Abstract
Elevated prolactin (PRL) has been associated with the expression of social and cooperative behaviours in a number of vertebrate species, as well as suppression of reproduction. As social mole-rats exhibit both of these traits, PRL is a prime candidate in mediating their social phenotype. While naked and Damaraland mole-rats (NMRs and DMRs) have evolved eusociality independently within their family, both species exhibit an extreme skew in lifetime reproductive success, with breeding restricted to a single female and one or two males. Non-breeding NMRs of both sexes are physiologically inhibited from reproducing, while in DMRs only the non-breeding females are physiologically suppressed. Newly emerging work has implicated the dopamine system and PRL as a component in socially induced reproductive suppression and eusociality in NMR, but the DMR remains unstudied in this context. To investigate evolutionary convergence in the role of PRL in shaping African mole-rat eusociality, we determined plasma PRL concentrations in breeders and non-breeders of both sexes, comparing DMRs with NMRs. Among samples from non-breeding NMRs 80% had detectable plasma PRL concentrations. As a benchmark, these often (37%) exceeding those considered clinically hyperprolactinaemic (25 ng ml−1) in humans: mean ± s.e.m.: 34.81 ± 5.87 ngml−1; range 0.00–330.30 ng ml−1. Conversely, 85% of non-breeding DMR samples had undetectable values and none had concentrations above 25 ng ml−1: 0.71 ± 0.38 ng ml−1; 0.00–23.87 ngml−1. Breeders in both species had the expected variance in plasma PRL concentrations as part of normal reproductive function, with lactating queens having significantly higher values. These results suggest that while elevated PRL in non-breeders is implicated in NMR eusociality, this may not be the case in DMRs, and suggests a lack of evolutionary convergence in the proximate control of the social phenotype in these mole-rats.
Keywords: prolactin, African mole-rats, reproductive suppression, cooperative breeding
1. Introduction
Prolactin (PRL) has the potential to play a key role in a number of components of mammalian sociality and cooperative breeding. Elevated PRL has been associated with the expression of social and cooperative behaviours in both sexes in a number of vertebrates [1–4]. A principal feature of cooperative breeding strategies is a reproductive division of labour, in some cases maintained by a socially induced suppression of fertility [5]. Elevated circulating PRL (hyperprolactinemia) is well known to be a major cause of infertility in mammals (males and females), and mediates natural suppression of reproduction that occurs during lactation [6,7]. Owing to medical implications, elevated PRL has been well studied in humans, where PRL concentrations above 25 ng ml−1 for women and 20 ng ml−1 for men are considered as clinical hyperprolactinemia [8].
Naked and Damaraland mole-rats (NMRs and DMRs) are divergent species within the African mole-rat clade, and have convergently evolved highly social cooperative breeding systems similar to those seen in eusocial insects [5]. A characteristic of these societies is an extreme reproductive division of labour and skew in lifetime reproductive success, with breeding restricted to a single female and one or two males, with more than 90% of individuals never having the opportunity to reproduce [8]. While non-breeding NMRs of both sexes are physiologically inhibited from reproducing, in DMRs only non-breeding females are physiologically suppressed. Differences between species in the underlying mechanism of female suppression are also evident, as the ovaries of non-breeding NMRs are prepubescent, while those of DMRs are fully developed [5,9–12]. In both species, socially induced physiological suppression is mediated centrally via the hypothalamic GnRH system, with GnIH (RF amide-related protein 3) and kisspeptin also implicated in the mechanism in NMRs [13–15]. Newly emerging work has implicated the dopamine system and raised levels of PRL in non-breeders as another potential component in reproductive suppression and eusociality in NMRs [16]. Despite the potential for PRL in playing a part in cooperative breeding in African mole-rats, either in suppression of reproduction, or in expression of alloparental behaviour, its possible role has been largely unexplored. This study aims to compare and contrast plasma PRL concentrations in eusocial DMRs with NMRs, examining breeders and non-breeders of both sexes. We predict that if the proximate control of sociality in these species has, together with a eusocial lifestyle, convergently evolved in both species, then plasma PRL should be elevated in non-breeders in both NMRs and DMRs.
2. Material and methods
(a). Sampling
Blood samples were obtained from 112 NMRs (two lactating and 10 non-lactating queens, seven breeding males, 51 non-breeding females and 42 non-breeding males) from among 13 captive colonies from the University of Pretoria and Queen Mary University of London. In total, 132 DMRs were sampled (six lactating and 18 non-lactating queens, 20 breeding males, 41 non-breeding females and 47 non-breeding males), from among 31 colonies from Winton and the Kalahari Research Centre, South Africa. The blood was centrifuged at 500g and the resulting plasma decanted and stored at −80°C until hormone analysis. Further details are given in the electronic supplementary material, tables S1 and S2.
(b). Prolactin assay
Plasma PRL concentrations were validated for mole-rats and determined using a commercial enzyme-linked immunosorbent assay (Elabscience© guinea pig prolactin ELISA kit, Catalogue no. E-EL-GP0358) according to the manufacturer's instructions. Data were analysed using a general linear model and results plotted using R statistical software [17,18] (see the electronic supplementary material).
(c). Results
The mean, 95% highest density intervals values and a density plot of plasma PRL concentrations for status groups in both species are displayed in figure 1. For DMRs, only 13 of 88 non-breeders of both sexes (15%) had detectable concentrations of PRL, and of these none had values exceeding 25 ng ml−1 (that would be considered as clinically hyperprolactinemic; see the electronic supplementary material for justification of the use of this value as a benchmark): non-breeding females (mean ± s.e.m.), 0.65 ± 0.58 ng ml−1; n = 41; range, less than 0.03–23.87 ng ml−1; non-breeding males, 0.77 ± 0.49 ng ml−1; n = 47; range, less than 0.03–20.22 ng ml−1. For breeding queens (six of which were lactating), 13 out of the 24 samples had detectable PRL values: 4.98 ± 2.20 ng ml−1; range less than 0.03–47.65 ng ml−1. In breeding males only three out of 20 samples had detectable concentrations of PRL: 0.12 ± 0.07 ng ml−1; range less than 0.03–1.19 ng ml−1. When the DMR data were analysed separately to the NMRs, the difference between breeders and non-breeders was significant (F = 4.530; d.f. = 1, 124; p = 0.0353; figure 1; electronic supplementary material).
Figure 1.
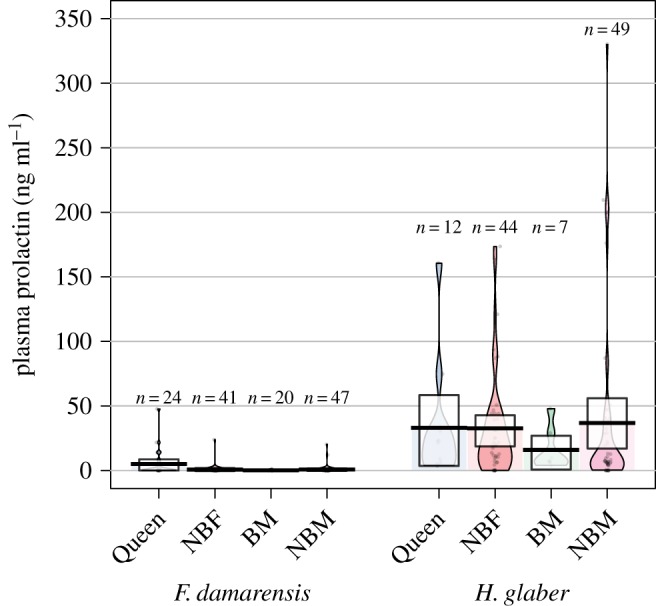
Pirate plot showing plasma PRL concentrations for Damaraland and naked mole-rats, respectively, by reproductive status: BM, breeding male; NBF, non-breeding female; NBM, non-breeding male; Q, breeding queen. Raw data for each group are displayed as an open circle with the points randomly jittered horizontally for ease of viewing, together with the densities (how crowded or sparse the data are at every possible value) shaded in colour. The mean is shown as a dark horizontal line, with boxes showing the 95% highest density intervals of the mean of each group (the interval spanning 95% of the distribution [18]). (Online version in colour.)
By contrast, 75 of 93 non-breeding NMRs (80%) had detectable concentrations of PRL, and these often reached distinctly higher levels than DMRs (F = 50.325; d.f. = 1, 236; p = 1.51 × 10−11). None of the interactions in the GLM were significant at α = 0.05 (electronic supplementary material). Thirty-seven per cent exceeded concentrations of greater than 25 ng ml−1: non-breeding females 32.64 ± 6.13 ng ml−1; n = 43; range, less than 0.03–23.87 ng ml−1; non-breeding males 36.77 ± 9.81 ng ml−1; n = 4; range, less than 0.03–330.30 ng ml−1. As with DMRs, breeding queens had the expected variance in plasma PRL concentrations as part of normal reproductive function (ovarian cyclicity, pregnancy and lactation): 33.02 ± 12.94 ng ml−1; n = 12; range, 3.60–160.80 ng ml−1. Two values were obtained from lactating queens, 21.14 ng ml−1 (23 days post-partum, at the end of the period of lactation) and 160.80 ng ml−1 (7 days post-partum), the latter being the highest concentration recorded among the breeding female samples. Only a small number of breeding males were sampled as they are often difficult to identify with certainty, and these had comparatively low plasma PRL concentrations, 15.91 ± 6.21 ng ml−1; n = 7; range, 3.92–47.92 ng ml−1.
Analysis of breeding queens revealed an overall significant increase in PRL in lactating versus non-lactating females (F = 11.22; d.f. = 1, 32; p = 0.002), and a significant interaction (F = 7.33; d.f. = 1, 32; p= 0.011), indicating a species difference (although only two samples were obtained from lactating NMRs; see electronic supplementary material, figure S2).
3. Discussion
Elevated PRL is well known to suppress fertility, and has also been implicated in many studies (including birds and mammals) as a factor mediating both parental and alloparental care, a key feature of cooperatively breeding societies, together with affiliative and other sociosexual behaviours (e.g. [1–4] and references therein). For example, in a study of zebra finches, plasma PRL was found to be positively correlated with parental behaviour, the number of chicks hatched and chick survival [19]. Further, in one of the classic examples of avian cooperative breeding, non-breeding Florida scrub jay helpers (Aphelocoma coerulescens) have been shown to have PRL levels that positively correlated with the rate of their nestling provisioning [1]. In one of the relatively few studies of mammalian cooperative breeders, elevated levels of PRL in male meerkats preceded bouts of helping behaviour in the form of babysitting [4].
Patterns of plasma PRL differed markedly between DMRs and NMRs. As expected for a reproductively active mammal, circulating PRL was detected in breeding queens in both species, with high values recorded during pregnancy and lactation (electronic supplementary material, tables S1 and S2; figure S2). However, most non-breeding NMRs (80%) of both sexes had detectable plasma PRL concentrations, often exceeding those considered clinically hyperprolactinaemic. Conversely, 85% of non-breeding DMR samples of both sexes had undetectable values and none reached clinically high values. These results suggest that while elevated PRL in non-breeders may be an important component in NMR eusociality, this is apparently not the case in convergently eusocial DMRs.
Recent transcriptome profiling of the NMR brain has shown that breeding animals have increased expression of genes involved in dopamine metabolism, (dopamine inhibits PRL secretion), compared with non-breeders. This is consistent with the observed high levels of PRL in non-breeding NMRs and strongly suggests a role for hyperprolactinaemia as a component in socially induced reproductive suppression [16]. The highly elevated levels of plasma PRL observed in non-breeding NMRs could function to inhibit the release of GnRH, and thus LH, FSH, oestrogen and testosterone, leading to the well-characterized block to follicular development (in females) and spermatogenesis (in males). In addition to a possible role in suppressing reproduction, it is tempting to speculate that elevated PRL in NMRs may also play a part in the mechanisms eliciting cooperative behaviour. It is, therefore, intriguing that apart from the breeding queen, almost all monitored DMRs in this study had little or no detectable PRL. This may reflect the fact that unlike NMRs, the ovaries of female DMRs have varying levels of follicular development, although they also do not ovulate.
In this first major cross-species study of PRL in African mole-rats, our data suggest that divergent mechanisms have evolved in a role for this hormone in convergently eusocial mole-rats. At present, the results, although highly persuasive, are of an associative nature and functional studies on both species are now needed to confirm cause and effect, and to establish the mechanistic details.
Supplementary Material
Acknowledgements
We thank Northern Cape Nature Conservation for permits to trap animals at Winton, and Tim Clutton-Brock, Philippe Vullioud and the Kalahari Research Centre. P.V. also organized the sample collection and commented on the draft manuscript. Thanks also to Steve Le Comber and Richard Nichols for advice and help with statistical analysis.
Ethics
All procedures involving live animals and sample collection described in this manuscript were conducted in accordance with appropriate national and provincial guidelines, permits and regulations. The protocol used in this study was approved by the animal ethics committee of the University of Pretoria EC0 84-15.
Data accessibility
All raw data are supplied in the electronic supplementary material.
Authors' contributions
N.C.B. and C.G.F. conceptualised the project. N.C.B., J.U.M.J., M.Z. and C.G.F. collected samples. S.G., A.G. and C.G.F. undertook the assays. C.G.F. analysed the data and C.G.F. and N.C.B. refined several drafts of the manuscript. All authors contributed towards revision of the article, approve the final version of the manuscript, and agree to be held accountable for the content therein.
Competing interests
The authors have no competing interests to declare.
Funding
This study was funded by SARCHI Chair to N.C.B. (grant no. 64756). The study population at the KRC was maintained by a European Research Council grant (294494) to Tim Clutton-Brock.
References
- 1.Schoech SJ, Mumme RL, Wingfield JC. 1996. Prolactin and helping behaviour in the cooperatively breeding Florida scrub-jay, Aphelocoma c. coerulescens. Anim. Behav. 52, 445–456. ( 10.1006/anbe.1996.0189) [DOI] [Google Scholar]
- 2.Angelier F, Chastel O. 2009. Stress, prolactin and parental investment in birds: a review. Gen. Comp. Endocrinol. 163, 142–148. ( 10.1016/j.ygcen.2009.03.028) [DOI] [PubMed] [Google Scholar]
- 3.Snowdon CT, Ziegler TE. 2015. Variation in prolactin is related to variation in sexual behavior and contact affiliation. PLoS ONE 10, e0120650 ( 10.1371/journal.pone.0120650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson AA, Russell AF, Young AJ, Jordan NR, McNeilly AS, Parlow AF, Clutton-Brock T. 2006. Elevated prolactin levels immediately precede decisions to babysit by male meerkat helpers. Horm. Behav. 50, 94–100. ( 10.1016/j.yhbeh.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 5.Faulkes CG, Bennett NC. 2013. Plasticity and constraints on social evolution in African mole-rats: ultimate and proximate factors. Phil. Trans. R. Soc. B 368, 1618 ( 10.1098/rstb.2012.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RS, Herbison AE, Grattan DR. 2014. Prolactin regulation of kisspeptin neurones in the mouse brain and its role in the lactation-induced suppression of kisspeptin expression. J. Neuroendocrinol. 26, 898–908. ( 10.1111/jne.12223) [DOI] [PubMed] [Google Scholar]
- 7.Kauppila A, Martikainen H, Puistola U, Reinila M, Ronnberg L. 1988. Hypoprolactinemia and ovarian function. Fertil. Steril. 49, 437–441. ( 10.1016/S0015-0282(16)59769-6) [DOI] [PubMed] [Google Scholar]
- 8.Majumdar A, Mangal NS. 2013. Hyperprolactinemia. J. Hum. Reprod. Sci. 6, 168–175. ( 10.4103/0974-1208.121400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faulkes CG, Abbott DH, Jarvis JUM. 1990. Social suppression of ovarian cyclicity in wild colonies of naked mole-rats, Heterocephalus glaber. J. Reprod. Fertil. 88, 559–568. ( 10.1530/jrf.0.0880559) [DOI] [PubMed] [Google Scholar]
- 10.Faulkes CG, Abbott DH, Jarvis JUM. 1991. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J. Reprod. Fertil. 91, 593–604. ( 10.1530/jrf.0.0910593) [DOI] [PubMed] [Google Scholar]
- 11.Faulkes CG, Trowell SN, Jarvis JUM, Bennett NC. 1994. Investigation of sperm numbers and motility in reproductively active and socially suppressed males of two eusocial African mole-rats, the naked mole-rat (Heterocephalus glaber), and the Damaraland mole-rat (Cryptomys damarensis). J. Reprod. Fertil. 100, 411–416. ( 10.1530/jrf.0.1000411) [DOI] [PubMed] [Google Scholar]
- 12.Bennett NC. 1994. Reproductive suppression in social Cryptomys damarensis colonies—a lifetime of socially-induced sterility in males and females. J. Zool. Lond. 234, 25–39. ( 10.1111/j.1469-7998.1994.tb06054.x) [DOI] [Google Scholar]
- 13.Bennett NC, Jarvis JUM, Faulkes CG, Millar RP. 1993. LH responses to single doses of exogenous GnRH by freshly captured Damaraland mole-rats, Cryptomys damarensis. J. Reprod. Fertil. 99, 81–86. ( 10.1530/jrf.0.0990081) [DOI] [PubMed] [Google Scholar]
- 14.Peragine DE, Pokarowski M, Mendoza-Viveros L, Swift-Gallant A, Cheng H-YM, Bentley GE, Holmes MM. 2017. RFamide-related peptide-3 (RFRP-3) suppresses sexual maturation in a eusocial mammal. Proc. Natl Acad. Sci. USA 114, 1207–1212. ( 10.1073/pnas.1616913114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Holmes MM, Forger NG, Goldman BD, Lovern MB, Caraty A, Kallo I, Faulkes CG, Coen CW. 2013. Socially regulated reproductive development: analysis of GnRH-1 and kisspeptin neuronal systems in cooperatively breeding naked mole-rats (Heterocephalus glaber). J. Comp. Neurol. 521, 3003–3029. ( 10.1002/cne.23327) [DOI] [PubMed] [Google Scholar]
- 16.Mulugeta E, Marion-poll L, Gentie D, Ganswindt SB, Ganswindt A, Bennett NC, Blackburn EH, Faulkes CG, Heard E. 2017. Molecular insights into the pathways underlying naked mole-rat eusociality. bioRxiv 209932 ( 10.1101/209932) [DOI] [Google Scholar]
- 17.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 18.Kampstra P. 2008. Beanplot: a boxplot alternative for visual comparison of distributions. J. Stat. Softw. 28, 1–9. (http://www.jstatsoft.org/v28/c01/)27774042 [Google Scholar]
- 19.Smiley KO, Adkins-Regan E. 2016. Prolactin is related to individual differences in parental behavior and reproductive success in a biparental passerine, the zebra finch (Taeniopygia guttata). Gen. Comp. Endocrinol. 234, 88–94. ( 10.1016/j.ygcen.2016.03.006) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data are supplied in the electronic supplementary material.


