Abstract
Smoking is a significant risk factor for morbidity and mortality, particularly among patients with tuberculosis (TB). Although smoking cessation is recommended by the World Health Organization and the International Union Against Tuberculosis and Lung Disease, there has been no published evaluation of smoking cessation interventions among people with TB. The purpose of this review was to synthesize the evidence on interventions and suggest practice, research and policy implications. A systematic review of the literature identified 14 peer-reviewed studies describing 13 smoking cessation interventions between 2007 and 2017. There were five randomized controlled trials, three non-randomized interventions, and five prospective cohort studies. The primary types of interventions were brief advice (n = 9), behavioral counseling (n = 4), medication (n = 3), and community-based care (n = 3). A variety of health care workers (HCWs) implemented interventions, from physicians, nurses, clinic staff, community health workers (CHWs), as did family members. There was significant heterogeneity of design, definition of smoking and smoking abstinence, and implementation, making comparison across studies difficult. Although all smoking interventions increased smoking cessation between 15% and 82%, many studies had a high risk for bias, including six without a control group. The implementing personnel did not make a large difference in cessation results, suggesting that national TB programs may customize according to their needs and limitations. Family members may be important supporters/advocates for cessation. Future research should standardize definitions of smoking and cessation to allow comparisons across studies. Policy makers should encourage collaboration between tobacco and TB initiatives and develop smoking cessation measures to maximize results in low-resource settings.
Keywords: tobacco use, tobacco use cessation, smoking cessation products, TB
Abstract
Le tabac constitue un facteur de risque significatif en termes de morbidité et de mortalité, particulièrement pour les patients atteints de tuberculose (TB). L'arrêt du tabac a été recommandé par l'Organisation Mondiale de la Santé et l'Union Internationale contre la Tuberculose et les Maladies Respiratoires ; aucune évaluation n'a cependant été publiée à propos des interventions de sevrage du tabac parmi les personnes atteintes de TB. Le but de cette revue a été de synthétiser les données probantes relatives à ces interventions et de suggérer les implications en matière de pratique, de recherche et de politique. Une revue systématique de la littérature a identifié 14 études revues par des pairs, décrivant 13 interventions d'arrêt du tabac entre 2007 et 2017 : 5 essais randomisés contrôlés, 3 interventions non randomisées et 5 études prospectives de cohorte. Les types principaux d'intervention ont consisté en brefs conseils (n = 9), en conseil comportemental (n = 4), en médicaments (n = 3) et en prise en charge communautaire (n = 3). Les interventions ont été mises en œuvre par toute une gamme de personnel de santé—médecins, infirmiers, personnel des dispensaires, travailleurs de santé communautaire—et par des membres de la famille. Ces interventions ont été significativement hétérogènes en matière de schéma, de définition du tabagisme et de l'abstinence et de mise en œuvre, ce qui a rendu difficiles les comparaisons entres les études. Dans l'ensemble, toutes les interventions ont accru le taux d'arrêt du tabac de 15% à 82%, mais de nombreuses études sont très sujettes aux biais, notamment les six études dépourvues de groupe témoin. Le type de personnel de mise en œuvre n'a pas entrainé de modifications majeures en termes de résultats, ce qui suggère que les programmes nationaux TB peuvent adapter la mise en œuvre de ces interventions à leurs besoins et à leurs limites. Les membres de la famille semblent jouer un rôle important en matière de soutien et de plaidoyer. Des recherches ultérieures devraient standardiser les définitions de la consommation et de l'arrêt du tabac afin de permettre des comparaisons entre les études. Les décideurs politiques devraient encourager la collaboration entre les initiatives liées au tabac et celles liées à la TB et élaborer des mesures d'arrêt du tabac pour maximiser les résultats dans les contextes de faibles ressources.
Abstract
El tabaquismo representa un importante factor de riesgo de morbilidad y mortalidad, sobre todo para los pacientes con tuberculosis (TB). La Organización Mundial de la Salud y la Unión Internacional contra la Tuberculosis y las Enfermedades Respiratorias han recomendado que se promueva la deshabituación tabáquica, pero aún no se ha publicado una evaluación de las intervenciones que favorecen el abandono del tabaquismo en las personas con diagnóstico de TB. La finalidad de la presente revisión consistió en reunir la evidencia existente sobre estas intervenciones y proponer los corolarios que se podrían aplicar en la práctica, la investigación y la formulación de políticas. En una revisión sistemática de artículos científicos se encontraron 14 estudios publicados del 2007 al 2017 en revistas con comité de lectura que describían 13 intervenciones de deshabituación tabáquica. Los artículos abordaban 5 ensayos aleatorizados, 3 intervenciones no aleatorizadas y 5 estudios de cohortes prospectivos. Los principales tipos de intervenciones consistieron en asesoramiento breve (n = 9), orientación conductual (n = 4), tratamiento médico (n = 3) y atención al nivel comunitario (n = 3). Diversos profesionales de salud participaron en la ejecución de las intervenciones como miembros del personal médico, de enfermería, auxiliares clínicos, agentes de salud comunitarios y miembros de la familia. Se observó una gran heterogeneidad con respecto al diseño de los estudios, la definición de tabaquismo y de la abstinencia de tabaco y a la ejecución, que dificultó las comparaciones entre los estudios. En general, todas las intervenciones de deshabituación tabáquica aumentaron el abandono del tabaco de 15% a 82%, pero en muchos de los artículos existía la probabilidad de sesgo como en seis estudios que no contaban con un grupo testigo. El tipo de personal que ejecutaba la intervención no tuvo un efecto notorio en los resultados de abandono, lo cual indica que los programas nacionales contra la TB pueden adaptar las iniciativas a sus necesidades y limitaciones. Los miembros de la familia pueden cumplir una función importante de apoyo o promoción del abandono del tabaco. En las investigaciones futuras es preciso normalizar las definiciones de tabaquismo y de abandono del tabaco con el fin de facilitar las comparaciones entre los estudios. Las instancias normativas deben fomentar la colaboración entre las iniciativas contra el tabaquismo y contra la TB y formular medidas encaminadas a la deshabituación tabáquica que optimicen sus resultados en los entornos con bajos recursos.
Tuberculosis (TB) is the leading cause of death from infectious disease worldwide.1 Smoking is a significant driver of the TB epidemic, accounting for 8% of TB cases among the 30 countries with the highest TB burden.1,2 Smokers have increased risk for developing TB and negative treatment outcomes.3,4 This may be due to biologic processes that impact lung health, as well as social factors associated with tobacco use, such as alcohol use.3,5 Ongoing tobacco use increases the risk of negative TB outcomes, primarily TB relapse or recurrence.4,6
Of the one billion smokers worldwide, 80% reside in low- and middle-income countries (LMICs), many of which also have a high TB burden.7 Provision of smoking cessation services during anti-tuberculosis treatment is critical to reduce the negative effects of smoking on TB treatment and lifelong health; patients are also more likely to change their smoking behavior during TB treatment, underscoring this period as a critical intervention opportunity for cessation.8–10 In 2007, the World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (The Union) recommended that smoking cessation interventions be added to National TB Programs (NTPs) using the ‘5As’ approach: 1) Ask the patient about smoking; 2) Advise about the risk of smoking; 3) Assess willingness to stop smoking; 4) Assist patient to stop smoking; and 5) Arrange for follow-up; or a modified version called the ABC approach, i.e., A, Ask about smoking; B, provide Brief advice; C, provide Cessation support.9,11 These frameworks provide a foundation for NTPs to integrate smoking cessation interventions within TB care.
Smoking interventions have been implemented as part of NTPs since 2007 in LMICs such as Sudan, Pakistan, and South Africa, but no systematic review has explored the impact of these programs on smoking cessation among TB patients. The purpose of this systematic review was to consolidate existing evidence on smoking cessation interventions among TB patients in LMICs and summarize the practice, policy, and research implications of these findings to improve smoking cessation efforts.
METHODS
Search strategy
A systematic review of peer-reviewed literature on smoking cessation interventions among TB patients was conducted using PRISMA (Preferred reporting items for systematic reviews and meta-analyses) guidelines in May 2017.12 The following databases were used: PubMed, the Cumulative index to Nursing and Allied Health Literature, SCOPUS, Web of Science, Cochrane, and Embase. Search criteria were developed using Medical Subject Headings (MeSH) and non-Mesh terms which were adapted to the specific database as follows:
TB: tuberculosis[Mesh] OR tuberculo* OR ‘TB’
Smoking cessation: ‘Tobacco Use Cessation’[Mesh] OR ‘Smoking Cessation’[Mesh] OR ‘Tobacco Use Cessation Products’[Mesh] OR ‘Smoking/prevention and control’[Mesh] OR ‘Smoking/therapy’[Mesh] OR ‘Bupropion’[Mesh] OR ‘Varenicline’[Mesh] OR ‘Clonidine’[Mesh] OR ‘Nortriptyline’[Mesh] OR ‘smoking cessation’[tiab] OR ‘smoking cessations’[tiab] OR ((‘tobacco products’[Mesh] OR ‘Tobacco Use’[Mesh] OR ‘Smoking’[Mesh] OR ‘vaping’[Mesh] OR ‘nicotine’[mesh] OR smok*[tiab] OR ‘tobacco’[tiab] OR cigar*[tiab] OR e-cig*[tiab] OR ‘nicotine’[tiab] OR ‘hookah’[tiab] OR ‘pipe’[tiab] OR ‘vaping’[tiab] OR ‘vape’[tiab]) and (quit*[tiab] OR ceas*[tiab] OR cessation*[tiab] OR stop*[tiab] OR suspend*[tiab] OR desist*[tiab] OR end*[tiab] OR break*[tiab] OR cutoff*[tiab] OR termin*[tiab] OR discontinu*[tiab] OR abstin*[tiab] OR ‘dehabituation’[tiab] OR ‘Nicorette’[tiab] OR ‘gum’[tiab] OR ‘NRT’[tiab] OR patch*[tiab] OR ‘bupropion’[tiab] OR ‘varenicline’[tiab] OR ‘nortriptyline’[tiab] OR ‘clonidine’[tiab] OR ‘chantix’[tiab] OR ‘champix’[tiab] OR ‘well-butrin’[tiab] OR ‘Zyntabac’[tiab] OR ‘Quomen’[tiab] OR ‘Zyban’[tiab] OR ‘Amfebutamone’[tiab]))
Inclusion criteria
Peer-reviewed journal articles were included if they evaluated any smoking cessation intervention among patients with suspected or confirmed TB. Any studies that did not report on smoking cessation outcomes were excluded. The search included publications written in English, French, Spanish, Portuguese, and Korean.
Procedure
All retrieved citations were imported into Covidence® (Cochrane, Melbourne, VIC, Australia) and duplicates were removed. Two reviewers (EW, JL) independently reviewed titles and abstracts. Differences concerning full-text inclusion were resolved through consensus. The reviewers then independently extracted data on participant characteristics, intervention characteristics, smoking cessation outcome, and other qualitative or quantitative information on the implementation of the intervention. Risk of bias was assessed using the Cochrane Risk of Bias tool for intervention studies.13 Results were compared and discrepancies were resolved through discussion. Due to the heterogeneity of comparison groups among the randomized controlled trials, the results were synthesized, but a meta-analysis was not conducted.
Ethics
Institutional review board approval was not required for this literature review.
RESULTS
After removing duplicates, the search resulted in 1646 articles for review (Figure). A total of 14 articles were included based on the inclusion/exclusion criteria for this review. Although there were no country-based exclusion criteria, all studies took place in LMICs. As two of the articles were based on the same intervention but compared outcomes for different groups, there were a total of 13 different interventions.
FIGURE.
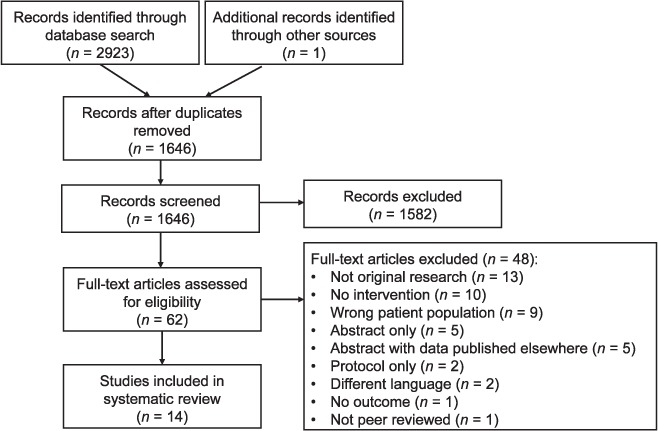
Literature review flowchart based on PRISMA guidelines12
Study characteristics
Included studies were conducted across 11 different countries and published between 2007 and 2017. Study designs included three randomized controlled trials,14–16 two cluster randomized controlled trials,17,18 five prospective cohort studies,19–23 and three non-randomized intervention studies24–26 (Table 1). Three of the studies were intended as feasibility or pilot studies focusing on initial implementation of smoking cessation.19,24,25
TABLE 1.
Study and participant characteristics *
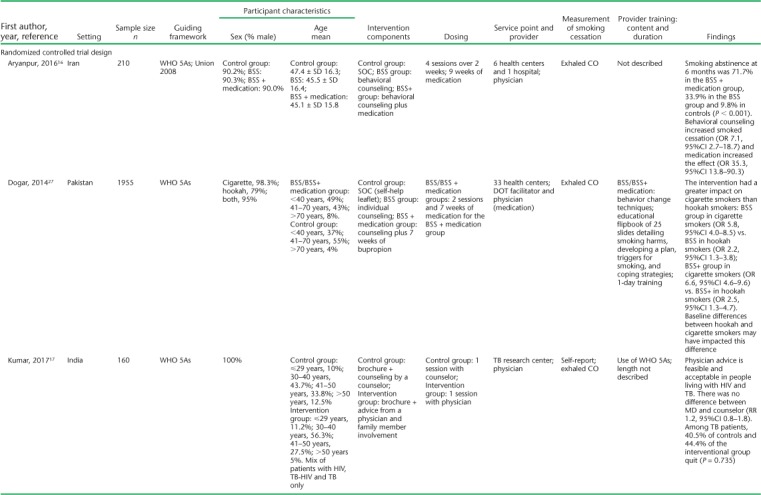
TABLE 1.
(continued)
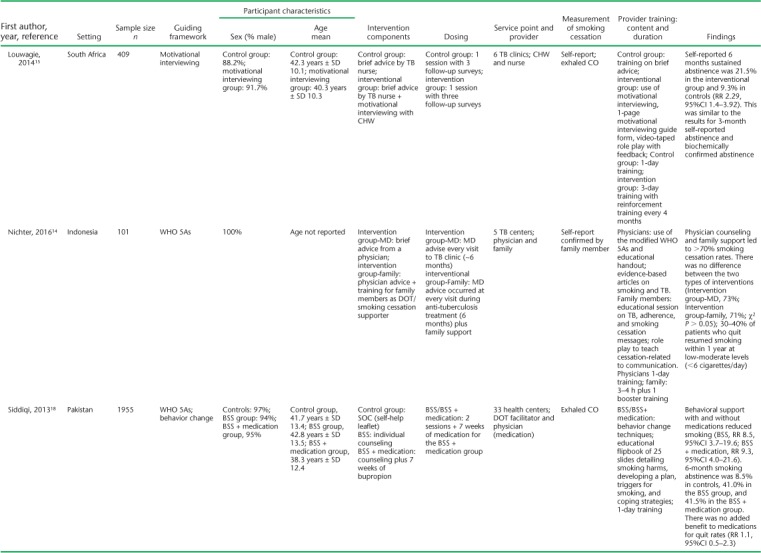
TABLE 1.
(continued)
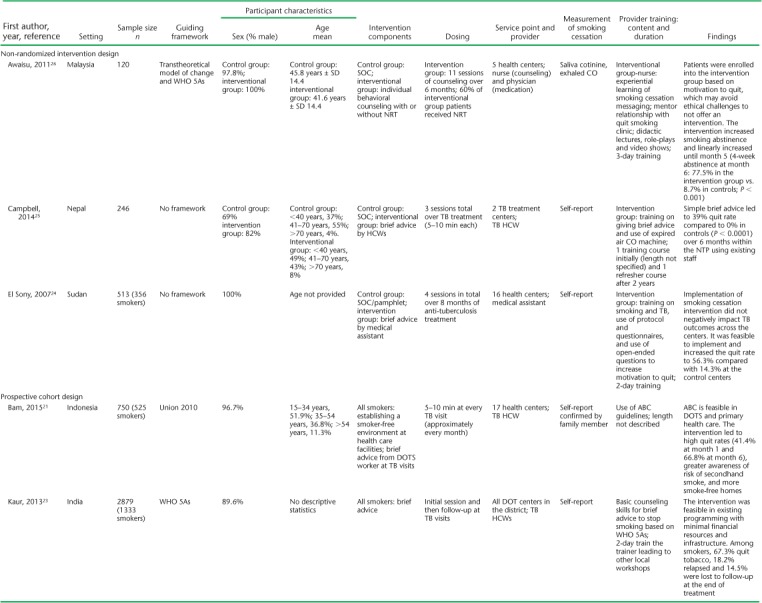
TABLE 1.
(continued)
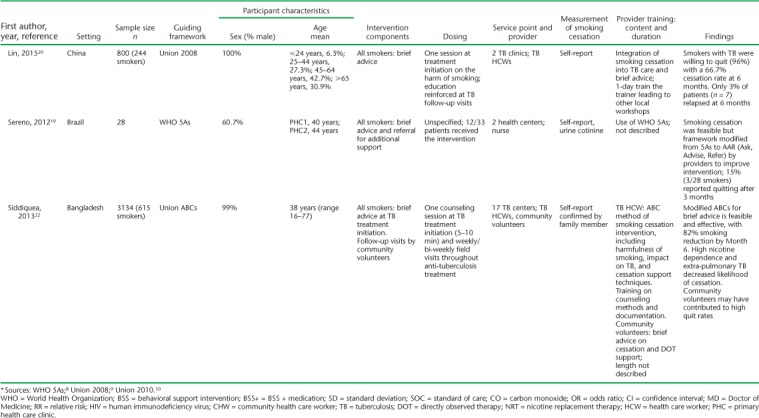
Settings for the studies varied, with the majority (n = 7) conducted in primary health centers. All but one (Kumar et al.17) were conducted as multisite studies, and most (n = 11) included patients with TB on treatment. Only Kumar et al.17 included some patients living with the human immunodeficiency virus (HIV) with no TB in addition to TB patients. Siddiqi et al. included patients with presumptive but not confirmed TB.18 Study sample sizes ranged from 28 to 1955. The mean age of the participants, where reported, ranged from 38 to 47 years. Most participants (range 60.7–100%) were male; five studies included only male participants. Only two studies included TB outcome measures. Awaisu et al. found higher rates of successful TB treatment outcomes in the intervention group (79.5% vs. 78.3%, P = 0.0031),26 whereas El Sony et al. found no differences.24
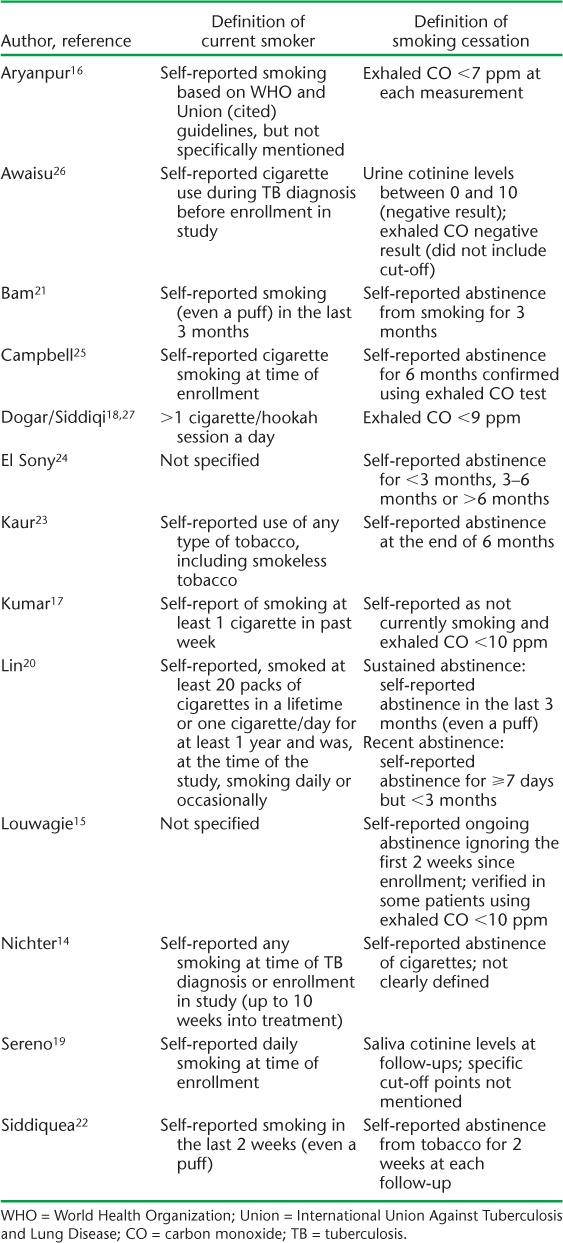
The operational definition of smoking ranged from any self-reported smoking to those who had smoked at least 20 packs in their lifetime (Table 2). The majority (n = 11) included patients who smoked cigarettes or tobacco only, while three included hookah as well.18,23,27 The outcome definition of smoking cessation was highly variable (Table 2). Six of the 14 studies used exhaled carbon monoxide (CO) to define smoking cessation, some in addition to self-report and cotinine. Two studies used cotinine measurement, one in saliva and the other urine, to confirm self-reported smoking status. The remaining seven studies used patient self-report of smoking cessation, with two of those studies asking for family member confirmation of smoking status when possible.
TABLE 2.
Definition of current smoker and smoking abstinence by study
Intervention characteristics
All interventions were based generally on WHO or Union smoking cessation guidelines; what varied across studies was the interventionist, the method, and the frequency. Only El Sony et al. and Nichter et al. followed patients beyond anti-tuberculosis treatment, for 12 months in total.14,24 The other studies followed patients for 1 month (n = 1),17 3 months (n = 1),19 and 6 months or to the end of anti-tuberculosis treatment (n = 10).15,16,18,20–23,25–27 Awaisu et al. and Siddiqi et al. were the only published protocol papers that described the process of developing smoking cessation tools for the local training context and implementing a training program for staff.27,30 A variety of HCWs delivered the interventions; many of these were directly observed therapy (DOT) providers. Physicians most often prescribed smoking cessation medications in intervention studies,16,18,26 but two studies specifically evaluated the added effect of brief advice provided by a physician.14,17 Of the remaining studies, the interventionists were nurses (n = 2),19,26 trained TB staff/DOT facilitators (n = 6),18,20,21,23–25 and community CHWs (n = 2);15,22 one included trained family member supporters.14
There were four general categories of intervention: brief advice, behavioral counseling, medication, and community-based care/family support. Many of the smoking cessation services combined a number of these interventions (Table 3). Brief advice was the most common form of intervention (n = 9). This consisted typically of 5–10 min of advice on the harms of smoking, asking the person if he/she wanted to quit, and promoting cessation strategies with possible referral to smoking cessation services outside the TB clinic. The number of sessions ranged from one at the beginning of anti-tuberculosis treatment to expected brief advice sessions at every visit. Of the brief advice interventions occurring with every TB visit, only Sereno et al. reported on adherence, with 12 of 33 patients receiving any brief counseling.19
TABLE 3.
Summary of components for each type of intervention
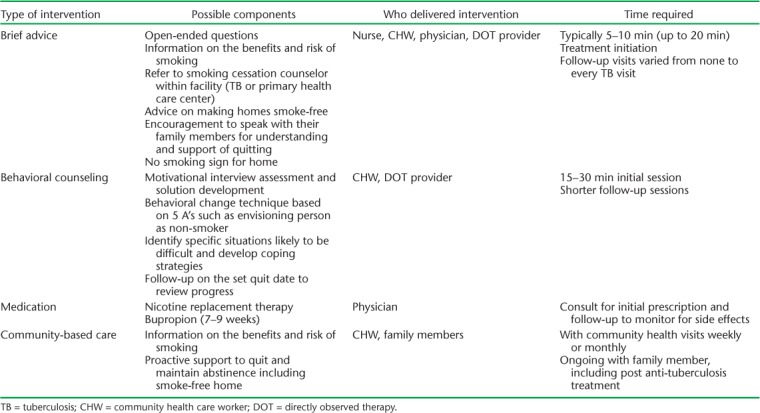
Behavioral counseling was the second most common form of intervention (n = 4). What differentiated behavioral counseling from brief advice was not well described, but included behavioral change training for staff, additional questions to elicit stronger patient involvement in behavior change, and longer but fewer sessions (15–30 min for typically 1–2 sessions). Awaisu et al. was the exception, with 11 behavioral counseling sessions across 6 months of anti-tuberculosis treatment.26 There did not appear to be any correlation between the number of sessions and the success of smoking cessation, although this was difficult to evaluate given the varied study designs.
Two studies prescribed medication (bupropion) for 7 or 9 weeks as a specific intervention arm in addition to counseling,16,18 while Awaisu et al. allowed providers to prescribe nicotine replacement therapy (NRT). Only 60% of participants received NRT.26
Finally, three studies involved community-based care, with either family members14,17 or CHWs providing cessation support in the community.22 Community-based care did not demonstrate any significant improvement in smoking cessation above routine provider advice; however, qualitative interviews suggest that family members provided sustained counseling beyond anti-tuberculosis treatment, which may have led to unmeasured improvements in quit rates.14
Implementation
There was a significant component of clinician training involved in the implementation of interventions (Table 1). Most training sessions lasted 1–2 days, although five of the studies did not state the duration of training. Kaur et al. and Lin et al. used ‘Train the trainer’ models to enable more staff to be trained at local TB clinics.20,23 For example, Kaur et al. trained over 1400 staff in smoking cessation messaging using this model. Training courses that involved more hands-on components, such as role-playing, appeared to last longer. However, as many of the studies did not detail how knowledge and skills regarding smoking cessation messages were taught in the training courses, it is difficult to draw any conclusions. Only Louwagie et al. provided specific follow-up training for staff to reinforce smoking cessation messages; Nichter et al. provided follow-up training for family members.14,15 Few studies provided evidence on the effectiveness or acceptability of the training by staff. El Sony et al. noted an increase in the use of smoking abstinence messaging after the training; however, the difference between the control and intervention staff was not statistically significant.24 Lin et al. and Sereno et al. noted challenges in implementation due to busy clinic schedules or clinicians who smoked and who did not believe smoking cessation was important.19,20 However, three other studies reported positive responses by staff, including the fact that staff and families were not always aware of the connection between smoking and TB and appreciated the training.14,19,21
Effectiveness of interventions
One of the challenges of evaluating effectiveness is that six of the studies did not use a control group. In addition, studies with a control group varied greatly as regards the standard of care provided to the control group. The standard of care ranged from asking about smoking status24,25 to receiving standard DOT care that may or may not have included smoking cessation messages,16,26 brochures,17,18 or even brief advice or counseling by a smoking cessation counselor.15,17 This made comparisons across different types of intervention very difficult.
Randomized controlled trials
Among the five randomized controlled trials, those that provided both advice by a health care provider and advice plus medication improved smoking cessation. Among the studies that reported a relative risk (RR) (n = 3), the RR for smoking abstinence ranged from 2.3 to 8.5 at the end of the follow-up period for counseling (3 studies)15,16,18 and from 9.3 to 35.3 (odds ratio) for the combination of counseling and medication (2 studies).16,18 In both studies where bupropion was added to counseling, there was an increase in cessation; however, this was either not evaluated statistically or was statistically non-significant. In the additional comparison of hookah smokers with cigarette smokers (1 study), smoking cessation interventions had less impact on hookah smokers; the chance of smoking abstinence in this group was nevertheless doubled (RR 2.2–2.5). Neither Kumar et al.17 nor Nichter et al.14 had standard of care control groups, but compared counseling or trained family support, respectively, with physician advice alone. In these two studies, quit rates were comparable between groups, suggesting that advice given specifically by a non-physician, including a family member, may be as effective as provider advice. Kumar et al. reported quit rates of 40.5–44% among TB patients,17 while Nichter et al. reported 71–73% quit rates.14 The intervention evaluated by Nichter et al. may have been more successful, as it continued for the entire treatment period,14 whereas Kumar et al. had only one advice session.17
Non-randomized interventions
Three of the 14 interventions were non-randomized, but included a control group receiving standard of care. All three studies reported that more participants receiving the intervention quit compared with the control group, with a quit rate ranging from 39% to 82.5% compared with 0% to 14.3%, respectively.24–26 Awaisu et al. reported the highest level of smoking cessation compared with controls (78% vs. 9%).26 However, the study recruited patients who were already motivated to quit (based on the transtheoretical model stages of change), whereas the control group was made up of smokers unwilling to quit. Awaisu et al. was the only study to use NRT along with counseling, but they did not analyze differences between patients who received NRT and those who did not. Campbell et al.25 and El Sony et al.24 both reported on pilot/feasibility studies taking place within the existing NTP structures, where TB treatment staff received training on brief smoking advice.
Prospective cohort studies
Five studies were based on the implementation of smoking cessation interventions among all smokers within existing NTPs, with no control group comparison.19–23 The percentage of smokers who quit at the end of the follow-up period ranged from 66.8% to 82%. Most of these interventions consisted of 5–10 min of brief advice by TB center staff at initiation, with follow-up sessions intended for each return TB visit. The highest quit rate was 82%, in a study where community volunteers were trained in smoking cessation brief advice and provided weekly or bimonthly follow-up advice in the community.22
Quality of articles
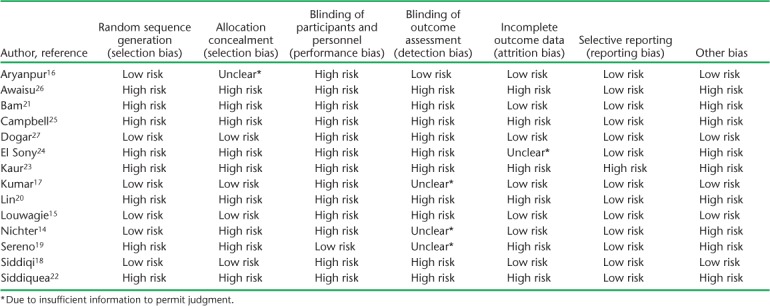
All the studies were evaluated using the Cochrane Risk for Bias for Interventional Studies tool (Table 4).13 Most studies had significant risk for bias, as they did not have a control group or participants who were not randomized to the intervention. Many studies did not have blinding of study participants or study personnel both in assignment of intervention and during data collection, leading to a higher risk of bias in the outcome. However, it is to be noted that the nature of this behavioral intervention makes it difficult to blind participants and clinicians to the intervention.
TABLE 4.
Cochrane Risk of Bias Assessment Tool * 13
DISCUSSION
The addition of smoking cessation interventions to routine TB case management is feasible and effective in reducing smoking rates among patients during anti-tuberculosis treatment. While all the studies in this review reported at least some reduction in smoking, there remains a need for clearer evidence to guide the operationalization and scale-up of smoking cessation strategies within NTPs.
Practice implications
This review suggests that NTPs can implement smoking cessation interventions using existing staff for advice or counseling.19,24 However, clinicians in LMICs are often in high demand to provide clinical care, and thus have limited time.19,30 This review suggests that, in addition to physicians and nurses, lay counselors and HCWs can provide ongoing smoking cessation counseling.15,17 There is some evidence that physicians may play an important role in smoking cessation messaging.31 Physicians and nurses could incorporate short cessation messages that would be followed up with ongoing support provided by CHWs or HCWs. A combination of HCWs reinforcing smoking cessation messaging could complement a more holistic approach to health messaging around smoking and other related topics in TB care. Standardized patient education materials could make it more feasible for this messaging to be provided consistently.
Family members are a critical component of smoking cessation that could be more systematically included during smoking cessation interventions. As smoking may resume after anti-tuberculosis treatment is completed, this is particularly important for preventing relapse.14,20,32 This review found that family members enjoyed learning more about TB and smoking cessation messages and requested additional topics for training in the future.14 When considering who participates in the family, an intergenerational approach to engaging older and younger generations could facilitate stronger messaging.33,34 Research on smoking cessation in non-TB-specific contexts has demonstrated the influence of both social networks and family members on smoking habits and cessation efforts.35–37 Despite this, a review of smoking cessation interventions involving family members found few studies that directly compared individual vs. family-based interventions, limiting the ability to draw conclusions as to whether family member involvement had an additive effect.38 In addition, all of these studies were from Europe and North America, and family dynamics may vary across countries. This review suggests that family members may have a positive influence on TB patients, but additional research is needed to identify how to maximize family support. In addition, as secondhand smoke is a serious health concern, not only for TB patients but their families, counseling TB patients and any other family members within the household who smoke may help to reduce the risk of exposure to secondhand smoke and the risk of development of TB for household members.39,40
Training and supervision are a key requisite for integrating smoking cessation messaging into routine NTPs.41,42 HCWs themselves may smoke and not recognize the importance of smoking cessation messages, particularly for TB patients.30,41 In addition, even if HCWs agree with smoking cessation, they may not have adequate knowledge to provide tailored advice for TB patients.30,41,42 For example, one study noted that TB nurses asked patients about smoking habits and gave advice 87% of the time, but did not provide TB-specific messaging that could increase the likelihood of quitting.15 Most interventions required a 1-day training course for providers, with some utilizing a training-of-trainers approach to increase reach. More in-depth techniques, such as motivation interviewing, could be incorporated into the training if time and resources allow. Motivational interviewing has been found to be effective in the case of counseling on a variety of topics, from medication adherence to chronic disease management to smoking cessation.43 These techniques may help TB providers target patient barriers to smoking cessation during anti-tuberculosis treatment and at the same time increase TB treatment success rates.15,26
Cessation counseling may be accompanied by medications such as NRT or anti-depressants used to reduce cravings, such as bupropion. Of the three studies that included medications, only one compared the effect of advice with advice plus medication, and found no difference. A systematic review by Cochrane suggested that anti-depressants such as bupropion may not be any more effective than NRTs.44 In addition, a separate review found that combined behavioral interventions with medication (NRTs or bupropion) worked better than usual care in the general population, but they did not explore the separate impacts of medication and intensive behavioral therapy on smoking cessation.45 However, given that NRT and other pharmaceuticals can be costly and add to the pill and side effect burden, providers may try other methods of promoting smoking cessation before routinely prescribing medications. Further studies are needed to determine which patients may derive the greatest benefit from medications to guide prescribers. In addition, it is important for TB and tobacco control programs to be able to evaluate whether it would be beneficial to include medications in their programming choices, or whether other cessation methods provide greater benefit, and have a larger impact on community smoking norms and behaviors.
None of the studies in this review used technology to promote smoking cessation. As more people have access to mobile and smartphones, this may be a way to provide additional information and smoking cessation messages for smokers and their families. There is some evidence that mobile phone use in HIV care has improved treatment adherence, and mixed results about whether text reminders could also improve adherence to TB treatment.46–48 In general populations, mobile technology, including short messaging service text reminders, has been effective in promoting and sustaining smoking cessation for up to 6 months, although most such studies have been conducted in high-income countries.49,50 However, as mobile phone use increases in LMICs, technology will continue to be a possible source of cessation or medication adherence messaging.
Research implications
This review provides initial evidence that smoking cessation interventions can reduce smoking rates in TB patients. However, there were few randomized trials, and most studies did not have a control group for comparison. The risk for bias was thus high, and there is a need for additional studies such as adaptive clinical trials to evaluate the effectiveness of smoking cessation interventions in this population.51 Both qualitative and quantitative studies of TB patients who have and those who have not successfully quit smoking would further elucidate what was most helpful about specific interventions, how interventions impact patients beyond just smoking cessation, such as improving overall quality of life, and other factors that contribute to smoking cessation, such as self-efficacy.52,53 Nichter et al. included qualitative interviews of patients who continued smoking after the 6-month TB treatment period and intervention.14 These interviews suggested that the smokers did not see low-level smoking as harmful and that they resumed smoking to demonstrate that they were healthy enough to smoke. This also suggests a critical need for studies that follow patients after the end of anti-tuberculosis treatment, because patients who quit may resume smoking once they have completed treatment, and such patients have been shown to be at greater risk of recurrent TB.6,14 In this review, only two studies assessed smoking cessation after the end of anti-tuberculosis treatment. Studies with longer follow-up and using more qualitative research methods are therefore critical to facilitating and maintaining smoking abstinence.
While the recommendations for integrating smoking cessation messages into TB care are clear, the most cost-effective methods for improving cessation are not known. Only two of the studies in this review mentioned cost, and none did a cost-analysis.18,23 Siddiqi et al. reported that behavioral support cost US$2.50 per person, while medication cost US$20.90 per person.18 Kaur et al. reported that the entire program—training 1436 staff and counseling 1333 smokers among 2879 registered TB patients—was conducted between October 2010 and June 2011 at a cost of US$7000, and could therefore be incorporated into the NTP and smoking cessation budget.23 Future studies should focus on the direct and indirect costs of smoking cessation interventions to assist programs in deciding how to effectively use scarce resources.
Policy implications
Both the WHO and The Union have recommended the integration of smoking cessation messages into TB care for over a decade. However, this review highlights the challenges of implementing effective smoking cessation interventions in resource-limited TB care programs. While the WHO and The Union guidelines provide broad overviews of smoking cessation techniques and messaging, additional resources based on empirical research that can be adapted to different settings are needed. In addition, encouraging standardization of tools and definitions in research and practice will allow for better comparisons of the various smoking cessation interventions. This review also highlights the richness of the data from studies outside of randomized controlled trials. Although randomized controlled trials are the standard, particularly in biomedical research, they may not be the most useful for developing effective pragmatic behavioral interventions and programming, particularly when smoking cessation messaging should be the standard of care.54,55
National and local TB programs should look for ways to integrate smoking cessation training and messaging into existing programs. While smoking cessation interventions can feasibly be introduced in TB clinics, these programs do require resources—smoking cessation materials, training of personnel, changing TB protocols and forms—to include ongoing screening for smoking. As recommended by the WHO, the incorporation of smoking indicators into TB clinical documentation tools could increase the accountability of providers in implementing smoking cessation interventions. These indicators also provide important feedback on the programs' success. In addition, as reported by Kaur et al., integration of national tobacco control programming with TB programming can be successful, especially when TB clinics are located within primary health clinics.23 National and international policy should encourage collaboration between chronic disease prevention and health promotion with TB programming to maximize the impact of messaging.
Strength and limitations of the studies
Most of the studies reviewed were non-randomized or observational, with no control groups. As noted in Table 4, the risk of bias in many of the studies was high. Additional limitations were small sample size, high rates of attrition among participants, and measurement error related to self-reporting of smoking status. Furthermore, most studies followed patients only up to the end of anti-tuberculosis treatment, which fails to capture longer-term relapse and intervention impact. In addition, differing definitions of smokers and smoking cessation made it difficult to compare results across studies.
A major strength of these studies is that they were largely pragmatic interventions situated in existing NTPs. They all suggest that it is feasible to integrate smoking cessation into TB care, and that this can successfully increase the number of patients who quit smoking. In addition, half of the studies used biometric methods (exhaled CO or cotinine) to validate self-reported smoking status, increasing the validity of the results.
Strength and limitations of this review
A limitation of this review was that it included only peer-reviewed journal articles, while program reports and grey literature such as WHO or national reports were excluded. NTPs implementing smoking cessation interventions may have important findings that have not been published in peer-reviewed journals. This review specifically included a variety of study designs, making comparison of outcomes difficult. The strength of this method was the inclusion of more interventions beyond randomized controlled trials to understand the qualitative aspects of interventions that may otherwise have been excluded.
CONCLUSIONS
Smoking cessation interventions can be incorporated into TB treatment and care programs across hospitals and clinics. Increased access to smoking intervention services within NTPs can play a critical role in reducing tobacco use among patients, which could improve TB cure rates and reduce the risk of subsequent morbidity and mortality.3 However, to facilitate the integration of behavioral smoking cessation interventions into NTPs as standard practice, greater policy and program guidance is needed. In addition to research on the effectiveness of smoking cessation strategies in TB patients, a greater focus on the feasibility and cost requirements of various intervention approaches is critically needed to guide implementation.
Acknowledgments
The authors would like to thank M Truskey, information specialist at the Welch Medical Library, Baltimore, MD, USA, for her assistance in developing and implementing the search strategy.
This manuscript was supported by the National Institutes of Health, Bethesda, MD, USA, under award number F31-NR016909 (EW, National Institute of Nursing Research), R01-AI104488 (JF, National Institute of Allergy and Infectious Diseases), and R01-DA030276 (JG, National Institute on Drug Abuse).
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2017. WHO/HTM/TB/2017.23 Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 2. Lönnroth K, Castro K G, Chakaya J M, . et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet 2010; 375: 1814– 1829. [DOI] [PubMed] [Google Scholar]
- 3. Lin H-H, Ezzati M, Murray M.. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLOS Med 2007; 4: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leung C C, Yew W W, Chan C K, . et al. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J 2015; 45: 738– 745. [DOI] [PubMed] [Google Scholar]
- 5. O'Leary S M, Coleman M M, Chew W M, . et al. Cigarette smoking impairs human pulmonary immunity to Mycobacterium tuberculosis. Am J Respir Crit Care Med 2014; 190: 1430– 1436. [DOI] [PubMed] [Google Scholar]
- 6. Yen Y-F, Yen M-Y, Lin Y-S, . et al. Smoking increases risk of recurrence after successful anti-tuberculosis treatment: a population-based study. Int J Tuberc Lung Dis 2014; 18: 492– 498. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. . Tobacco. Geneva, Switzerland: WHO, 2018. http://www.who.int/mediacentre/factsheets/fs339/en/ Accessed April 2018. [Google Scholar]
- 8. World Health Organization. . A WHO/The Union Monograph on TB and Tobacco Control. Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 9. Slama K, Chen-Yuan C, Enarson D A.. Tobacco cessation interventions for tuberculosis patients: a guide for low-income countries. Paris, France: International Union Against Tuberculosis and Lung Disease, 2008. [Google Scholar]
- 10. Bissell K, Fraser T, Chiang C-Y, Enarson D.. Smoking Cessation and smokefree environments for tuberculosis patients. 2nd ed Paris, France: International Union Against Tuberculosis and Lung Disease, 2010. [Google Scholar]
- 11. World Health Organization. . A guide for tuberculosis patients to quit smoking. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman D G; PRISMA Group. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins J, Green S, . Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London, UK: The Cochrane Collaboration, 2011. http://handbook.cochrane.org. Accessed April 2018. [Google Scholar]
- 14. Nichter M, Padmawati S, Ng N.. Introducing smoking cessation to Indonesian males treated for tuberculosis: The challenges of low–moderate level smoking. Soc Sci Med 2016; 152: 70– 79. [DOI] [PubMed] [Google Scholar]
- 15. Louwagie G M C, Okuyemi K S, Ayo-Yusuf O A.. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: a randomized controlled trial. Addiction 2014; 109: 1942– 1952. [DOI] [PubMed] [Google Scholar]
- 16. Aryanpur M, Hosseini M, Masjedi M R, . et al. A randomized controlled trial of smoking cessation methods in patients newly-diagnosed with pulmonary tuberculosis. BMC Infect Dis 2016; 16: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar S R, Pooranagangadevi N, Rajendran M, . et al. Physician's advice on quitting smoking in HIV and TB patients in south India: a randomised clinical trial. Public Health Action 2017; 7: 39– 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siddiqi K, Khan A, Ahmad M, . et al. Original research action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trial. Ann Intern Med 2013; 158: 667– 675. [DOI] [PubMed] [Google Scholar]
- 19. Sereno A B, Soares E C C, Lapa E Silva J R, . et al. Feasibility study of a smoking cessation intervention in directly observed therapy short-course tuberculosis treatment clinics in Rio de Janeiro, Brazil. Rev Panam Salud Publica 2012; 32: 451– 456. [PubMed] [Google Scholar]
- 20. Lin Y, Wang L-X, Qiu L-X, . et al. A smoking cessation intervention among tuberculosis patients in rural China. Public Health Action 2015; 5: 183– 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bam T S, Aditama T Y, Chiang C-Y, Rubaeah R, Suhaemi A.. Smoking cessation and smokefree environments for tuberculosis patients in Indonesia-a cohort study. BMC Public Health 2015; 15: 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siddiquea B N, Islam M A, Bam T S, . et al. High quit rate among smokers with tuberculosis in a modified smoking cessation programme in Dhaka, Bangladesh. Public Health Action 2013; 3: 243– 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaur J, Sachdeva K, Modi B, . et al. Promoting tobacco cessation by integrating ‘brief advice’ in tuberculosis control programme. WHO South East Asia J Public Health 2013; 2: 28. [DOI] [PubMed] [Google Scholar]
- 24. El Sony A, Slama K, Salieh M, . et al. Feasibility of brief tobacco cessation advice for tuberculosis patients: a study from Sudan. Int J Tuberc Lung Dis 2007; 11: 150– 155. [PubMed] [Google Scholar]
- 25. Campbell I A, Chaudhary R D, Holdsworth G M C, Lyne O D.. Brief advice to tuberculosis patients in Nepal to stop smoking: a pilot study by the Britain Nepal Medical Trust. Int J Tuberc Lung Dis 2014; 18: 1438– 1442. [DOI] [PubMed] [Google Scholar]
- 26. Awaisu A, Nik Mohamed M H, Mohamad Noordin N, . et al. The SCIDOTS Project: Evidence of benefits of an integrated tobacco cessation intervention in tuberculosis care on treatment outcomes. Subst Abus Treat Prev Policy 2011; 6: 1– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dogar O, Jawad M, Shah S K, . et al. Effect of cessation interventions on hookah smoking: post-hoc analysis of a cluster-randomized controlled trial. Nicotine Tob Res 2014; 16: 682– 688. [DOI] [PubMed] [Google Scholar]
- 28. Siddiqi K, Khan A, Ahmad M, Shafiq-ur-Rehman. . An intervention to stop smoking among patients suspected of TB—evaluation of an integrated approach. BMC Public Health 2010; 10: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Awaisu A, Sulaiman S A S, Mohamed M H N, . et al. Potential impact of a pilot training program on smoking cessation intervention for tuberculosis DOTS providers in Malaysia. J Public Health (Bangkok) 2010; 18: 279– 288. [Google Scholar]
- 30. Shin S S, Xiao D, Cao M, . et al. Patient and doctor perspectives on incorporating smoking cessation into tuberculosis care in Beijing, China. Int J Tuberc Lung Dis 2012; 16: 126– 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stead L F, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T.. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013; 5: CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ng N, Padmawati R S, Prabandari Y S, Nichter M.. Smoking behavior among former tuberculosis patients in Indonesia: Intervention is needed. Int J Tuberc Lung Dis 2008; 12: 567– 572. [PubMed] [Google Scholar]
- 33. Yang J J, Song M, Yoon H-S, . et al. What are the major determinants in the success of smoking cessation: results from the health examinees study. PLOS ONE 2015; 10: e0143303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vandewater E A, Park S E, Carey F R, Wilkinson A V.. Intergenerational transfer of smoking across three generations and forty-five years. Nicotine Tob Res 2014; 16: 11– 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rüge J, Ulbricht S, Schumann A, Rumpf H J, John U, Meyer C.. Intention to quit smoking: Is the partner's smoking status associated with the smoker's intention to quit? Int J Behav Med 2008; 15: 328– 335. [DOI] [PubMed] [Google Scholar]
- 36. Blok D J, de Vlas S J, van Empelen P, van Lenthe F J.. The role of smoking in social networks on smoking cessation and relapse among adults: a longitudinal study. Prev Med (Baltim) 2017; 99: 105– 110. [DOI] [PubMed] [Google Scholar]
- 37. Saari A J, Kentala J, Mattila K J.. The smoking habit of a close friend or family member—how deep is the impact? A cross-sectional study. BMJ Open 2014; 4: e003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hubbard G, Gorely T, Ozakinci G, Polson R, Forbat L.. A systematic review and narrative summary of family-based smoking cessation interventions to help adults quit smoking. BMC Fam Pract 2016; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patra J, Bhatia M, Suraweera W, . et al. Exposure to second-hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta-analysis of 18 observational studies. PLOS Med 2015; 12: e1001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dogar O F, Pillai N, Safdar N, Shah S K, Zahid R, Siddiqi K.. Second-hand smoke and the risk of tuberculosis: a systematic review and a meta-analysis. Epidemiol Infect 2015; 143: 3158– 3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Magee M J, Darchia L, Kipiani M, . et al. Smoking behavior and beliefs about the impact of smoking on anti-tuberculosis treatment among health care workers. Int J Tuberc Lung Dis 2017; 21: 1049– 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dogar O, Elsey H, Khanal S, Siddiqi K.. Challenges of integrating tobacco cessation interventions in TB programmes: case studies from Nepal and Pakistan. J Smok Cessat 2016; 11: 108– 115. [Google Scholar]
- 43. Lundahl B, Moleni T, Burke B L, . et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 2013; 93: 157– 168. [DOI] [PubMed] [Google Scholar]
- 44. Hughes J R, Stead L F, Hartmann-Boyce J, Cahill K, Lancaster T.. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2014; 1: CD000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stead L F, Koilpillai P, Fanshawe T R, Lancaster T.. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016; 3: CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lester R T, Ritvo P, Mills E J, . et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010; 376: 1838– 1845. [DOI] [PubMed] [Google Scholar]
- 47. Iribarren S, Beck S, Pearce P F, . et al. TextTB: a mixed method pilot study evaluating acceptance, feasibility, and exploring initial efficacy of a text messaging intervention to support TB treatment adherence. Tuberc Res Treat 2013; 2013: 1– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nglazi M D, Bekker L G, Wood R, Hussey G D, Wiysonge C S.. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review. BMC Infect Dis 2013; 13: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y.. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2016; 4: CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bock B, Heron K, Jennings E, . et al. A text message delivered smoking cessation intervention: the initial trial of TXT-2-Quit: randomized controlled trial. JMIR MHealth UHealth 2013; 1: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chow S-C. Adaptive clinical trial design. Annu Rev Med 2014; 65: 405– 415. [DOI] [PubMed] [Google Scholar]
- 52. Louwagie G M C, Ayo-Yusuf O A.. Predictors of tobacco smoking abstinence among tuberculosis patients in South Africa. J Behav Med 2015; 38: 472– 482. [DOI] [PubMed] [Google Scholar]
- 53. Awaisu A, Nik Mohamed M H, Noordin N, . et al. Impact of connecting tuberculosis directly observed therapy short-course with smoking cessation on health-related quality of life. Tob Induc Dis 2012; 10: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanson-Fisher R W, Bonevski B, Green L W, D'Este C.. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med 2007; 33: 155– 161. [DOI] [PubMed] [Google Scholar]
- 55. Cartwright N, Munro E.. The limitations of randomized controlled trials in predicting effectiveness. J Eval Clin Pract 2010; 16: 260– 266. [DOI] [PubMed] [Google Scholar]


