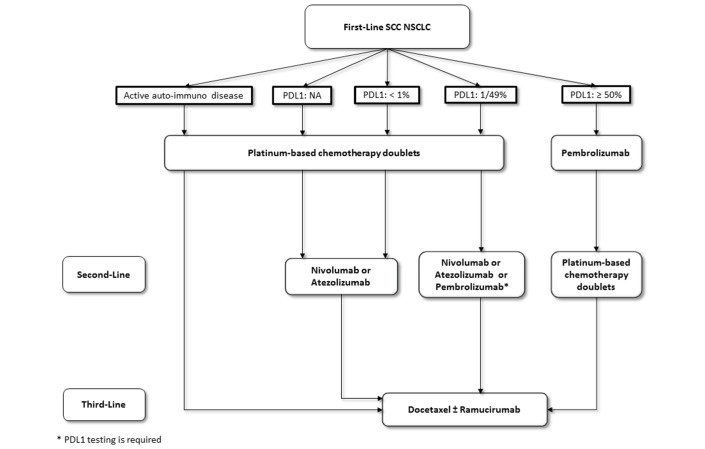
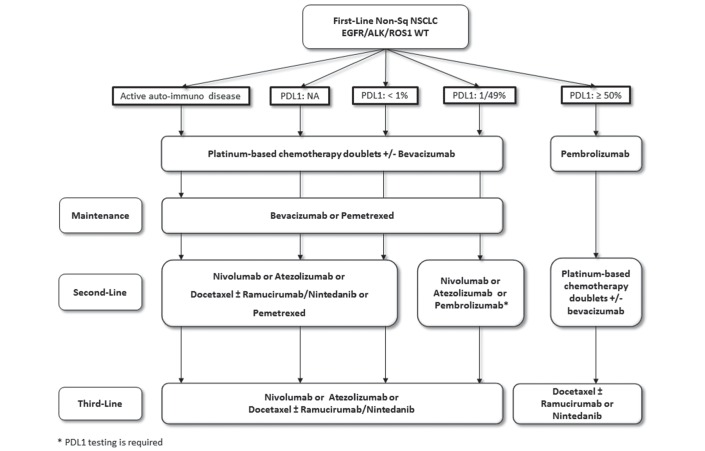
Based on the results of the discussed trials, the algorithm for the treatment of advanced or metastatic NSCLC changed substantially since the last update. Figures 1 and 2 showed the updated algorithm based on the phase 3 trials (figures 1 and 2).
Figure 1.
The updated algorithm based on the phase 3 trials. NSCLC, non-small cell lung cancer.
Figure 2.
The updated algorithm based on the phase 3 trials. NSCLC, non-small cell lung cancer.
Patients selection for first-line treatment require PD-L1 analysis in squamous and non-squamous histology. Based on the results of KEYNOTE-024, all patients with PD-L1 ≥50% on TCs should be receive pembrolizumab as front-line therapy.
For patients with PD-L1 expression <50%, immune checkpoint inhibitors did not show a great survival benefit and nowadays are not approved in monotherapy in first-line setting.
Combination treatment with carboplatin-pemetrexed plus pembrolizumab received accelerated approval from FDA based on the results of a small phase 2 trial KEYNOTE-021, but this combination is not be considered as a valid therapeutic option for the moment. The results of the confirmatory phase 3 trial are awaited.
For patients with non-squamous NSCLC in the second-line setting, immunotherapy should be considered the new standard of treatment independent of PD-L1 expression. Atezolizumab, nivolumab and pembrolizumab showed to be very effective compared with standard docetaxel and should be considered as a new cornerstone class of agents in this setting. Pembrolizumab treatment requires PD-L1 analysis ≥1% performed with companion test as required by FDA and EMA registration; otherwise, considering the overall benefit achieved by atezolizumab and nivolumab in all the subgroups of patient population evaluated (PD-L1 negative and positive), PD-L1 expression is not required for patients selection. Patients selection between immunotherapy (docetaxel±nintedanib or immunotherapy) is required considering ECOG Performance Status and comorbidities for the choice of the best treatment.
Although PD-L1 is an imperfect biomarker, it is the only approved predictive biomarker that is validated for the use in clinical practice for patients’ selection. PD-L1 analysis is mandatory in first-line setting, and in second-line setting, it is highly recommended but not required by FDA and EMA for all different checkpoint inhibitors.
Based on the available scientific data, patients with active autoimmune disease cannot be considered candidate to receive immune checkpoint inhibitor. Otherwise, the presence of inactive autoimmune disease is not a contraindication for immunotherapy treatment.
Conclusion
In the recent years, the increasing knowledge on tumour biology and immune system allows us to improve survival and safety of patients affected by NSCLC, with or without driver mutations.
Until recently, for patients without EGFR, ALK or ROS1 alterations, chemotherapy with or without antiangiogenics agents was the only available option in first line and second line, with limited results and a well-known safety profile characterised by high rate of nausea, vomiting and bone marrow toxicities.
Lately, different immune check-point agents were tested at first in second-line setting and then were moved to the first-line clinical trials.
Nivolumab, pembrolizumab and atezolizumab achieved amazing results for patients who progressed to a first-line setting with squamous and non-squamous histology, confirming the trilling premises long-waited by thoracic oncologist. All of these three agents improve survival when compared with standard docetaxel, although with different and discussed results about the correlation with PD-L1 expression.
The development of these interesting immunotherapy agents was moved from the second-line to the front-line setting in which only pemetrexed showed to increase with a statistical significant benefit survival when compared with platinum-based chemotherapy.
Nowadays, a multitude of clinical trials are ongoing to evaluate different immune checkpoint agents in first line, in monotherapy or in combination therapy with chemotherapy or other immunotherapy agents with or without PD-L1 expression analysis as an inclusion criteria (table 2).
Table 2.
Ongoing phase 3 trials of first-line treatment with immune checkpoint inhibitors
| Trial | Agent | Histology (SQ or no-SQ) | No. of patients | Experimental arm | PD-L1 status |
| CheckMate 227 (NCT02477826) |
Nivolumab | Both | 1980 | Arm A: nivolumab. Arm B: nivolumab+ipilimumab. Arm C: SOC+nivolumab. |
All comers |
| KEYNOTE-042 (NCT02220894) |
Pembrolizumab | Both | 1240 | Arm A: pembrolizumab. Arm B: chemotherapy. |
Positive >1% |
| KEYNOTE-407 (NCT02775435) |
Pembrolizumab | SQ | 560 | Arm A: SOC. Arm B: SOC+pembrolizumab. |
All comers |
| KEYNOTE-189 (NCT02578680) |
Pembrolizumab | No-SQ | 580 | Arm A: SOC. Arm B: SOC+pembrolizumab. |
All comers |
| NEPTUNE (NCT02542293) |
Durvalumab | Both | 960 | Arm A: durvalumab+tremilimumab. Arm B: SOC. |
All comers |
| PEARL (NCT03003962) |
Durvalumab | Both | 440 | Arm A: durvalumab. Arm B: SOC. |
Positive≥25% |
| IMpower 110 (NCT02409342) |
Atezolizumab | Both | 570 | Arm A: SOC. Arm B: atezolizumab. |
Positive TC 2/3 or IC 2/3 |
| IMpower 130 (NCT02367781) |
Atezolizumab | No-SQ | 650 | Arm A: SOC. Arm B: SOC+atezolizumab. |
All comers |
| IMpower 131 (NCT02367794) |
Atezolizumab | SQ | 1025 | Arm A: SOC. Arm B: SOC+atezolizumab. |
All comers |
| IMpower 132 (NCT02657434) |
Atezolizumab | No-SQ | 568 | Arm A: SOC. Arm B: SOC+atezolizumab. |
All comers |
| IMpower 150 (NCT02366143) |
Atezolizumab | No-SQ | 1200 | Arm A: SOC+bevacizumab. Arm B: SOC+B+atezolizumab. |
All comers |
| JAVELIN LUNG 100 (NCT02576574) |
Avelumab | Both | 1095 | Arm A: SOC. Arm B: avelumab. |
Positive ≥1% |
SOC, standard of care; SQ, sqamous.
Overall, these interesting and changing practice results are only the tip of the iceberg of the immunotherapy era, that is, just at the beginning of its satisfying path.
Footnotes
Contributors: All authors contributed equally.
Funding: This study was funded by Italian Association of Thoracic Oncology (AIOT).
Competing interests: FdM received honoraria from BMS, Roche, Pfizer, AstraZeneca, Celgene, MDS, Boehringer and Novartis. PB has received research grants from or has served as a consultant for Merck, Bristol-Meyers Squibb, Polaris and Pfizer. MDH received honoraria from Genentech, Merck, AstraZeneca, Novartis, Janssen and Mirati. TSKM received honoraria from AstraZeneca, Roche Genentech, Eli Lilly, Bristol-Myers Squibb, Boehringer Ingelheim, Novartis, MSD and Pfizer. HL receiving honoraria from Novartis, Bristol-Myers Squibb, Lilly, Pierre Fabre Oncologie, Pfizer, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Roche and Amgen. LP-A receiving honoraria AstraZeneca, Pfizer, Bristol-Myers Squibb, MerckSharp & Dohme, Lilly, Roche, Merck Serono, Novartis and Boehringer Ingelheim. DR-A receiving advisory fees from MSD, Bristol-Myers Squibb, Roche and Boehringer Ingelheim.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Siegel RL, Miller KD, Jemal A, et al. CA Cancer J Clin 2015;2015:5–29. [DOI] [PubMed] [Google Scholar]
- 2. Liao BC, Lin CC, Lee JH, et al. Optimal management of EGFR-mutant non-small cell lung cancer with disease progression on first-line tyrosine kinase inhibitor therapy. Lung Cancer 2017;110:7–13. 10.1016/j.lungcan.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 3. Passaro A, Lazzari C, Karachaliou N, et al. Personalized treatment in advanced ALK-positive non-small cell lung cancer: from bench to clinical practice. Onco Targets Ther 2016;9:6361–76. 10.2147/OTT.S98347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchetti A, Barberis M, Di Lorito A, et al. JCO Precision. Oncology 2017. [DOI] [PubMed] [Google Scholar]
- 5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 7. Brahmer JR, Pardoll DM. Immune checkpoint inhibitors: making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res 2013;1:85–91. 10.1158/2326-6066.CIR-13-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brahmer JR. Immune checkpoint blockade: the hope for immunotherapy as a treatment of lung cancer? Semin Oncol 2014;41:126–32. 10.1053/j.seminoncol.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brahmer JR, Hammers H, Lipson EJ. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future Oncol 2015;11:1307–26. 10.2217/fon.15.52 [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garon EB, Rizvi NA, Hui R, et al. KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 12. Qiao M, Jiang T, Ren S, et al. Combination Strategies on the Basis of Immune Checkpoint Inhibitors in Non-Small-Cell LungCancer: Where Do We Stand? Clin Lung Cancer 2017;23. [DOI] [PubMed] [Google Scholar]
- 13. Tanvetyanon T, Gray JE, Antonia SJ. PD-1 checkpoint blockade alone or combined PD-1 and CTLA-4 blockade as immunotherapy for lung cancer? Expert Opin Biol Ther 2017;17:305–12. 10.1080/14712598.2017.1280454 [DOI] [PubMed] [Google Scholar]
- 14. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. Reck M, Rodríguez-Abreu D, Robinson AG, et al. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 18. Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415–26. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497–508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]