Abstract
Most birds sit on their eggs during incubation, a behaviour that likely evolved among non-avian dinosaurs. Several ‘brooding' specimens of smaller species of oviraptorosaurs and troodontids reveal these non-avian theropods sat on their eggs, although little is known of incubation behaviour in larger theropod species. Here we examine egg clutches over a large body size range of oviraptorosaurs in order to understand the potential effect of body size on incubation behaviour. Eggshell porosity indicates that the eggs of all oviraptorosaurs were exposed in the nest, similar to brooding birds. Although all oviraptorosaur clutches consist of radially arranged eggs in a ring configuration, clutch morphology varies in that the central opening is small or absent in the smallest species, becomes significantly larger in larger species, and occupies most of the nest area in giant species. Our results suggest that the smallest oviraptorosaurs probably sat directly on the eggs, whereas with increasing body size more weight was likely carried by the central opening, reducing or eliminating the load on the eggs and still potentially allowing for some contact during incubation in giant species. This adaptation, not seen in birds, appears to remove the body size constraints of incubation behaviour in giant oviraptorosaurs.
Keywords: dinosaur, egg, incubation, nest, Oviraptorosauria, Theropoda
1. Background
Egg incubation in nearly all extant birds involves an adult sitting on the eggs, a behaviour that likely first evolved among non-avian theropod dinosaurs [1,2]. The largest birds have a relatively small body mass (reaching 450 kg in the recently extinct elephant bird [3]) compared to non-avian theropods (up to 7000 kg [4]), potentially because the weight borne by the eggs while brooding constrains maximum adult body size [5]. Among non-avian theropods, species of oviraptorosaurs and troodontids within the size range of birds have been found in avian brood-like positions atop of their egg clutches [1,2,6–8]; the best-preserved specimen (Citipati: IGM 100/979) shows the adult partly in contact or nearly in contact with the clutch [7]. Also, consistent with these specimens is evidence that the eggs were laid exposed (at least partially) in open nests similar to brooding birds (e.g. low eggshell porosity [9,10], egg coloration [10] and taphonomic indicators [2,6]).
Unlike small maniraptoran theropods, little is known about incubation behaviours in large theropod species due to a scarcity of specimens; however, their high body mass may have precluded them from sitting atop their eggs. Recently, large egg clutches (Macroelongatoolithus) have been ascribed definitively to oviraptorosaur species of high body mass (greater than 1000 kg) [11], but differences in clutch configuration suggest these animals had different nesting styles relative to smaller species. In this study, we examine oviraptorosaur eggs and clutches from species representing a large range of body sizes (approx. 1560 kg) in order to infer the effect of increasing body size on their incubation behaviours.
2. Material and methods
Eggs and clutches assignable to the oofamily Elongatoolithidae [12,13] were studied (see electronic supplementary material) because they have been definitively ascribed to oviraptorosaurs based on numerous previous discoveries (e.g. [1,6–8,11]). Here, eggs less than 170 mm in length were assigned to the oogenus Elongatoolithus, 170–240 mm to Macroolithus and ≥240 mm to Macroelongatoolithus [14,15]. Measurements of inner and outer clutch diameters (figure 1a: n = 40), square value of eggshell thickness as a proxy for eggshell strength at the yield point ([16,17]: n = 41) and estimates of eggshell porosity (n = 10) were regressed against estimates of egg mass, using ordinary least-squares regressions. Formulae established in the literature were used to calculate egg mass/volume [18] and clutch volume [19]. Adult body mass was predicted from a phylogenetically generalized least-squares regression of clutch volume against adult body mass for living species of precocial birds and crocodylians [19,20], using the dataset and phylogenetic tree of [20]. Following the methodology of [9], eggshell porosity was estimated from eggshell thickness and pore area to predict nest types (i.e. covered or open nests) using linear discriminant analysis. All statistical analyses were implemented with IBM SPSS Statistics v. 25 (IBM SPSS Inc.) for non-phylogenetic approaches and with software platform R3.1.3 (http://www.r-project.org/) for phylogenetic approaches using ‘corBrownian' and ‘gls()' functions and additional packages (e.g. ‘ape', ‘nlme'). All values were log-transformed prior to analyses.
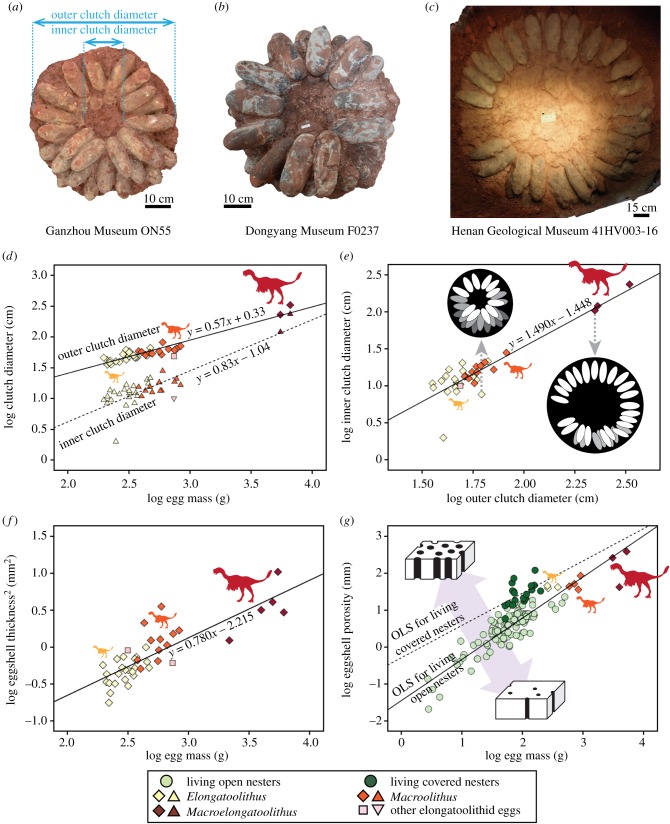
Figure 1.
Oviraptorosaur eggs and clutches showing: (a) Elongatoolithus, (b) Macroolithus, (c) Macroelongatoolithus (photogrammetric image), (d) comparison between clutch diameters and egg mass, (e) comparison between outer and inner clutch diameters, (f) comparison between eggshell strength and egg mass and (g) comparison between eggshell porosity and egg mass (data for extant species from [9]). OLS, ordinary least-squares.
3. Results
Oviraptorosaur eggs are elongated and highly variable in size, ranging from 197 to 6594 g (table 1: figure 1a–c: electronic supplementary material). Oviraptorosaur eggs are arranged radially in a ring-shaped clutch. Most oviraptorosaur clutches discovered are incomplete and preserve between 3 and 35 eggs; well-preserved complete clutches contain more than 30 eggs regardless of egg taxon.
Table 1.
Summary of measurements for oviraptorosaur eggs and clutches. Parentheses indicate number of clutches examined. See electronic supplementary material for the full descriptions of the results.
| ootaxon | Elongatoolithus | Macroolithus | Macroelongatoolithus |
|---|---|---|---|
| number of eggs in the best-preserved clutch | 34 | 35 | 32 |
| outer clutch diameter (cm) | 35.55–66.60 (20) | 51.10–82.00 (12) | 188.18–330.00 (3) |
| inner clutch diameter (cm) | 2.00–21.55 (21) | 10.85–28.10 (12) | 106.54–236.00 (3) |
| estimated egg mass (g) | 197.22–473.45 (26) | 380.50–911.00 (16) | 2177.59–6593.54 (8) |
| eggshell thickness (mm) | 0.420–0.996 (23) | 0.713–1.880 (11) | 1.109–3.228 (5) |
| eggshell porosity (mm) | 39.171–45.555 (3) | 43.809–85.364 (4) | 41.990–385.358 (3) |
| estimated adult body mass (kg) | 41.68–104.62 (25) | 85.73–196.43 (14) | 487.93–1563.03 (8) |
Adult body mass predicted from clutch volume ranges from 41 to 1563 kg, which is comparable to body mass estimates based on skeletal remains of small (e.g. Nomingia: 37 kg) to large known species of oviraptorosaurs (Gigantoraptor: 1400–2092 kg) [4,21,22].
Outer and inner clutch diameters are highly variable among oviraptorosaurs (table 1) but are correlated to egg mass (r = 0.93, p < 0.01 and r = 0.85, p < 0.01 for outer and inner clutch diameters, respectively: figure 1d). Outer clutch diameter ranged from 35.6 to 66.6 cm for Elongatoolithus, 51.1 to 82.0 cm for Macroolithus and 223.0 to 330.0 cm for Macroelongatoolithus, whereas inner clutch diameter ranged from 2.0 to 21.6 cm for Elongatoolithus, 10.9 to 28.1 cm for Macroolithus and 104.4 to 236.0 cm for Macroelongatoolithus. Regardless of nest size, all eggs are inclined and arranged in a radial pattern within a ring-shaped clutch (figure 1a–c). While clutches of Elongatoolithus and Macroolithus consist of two or three layers of eggs, those of Macroelongatoolithus mostly consist of a single layer although sometimes a second layer is present. Inner and outer clutch diameters are positively correlated (r = 0.90, p < 0.01: figure 1e) with a slope of 1.49, indicating a positive allometric change in clutch morphology where the central opening of the large clutches (Macroelongatoolithus: figure 1c) represents a much greater proportion of the nest area when compared to the central opening of the smaller clutches (Elongatoolithus and Macroolithus; figure 1a,b).
The square of eggshell thickness is significantly correlated to egg mass (r = 0.83, p < 0.01: figure 1f) with a slope of 0.78, indicating that larger eggs tend to have relatively thinner eggshell and thus were probably structurally weaker than smaller eggs.
The eggshell of all examined oviraptorosaur eggs has low porosity values comparable to those of extant brooding birds (figure 1g), which are only 6–69% of porosity values for living covered nesters (i.e. crocodylians and megapode birds; calculation based on the regression of living covered nesters [9]). Linear discriminant analysis classified all oviraptorosaur egg taxa into open nesters with high posterior probabilities (greater than 0.64), except for one, Elongatoolithus elongatus.
4. Discussion
Our study demonstrates that characteristics of oviraptorosaur eggs and clutches provide new insight into incubation methods for a large range of non-avian theropod body sizes. Although taphonomic evidence reveals that sediment was built up partially around the eggs to support their inclined orientation in the clutch [6,10], a relatively low eggshell porosity indicates oviraptorosaur eggs were exposed in the nest [9,10]. Exposure of eggs in the nest is always associated with brooding in living birds where an adult sits on the eggs for incubation [23]. Among brooding birds, the eggs are strong enough relative to the body mass of the incubator to allow the animal to sit entirely or partly on the clutch [5,17]. Oviraptorosaurs appear to have adapted to sitting on their nests by somewhat modifying the clutch configuration as species increased in body size, probably because egg mass becomes relatively smaller and eggshell thickness (relative to egg mass) becomes relatively thinner, resulting in a structurally weaker egg (also the case in birds [16,17]). Among the smallest oviraptorosaur clutches, where little (less than 10 cm in diameter) or no central opening is found, the clutch of greater than 30 eggs was presumably strong enough to bear the weight of the adult (figure 2). As the clutch diameter and body size increased among species, the central opening in the clutch (as seen in the ‘brooding' Citipati specimen [1,7]) became larger and would have likely carried at least some of the adult's weight, thus reducing the load on the eggs. In the largest oviraptorosaur clutches (Macroelongatoolithus), the central opening represents most of the total clutch area, likely allowing giant-sized species to rest most or all of their weight on this area so as not to crush the eggs (figure 2). Whereas birds may have had constraints on body size owing to their clutch configuration and incubation style [5], the presence of a central opening in the oviraptorosaur clutch and its increasing proportion of total nest diameter with increasing body size appears to be a unique adaptation of oviraptorosaurs among dinosaurs (including birds) for egg incubation. This adaptation may have allowed for an adult to sit on the nest and potentially even allow some contact with the eggs in the largest oviraptorosaurs. As in brooding birds, this behaviour may have been related to protection, shelter or thermoregulation of the eggs in oviraptorosaurs. If related to heat transfer, this brooding behaviour may have been less effective in large species because there may have been less contact with the eggs due to the modified configuration of their clutches.
Figure 2.
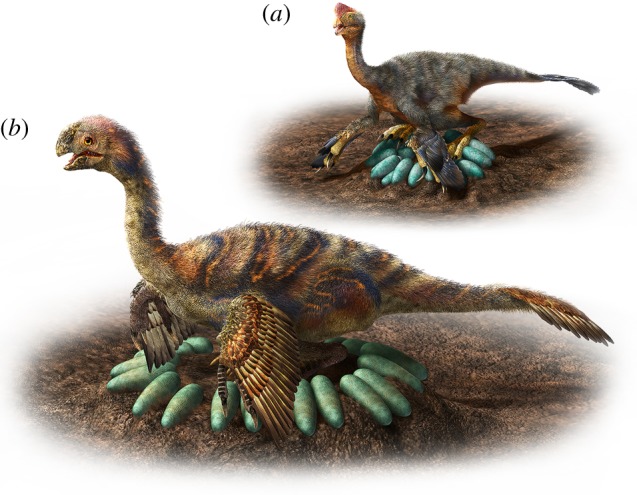
Reconstruction of egg incubation in oviraptorosaurs showing small species sat on the eggs (a), whereas giant species rested in the central opening of the clutch (b). Illustration is drawn by Mr Masato Hattori.
Supplementary Material
Acknowledgements
We would like to thank Hua Li, Shaohui Fan, Zhanfu Shao, Li Xu, and Jiming Zhang for specimen access, and to François Therrien, Shin-Ichi Fujiwara, and three anonymous reviewers for their valuable advice.
Data accessibility
Datasets used in this study are available in the electronic supplementary material.
Authors' contributions
K.T., D.K.Z. and J.L. conceived/designed the project; L.Y., S.J., F.D., M.X., D.L., C.S. and R.C. organized collection and preparation for the specimens; K.T., D.K.Z., J.L. and C.L.D. collected the data, completed analyses and wrote the manuscript. All authors discussed the results and wrote/revised the manuscript, gave final approval for publication and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Japan Society for the Promotion of Science (Project ID: 17J00224) to K.T., an NSERC Discovery Grant (Project ID: 327513-2009) to D.K.Z., and National Natural Science Foundation of China (Project ID: 41672019; 41688103), the Fundamental Research Funds for the Chinese Academy of Geological Sciences (Project ID: JB1504) and the China Geological Survey (Project ID: DD20160126) to J.L.
References
- 1.Norell MA, Clark JM, Chiappe LM, Demberelyin D. 1995. A nesting dinosaur. Nature 378, 774–776. ( 10.1038/378774a0) [DOI] [Google Scholar]
- 2.Varricchio DJ, Jackson F, Borkowski JJ, Horner JR. 1997. Nest and egg clutches of the dinosaur Troodon formosus and the evolution of avian reproductive traits. Nature 385, 247–250. ( 10.1038/385247a0) [DOI] [Google Scholar]
- 3.Hansen DM, Galetti M. 2009. The forgotten megafauna. Science 324, 42–43. ( 10.1126/science.1172393) [DOI] [PubMed] [Google Scholar]
- 4.Campione NE, Evans DC, Brown CM, Carrano MT. 2014. Body mass estimation in non-avian bipeds using a theoretical conversion to quadruped stylopodial proportions. Methods Ecol. Evol. 5, 913–923. ( 10.1111/2041-210X.12226) [DOI] [Google Scholar]
- 5.Deeming DC, Birchard GF. 2008. Why were extinct gigantic birds so small? Avian Biol. Res. 1, 187–194. ( 10.3184/175815508X402482) [DOI] [Google Scholar]
- 6.Dong ZM, Currie PJ. 1996. On the discovery of an oviraptorid skeleton on a nest of eggs at Bayan Mandahu, Inner Mongolia, People's Republic of China. Can. J. Earth Sci. 33, 631–636. ( 10.1139/e96-046) [DOI] [Google Scholar]
- 7.Clark JM, Norell MA, Chiappe LM. 1999. An oviraptorid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avianlike brooding position over an oviraptorid nest. Am. Mus. Novit. 3265, 1–35. [Google Scholar]
- 8.Fanti F, Currie PJ, Badamgarav D. 2012. New specimens of Nemegtomaia from the Baruungoyot and Nemegt formations (Late Cretaceous) of Mongolia. PLoS ONE 7, e31330 ( 10.1371/journal.pone.0031330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka K, Zelenitsky DK, Therrien F. 2015. Eggshell porosity provides insight on evolution of nesting in dinosaurs. PLoS ONE 10, e0142829 ( 10.1371/journal.pone.0142829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiemann J, Yang T, Sander PN, Schneider M, Engeser M, Kath-Schorr S, Müller CE, Sander PM. 2017. Dinosaur origin of egg color: oviraptors laid blue-green eggs. PeerJ 5, e3706 ( 10.7717/peerj.3706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pu H, et al. 2017. Perinate and eggs of a giant caenagnathid dinosaur from the Late Cretaceous of central China. Nat. Commun. 8, 14952 ( 10.1038/ncomms14952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao ZK. 1975. Microstructures of dinosaurian eggshells of Nanxiong, Guangdong, and the problem in egg classification. Vertebr. Palasiat. 13, 105–117. [Google Scholar]
- 13.Mikhailov KE. 1991. Classification of fossil eggshells of amniotic vertebrates. Acta Palaeontol. Pol. 36, 193–238. [Google Scholar]
- 14.Mikhailov K. 1994. Theropod and protoceratopsian dinosaur eggs from the Cretaceous of Mongolia and Kazakhstan. Paleontol. J. 28, 101–120. [Google Scholar]
- 15.Fang X, Wang Y, Jiang Y. 2000. On the Late Cretaceous fossil eggs of Tiantai, Zhejiang. Geol. Rev. 46, 107–112. [Google Scholar]
- 16.Ar A, Rahn H, Paganelli CV. 1979. Avian egg: mass and strength. Condor 81, 331–337. ( 10.2307/1366955) [DOI] [Google Scholar]
- 17.Birchard GF, Deeming DC. 2009. Avian eggshell thickness: scaling and maximum body mass in birds. J. Zool. 279, 95–101. ( 10.1111/j.1469-7998.2009.00596.x) [DOI] [Google Scholar]
- 18.Hoyt DF. 1979. Practical methods of estimating volume and fresh weight of bird eggs. Auk 96, 73–77. [Google Scholar]
- 19.Varricchio DJ, Moore JR, Erickson GM, Norell MA, Jackson FD, Borkowski JJ. 2008. Avian paternal care had dinosaur origin. Science 322, 1826–1828. ( 10.1126/science.1163245) [DOI] [PubMed] [Google Scholar]
- 20.Moore JR, Varricchio DJ. 2016. The evolution of diapsid reproductive strategy with inferences about extinct taxa. PLoS ONE 11, e0158496 ( 10.1371/journal.pone.0158496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Tan Q, Wang J, Zhao X, Tan L. 2007. A gigantic bird-like dinosaur from the Late Cretaceous of China. Nature 447, 844–847. ( 10.1038/nature05849) [DOI] [PubMed] [Google Scholar]
- 22.Benson RBJ, Campione NE, Carrano MT, Mannion PD, Sullivan C, Upchurch P, Evans DC. 2014. Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 12, e1001853 ( 10.1371/journal.pbio.1001853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skutch AF. 1976. Parent birds and their young. Austin, TX: University of Texas Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets used in this study are available in the electronic supplementary material.