Abstract
Setting: Community health workers (CHWs) increasingly deliver community-based human immunodeficiency virus (HIV) counselling and testing (HCT) services. Less is known about how this strategy performs when integrated with household tuberculosis (TB) contact investigations.
Objective: We conducted a prospective mixed-methods study to evaluate the feasibility and quality of CHW-facilitated, home-based HCT among household TB contacts.
Design: CHWs visited households of consenting TB patients to screen household contacts for TB and HIV. They performed HIV testing using a serial enzyme-linked immunosorbent assay rapid-antibody testing algorithm. Laboratory technicians at health facilities re-tested the samples and coordinated quarterly HIV panel testing for CHWs. We conducted focus group discussions (FGDs) with CHWs on their experiences in carrying out home-based HCT.
Results: Of 114 household contacts who consented to and underwent HIV testing by CHWs, 5 (4%) tested positive, 108 (95%) tested negative, and 1 (1%) had indeterminate results; 110 (96%) samples had adequate volume for re-testing. Overall agreement between CHWs and laboratory technicians was 99.1% (κ = 0.90, 95%CI 0.71–1.00, P < 0.0001). In FGDs, CHWs described context-specific social challenges to performing HCT in a household setting, but said that their confidence grew with experience.
Conclusion: Home-based HCT by CHWs was feasible among household TB contacts and produced high-quality results. Strategies to address social challenges are required to optimize yield.
Keywords: tuberculosis, community health workers, home-based HIV testing
Abstract
Contexte : Les travailleurs de santé communautaire (CHW) offrent de plus en plus de services de conseil et de test communautaires relatifs au virus de l'immunodéficience humaine (HCT). On sait moins quel est le degré de performance de cette stratégie quand elle est intégrée à des visites à domicile à la recherche de contacts de tuberculose (TB).
Objectif : Nous avons réalisé une étude prospective à méthodes variées afin d'évaluer la faisabilité et la qualité de CHW à domicile, facilité par des CHW dans les foyers des contacts de TB.
Schéma : Les CHW ont visité les foyers des patients TB consentants afin de dépister les contacts domiciliaires de TB et du virus de l'immunodéficience humaine (VIH). Ils ont réalisé des tests VIH grâce à un algorithme de test rapide de recherche d'anticorps en série par titrage avec immunoadsorbant lié à une enzyme. Les techniciens de laboratoire des structures de santé ont re-testé les échantillons et coordonné un test VIH en groupe trimestriel pour les CHW. Nous avons réalisé des discussions en groupe focal (FGD) avec les CHW à propos de leurs expériences de HCT à domicile.
Résultats : Ont été consentants 114 contacts domiciliaires qui ont été testés à la recherche du VIH par les CHW : 5 (4%) ont eu un test positif, 108 (95%) ont eu un test négatif et 1 seul (1%) a eu des résultats indéterminés ; 110 (96%) échantillons avaient un volume suffisant pour un deuxième test. Au total, l'accord entre les CHW et les techniciens de laboratoire a été de 99,1% (κ = 0,90 ; IC95% 0,71–1,00 ; P < 0,0001). Lors des FGD, les CHW ont décrit les défis sociaux spécifiques du contexte de la réalisation du HCT dans le cadre d'un foyer, mais ont affirmé que leur confiance en eux avait augmenté avec leur expérience.
Conclusion : Le HCT à domicile par les CHW s'est avéré faisable parmi les contacts domiciliaires de TB et a produit des résultats de très bonne qualité. Des stratégies visant à résoudre les défis sociaux sont requises afin d'optimiser le rendement.
Abstract
Marco de referencia: Los agentes de salud comunitarios (CHW) prestan cada vez con mayor frecuencia servicios de asesoramiento y pruebas de detección del virus de la inmunodeficiencia humana (HCT) en las comunidades. Se conoce poco sobre la eficacia de esta estrategia cuando se integra en la investigación de contactos domiciliarios de los pacientes con tuberculosis (TB).
Objetivo: Se llevó a cabo un estudio prospectivo con métodos mixtos, con el objeto de evaluar la factibilidad y la calidad de los servicios de HCT prestados por los CHW a los contactos de los casos de TB en los hogares.
Método: Los CHW visitaron los hogares de los pacientes con TB que dieron su consentimiento, con el fin de realizar el HCT en los contactos domiciliarios. Los CHW practicaron la investigación de la infección por el virus de la inmunodeficiencia humana (VIH) mediante un algoritmo de pruebas rápidas seriadas de anticuerpos de tipo inmunoabsorbente ligado a la enzima. Los auxiliares de laboratorio en los establecimientos de salud practicaban de nuevo las pruebas en las muestras y coordinaban la realización trimestral de series de pruebas por parte de los CHW. Se realizaron sesiones de grupos de opinión (FGD) con estos profesionales, a fin de compartir sus experiencias en HCT en los hogares.
Resultados: Tras recibir su consentimiento, los CHW practicaron las pruebas del VIH a 114 contactos domiciliarios. Cinco contactos obtuvieron un resultado positivo (4%), en 108 el resultado fue negativo (95%) y en un caso el resultado fue indeterminado (1%). El volumen de 110 muestras (96%) fue suficiente para repetir las pruebas. La concordancia global entre los CHW y los auxiliares de laboratorio fue 99,1% (κ = 0,90; IC95% 0,71–1,00; P < 0,0001). En las FGD, los CHW describieron las dificultades sociales específicas del contexto que tuvieron que afrontar al prestar estos servicios en los hogares, pero afirmaron que con la práctica habían adquirido mayor confianza.
Conclusión: La práctica domiciliaria del HCT a los contactos de los casos de TB por parte de los CHW fue factible y se obtuvieron resultados de gran calidad. Se precisan estrategias que respondan a las dificultades sociales encontradas con el propósito de optimizar el rendimiento.
Efforts to integrate diagnostic evaluation and linkage to care for human immunodeficiency virus (HIV) and tuberculosis (TB) have improved,1,2 and active case-finding initiatives increasingly include screening for both diseases. International recommendations suggest that all patients undergoing TB evaluation should be tested for HIV;3 HIV counselling and testing (HCT) is an indicator of the quality of household TB contact investigation.4 Community-based initiatives that integrate HCT into TB contact investigation can potentially improve case finding for both diseases.5 However, a shortage of HIV counselors and laboratory technicians makes it challenging to provide HCT outside health facilities.6 Public health programs are therefore shifting these tasks to community health workers (CHWs).7
Guidance from the World Health Organization (WHO) supports task-shifting to CHWs and integrating testing services for TB and HIV to promote the uptake of testing.8 Previous studies have suggested that household HCT led by CHWs is efficient and preferred by clients over testing by formal health workers.9–11 In Uganda, however, the appropriate role of CHWs in HIV case finding remains uncertain.12,13 Moreover, existing guidelines in Uganda and other countries do not address training or quality assurance (QA) procedures for CHW-conducted HCT.12
We conducted a mixed-methods study to determine the feasibility and quality of home-based HCT by CHWs and to collect CHW perspectives on delivering HCT during a household TB contact investigation in Kampala, Uganda.
METHODS
Study design and setting
We carried out a prospective, cross-sectional study to collect quantitative and qualitative data on the delivery of CHW-led HCT within a household-randomized controlled trial of home-based TB evaluation (Pan-African Trials Registry #20150900877140). This study was carried out in communities surrounding seven public primary care clinics providing TB services. At each clinic, we recruited 1–3 CHWs involved in TB-related activities. CHWs spent half their time supporting TB evaluation and treatment in clinics, with the other half spent visiting homes for TB contact tracing.
Human immunodeficiency virus counselling and testing training for community health workers
Fifteen CHWs attended a 2-week HCT training course conducted according to national HCT guidelines (Appendix 1).13 All trainees completed a week of supervised HCT at the AIDS Information Centre, a community-based non-governmental organization. CHWs completed 1 additional week of practice at study sites conducting routine HCT under the supervision of experienced laboratory technicians.
Recruitment of household contacts
After obtaining written informed consent from TB patients, CHWs visited their homes to screen contacts for TB.14,15 The CHWs provided group TB education, including the need for HCT, and then screened all contacts for TB symptoms and risk factors. Contacts were eligible for the parent study if they resided under the same roof as the TB patient, were not taking medication for TB, had access to a phone, were willing to receive SMS communications, and spoke English or Luganda. Contacts were eligible to receive home-based HCT if they resided in a household randomized to receive home-based services. We excluded individuals aged <15 years and people living with HIV (PLHIV).
Home-based human immunodeficiency virus counselling and testing
After obtaining written informed consent, the CHWs provided group pre-test HIV education to the household. For those who consented to HIV testing, CHWs collected 400–500 μl of capillary blood into two microtainers containing ethylene-diamine triacetic-acid anticoagulant. CHWs used one aliquot for primary testing and delivered the second for re-testing at the clinic within 72 h. CHWs used a serial rapid diagnostic test algorithm:13 Determine HIV-1/2 (Abbott Laboratories, Abbott Park, IL, USA) was used as the screening test, HIV-1/2 Stat-Pak (Chembio, Medford, NY, USA) as the confirmatory test, and Uni-Gold Recombigen HIV (Trinity Biotech, Wicklow, Ireland) as the gold standard for discordant results. The CHWs re-tested samples with indeterminate results using new test kits. Individual results and post-test counseling were offered privately to each contact. Contacts who tested HIV-positive were referred to the clinic of their preference for HIV treatment.
Quality assurance procedures
Clinic-based laboratory technicians re-tested samples obtained by CHWs using the same HIV testing algorithm and test-kit lots. A study laboratory supervisor accompanied each CHW on one home visit every month to provide supportive supervision by observing and ensuring adherence to procedures. Finally, the Uganda Virus Research Institute's (UVRI's) external quality assurance (QA) program provided quarterly HIV panel testing for all CHWs. Each panel consisted of six blinded, randomly numbered, dried-tube specimens with pipettes, buffer solution, and instructions for reconstitution. After testing each sample under direct observation by a laboratory technician, the CHWs forwarded results to the UVRI, which returned graded performance reports within 1 month.
Data collection
The CHWs collected clinical data on electronic tablets using a survey application (CommCare, Dimagi, Cambridge, MA, USA). Study staff entered the results of HIV re-testing and external QA reports from clinic records.
At the end of the study, two bilingual researchers led focus group discussions (FGDs) with study CHWs in two groups. The FGD guide (Appendix 2) probed CHW experiences carrying out home-based HCT. This complements previous work describing contact perspectives on the acceptability of CHW-led HCT in the same study.16 The FGDs were digitally recorded, transcribed, translated, and entered into Atlas.ti (ATLAS.ti Scientific Software Development, Berlin, Germany) for analysis.
Data analysis
Continuous variables using medians and interquartile ranges (IQRs) were summarized, as were categorical variables using proportions. We characterized CHWs by age, sex, education, marital status and work experience. Feasibility was defined as the proportion of individual rapid HIV tests conducted in accordance with the algorithm. We defined quality as the proportion of participants for whom results obtained by CHWs were concordant with those obtained by laboratory technicians on re-testing in clinic laboratories. Concordance of overall HIV testing results was calculated using Cohen's κ coefficient. We calculated 95% confidence intervals (95%CIs) using robust standard error estimation. All quantitative data were analyzed using STATA v 14.2 (StataCorp, College Station, TX, USA).
We used inductive-content analysis to code FGD responses in Atlas.ti and identify emergent themes related to CHW experiences carrying out home-based HCT. The research team discussed emergent themes. A social scientist (MAH) selected illustrative excerpts for each theme.
Ethical considerations
The study was approved by the Makerere School of Medicine Research Ethics Committee, Kampala; the Uganda National Council for Science and Technology, Kampala, Uganda; and the Yale University Human Investigation Committee, New Haven, CT, USA.
RESULTS
Home-based human immunodeficiency virus testing results
Between July 2016 and June 2017, CHWs offered home-based HCT to 216 of 217 eligible contacts (Figure) after screening them for TB symptoms; 114 (53%) consented and were tested using the Determine test. Of 102 (47%) contacts who declined HIV testing, 87 (85%) had previously tested negative. Of those tested, 95% (n = 108) had negative HIV results, which CHWs reported to contacts as final results. Five individuals (4%) had positive HIV results using the Determine test; all five subsequently tested positive using the Stat-Pak test, resulting in a yield of 2.3%, or one new HIV diagnosis for every 44 contacts screened. CHWs reported these positive results in the home and referred them for HIV care. All five were enrolled in care and initiated antiretroviral therapy within 2 months. No Uni-Gold tests were performed because the Determine and Stat-Pak tests were concordant in all cases. Of 119 tests performed, 118 (99.2%) were conducted in accordance with the HIV testing algorithm. There was one (1%) indeterminate HIV test result, in which the CHW terminated testing after the Determine result. Instead of re-testing the contact per protocol, the CHW referred the participant to the health facility for re-testing.
FIGURE.
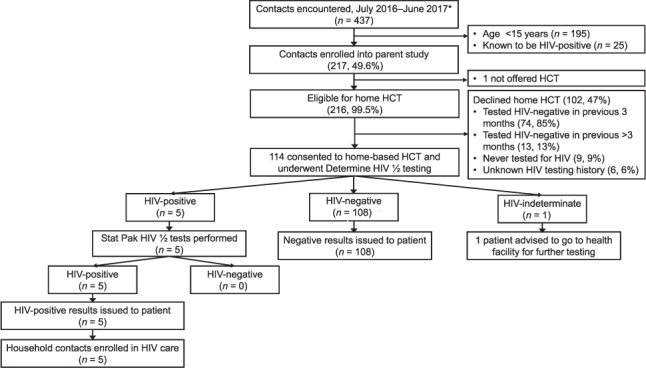
Enrollment of household TB contacts and results of HIV testing algorithm. * Median number of contacts per household of 3 (interquartile range 2–5). HIV = human immunodeficiency virus; HCT = HIV counseling and testing; TB = tuberculosis.
Quality of home-based community health worker-led human immunodeficiency virus counselling and testing
After the CHWs had tested individuals in their homes, 110 (96%) samples were re-tested by laboratory technicians. Four (2.7%), samples were reported to have an inadequate amount of blood for re-testing. There was 99.1% agreement between CHWs and laboratory technicians when testing using the Determine test, resulting in a κ of 0.90 (95%CI 0.71–1.0, P < 0.0001). The one instance of disagreement (0.9%) was when the CHW received an indeterminate test result, whereas laboratory technicians received a negative result. There was 100% agreement between CHWs and laboratory technicians when using Stat-Pak. This resulted in 99.1% overall agreement between the CHWs and laboratory technicians (κ = 0.90, 95%CI 0.71–1.00, P < 0.0001). All 15 CHWs completed four quarterly rounds of HIV panel testing, resulting in 336 proficiency tests. There were no inaccurate results during proficiency testing.
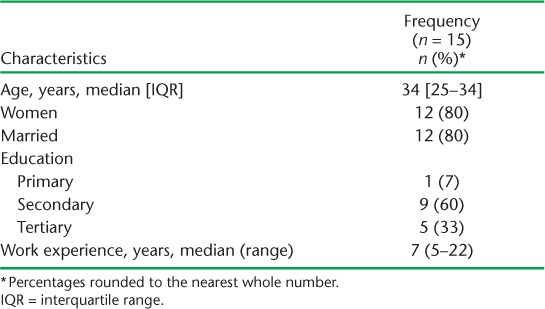
Characteristics of community health workers
Of 15 CHWs, 12 (80%) were women (Table). The median age was 34 years (IQR 25–34). The majority (n = 12, 80%) were currently married. One (7%) had left school after primary education, nine (60%) after secondary education, and five (33%) after tertiary education. CHWs had a median of 7 years (range 5–22) experience in HIV and/or TB-related community health work. CHWs earned ~US$42/month and received transport allowances of US$3 per home visited. The CHWs spent on average 23 min/patient (range 1–145) on TB-HIV home services.
TABLE.
Characteristics of community health workers
Community health worker experiences with home-based human immunodeficiency virus counselling and testing
Thirteen (87%) CHWs participated in FGDs, each of which lasted ~90 min. In the discussions, two themes emerged regarding CHW-led HCT in the home. First, the CHWs described unique social challenges posed by performing HCT in homes rather than clinics.
Sometimes we have challenges; we get patients from the clinic and follow them at home but you may find that the patient is a minor and you need consent from an adult or household head…if you need to do an HCT you need to make sure that everyone has his/her privacy and you need to be so careful otherwise you may leave the home when you have caused divisions…that is why sometimes we do our work slowly to make sure you handle each case well to avoid separations, but we always try our best. (CHW 4, female, 5 years' experience)
CHWs in both focus groups echoed this respondent's concern about causing divisions in the home. Some described instances where they referred contacts to clinics rather than carry out home-based HCT because they observed conflict regarding disclosure of results and were worried they would not be able to protect the participants' privacy. For example, the respondent quoted above cited a home visit in which a 17-year-old girl wanted to test for HIV, but her father insisted that he be informed of the results. Because the 17-year-old did not wish to disclose, the CHW referred her to a clinic for HCT rather than risk conflict by testing her at home.
Second, CHWs said they were initially apprehensive about carrying out HCT but became more confident over time. One respondent explained,
I have managed to learn how to counsel people and whenever we keep doing it we learn new things on how to do it better. In the beginning, we used to fear giving people positive results but now we have learnt and it is something we find easy to do and I like it. We have learnt how to do it and we are able to test, give results, and make them come to the clinic to start treatment, which in the end makes us feel good, seeing that you helped someone who is now looking good. (CHW 3, female, 5 years' experience)
The CHWs' initial apprehension was related to counseling clients receiving positive results, not their ability to carry out the testing itself. They therefore cited experience—rather than formal training—when describing the source of their growing confidence. Carrying out HCT during home visits with supportive supervision also helped increase their confidence. CHWs unanimously agreed that they enjoyed providing home-based HCT to contacts.
DISCUSSION
CHW-led, home-based HCT has the potential to improve HIV testing rates and linkage to HIV treatment. CHWs can approach at-risk populations in ways that may be more acceptable and convenient for clients, while simultaneously shifting responsibility away from overburdened health workers in facilities.17 However, it is essential that these services be provided competently. Here, we have demonstrated that CHW-led, home-based HCT among high-risk TB contacts can provide results similar to those obtained in laboratory settings.
We found that CHWs adhered to the HIV testing algorithm. Moreover, the results of CHW-led tests had a high level of concordance with re-testing by laboratory personnel. While other studies have found gaps in adherence to confirmatory testing among CHWs, the CHWs in our study had very high rates of adherence, which is associated with accurate results.18,19 Our findings are in agreement with a recent large study of community members tested in rural Uganda, where the quality of the CHW-led, home-based HCT provided was extremely high.20 In that study, no false-positives or negatives were reported among a subset of samples selected for re-testing. Together, these studies suggest that it is possible to achieve high-quality, CHW-led testing in a variety of settings if training and QA mechanisms are in place.
We also found that the CHWs' confidence grew as they gained field experience in carrying out home-based HCT, and that they valued the continued supportive supervision beyond the initial training period. Continuing support at regular intervals may help CHWs achieve high fidelity to testing algorithms and a high degree of accuracy. However, further research is needed to develop affordable, scalable QA programs for home-based HCT.
As described in a previous study, the test acceptance rates observed are consistent with other reports from the region.16,21 In this study, CHWs described unique social barriers affecting their ability to deliver home-based HCT, citing participant expectations about disclosure, consent, and privacy that were different from those they encountered in clinical settings. We have presented evidence elsewhere from interviews with contacts that household settings may contribute to communal decision-making or complicate individual decisions about testing.16 Additional research evaluating interventions addressing these unique social challenges is required if home-based HCT is to be integrated into household contact investigation.
The study had some limitations. First, we employed an intensive approach to HCT training: a laboratory supervisor joined CHWs on household visits every month for supportive supervision. This approach may not be generalizable or scalable in resource-constrained settings. Future studies should evaluate whether similarly high proficiency can be obtained with shorter training periods, and determine the optimal spacing of supportive supervision. Second, overall HIV prevalence was low, leading to fewer opportunities for assessing CHW and laboratory concordance among PLHIV. It is thus possible that we would have observed some false-negative results by CHWs with more testing. However, CHWs achieved high levels of accuracy during panel testing, implying that our results are unlikely to change significantly. Third, as our inclusion criteria included only those aged ⩾15 years, these results may not be generalizable to all household contacts. However, HIV prevalence in those aged <15 years is thought to be minimal, and testing procedures should not differ in this population. Finally, oral HIV testing represents an exciting, although costly, alternative to blood-based HIV testing.22,23 While oral testing may simplify the home HCT process, it would also reduce the relevance of the training and specific QA mechanisms that we evaluated, which were focused on blood testing. However, the overall process of assuring quality through training, supervision, and external QA would likely apply to oral HIV testing.
The study also has several strengths. First, we examined the success of bundling home-based HCT with TB contact-tracing activities, a relatively innovative approach that targets individuals at high risk for both diseases. This is important because although HCT is a core element of high-quality TB household contact investigation,3,24 few implementation studies have assessed the integrated provision of these services. Second, our study adds to existing literature by looking at the quality of HIV testing conducted by CHWs in a low-income, urban setting.20
By utilizing CHWs, HIV testing can be feasibly integrated into household TB contact screening, potentially facilitating faster identification of those infected. Future research should identify strategies for implementing standardized and cost-effective QA programs.
Acknowledgments
The authors would like to thank the study participants, the National Tuberculosis and Leprosy Programme (Kampala), the Kampala Capital City Authority (Kampala, Uganda), and the community health workers who aided in this study; and the administrative team at the Uganda TB Implementation Research Consortium at the Makerere College of Health Sciences (Kampala, Uganda). Funding was provided by National Institutes of Health (Bethesda, MD, USA) grant R01AI104824 (JLD) and the Nina Ireland Program in Lung Health at the University of California San Francisco, San Francisco, CA, USA (JLD).
APPENDIX
APPENDIX 1.
Syllabus and schedule for training of CHWs in HCT *

APPENDIX 2 FGD guide and debriefing tool
Home-based sputum collection: FGD guide
(Introducing questions in bold; probes in bullet points)
Thank you for joining this discussion. My name is ___________ and I am ________. This is [introduce notetaker]. Our discussion topic today is home-based procedures. We would like to learn about visiting TB contacts at home, asking them to submit sputum, and collecting sputum. This discussion will help us learn more about being a CHW.
There are no right or wrong answers. We are interested in everyone's views, so please share your opinion even if it's different from others. Because we will be recording the discussion, please speak one at a time. But it's also good to talk to one another and have a discussion. I will help guide the discussion.
CHW role
What is it like being a CHW?
How would you describe working as a CHW?
How do you feel about being a CHW?
Visiting TB contacts at home
What is it like to go to people's homes and do these activities?
Tell me about your interactions with TB contacts at home.
What do you like about carrying out extended procedures on home visits?
What don't you like about carrying out extended procedures home visits?
Sputum collection and HIV testing at home
What is it like to collect sputum during home visits?
How does HIV testing at home compare to sputum collection at home?
Given everything we have talked about, if you had the opportunity to change the ways these activities are done, what would you pay attention to first?
Is there anything that you would like me to know?
TABLE.
FGD characteristics
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS. . Global HIV Statistics—July 2017. Geneva, Switzerland: UNAIDS, 2017. [Google Scholar]
- 3. TB CARE I, Joint United Nations Programme on HIV/AIDS. . International standards for tuberculosis care. The Hague, The Netherlands: World Health Organization, 2014. [Google Scholar]
- 4. Fair E, Miller C R, Ottmani S E, Fox G J, Hopewell P C.. Tuberculosis contact investigation in low- and middle-income countries: standardized definitions and indicators. Int J Tuberc Lung Dis 2015; 19: 269– 272. [DOI] [PubMed] [Google Scholar]
- 5. Sekandi J N, Dobbin K, Oloya J, Okwera A, Whalen C C, Corso P S.. Cost-effectiveness analysis of community active case finding and household contact investigation for tuberculosis case detection in urban Africa. PLOS ONE 2015; 10: e0117009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanjana P, Torpey K, Schwarzwalder A, . et al. Task-shifting HIV counselling and testing services in Zambia: the role of lay counsellors. Hum Resour Health 2009; 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ledikwe J H, Kejelepula M, Maupo K, . et al. Evaluation of a well-established task-shifting initiative: the lay counselor cadre in Botswana. PLOS ONE 2013; 8: e61601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization Stop TB Partnership. . Interim policy on collaborative TB/HIV activities. WHO/HTM/TB/2004.330. WHO/HTM/HIV/2004.1 Geneva, Switzerland: WHO, 2004. [Google Scholar]
- 9. Kennedy C E, Yeh P T, Johnson C, Baggaley R.. Should trained lay providers perform HIV testing? A systematic review to inform World Health Organization guidelines. AIDS Care 2017; 29: 1473– 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. . Consolidated guidelines on HIV testing services: 5Cs: consent, confidentiality, counselling, correct results and connection. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 11. Chamie G, Kwarisiima D, Clark T D, . et al. Uptake of Community-based HIV testing during a multi-disease health campaign in rural Uganda. PLOS ONE 2014; 9: e84317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uganda Ministry of Health. . National policy guidelines for TB/HIV collaborative activites in Uganda. Kampala, Uganda: Uganda MoH, 2006. [Google Scholar]
- 13. Uganda Ministry of Health. . National implementation guidelines for HIV counselling and testing in Uganda. Kampala, Uganda: Uganda MoH, 2010. [Google Scholar]
- 14. National Tuberculosis and Leprosy Programme. . Manual of the National Tuberculosis and Leprosy Programme. Kampala, Uganda: Uganda MoH, 2010. [Google Scholar]
- 15. World Health Organization. . Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. WHO/HTM/TB/2012.9 Geneva, Switzerland: WHO, 2012. [PubMed] [Google Scholar]
- 16. Armstrong-Hough M, Ggita J, Ayakaka I, . et al. Brief report: ‘Give me some time’ facilitators of and barriers to uptake of home-based HIV testing during household contact investigation for tuberculosis in Kampala, Uganda. J Acquir Immune Defic Syndr 2018; 77: 400– 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph J K, Rigodon J, Cancedda C, . et al. Lay health workers and HIV care in rural Lesotho: a report from the field. AIDS Patient Care STDS 2012; 26: 141– 147. [DOI] [PubMed] [Google Scholar]
- 18. Mwangala S, Musonda K G, Monze M, Musukwa K K, Fylkesnes K.. Accuracy in HIV rapid testing among laboratory and non-laboratory personnel in Zambia: observations from the national HIV proficiency testing system. PLOS ONE 2016; 11: e0146700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mwisongo A, Peltzer K, Mohlabane N, Tutshana B.. The quality of rapid HIV testing in South Africa: an assessment of testers' compliance. Afr Health Sci 2016; 16: 646– 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asiimwe S, Ross J M, Arinaitwe A, . et al. Expanding HIV testing and linkage to care in southwestern Uganda with community health extension workers. J Int AIDS Soc 2017; 20 Suppl 4: 21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heunis J C, Wouters E, Norton W E, . et al. Patient- and delivery-level factors related to acceptance of HIV counseling and testing services among tuberculosis patients in South Africa: a qualitative study with community health workers and program managers. Implement Sci 2011; 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delaney K P, Branson B M, Uniyal A, . et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. AIDS 2006; 20: 1655– 1660. [DOI] [PubMed] [Google Scholar]
- 23. Semá Baltazar C, Raposo C, Jani I V, . et al. Evaluation of performance and acceptability of two rapid oral fluid tests for HIV detection in Mozambique. J Clin Microbiol 2014; 52: 3544– 3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. TB CARE I, US Agency for International Development, World Health Organization. . Adaptation and implementation guide for recommendation for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. The Hague, The Netherlands: WHO, 2015. [PubMed] [Google Scholar]


