Abstract
By using blocked and rapid event-related functional MRI studies of memory, we explored the implications of using rest periods as a baseline condition in functional MRI studies. Activity in the medial temporal lobe (as well as in other brain regions) was substantially higher during rest than during several alternative baseline conditions. The effect of this elevated activity during rest was to reduce, eliminate, or even reverse the sign of the activity during task conditions relevant to memory functions. The results demonstrate that periods of rest are associated with significant cognitive activity and, therefore, provide a nonoptimal baseline for memory tasks. These results were observed not only when relatively long blocks of rest were used (experiment 1), but also when rest consisted of the short null trials typically used in rapid event-related designs (experiment 2). The findings have important implications for the design and interpretation of a wide range of fMRI studies of cognition.
Although there is no inherent baseline associated with the blood oxygen-level-dependent (BOLD) signal that is measured in traditional functional MRI (fMRI) studies, researchers often have attempted to establish such a baseline by using periods of rest. Rest periods may be 10- to 30-s long blocks of rest or fixation (blocked fMRI), the final seconds of long intertrial intervals (ITIs; in the case of slow, or nonoverlapping, event-related fMRI), or 2- to 4-s null trials (in the case of rapid event-related fMRI). Because no task is being performed during rest, it has seemed reasonable to assume that this baseline represents something akin to a zero-activity condition that then can be compared with activity during cognitive tasks. Therefore, when activity in a particular region of the brain during a cognitive task is no greater than during rest, it often has been supposed that this particular region of the brain is not involved in the task.
Yet, in recent years, a number of neuroimaging studies have reported that activity during rest is greater than in some cognitive tasks (see ref. 1 for review). The most likely interpretation is that what is measured during rest periods (at least, when they are relatively long) may reflect significant mental activity and more activity than in some formal tasks (1–4). These studies have focused on prefrontal areas and have explored the idea that activity during rest might reflect unconstrained thought or other self-referential aspects of consciousness. Additionally, some of these studies have reported increased activity during rest in the parahippocampal gyrus (1, 2).
Here, we explore a strong implication of these findings suggested by Martin (5). If activity resulting from unconstrained cognitive activity is present in the brain during periods of rest, rest should not be used as a baseline in fMRI and should not be viewed as a zero activity condition. Further, alternative baseline conditions that constrain cognitive activity may provide more appropriate comparison tasks for fMRI studies of cognition. We have used a memory-encoding paradigm (6) and five different comparison conditions (including rest) to examine this hypothesis.
Numerous fMRI studies have used this paradigm to demonstrate that during encoding there is greater activity bilaterally in the parahippocampal gyrus (and sometimes also in the hippocampal region) in association with viewing novel pictures relative to frequently presented, familiar pictures (e.g., refs. 6–12). fMRI activity in the medial temporal lobe also has been reported during recognition memory tasks in association with previously studied, and now familiar, pictures relative to unstudied novel pictures (8, 13–15). However, because of the ease and reliability with which medial temporal lobe activity can be observed during memory encoding, we have chosen to use encoding rather than retrieval as a benchmark task.
By using both blocked (experiment 1) and rapid event-related (experiment 2) studies of memory encoding, we demonstrate that activity during rest periods can reduce, eliminate, or even reverse the sign of effects related to memory functions. The effect was observed not only when relatively long (21 s) blocks of rest were used (experiment 1), but also when the rest periods were relatively short (3 s), comprising the null events typically used in event-related fMRI (experiment 2). Together, these results demonstrate that rest provides a nonoptimal baseline for memory tasks and have important implications for the design and interpretation of fMRI studies of cognition.
Methods
Participants.
Eight healthy volunteers (3 men, 5 women; mean age = 27.6 years; range = 24–31 years) gave written informed consent before participating in the study. The same eight volunteers participated in both experiment 1 and experiment 2, which were separated by approximately 2 weeks.
Behavioral Tasks.
In experiment 1, seven tasks were administered to participants using a blocked design. In the first task (Novel Pictures), participants were shown novel color outdoor scenes [duration = 2.5 s, ITI = 0.5 s, ≈10° of visual angle]. Using their right hands, participants pressed one button on a response box to indicate that the picture was oriented vertically (portrait orientation) and another to indicate that the picture was presented horizontally (landscape orientation). In the second task (Familiar Pictures), participants were shown repeatedly a single familiar picture (duration = 2.5 s, ITI = 0.5 s, ≈10°) with the same instructions. Participants had been prefamiliarized to this picture (it was present on the screen for ≈5 min. before scanning), and it was used in both experiments. In the third task (Noise Detection), participants were asked to indicate whether a pale green “X” or “T” (≈0.7°) was embedded in a visual white noise mask (≈11°). In the fourth task (Odd/Even Digits), participants were shown single digits (1–9, ≈0.5°) and asked to indicate whether the digit was odd or even. In the fifth task (Arrows), participants were shown a white arrow (≈1.5°) and asked to indicate whether it pointed left or right. In the sixth task (Moving Fixation), participants were asked to maintain fixation on a white dot (≈0.4°) that randomly moved after a variable delay (0.5–1.5 s). In the seventh task (Rest), participants were instructed to relax and wait for the next task to appear. For each task, a brief instruction (e.g., “Odd or Even?” or “Rest”) was displayed at the top of the screen.
Within each scan, all seven tasks were presented three times in a fixed, pseudorandom order for a total of 21 blocks. Each block lasted 21 s. There were seven trials within each block of Novel or Familiar Pictures, and a variable number of trials (self-paced) within each of the other blocks. Four complete scans were administered in succession to each participant, for a total of 12 blocks of each task.
In experiment 2, a rapid event-related (16, 17) design was used in which there were three types of trials: Novel Pictures (2.5-s duration, 0.5-s ITI), Familiar Pictures (2.5-s duration, 0.5-s ITI), and baseline trials. Two baseline conditions were used, and each was administered twice each to each participant. In one condition (Rest Baseline), baseline trials consisted of a 3-s blank screen (a conventional baseline or null trial). In the other condition (Odd/Even Baseline), baseline trials consisted of 3 s of the Odd/Even Digit task. Participants alternated between scans using each baseline condition. Within each scan, 48 Novel Pictures, 48 Familiar Pictures, and 48 Baseline trials were presented in a predetermined, pseudorandom order (17, 18). There were a total of 96 trials of each type in the Rest Baseline condition and 96 trials of each type in the Odd/Even Baseline condition. Unlike experiment 1, no instructions were displayed at the top of the screen during the Rest trials, and the screen was completely blank.
Imaging Parameters.
Imaging was performed on a Siemens (Erlangen, Germany) 1.5T Vision clinical MRI scanner equipped with a large, clinical flex coil and a bite bar. Functional, whole-brain T2*-weighted images were acquired by using an echoplanar, single-shot pulse sequence with a matrix size of 64 × 64, a TE (echo time) of 43 msec, a flip angle of 90°, and an in-plane resolution of 4 × 4 mm. For each scanning run, 153 oblique axial images were acquired for each of 32 4-mm-thick slices aligned with the principle axis of the hippocampus. Images were acquired in an interleaved fashion with a TR (repetition time) of 3 s. Stimulus presentation began on the 5th image and ended on the 148th image, allowing for the initial stabilization of the MR signal (four images) and for its return to baseline at the end of the task (five images). After the last fMRI scan, a high-resolution (1 mm3) MP-RAGE (magnetization prepared-rapid gradient echo) structural scan was acquired for anatomical localization.
Image Analysis.
Images were first coregistered through time by using a three-dimensional registration algorithm (19). Within each run, voxels were eliminated if the signal magnitude changed >8% between two samples or if the mean signal level was below a threshold defined by the inherent noise in the data acquisition. Images were then spatially smoothed by using a two-dimensional (in-plane) Gaussian kernel (full-width half-maximum = 1.5 voxels).
In experiment 1, each participant's fMRI data from the four blocked scans was then averaged. A general linear model (GLM) of the activity in each voxel then was constructed by using a multiple-regression technique with eight regressors. Six of the regressors coded for the six nonrest block types, adjusting for typical values of the hemodynamic response. The other two regressors coded for linear and second-order drift in the MR signal. Six statistical maps resulted from this analysis, each indicating the percent signal change in each voxel for a particular task relative to the rest task. These maps of percent change were then used in calculating cross-participant t tests and repeated-measures ANOVAs.
In experiment 2, the fMRI data from the two scans of each of the two baseline conditions were concatenated. Separate GLMs then were constructed for each baseline condition. For each GLM, 10 vectors were used to model activity within each voxel. Two vectors coded for Novel Pictures and Familiar Pictures, six vectors coded the amount of three-dimensional motion detected during image registration, and two vectors coded for drift (first and second order) in the MR signal. Each GLM was constructed by using a deconvolution technique (D. Ward, http://afni.nimh.nih.gov/afni) to estimate the impulse–response function (here, based on five time points, 0–15 s) associated with both Novel Picture trials and Familiar Picture trials relative to each of the baseline conditions. Cubic spline interpolation was used to align the impulse–response functions in different slices to a common time base. The mean percent change in the second and third acquisitions (3–9 s) was taken as the magnitude of the response when calculating cross-participant t tests.
Regions of Interest (ROI) Analysis.
Ten ROIs were identified in each participant's high-resolution magnetization prepared-rapid gradient echo (MP-RAGE) anatomical MRI from experiment 1. Each participant's perirhinal cortex, temporopolar cortex, and entorhinal cortex were defined bilaterally according to the technique described by Insausti et al. (20). The parahippocampal cortex was defined bilaterally as the portion of the parahippocampal gyrus caudal to the perirhinal cortex and rostral to the splenium of the corpus callosum. Finally, the hippocampal region (the CA fields of the hippocampus, the dentate gyrus, and the subiculum) was defined bilaterally.
A six-parameter (rigid-body) transformation matrix was calculated to align each volunteer's structural MRI from experiment 1 to the scans from experiment 2. This transformation matrix allowed the same anatomically defined ROIs used in experiment 1 to be used in experiment 2. For each experiment, the ROIs were resampled to be in alignment with the functional data, and the results from the GLMs were averaged for each ROI. The mean percent change within each ROI was used in calculating cross-participant t tests.
Whole-Brain Analysis.
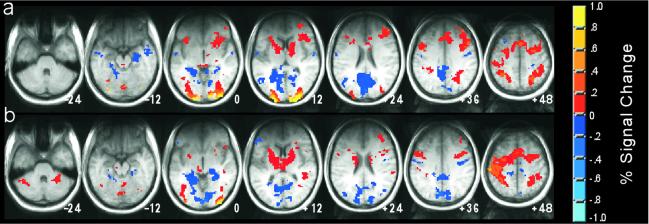
For the whole-brain analysis presented in Fig. 3, a nine-parameter transformation matrix was calculated to transform (21) the structural MRI from each volunteer's first scanning session to conform to the atlas of Talairach and Tournoux (22). The percent signal-change statistical maps then were transformed and resampled to 2.5 mm3 with this transformation matrix. Group analyses were conducted by performing voxel-wise t tests on the resulting statistical maps by using an α-threshold of P < 0.01 (t > 3.5, two-tailed) for each voxel and a minimum cluster size of 250 mm3 of such voxels (16 resampled 2.5-mm3 voxels).
Figure 3.
fMRI data from two of the baseline tasks used in experiment 1, Noise Detection (a) and Odd/Even Digits (b), are shown in axial sections as colored overlays on the average structural images (transformed to the atlas of Talairach and Tournoux, ref. 22). Regions shown in yellow and orange exhibited greater activity in the baseline task than in Rest. Regions shown in blue exhibited greater activity in Rest than in the baseline condition. Deactivations relative to rest were observed not only in the medial temporal lobes, but in many regions throughout the brain. The relative absence of significant activity in the frontal lobe may be the result of the limited coverage of the radio frequency (RF) coil that was used.
Results
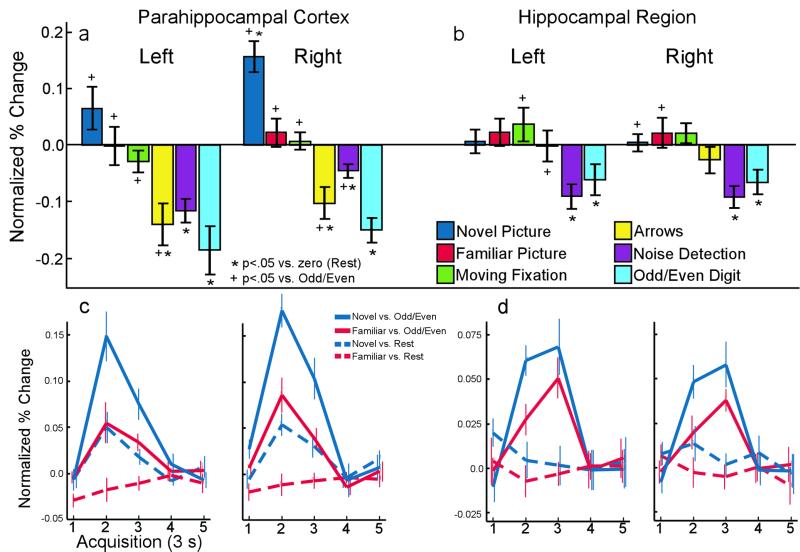
In the blocked fMRI design of experiment 1, activity in 6 of the 10 medial temporal lobe ROIs (left and right parahippocampal cortex, left and right hippocampal region, right perirhinal cortex, and right entorhinal cortex) varied as a function of task. In four of these six regions (Fig. 1 a and b), there was significantly greater activity during Rest than during several alternative baseline conditions. Task-related activity in the left [F(5, 35) = 8.3; P < 0.001] and right [F(5, 35) = 22.2; P < 0.001] parahippocampal cortex is shown in Fig. 1a, where activity during each task is plotted relative to the mean signal during the Rest task. In the left parahippocampal cortex, the Rest condition was associated with more activity than the Arrows task [t(7) = 3.8; P < 0.01], the Noise Detection task [t(7) = 5.5; P < 0.001], and the Odd/Even Digits task [t(7) = 4.3; P < 0.005]. In the right parahippocampal cortex, the Rest condition was associated with less activity than the Novel Picture task [t(7) = 5.8; P < 0.001] but more activity than the Arrows task [t(7) = 3.6; P < 0.01], the Noise Detection task [t(7) = 3.8; P < 0.01], and the Odd/Even Digits task [t(7) = 6.9; P < 0.001].
Figure 1.
(a and b) Activity within anatomically defined ROIs in the medial temporal lobe during six tasks from experiment 1. Bars show the mean percent signal change during each task relative to the mean signal during the Rest task. (Error bars = SEM). (a) Activity in the left and right parahippocampal cortex. The Novel Picture task was associated with increased activity relative to rest in the right parahippocampal cortex. Bilaterally, activity during three of the tasks (Arrows, Noise Detection, and Odd/Even Digits) was significantly less than activity during Rest. The Familiar Picture task was not associated with detectable activity when compared with Rest. When compared with the Odd/Even Digit task, viewing novel or familiar pictures was associated with bilateral activity. (b) Activity in the left and right hippocampal region. Bilaterally, there was significantly less activity during two of the tasks (Noise Detection and Odd/Even Digits) than during Rest. Neither the Novel Picture nor the Familiar Picture task was associated with more activity than Rest, but activity was detected in comparison with the Odd/Even Digits task. (c and d) Hemodynamic response from experiment 2 showing activity over time (3 s per sample) within anatomically defined parahippocampal cortex (c) and hippocampal (d) ROIs. When the Odd/Even Digit task was used as the baseline for activity in a rapid event-related design, both Novel and Familiar Pictures were associated with a significant response in both the parahippocampal cortex and the hippocampus, bilaterally. In contrast, when Rest was used as a baseline for activity in a rapid event-related design, a significant response was found only for Novel Pictures in the parahippocampal cortex.
Task-related activity in the left [F(5, 35) = 4.4; P < 0.005] and right [F(5, 35) = 6.0; P < 0.001] hippocampal region is shown in Fig. 1b. In the left hippocampal region, the Rest condition was associated with more activity than the Noise Detection task [t(7) = 4.1; P < 0.005] and the Odd/Even Digits task [t(7) = 2.4; P < 0.05]. Likewise, in the right hippocampal region, the Rest condition was associated with more activity than the Noise Detection task [t(7) = 4.7; P < 0.005] and the Odd/Even Digits task [t(7) = 3.0; P < 0.05].
The two remaining medial temporal lobe ROIs that exhibited task-related activity were the right perirhinal and entorhinal cortices (data not shown). In the right perirhinal cortex, the Rest condition was associated with less activity than the Familiar Picture task [t(7) = 4.1; P < 0.005] and marginally less activity than the Novel Picture task [t(7) = 2.0; P = 0.08]. In the right entorhinal cortex, the Rest condition was associated with less activity than the Moving Fixation task [t(7) = 3.8; P < 0.01].
Activity During Rest Periods Masks Activity Related to Novel and Familiar Pictures.
Although the Novel Picture task was associated with activity in the right parahippocampal cortex when Rest was used as a baseline, the right parahippocampal cortex was the only medial temporal area that demonstrated such activity. In addition, no medial temporal ROIs demonstrated activity in association with the Familiar Picture task. If Rest were the only baseline available, one would conclude that the presentation of a familiar picture does not result in activity in the medial temporal lobe and that medial temporal lobe activity associated with encoding novel pictures is limited to the right parahippocampal cortex.
The data in Fig. 1 a and b suggest that such a conclusion is inappropriate. When either the Odd/Even Digit task or the Noise Detection task was used as a baseline, substantial medial temporal lobe activity was observed in association with both the Novel and Familiar Picture tasks. For example, with the Odd/Even Digit task as a baseline, pairwise t tests revealed significant bilateral activity in the parahippocampal cortex associated with both the Novel and Familiar Picture tasks and right unilateral activity in the hippocampal region associated with the Novel Picture task (all P < 0.05). Within the left and right hippocampal regions, activity associated with the Familiar Picture task fell just short of significance (left, P = 0.1; right, P = 0.06), as did activity within the left hippocampal region in association with the Novel Picture task (P = 0.08). With the Noise Detection task as a baseline, pairwise t tests revealed significant bilateral activity during the Novel and Familiar Picture tasks in both the parahippocampal cortex and the hippocampal region (activity in the right parahippocampal cortex during the Familiar Picture task, P = 0.1). Similar results were obtained in the parahippocampal cortex when the Arrow task was used as a baseline. Thus, rather than activity being restricted to the right parahippocampal cortex during the Novel Picture task, as would be suggested if Rest were used as a baseline, it seems that the presence of activity during the Rest condition masked activity during both the Novel and Familiar Picture tasks throughout large portions of the medial temporal lobe.
Activity Is Present During Rest Even When Rest Is Used as a Null Event in Rapid Event-Related fMRI.
The studies to date that have examined activity during rest have used blocked designs in which the rest condition is relatively long (e.g., 21 s in experiment 1). Thus, the results from these experiments and from experiment 1 have clear implications for the design and interpretation of both blocked and nonoverlapping or slow event-related experiments (i.e., those in which trials are spaced 16–24 s apart, and the final seconds of the relatively long rest period between trials are used as a baseline).
If the activity observed during rest is the result of unconstrained cognitive activity (e.g., one's mind wandering), it is possible that the activity detected during rest might be absent if shorter rest periods were used. In rapid event-related fMRI designs, trials (including null or baseline trials such as rest conditions) are typically spaced only 2–4 s apart. Given such short trial durations, it is possible that active mentation might not occur during rest. Alternatively, it is possible that significant cognitive activity readily occurs during short rest trials (or during the randomly occurring groups of consecutive rest trials that will occur in such designs).
In experiment 2, two rapid event-related designs (3 s per trial) were used to test these possibilities. In one case, trials consisted of the Novel Picture task, the Familiar Picture task, and Rest. This design can be viewed as a conventional event-related design, wherein a blank screen (Rest) is used as a null or baseline trial. In the other case, trials consisted of the Novel Picture task, the Familiar Picture task, and the Odd/Even Digit task. Each participant was tested with each design, and the designs differed only with respect to the choice of which condition served as the baseline or null task.
The magnitude of the response in the medial temporal lobe to both the Novel and Familiar Pictures was substantially greater when the Odd/Even Digit task was used as a baseline than when Rest was used as a baseline. The results for the parahippocampal cortex are shown in Fig. 1c, and the results for the hippocampal region are shown in Fig. 1d. When Rest was used as a baseline (dashed lines), a significant response to Novel Pictures was detected in both the left [t(7) = 3.1; P < 0.05] and right [t(7) = 4.2; P < 0.005] parahippocampal cortex. However, no response to Novel Pictures was detected in the hippocampal region [P > 0.3]. Further, no response to Familiar Pictures was detected in either the parahippocampal cortex [P > 0.18] or in the hippocampal region [P > 0.4]. Indeed, no response to Familiar Pictures was detected in any of the 10 medial temporal lobe ROIs.
When the Odd/Even Digit task was used as a baseline (Fig. 1 c and d, solid lines), the results were strikingly different. Of the 10 ROIs, 8 exhibited a significant response to Novel Pictures (all P < 0.05 except left entorhinal cortex, P = 0.5, and right entorhinal cortex, P = 0.095). Of the 10 ROIs, 7 exhibited a significant response to Familiar Pictures (all P < 0.05, except left temporopolar cortex, P = 0.056; right perirhinal cortex, P = 0.055; left entorhinal cortex, P = 0.39). Therefore, the effect of activity during the Rest trials was to reduce the fMRI response, often to the point of apparently eliminating the effect of presenting novel or familiar pictures.
Using Rest as a Null Task in Rapid Event-Related fMRI Can Reverse the Sign of Activity.
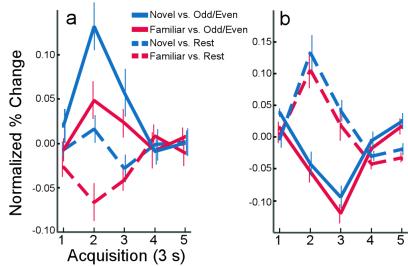
It is worth noting that not only can activity during a baseline condition serve to reduce or eliminate the fMRI response (Fig. 1 c and d), but also activity during a baseline condition can reverse its sign. To illustrate this point, a region within the left parahippocampal gyrus was identified in which the response in experiment 1 to Familiar Pictures was less than the response to Rest. The data from experiment 2 within this functionally defined ROI are shown in Fig. 2a. When the Odd/Even digit task was used as a baseline, the Familiar Picture task was associated with a marginal increase in activity [t(7) = 2.2; P = 0.06]. In contrast, when Rest was used as a baseline, the Familiar Picture task was associated with a significant decrease in activity [t(7) = 4.8; P < 0.005]. This reversal of sign can be attributed to greater activity within this ROI during the Rest task than during the Familiar Picture task.
Figure 2.
Hemodynamic response showing activity over time (3 s per sample) within functionally defined ROIs in experiment 2. (a) Activity in a subregion of the left parahippocampal cortex functionally defined from experiment 1 (see text). When the Odd/Even Digit task was used as the baseline for activity in a rapid event-related design, both Novel and Familiar Pictures were associated with increased activity. When Rest was used as a baseline for activity, Novel Pictures were not associated with detectable activity, and Familiar Pictures were associated with decreased activity. (b) Activity in a subregion (see text) of the left motor cortex where the button pushes in the Odd/Even Digit task would be expected to be associated with significant activity. Here, the activity associated with Novel and Familiar Pictures was greater when Rest was used as a baseline than when the Odd/Even Digit task was used as a baseline. Thus, the effect of activity during a baseline task can be to reduce, eliminate, or even reverse the sign of the activity during the conditions of interest.
Although the Odd/Even Digit task was associated with less activity in the medial temporal lobe than Rest (and, therefore, likely represents a more appropriate baseline), the findings were not the same in all brain regions. For example, with its motoric component, the Odd/Even Digit task would be a poor baseline for studies of motor cortex. In Fig. 2b, activity from experiment 2 within a region of the left motor cortex is shown (functionally defined from experiment 1). Here, the hemodynamic response associated with both Novel and Familiar Pictures is positive when Rest is used as a baseline and negative when the Odd/Even Digit task is used as a baseline. In this case, the presence of significant activity during the Odd/Even baseline task (presumably related to motor activity during button presses) has served to reverse the sign of the activity during the conditions of interest.
Results Throughout the Brain.
Although the data analysis so far has focused on the medial temporal lobe, whole-brain analyses indicate that elevated activity during Rest was present in many other regions as well. Comparisons from experiment 1 between two of the baseline tasks (Noise Detection and Odd/Even Digits) and Rest are shown in Fig. 3. Results with the Arrows task were similar (data not shown). A remarkably similar pattern of increased and decreased activity was observed throughout the brain. Decreases in activity relative to rest were not limited to the medial temporal lobe, but also were observed in frontal, parietal, lateral temporal, and occipital regions. Perhaps most striking was the consistent observation of decreased occipital activity relative to rest in the cuneus, precuneus, and lingual gyrus. The finding that activity in visual areas during Rest (in which no stimulus other than the word “Rest” was presented) was greater than activity during the other baseline tasks (in which digits or a large field of white noise was presented) is intriguing. Although the fMRI scanning room was relatively dark, it is possible that volunteers were able to look around the room and see enough during the Rest task to elicit this visual activity. Additionally, the finding of widespread suppression of visual cortical activity outside the central focus of attention (23) might account for these results. Alternatively, it is possible that the activity reflects the presence of visual imagery during Rest (24).
It should be noted that the coverage of the RF (radio frequency) coil used in this experiment was not uniform throughout the brain. In particular, there was a significant loss of signal in the frontal lobes (which can be seen as darkening in the structural images in Fig. 3). The resulting reduction in the signal-to-noise ratio made it more difficult to detect frontal activity, regardless of whether it was increased or decreased relative to rest.
Discussion
fMRI studies often have used rest periods to identify a baseline level of brain activity that then can be compared with activity during cognitive tasks. These periods may be relatively long blocks of rest or fixation (blocked fMRI), the final seconds of long ITIs (nonoverlapping event-related fMRI), or null trials (rapid event-related fMRI). Because no task is being performed during rest, failures to find activity in some brain region in relation to rest (e.g., failures to observe a hemodynamic response in an event-related design), often have been taken to mean that this particular region of the brain is not involved in the task.
Here, we explored the validity of this conclusion for both blocked and rapid event-related fMRI by using a memory encoding paradigm to evoke activity in the medial temporal lobe. In experiment 1, a blocked design was used to assess activity during seven tasks: (i) deciding whether a novel picture was oriented vertically or horizontally, (ii) deciding whether a familiar picture was presented vertically or horizontally, (iii) deciding whether a digit was odd or even, (iv) deciding whether an arrow pointed left or right, (v) deciding whether an “X” or a “T” was present in a visual noise mask, (vi) following a moving fixation dot, and (vii) rest. Activity in the medial temporal lobe (both in the parahippocampal cortex and the hippocampal region) was substantially higher during rest than during the odd/even task, the arrow task, and the visual-noise task. Moreover, familiar pictures were not associated with more activity than the rest condition.
In experiment 2, two rapid event-related designs were used. In one design, trials consisted of novel pictures, familiar pictures, and rest. In the other design, the odd/even task was used instead of rest. The magnitude of the response in the medial temporal lobe to both novel and familiar pictures was substantially greater when the odd/even task was used as a baseline than when rest was used as a baseline. When rest was used as a baseline, no response to novel pictures was detected in the hippocampal region, and no response to familiar pictures was detected in either the parahippocampal cortex or the hippocampal region. Indeed, regions could be identified in which the response to familiar pictures was negative when rest was used as a baseline. In contrast, when the odd/even task was used as a baseline, both novel and familiar pictures were associated with significant activity throughout most of the medial temporal lobe.
Together, these results demonstrate that even relatively short periods of rest provide a nonoptimal baseline for assessing activity in fMRI studies. Because fMRI is an inherently contrastive methodology, the presence of activity during the baseline condition can seriously compromise the integrity of the analysis. For example, if rest were used as a baseline, one might conclude that the only structure in the medial temporal lobe that responds to viewing novel pictures is the parahippocampal cortex (Fig. 1), and that no structures in the medial temporal lobe respond to viewing familiar pictures. Although the data would seem to support this conclusion (e.g., in Fig. 1d, none of the dashed lines reveal a hemodynamic response), this conclusion would be inappropriate.
The fact of the matter is that, throughout much of the medial temporal lobe, rest was associated with approximately the same level of activity as the viewing of both novel and familiar pictures. Thus, rest is apparently an active condition associated with significant cognitive activity. Some early failures to observe activity in the medial temporal lobe during encoding (25) or retrieval (26) may have occurred because memory tasks were contrasted with rest, simple visual fixation, or similar baseline conditions. Even in recent studies that have successfully demonstrated activity in the hippocampal region in association with recollective success (13, 14), the activity during rest has been in between the activity associated with the two task conditions being compared (e.g., targets and foils). Using a rest condition as a baseline often has made it difficult to observe activity during memory tasks, and difficult to interpret activity when it is observed. The present results indicate that, when alternative baseline tasks were used (e.g., the Odd/Even Digit task), robust activity could be detected in association with conventional memory tasks, in this case, during the incidental encoding of novel pictures and during the incidental retrieval and further encoding of familiar pictures.
These results highlight an important limitation of traditional BOLD fMRI—that it is fundamentally a contrastive methodology with no true baseline-signal level. Although the present data demonstrate that several tasks (e.g., the Odd/Even Digit task) are associated with less activity than Rest, it is not possible to determine how much activity is associated with either condition alone. One can determine only the relative level of activity and cannot know how close either is to a zero or tonic level of activity. For that matter, from BOLD fMRI data, one cannot know whether decreases in activity result from a reduction in excitatory activity or from active inhibition. Recent efforts to uncover the neurophysiological basis of the BOLD fMRI effect (27) may help in this regard, but the effect of inhibitory activity on the BOLD signal is not well understood. In any case, the central conclusions of the present paper stand, regardless of whether reduced medial temporal lobe activity (relative to Rest) during tasks like the Odd/Even Digit tasks results from a failure to engage the medial temporal lobe or from an active suppression (3, 4). The important finding is that neural activity during rest periods (even short, 3 s rest periods) can reduce, eliminate, or even reverse the sign of activity during a cognitive task.
We should emphasize that, although the present study has used a memory task and focused on the medial temporal lobe, the rest condition seems to activate many brain areas (Fig. 3). Specifically, in comparison to three of our tasks, the rest condition seemed to activate regions in the frontal, parietal, and occipital lobes. The rest condition presumably provides an opportunity for day dreaming, self-reflection, problem solving, and other active mental states. The ambiguity of such a condition makes it not ideal to serve as a baseline condition for comparison to cognitive tasks. In contrast, tasks such as making repetitive odd-even judgments may provide the kind of mindless task that is a more appropriate comparison condition for studies of cognition.
Acknowledgments
We thank Shauna Stark, Jennifer Frascino, and Joyce Zouzounis for their assistance with data collection and Kalina Christoff for helpful discussion of her studies with the Arrows task. This research was supported by the Medical Research Service of the Department of Veterans Affairs, National Institute of Mental Health Grants MH24600 and MH12278, the National Alliance for Research on Schizophrenia and Depression, and the Metropolitan Life Foundation.
Abbreviations
- BOLD
blood oxygen-level-dependent
- fMRI
functional magnetic resonance imaging
- ITI
intertrial interval
- GLM
general linear model
- ROI
region of interest
References
- 1.Schulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Binder J R, Frost J A, Hammeke T A, Bellgowan P S F, Rao S M, Cox R W. J Cognit Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 3.Gusnard D A, Akbudak E, Shulman G L, Raichle M E. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. . (First Published March 20, 2001; 10.1073/pnas.071043098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raichle M E, MacLeod A M, Snyder A Z, Powers W J, Gusnard D A, Schulman G L. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin A. Hippocampus. 1999;9:62–70. doi: 10.1002/(SICI)1098-1063(1999)9:1<62::AID-HIPO7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Stern C E, Corkin S, Gonzalez R G, Guimaraes A R, Baker J R, Jennings P J, Carr C A, Sugiura R M, Vadantham V, Rosen B R. Proc Natl Acad Sci USA. 1996;93:8600–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewer J B, Zhao Z, Glover G H, Gabrieli J D E. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 8.Gabrieli J D E, Brewer J B, Desmond J E, Glover G H. Science. 1997;11:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- 9.Kelly W, Miezin F, McDermott K, Buckner R L, Raichel M E, Cohen N J, Ollinger J M, Akbudak E, Contura T E, Snyder A Z, Peterson S E. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 10.Kirchoff B A, Wagner A D, Maril A, Stern C E. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rombouts S A, Scheltens P, Machielsen W C, Barkof F, Hoogenraad F G, Veltman D J, Valk J, Witter M P. Hippocampus. 1999;9:637–643. doi: 10.1002/(SICI)1098-1063(1999)9:6<637::AID-HIPO4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Rombouts S A, Machielsen W C, Witter M P, Barkof F, Lindeboom J, Scheltens P. Hippocampus. 1997;7:594–601. doi: 10.1002/(SICI)1098-1063(1997)7:6<594::AID-HIPO2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Eldridge L L, Knowlton B J, Furmanski C S, Bookheimer S Y, Engel S A. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 14.Stark C E L, Squire L R. J Neurosci. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark C E L, Squire L R. Hippocampus. 2000;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Dale A M, Buckner R L. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Dale A M. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friston K J, Zarahn E, Josephs O, Henson R N, Dale A M. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- 19.Cox R W. Comp Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 20.Insausti R I, Juottonen K, Soininen H, Insausti A M, Partanen K, Vainio P, Laakso M P, Pitkanen A. Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 21.Collins D L, Neelin P, Peters T M, Evans A C. J Comp Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 22.Talairach J, Tournoux P. A Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 23.Smith A T, Singh K D, Greenlee M W. NeuroReport. 2000;11:271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- 24.Kosslyn S M, Pascual-Leone A, Felician O, Camposano S, Keenan J P, Thompson W L, Ganis G, Sukel K E, Alpert N M. Science. 1999;284:167–170. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- 25.Schallice T, Fletcher P, Frith C D, Grasby P, Frackowiak R S J, Dolan R J. Nature (London) 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 26.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logothetis N K, Pauls J, Augath M, Trinath T, Oeltermann A. Nature (London) 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]