Abstract
The treatment of brain diseases with gene therapy requires the gene to be expressed throughout the central nervous system, and this is possible by using gene targeting technology that delivers the gene across the blood–brain barrier after i.v. administration of a nonviral formulation of the gene. The plasmid DNA is targeted to brain with pegylated immunoliposomes (PILs) using a targeting ligand such as a peptidomimetic mAb, which binds to a transporting receptor on the blood–brain barrier. The present studies adapt the PIL gene targeting technology to the mouse by using the rat 8D3 mAb to the mouse transferrin receptor. Tissue-specific expression in brain and peripheral organs of different exogenous genes (β-galactosidase, luciferase) is examined at 1–3 days after i.v. injection in adult mice of the exogenous gene packaged in the interior of 8D3-PIL. The expression plasmid is driven either by a broadly expressed promoter, simian virus 40, or by a brain-specific promoter taken from the 5′ flanking sequence of the human glial fibrillary acidic protein (GFAP) gene. The transgene is expressed in both brain and peripheral tissues when the simian virus 40 promoter is used, but the expression of the exogenous gene is confined to the brain when the transgene is under the influence of the brain-specific GFAP promoter. Confocal microscopy colocalizes immunoreactive bacterial β-galactosidase with immunoreactive GFAP in brain astrocytes. These studies indicate that tissue-specific gene expression in brain is possible after the i.v. administration of a nonviral vector with the combined use of gene targeting technology and tissue-specific gene promoters.
Keywords: blood–brain barrier‖gene therapy‖transferrin receptor‖ β-galactosidase‖liposomes
Brain-specific expression of a therapeutic gene requires the use of (i) organ-specific promoter elements and (ii) a transcellular targeting system that enables delivery of the transgene across both the blood–brain barrier (BBB) and the brain cell plasma membrane. Prior work has demonstrated the widespread expression of exogenous genes in the brain of rats after the i.v. injection of pegylated immunoliposomes (PILs) that carry the plasmid DNA in the interior of the nanocontainer (1). The PIL/DNA was targeted to brain cells with a peptidomimetic mAb to the rat transferrin receptor (TfR). Owing to the abundance of the TfR on the brain capillary endothelium, which forms the BBB in vivo, the mAb targeted the exogenous gene to brain cells in vivo in the rat (1). However, the TfR is also abundant in some peripheral tissues, such as liver and spleen, and the exogenous gene, driven by the simian virus 40 (SV40) promoter, also was expressed in liver and spleen after the i.v. injection of the PIL (2).
The present studies test the hypothesis that the expression of an exogenous gene can be restricted to the brain with the use of both the PIL gene targeting technology and a brain-specific promoter. The expression in brain and peripheral organs is measured for either a luciferase or a β-galactosidase exogenous gene. The plasmid DNA encapsulated in the 8D3-PIL is administered i.v., and the exogenous gene is under the influence of either the SV40 promoter or the human glial fibrillary acidic protein (GFAP) promoter (3, 4). A second goal of this work was to develop a gene targeting system specific for the mouse, given the availability of transgenic mouse models of human disease. Prior work in rats used the mouse OX26 mAb to the rat TfR (1, 2), but the OX26 mAb is not active in mice (5). The rat 8D3 mAb to the mouse TfR is an active BBB transport vector in mice (5), and the present studies describe the production of PILs targeted with the 8D3 mAb.
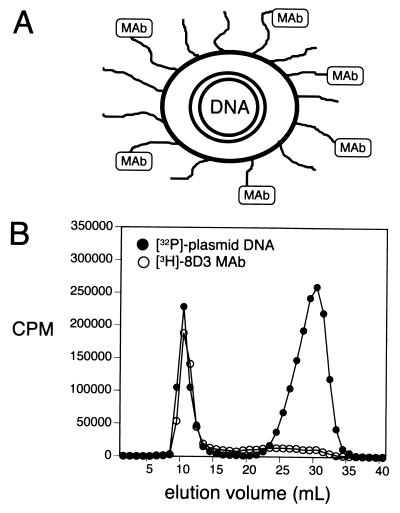
The structure of the PIL gene targeting system is shown in Fig. 1A, and this formulation is to be contrasted with conventional cationic lipid/DNA complexes. The PIL has a net negative charge (2) and internalizes the DNA in the interior of the liposome to render the plasmid DNA resistant to the endogenous endonucleases, which are ubiquitous in vivo (6). Any exteriorized DNA is removed from the PIL formulation by nuclease treatment and gel filtration chromatography (1, 2). The liposome surface is decorated with 2,000–3,000 strands of 2,000-Da polyethylene glycol, designated PEG2000. The “pegylation” of the liposome prevents absorption of serum proteins to the surface of the structure and minimizes uptake of the liposome by cells lining the reticuloendothelial system (7). The pegylated liposome is directed to tissues in vivo by tethering to the tips of 1–2% of the PEG strands a targeting peptidomimetic mAb (Fig. 1A). The anti-TfR mAb binds to the BBB TfR to trigger receptor-mediated transcytosis across the BBB in vivo, and this process is followed by Tfr-mediated endocytosis of the PIL into brain cells (1, 2).
Figure 1.
(A) A double-stranded supercoiled plasmid DNA is encapsulated in the interior of 75- to 85-nm liposomes, which are pegylated with PEG2000, and 1–2% of the PEG strands are conjugated with a targeting ligand, such as a mAb (1, 2). (B) Elution of 8D3 PILs through a Sepharose CL-4B gel filtration column allowed for separation of the PILs from unconjugated mAb and from exteriorized nuclease-digested DNA. The comigration of the interiorized [32P]-DNA and the [3H]8D3 mAb demonstrated the plasmid DNA and the targeting mAb were incorporated in the same structure.
Experimental Procedures
Materials.
Adult male BALB/c mice (25–30 g) were purchased from Harlan Breeders, Indianapolis. dCTP (specific activity: 800 Ci/mmol) was from Perkin–Elmer. N-succinimidyl[2,3-3H] propionate (specific activity: 97.0 Ci/mmol) was supplied by Amersham Pharmacia. DNase I, exonuclease III, and the Nick Translation Kit were from GIBCO/BRL. Traut's reagent was purchased from Pierce. Lipids for liposome synthesis were supplied by Avanti Polar Lipids. The LiposoFAST-Basic extruder and polycarbonate filters were from Avestin, Ottawa. 5-Bromo-4-chloro-3-indoyl-β-d-galactoside, rat IgG, and all other chemicals were purchased from Sigma. The pSV-β-galactosidase expression plasmid driven by the SV40 promoter, the pGL3-control luciferase expression plasmid driven by the SV40 promoter, the luciferase reagent, and recombinant luciferase were obtained from Promega. The pGfa-lacZ β-galactosidase expression plasmid was provided by Jose Segovia, Centro de Investigacìon y de Estudios Avanzados, San Pedro Zacatenco, Mexico (3, 4). In this plasmid, the lacZ gene is driven by the human GFAP promoter (nucleotides −2163 to +47). The GFAP ATG at position +15 was mutated to TTG, so translation begins at the lacZ ATG initiation codon. The 3′ untranslated region (UTR) following the lacZ gene is derived from the mouse protamine gene-1 3′ UTR, and contains an intron and poly(A) sequence (3, 4). The orientation of the GFAP promoter within the plasmid was confirmed by DNA sequencing. The 8D3 hybridoma line, secreting a rat IgG to the mouse Tfr (8), was obtained from Britta Engelhardt, Max Planck Institute, Bad Naoheim, Germany.
Plasmid DNA Preparation and Radiolabeling.
β-Galactosidase or luciferase plasmid DNA was purified from Escherichia coli with the maxiprep procedure and desalted by using the Qiagen (Chatsworth, CA) Plasmid Maxi Kit and QIAquick PCR purification kit, respectively. The size of the DNA was confirmed by 0.8% agarose gel electrophoresis. DNA was labeled with 32P-dCTP using nick translation. The specific activity of 32P-DNA was 15–20 μCi/μg. The purity measured by trichloroacetic acid precipitability was 99%.
mAb Purification and Radiolabeling.
The rat 8D3 anti-mouse TfR mAb was purified with protein G Sepharose affinity chromatography from ascites generated in nude mice. All animal procedures were performed with protocols approved by the University of California Los Angeles Animal Research Committee. The 8D3 hybridoma was grown on a feeder layer of newborn mouse thymocytes and peritoneal cells, as described by Kissel et al. (8). The antibody was tritiated with N-succinimidyl[2,3-3H] propionate as described (9). The specific activity and trichloroacetic acid precipitability of the 3H-8D3 mAb were 100–150 μCi/mg and 95%, respectively.
PIL Synthesis.
PILs were synthesized by using a total of 20 μmmol of lipids, including 18.8 μmmol of 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC), 0.4 μmmol of didodecyldimethylammonium bromide (DDAB), 0.6 μmmol of distearoylphosphatidylethanolamine (DSPE-PEG), and 200 nmol of DSPE-PEG-maleimide, as described (1, 2). The supercoiled plasmid DNA (150 μg) and 2 μCi of 32P-plasmid DNA were encapsulated in the pegylated liposomes, and exteriorized DNA was degraded by nuclease digestion, as described (1, 2). The thiolated 8D3 mAb was conjugated to the pegylated liposome overnight at room temperature. The 8D3 PIL/DNA was separated from degraded DNA absorbed to the exterior of the liposome, and from unconjugated mAb, using a 1.6 × 18 cm column of Sepharose CL-4B (Fig. 1B). The average number of 8D3 mAbs conjugated on one liposome was 50 and was computed from the measurement of radioactive mAb, as described (10). The DNA encapsulation efficiency was 20%. The control rat IgG PIL/β-galactosidase plasmid DNA was prepared by following the same procedure.
In Vivo Administration and Gene Expression Assays.
Male BALB/c mice weighing 25–30 g were anesthetized with ketamine (50 mg/kg) and xylazine (4 mg/kg) i.p. PIL conjugated with either the 8D3 mAb or nonspecific rat IgG, and carrying either the pSV-β-galactosidase plasmid DNA, the pGfa-lacZ plasmid DNA, or the pGL3-control plasmid DNA was injected i.v. in mice via the jugular or femoral vein with a 30-g needle at a dose of 3–5 μg of PIL-encapsulated plasmid DNA per mouse. Mice were killed at 24, 48, and 72 h postinjection. Brain, liver, spleen, kidney, heart, and lung were removed and frozen in OCT embedding medium for β-galactosidase histochemistry, or homogenized in 4 vol of lysis buffer for measurements of organ luciferase activity, as described (1). For histochemistry, frozen sections of 18-μm thickness were fixed in 0.1% glutaraldehyde in 0.1 M NaH2PO4 for 5 min and incubated with 0.1% 5-bromo-4-chloro-3-indoyl-β-d-galactoside at pH = 7.0 at 37°C overnight, as described (1, 2). Whole-mount images of the organs were obtained by scanning the section with a UMAX PowerLookIII scanner with transparency adapter, and the image was cropped in Adobe PHOTOSHOP 5.5 on a G4 Power Macintosh computer. The luciferase enzyme activity was measured in organ supernatants with a Luminometer (Biolumat LB 9507, Berthold, Nashua, NH), as described (1). A standard curve was assayed in parallel with recombinant luciferase, and the relative light units were converted to pg of luciferase. The protein concentration in the tissue extract was determined with the BCA protein assay reagent (Pierce), and the luciferase activity in the organ was expressed as pg luciferase/mg protein.
Confocal Microscopy.
Mouse brain frozen sections (20 μm) were fixed with 100% acetone at −20°C for 20 min. The sections were incubated in primary antibody for 1 h at room temperature. The primary antibodies were mixed and included a goat anti-human/rodent GFAP 1:20 polyclonal antiserum (Research Diagnostics, Chicago), and a mouse mAb against E. coli βgalactosidase, 20 μg/ml (Promega). After a PBS wash, a rhodamine-conjugated donkey anti-goat IgG 1:200 (Research Diagnostics) secondary antibody was added for 30 min at room temperature. The slides were then washed and incubated with fluorescein-conjugated goat anti-mouse IgG (Sigma) for 30 min at room temperature. The sections were mounted on slides with Vector Shield (Vector Laboratories) mounting media, and viewed with a ×10 or ×100 objective and a Zeiss LSM 5 PASCAL confocal microscope with dual argon and helium/neon lasers. The sample was scanned with multitrack mode to avoid leakage of the fluorescein signal into the rhodamine channel. Sections were scanned at intervals of 0.48 μm and reconstructed with Zeiss lsm software. Control experiments used either a mouse IgG (Cappel) or reagent grade goat IgG (Sigma) as primary antibody in lieu of the anti-β-galactosidase or the anti-GFAP antibody, respectively.
Results
The organ luciferase activity at 48 and 72 h after a single i.v. injection of the pGL3 plasmid DNA encapsulated in the 8D3-PIL is shown in Table 1 and indicates the 8D3 mAb targets the gene to TfR-rich organs such as brain, liver, spleen, and lung, with very little gene expression in kidney or heart. The results with the 8D3 TfR mAb in the mouse replicate previous work with the OX26 TfR mAb in the rat (1).
Table 1.
Organ luciferase activity
| Organ | pg Luciferase/mg protein
|
|
|---|---|---|
| 48 h | 72 h | |
| Brain | 0.76 ± 0.09 | 0.50 ± 0.10 |
| Heart | 0.015 ± 0.001 | 0.018 ± 0.004 |
| Liver | 3.1 ± 0.5 | 1.4 ± 0.1 |
| Spleen | 1.1 ± 0.1 | 0.52 ± 0.11 |
| Lung | 0.74 ± 0.06 | 0.34 ± 0.09 |
| Kidney | 0.0092 ± 0.0009 | 0.0082 ± 0.0003 |
Data are mean ± SE (n = 5 mice per time point). Mice were administered a single i.v. injection of 5 μg/mouse of SV40 promoter-pGL3 plasmid DNA encapsulated in 8D3-PIL.
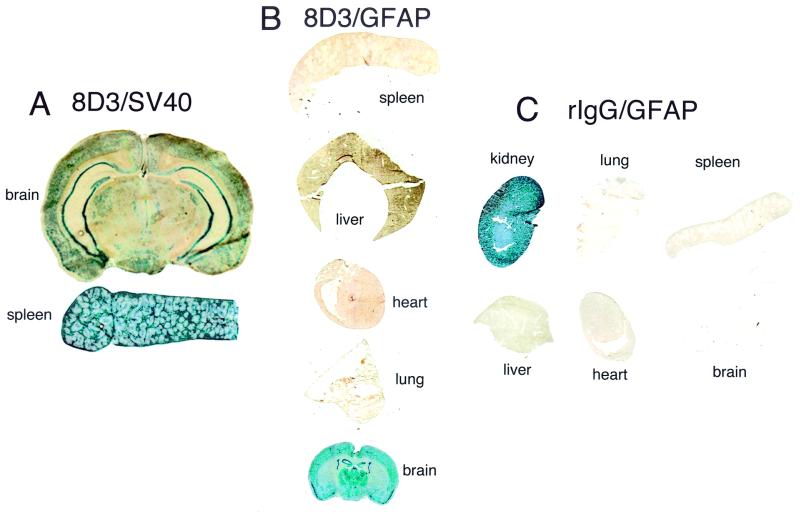
Administration of the pSV-β-galactosidase plasmid encapsulated in the 8D3-PIL resulted in gene expression in both brain and peripheral tissues, such as spleen (Fig. 2A). The pGfa-lacZ plasmid also was encapsulated in 8D3-PIL and injected i.v. in mice, and organs were removed 2 days later for β-galactosidase histochemistry (Fig. 2B). Whereas the GFAP/β-galactosidase gene was widely expressed throughout the mouse brain, there was no expression of the transgene in mouse spleen, liver, heart, or lung (Fig. 2B). Gene targeting of organs in vivo was determined by the specificity of the targeting mAb. When the 8D3 rat mAb to the murine TfR was replaced with nonspecific rat IgG (rIgG), and the GFAP/β-galactosidase plasmid was encapsulated in the interior of the rIgG-PIL, there was no gene expression in brain or peripheral tissues (lung, spleen, liver, or heart) (Fig. 2C). Owing to high expression of endogenous mammalian β-galactosidase in mouse kidney, this organ serves as a positive control for the histochemical assay (Fig. 2C). The β-galactosidase activity in mouse kidney represents endogenous mammalian enzyme, and this activity does not arise from expression of the bacterial β-galactosidase gene in kidney.
Figure 2.
(A) β-Galactosidase histochemistry was performed on organs removed 2 days after an i.v. injection of PILs carrying the pSV-β-galactosidase plasmid, driven by the SV40 promoter, and conjugated with the 8D3 rat mAb. (B) 5-Bromo-4-chloro-3-indoyl-β-d-galactoside histochemistry of organs removed from mice 2 days after a single i.v. injection of 8D3 PILs carrying the GFAP/β-galactosidase plasmid. (C) β-Galactosidase gene expression in mouse organs removed 48 h after a single i.v. injection of the GFAP/β-galactosidase plasmid incorporated in PILs that were targeted with nonspecific rat IgG (rIgG). All organs were negative except for kidney. The histochemical product in kidney is derived from endogenous mammalian β-galactosidase-like activity in that organ. Kidney removed from noninjected mice yielded similar β-galactosidase reaction product.
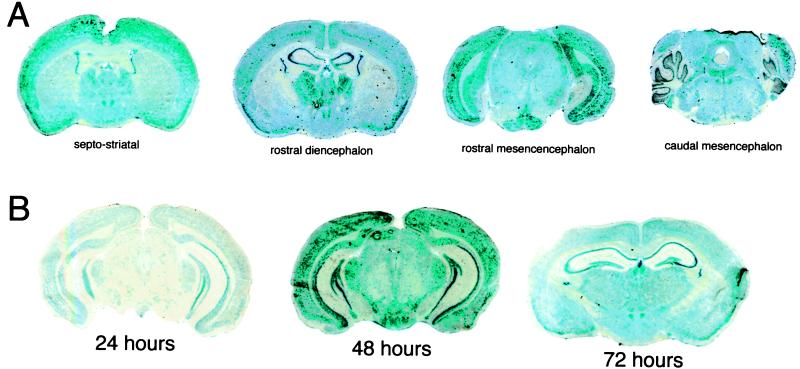
The extent to which the exogenous gene is expressed throughout the central nervous system is demonstrated with serial coronal sections through the septo-striatum, the rostral diencephalon, the rostral mesencephalon, and the caudal mesencephalon (Fig. 3A). Gene expression is detected widely throughout the cortical and subcortical structures at all levels of brain. In the septo-striatum, the GFAP/β-galactosidase gene is expressed in the caudate putamen nucleus and the epithelium of the choroid plexus lining the lateral ventricles and the third ventricle. At the level of the rostral diencephalon, there is gene expression in the parietal cortex, the hippocampus, and the thalamic nuclei (Fig. 3A). In the caudal mesencephalon, gene expression is observed throughout the cerebellum and the posterior colliculus anteriorly and various hindbrain nuclear structures posteriorly. The expression in mouse brain of the GFAP/β-galactosidase gene peaks at 48 h after a single i.v. injection as shown in Fig. 3B. Temporal studies were performed with a SV40/β-galactosidase plasmid, which showed a reduced gene expression at 24 h compared with the GFAP/β-galactosidase and a level of β-galactosidase gene expression in the brain at 72 h that was comparable to the activity at 48 h in Fig. 2A.
Figure 3.
(A) β-Galactosidase histochemistry in mouse brain removed 48 h after a single i.v. injection of the GFAP/β-galactosidase plasmid encapsulated in the interior of 8D3-targeted PILs. Coronal sections at different levels of the brain are shown. (B) β-Galactosidase histochemistry is shown for brain removed from mice at 24, 48, and 72 h after a single i.v. injection of the GFAP/β-galactosidase plasmid encapsulated in the interior of 8D3 PILs.
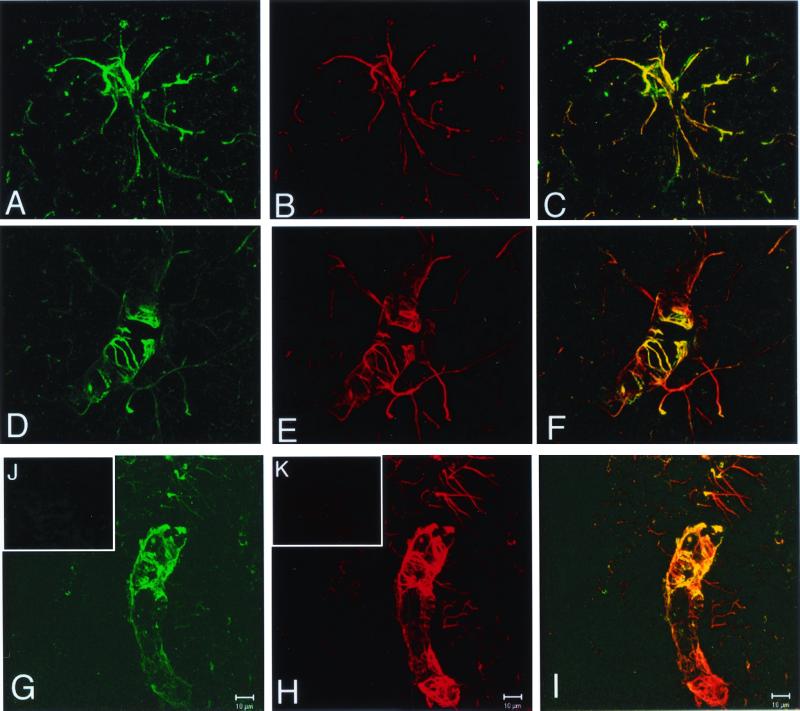
Confocal microscopy confirmed the expression of the β-galactosidase transgene in astrocytes (Fig. 4). The expression of immunoreactive β-galactosidase and immunoreactive GFAP in the same brain cell is shown in Fig. 4 A and B, respectively, and the yellow signal in the superimposed image demonstrates immune labeling of both the cell body and the processes of the astrocyte (Fig. 4C). Astrocyte foot processes invest the basement membrane surface of the cerebral microvasculature, and these structures express immunoreactive GFAP, as shown in Fig. 4 E and H. Immunoreactive bacterial β-galactosidase is also expressed in the astrocyte foot processes investing the microvasculature as shown in Fig. 4 D and G. The orange/yellow signal in the superimposed image demonstrates colocalization of the GFAP and the bacterial β-galactosidase in the astrocyte processes (Fig. 4 F and I).
Figure 4.
Confocal microscopy of mouse brain removed 48 h after a single i.v. injection of the GFAP/β-galactosidase plasmid encapsulated in the interior of 8D3 PILs. (A, D, and G) The confocal microscopy of immunoreactive β-galactosidase, stained with a fluorescein-labeled secondary antibody. (B, E, and H) Immunoreactive GFAP, stained with a rhodamine-labeled secondary antibody. (C, F, and I) The respective superimposed images showing colocalization of the β-galactosidase and the GFAP in astrocyte cell bodies (C) and astrocyte processes (F and I). The yellow/orange images in are the result of the superimposition of the green (β-galactosidase) and red (GFAP) images. No staining of brain was obtained with either mouse IgG/fluorescein-conjugated goat anti-mouse IgG (J) or goat IgG/rhodamine-conjugated donkey anti-goat IgG (K). (Scale bar: 10 μm.)
Discussion
These studies are consistent with the following conclusions. First, exogenous genes encoding for either luciferase or β-galactosidase and under the influence of the SV40 promoter are targeted to brain in the mouse using PILs and the rat 8D3 mAb to the mouse TfR. However, the transgene is also targeted to TfR-rich peripheral tissues such as spleen, liver, and lung (Table 1, Fig. 2A). Second, the expression of the exogenous gene in peripheral organs can be eliminated when the exogenous gene is under the influence of a brain-specific promoter, such as the 2 kb of the 5′ flanking sequence of the human GFAP gene (Fig. 2B). Third, the PIL gene targeting technology enables the widespread expression throughout the central nervous system of an exogenous gene after the noninvasive i.v. administration of a nonviral formulation of the gene (Fig. 3A). Fourth, the expression of the exogenous gene in brain persists for at least 3 days after the single i.v. administration (Table 1, Fig. 3B). Fifth, the β-galactosidase transgene, under the influence of the GFAP promoter, is widely expressed in both cell bodies and processes of astrocytes, as demonstrated by confocal microscopy (Fig. 4).
The 8D3-PIL delivers the exogenous gene both to brain and TfR-rich peripheral tissues (Table 1). However, when the transgene is under the influence of a brain-specific promoter, there is no gene expression in peripheral tissues (Fig. 2B). The absence of measurable expression of the transgene in lung (Fig. 2B) contrasts the PIL gene targeting system with conventional cationic lipid/DNA complexes. After the i.v. injection of cationic liposome/DNA complexes, the level of gene expression in the lung is log orders greater than in peripheral tissues such as liver or spleen, and there is no gene expression in brain (11). The absence of significant gene expression in liver (Fig. 2B) contrasts the PIL system with the adenovirus gene delivery system, wherein >90% of the exogenous gene is expressed in mouse liver after the i.v. administration of adenoviral vector systems (12).
Transgene expression peaks at 2 days after a single i.v. injection and starts to decline by 3 days, when the gene is driven by a GFAP promoter (Fig. 3B). The SV40 promoter gives a greater persistence of the transgene in both mice (Table 1) and rats (2). The level of gene expression does not decline to the 50% level until 6 days after i.v. administration of the SV40/β-galactosidase in rats, as measured by either histochemistry or Southern blotting (2). The persistence of a therapeutic gene may be increased with the use of expression plasmids that contain gene elements that enable extra-chromosomal replication of the plasmid DNA (13). In addition to persistence of gene expression, a goal of gene targeting is the organ specificity, and even cellular specificity, of transgene expression. This degree of specificity is possible with the use of organ- or cell-specific promoter elements.
The expression in the mouse of the β-galactosidase gene was restricted to the brain, if the β-galactosidase gene was under the influence of the GFAP promoter (Fig. 2B). The pattern of β-galactosidase brain histochemistry shown in Fig. 3 for adult mice administered the transgene i.v. is similar to the β-galactosidase histochemistry of the brain of transgenic mice expressing the β-galactosidase gene under the influence of the human GFAP promoter (3). The expression of the β-galactosidase transgene in astrocytes was confirmed in the present studies with confocal microscopy (Fig. 4). Three-dimensional reconstruction of the confocal images of peri-vascular astrocytes reveals the rosette-like structures that invest the basement membrane surface of the brain capillary (Fig. 4 E and H), and these structures express the bacterial β-galactosidase (Fig. 4 D and G). These rosette-like structures of the astrocyte foot processes are identical to those in normal rat brain, as described by Kacem et al. (14). The β-galactosidase transgene also is expressed in the cell bodies of astrocytes as shown in Fig. 4 A–C.
In summary, brain-specific expression of an exogenous gene is possible with the combined use of a brain-specific promoter and a gene targeting system using PILs. This gene delivery system offers the same advantages of viral delivery systems, such as (i) interiorization of the exogenous gene in a nanocontainer, and protection from ubiquitous endonucleases in vivo, and (ii) surface proteins that trigger uptake of the gene by target cells. However, humans have pre-existing immunity to either adenovirus or herpes simplex virus, and the single injection of either virus into either animal or human brain results in an inflammatory reaction in brain that can lead to demyelination (15, 16). In contrast, the immunogenicity of the PIL is restricted to the targeting mAb, and the antibody immunogenicity can be eliminated with the use of genetically engineered “humanized” mAbs. A mAb to the human insulin receptor (HIR) is an active BBB delivery system in Old World primates and humans and delivers 4% of the injected dose to the primate brain (17). This murine HIR mAb has been genetically engineered for use in humans (18). The reactivity of the genetically engineered form of the anti-HIR mAb is identical to that of the original murine HIR mAb, both with respect to binding to the HIR and in vivo targeting to the primate brain (18). The combined use of genetically engineered mAbs that are nonimmunogenic, and the PIL gene targeting system, may enable the widespread expression of a therapeutic gene throughout the brain in humans after i.v. administration. Brain specificity of gene expression can be controlled with the use of specific promoter and enhancer elements taken from brain-specific genes.
Acknowledgments
The pGfa-lacZ plasmid was provided by Dr. Jose Segovia, and the 8D3 rat hybridoma line was provided by Dr. Britta Engelhardt. Daniel Jeong skillfully prepared the manuscript. This work was supported by a grant from the University of California, Davis/Medical Investigation of Neurodevelopmental Disorders Institute Research Program, and the U.S. Department of Defense. The communicating member is a member of the Scientific Advisory Board of Lexrite Labs.
Abbreviations
- BBB
blood–brain barrier
- GFAP
glial fibrillary acidic protein
- PIL
pegylated immunoliposome
- PEG
polyethylene glycol
- TfR
transferrin receptor
- HIR
human insulin receptor
- SV40
simian virus 40
References
- 1.Shi N, Pardridge W M. Proc Natl Acad Sci USA. 2000;97:7567–7572. doi: 10.1073/pnas.130187497. . (First Published June 6, 2000; 10.1073/pnas.130187497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi N, Boado R J, Pardridge W M. Pharm Res. 2001;18:1091–1095. doi: 10.1023/a:1010910523202. [DOI] [PubMed] [Google Scholar]
- 3.Brenner M, Kisseberth W C, Su Y, Besnard F, Messing A. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segovia J, Vergara P, Brenner M. Neurosci Lett. 1998;242:172–176. doi: 10.1016/s0304-3940(98)00042-1. [DOI] [PubMed] [Google Scholar]
- 5.Lee H J, Engelhardt B, Lesley J, Bickel U, Pardridge W M. J Pharmacol Exp Ther. 2000;292:1048–1052. [PubMed] [Google Scholar]
- 6.Barry M E, Pinto-Gonzalez D, Orson F M, McKenzie G J, Petry G R, Barry M A. Hum Gene Ther. 1999;10:2461–2480. doi: 10.1089/10430349950016816. [DOI] [PubMed] [Google Scholar]
- 7.Papahadjopoulos D, Allen T M, Gabizon A, Mayhew E, Matthay K, Huang S K, Lee K D, Woodle M C, Lasic D D, Redemann C, et al. Proc Natl Acad Sci USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kissel K, Hamm S, Schulz M, Vecchi A, Garlanda C, Engelhardt B. Histochem Cell Biol. 1998;110:63–72. doi: 10.1007/s004180050266. [DOI] [PubMed] [Google Scholar]
- 9.Pardridge W M, Buckiak J L, Yoshikawa T. J Pharmacol Exp Ther. 1992;261:1175–1180. [PubMed] [Google Scholar]
- 10.Huwyler J, Wu D, Pardridge W M. Proc Natl Acad Sci USA. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osaka G, Carey K, Cuthbertson A, Godowski P, Patapoff T, Ryan A, Gadek T, Mordenti J. J Pharmacol Sci. 1996;85:612–618. doi: 10.1021/js9504494. [DOI] [PubMed] [Google Scholar]
- 12.Zinn K R, Douglas J T, Smyth C A, Liu H G, Wu Q, Krasnykh V N, Mountz J D, Curiel D T, Mountz J M. Gene Ther. 1998;5:798–808. doi: 10.1038/sj.gt.3300659. [DOI] [PubMed] [Google Scholar]
- 13.Pardridge W M. Brain Drug Targeting. Cambridge, U.K: Cambridge Univ. Press; 2001. [Google Scholar]
- 14.Kacem K, Lacombe P, Seylaz J, Bonvento G. GLIA. 1998;23:1–10. [PubMed] [Google Scholar]
- 15.Lawrence M S, Foellmer H G, Elsworth J D, Kim J H, Leranth C, Kozlowski D A, Bothwell A L, Davidson B L, Bohn M C, Redmond D E., Jr Gene Ther. 1999;6:1368–1379. doi: 10.1038/sj.gt.3300958. [DOI] [PubMed] [Google Scholar]
- 16.Dewey R A, Morrissey G, Cowsill C M, Stone D, Bolognani F, Dodd N J, Southgate T D, Klatzmann D, Lassmann H, Castro M G, Lowenstein P R. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 17.Pardridge W M, Kang Y-S, Buciak J L, Yang J. Pharm Res. 1995;12:807–816. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- 18.Coloma M J, Lee H J, Kurihara A, Landaw E M, Boado R J, Morrison S L, Pardridge W M. Pharm Res. 2000;17:266–274. doi: 10.1023/a:1007592720793. [DOI] [PubMed] [Google Scholar]