Abstract
The bacterial endosymbiont Wolbachia has been used to control insect pests owing to its ability to manipulate their life history and suppress infectious diseases. Therefore, knowledge on Wolbachia dynamics in natural populations is fundamental. The European cherry fruit fly, Rhagoletis cerasi, is infected with the Wolbachia strain wCer2, mainly present in southern and central European populations, and is currently spreading into wCer2-uninfected populations driven by high unidirectional cytoplasmic incompatibility. Here, we describe the distribution of wCer2 along two transition zones where the infection is spreading into wCer2-uninfected R. cerasi populations. Fine-scale sampling of 19 populations in the Czech Republic showed a smooth decrease of wCer2 frequency from south to north within a distance of less than 20 km. Sampling of 12 Hungarian populations, however, showed a sharp decline of wCer2 infection frequency within a few kilometres. We fitted a standard wave equation to our empirical data and estimated a Wolbachia wave speed of 1.9 km yr−1 in the Czech Republic and 1.0 km yr−1 in Hungary. Considering the univoltine life cycle and limited dispersal ability of R. cerasi, our study highlights a rapid Wolbachia spread in natural host populations.
Keywords: endosymbiont, European cherry fruit fly, modelling, cytoplasmic incompatibility
1. Introduction
Wolbachia is an endosymbiotic bacterium that is present in a wide range of arthropod and nematode species and can alter the reproduction of its host [1]. Being maternally inherited, Wolbachia is able to modify the reproduction of its host to its own advantage. The most efficient way is the induction of cytoplasmic incompatibility (CI) which results in embryonic mortality when the sperm of an infected male fertilizes the egg of a female that is not infected or is infected with a different Wolbachia strain [2]. Infected females, in contrast, produce viable offspring with both infected and uninfected males. This results in a reproductive advantage of infected over uninfected females and facilitates the spread of Wolbachia through host populations [3]. Although horizontal transmission within and among species is possible [4–6], Wolbachia mainly spreads vertically from females to their offspring via the egg cytoplasm [7].
Predicting Wolbachia spread through natural host populations is of considerable importance to understand how this bacterium invades new territory. Important parameters that influence the infection dynamics of Wolbachia are the strength of CI, the efficacy of maternal transmission, fitness effects on its host and the reproductive and dispersal potential of its host species [8]. The spread of the Wolbachia strain wRi in Drosophila simulans in California [3] and wAu in the same species over the eastern coast of Australia [8] are the best-studied examples of rapid Wolbachia spread in natural populations. These studies show that Wolbachia is able to provide fitness benefits to its host, enhancing the spatial spread from low initial infection frequencies [8]. By contrast, fecundity costs can prevent a range expansion of the endosymbiont. In this case, Wolbachia spreads as a bistable wave where a certain threshold frequency is necessary to get established [9]. This has been shown in Aedes aegypti artificially transinfected with wMel, where the Wolbachia infection causes fitness costs to its host that limit the spread of released populations [10].
The European cherry fruit fly, Rhagoletis cerasi, is an important agricultural pest of cherries that is distributed throughout Europe [11]. This tephritid is infected with at least five different Wolbachia strains [12,13]. All populations share one common strain, wCer1, whereas a second strain, wCer2, is mainly present in southern and central European populations [12]. This strain causes a high degree of CI between wCer2-infected males and wCer2-uninfected females, with egg mortality rates of up to 98% [14], and is currently spreading in central Europe [15].
The cherry fruit fly system provides an excellent model to study the invasion dynamics of Wolbachia: First, R. cerasi has a univoltine life cycle that allows an in-depth characterization of an ongoing spatial Wolbachia spread in natural populations. Second, dispersal rates of the fly are limited, with an average estimate of 200 m yr−1 and a few long distance dispersers migrating about 4 km yr−1 [16]. This allows the study of Wolbachia range expansion on a small geographical scale. Here, we characterize the infection frequency of wCer2 along two transition zones: one along a south–north axis in the Moravian region of the Czech Republic and the other along a west–east axis found in northern Hungary. We use a standard Barton–Turelli wave model [9] to estimate R. cerasi adult dispersal potential and approximate the width and speed of the Wolbachia travelling wave. Our results highlight a rapid ongoing Wolbachia spread in natural populations of R. cerasi.
2. Material and methods
(a). Collection and genetic analysis
Larvae and pupae of R. cerasi were collected in 2015 in Austria and the Czech Republic and in 2016 in Hungary from Prunus avium. All populations from each transect were sampled on the same day and each population was sampled from a single tree. Samples were stored in absolute ethanol at −20°C. Five hundred and forty-eight individuals of R. cerasi were collected along a south–north transect of 46 km from one population in Austria (CZ-1) and 18 populations in the Czech Republic (CZ-2 to CZ-19). Furthermore, 336 individuals were collected from 12 populations in Hungary (HU-1 to HU-12) along a 72 km west–east transect (figure 1; electronic supplementary material, S1). DNA was extracted using the GenElute Mammalian Genomic DNA miniprep kit (Sigma-Aldrich, St Louis, MO, USA). Wolbachia screening was performed on all collected samples using diagnostic polymerase chain reaction (PCR) with strain-specific primers targeting specific fragments of the Wolbachia surface protein gene (wsp) [13]. Since wCer1 is fixed in R. cerasi, wCer1-specific primers were used as positive controls for DNA quality.
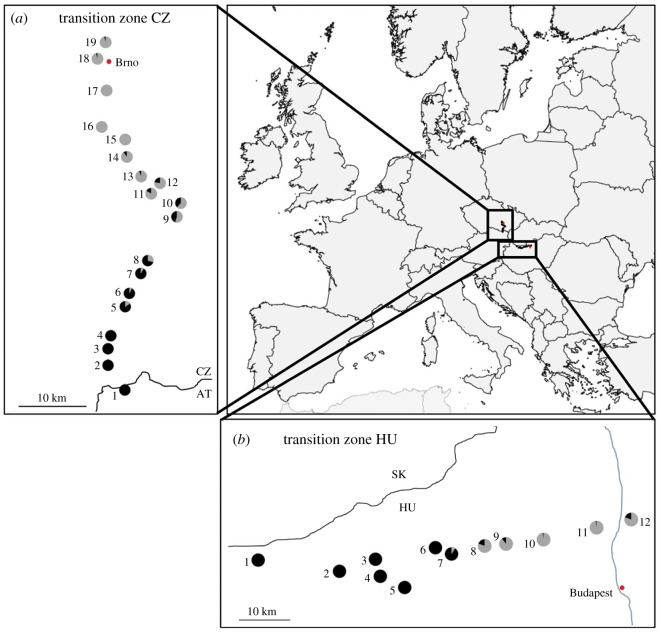
Figure 1.
Geographical location and spatial distribution of the two wCer2 transition zones of Rhagoletis cerasi. Each pie chart represents a single Prunus avium tree with ratio of wCer2-infected (black) and wCer2-uninfected (grey) individuals of R. cerasi in the Czech transition zone in 2015 (a) and in the Hungarian transition zone in 2016 (b). (Online version in colour.)
(b). Wave parameters
The spatial distribution of Wolbachia was modelled by a standard nonlinear equation that describes Wolbachia spread as a traveling wave [3]. Using the standard wave model, we estimated the adult fly dispersal potential and the Wolbachia wave width and speed in the Czech Republic and in Hungary (see details in electronic supplementary material, S2).
3. Results
(a). wCer2 infection frequencies
In the Czech transition zone, the four southernmost populations CZ-1 to CZ-4 were completely wCer2-infected. Populations CZ-5 and CZ-6 had high wCer2 infection frequencies of 85 and 93%, respectively. Similarly, 92% of the individuals from CZ-7 and 71% of the individuals from CZ-8 were wCer2-infected. A medium infection frequency was found in populations CZ-9 and CZ-10, with a wCer2 infection rate of 45 and 38%, respectively. Relatively low infection frequencies were found in CZ-11, with 17%, CZ-12 with 22%, CZ-13 with 6% and CZ-14 with 10% wCer2-infected individuals, and three populations further north (CZ-15 to CZ-17) were completely wCer2-uninfected. The two northernmost populations, CZ-18 and CZ-19, however, showed a wCer2 infection frequency of 4%, i.e. two wCer2-infected individuals (n = 48) in each population (figure 1; electronic supplementary material, S1).
In the Hungarian transition zone, the six westernmost populations (HU-1 to HU-6) were completely wCer2-infected. In HU-7 91% of the individuals were wCer2-infected, while 7 km further east in HU-8, wCer2 was present in just 20% of the individuals. In HU-9 a wCer2 infection rate of 11% was found, while HU-10 and HU-11 both had a low infection frequency of 2%. In the most eastern population, HU-12, 20% of the individuals were wCer2-infected (figure 1 and electronic supplementary material, S1).
(b). Wave parameters
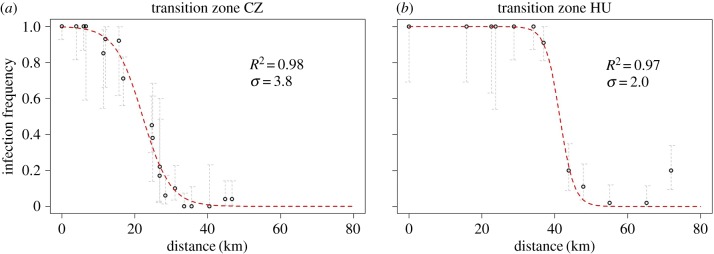
A nonlinear least-squares fit showed that the standard wave model fits well to our wCer2 infection frequency data with an R2 value of 0.98 in the Czech Republic and 0.97 in Hungary (figure 2). Rhagoletis cerasi adult dispersal (σ) estimated from the least-squares best fit was 3.8 km in the Czech Republic and 2.0 km in Hungary (figure 2). By using these two dispersal parameter values we estimated a wave width of 11.4 km and a wave speed of 1.9 km yr−1 in the Czech Republic, while in Hungary, we estimated a wave width of 5.8 km and a wave speed of 1.0 km yr−1.
Figure 2.
Predicted and observed wCer2 infection frequencies in the two transition zones of R. cerasi in the Czech Republic (a) and Hungary (b). Grey bars represent the 95% confidence intervals of infection frequency. (Online version in colour.)
4. Discussion
The classical textbook examples of Wolbachia spread in nature are the invasion of wRi in D. simulans populations in California [3] and Australia [8], and the release of artificially Wolbachia-infected Ae. aegypti mosquitos in Australia [10,17]. These studies showed how different Wolbachia-induced fitness effects influence its establishment and spread: while fitness costs hinder low-frequency infections from establishment and spread of Wolbachia in Ae. aegypti populations [10], positive fitness effects of Wolbachia resulted in a rapid spread of wRi in D. simulans of about 100 km yr−1 [3,8]. The dispersal rate of wMel in artificially transinfected mosquitoes in Australia, however, was multiple orders of magnitude lower, with a spatial spread of 100–200 m yr−1 [10].
The Wolbachia strain wCer2 has been shown to spread in R. cerasi within Central Europe, where the infection dynamics of its invasion were determined on a large scale [15]. Here we characterized the wCer2 frequency and estimated its spatial spread along two fine-scale transects of R. cerasi. We estimated an R. cerasi adult dispersal rate of 3.8 km per generation in the Czech Republic and 2.0 km per generation in Hungary. Differences in the estimated migration rates might have been influenced by dissimilarities in the landscape and the presence of hosts between the two transects. The estimated adult dispersal rate is in line with a capture–release maximal dispersal estimation of 4 km per generation [16]. We estimated a wave width of 11.4 km in the Czech Republic and 5.8 km in Hungary. This is in stark contrast to an estimated wave width of 170–260 km in a German wCer2 transition zone that might be influenced by long-dispersal migration of the fly [15]. The Wolbachia wave speed was estimated to be 1.9 km yr−1 in the Czech Republic and 1.0 km yr−1 in Hungary. Considering the univoltine biology of the fly, wCer2 is spreading with a rate of 1.9 km per generation in the Czech Republic and 1.0 km per generation in Hungary.
The infection frequency of wCer2 in the Hungarian transition zone has already been studied, in 1999, [12] and allowed a direct comparison with our data from 2016. Since the wave speed is defined as the distance travelled by an intermediate infection frequency over time (e.g. 50% infection rate), we measured the longitudinal distance between populations infected with greater than 50% in 1999 (HU-1; [12]) and 2016 (HU-7). HU-1 was 100% infected in 1999 while in 2016 wCer2 was present in 91% of the individuals from HU-7, 36 km further east, resulting in an estimated wave speed of 2.0 km yr−1. Although, we consider that this rough estimation might have overestimated the spread of wCer2 in Hungary, this direct comparison supports our estimated fast spatial spread of Wolbachia. Repeated fine-scale samplings over different years are needed to refine the estimated temporal and spatial dynamics of the wCer2 spread. In summary, considering the univoltine biology and the low dispersal rate of this fly, our study represents a new example of a rapid Wolbachia spread in nature.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Ferenc Lakatos, Zoltan Kovacs, Josef Janoušek and Petr Martinek for their help with sample collections and Susanne Krumböck for technical assistance.
Data accessibility
Data are provided in the electronic supplementary material.
Authors' contributions
C.S. and H.S. designed the project. V.B., M.S., H.S. and C.S. conducted fieldwork. V.B. performed the laboratory work. Modelling was done by V.B. and A.T. and all authors were involved in writing. All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, and revising the manuscript critically for important intellectual content and gave final approval of the version to be published. All authors agree to be held accountable for the content of the article.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was funded by the Austrian Science Fund (FWF) project P26749-B25 to C.S. H.S. was supported by FWF project J-3527-B22.
References
- 1.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Micro. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann AA, Turelli M. 1997. Cytoplasmatic incompatibility in insects. In Influential passengers: inherited microorgansisms and arthropod reproduction (eds SL O'Neill, AA Hoffmann, JH Werren), pp. 42–80. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Turelli M, Hoffmann AA. 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353, 440–442. ( 10.1038/353440a0) [DOI] [PubMed] [Google Scholar]
- 4.O'Neill SL, Giordano R, Colbert A, Karr T, Robertson H. 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl Acad. Sci. USA 89, 2699–2702. ( 10.1073/pnas.89.7.2699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuler H, et al. 2013. Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic Rhagoletis cerasi (Diptera: Tephritidae) to invasive Rhagoletis cingulata in Europe. Mol. Ecol. 22, 4101–4111. ( 10.1111/mec.12362) [DOI] [PubMed] [Google Scholar]
- 6.Baldo L, Ayoub NA, Hayashi CY, Russell JA, Stahlhut JK, Werren JH. 2008. Insight into the routes of Wolbachia invasion: high levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol. Ecol. 17, 557–569. ( 10.1111/j.1365-294X.2007.03608.x) [DOI] [PubMed] [Google Scholar]
- 7.Engelstädter J, Hurst GDD. 2009. The ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. Syst. 40, 127–149. ( 10.1146/annurev.ecolsys.110308.120206) [DOI] [Google Scholar]
- 8.Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR. 2013. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog. 9, e1003607 ( 10.1371/journal.ppat.1003607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton NH, Turelli M. 2011. Spatial waves of advance with bistable dynamics: cytoplasmic and genetic analogues of Allee effects. Am. Nat. 178, E48–E75. ( 10.1086/661246) [DOI] [PubMed] [Google Scholar]
- 10.Schmidt TL, et al. 2017. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 15, e2001894 ( 10.1371/journal.pbio.2001894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boller E, Prokopy R. 1976. Bionomics and management of Rhagoletis. Annu. Rev. Entomol. 21, 223–246. ( 10.1146/annurev.en.21.010176.001255) [DOI] [Google Scholar]
- 12.Riegler M, Stauffer C. 2002. Wolbachia infections and superinfections in cytoplasmically incompatible populations of the European cherry fruit fly Rhagoletis cerasi (Diptera, Tephritidae). Mol. Ecol. 11, 2425–2434. ( 10.1046/j.1365-294X.2002.01614.x) [DOI] [PubMed] [Google Scholar]
- 13.Arthofer W, Riegler M, Schneider D, Krammer M, Miller WJ, Stauffer C. 2009. Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae). Mol. Ecol. 18, 3816–3830. ( 10.1111/j.1365-294X.2009.04321.x) [DOI] [PubMed] [Google Scholar]
- 14.Boller E, Bush GL. 1974. Evidence for genetic variation in populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera: Tephritidae) based on physiological parameters and hybridization experiments. Entomol. Exp. Appl. 17, 279–293. ( 10.1111/j.1570-7458.1974.tb00345.x) [DOI] [Google Scholar]
- 15.Schuler H, et al. 2016. The hitchhiker's guide to Europe: the infection dynamics of an ongoing Wolbachia invasion and mitochondrial selective sweep in Rhagoletis cerasi. Mol. Ecol. 25, 1595–1609. ( 10.1111/mec.13571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boller E, Remund U. 1982. Field feasibility study for the application of SIT in Rhagoletis cerasi L. in northwest Switzerland (1976–79). In Fruit Flies of Economic Importance. Proc. CEC/IOBC Int. Symp. Athens, November 1982 (ed. Calvalloro R.), pp. 366–370. Rotterdam, The Netherlands: Balkema. [Google Scholar]
- 17.Hoffmann AA, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457. ( 10.1038/nature10356) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided in the electronic supplementary material.