Abstract
Setting and objectives: Tobacco use compromises tuberculosis (TB) treatment outcomes. Tobacco cessation is beneficial to TB patients at the individual level and from the perspective of a larger spectrum of non-communicable diseases associated with tobacco use. We assessed feasibility, effectiveness and provider perceptions on integrating brief tobacco cessation advice into routine TB care by DOTS providers from 27 TB treatment centres run by three non-governmental organisations (NGOs) in urban India.
Design: A mixed-methods study (triangulation design) involving analysis of programme data and semi-structured interviews (quantitative) and thematic analysis of focus group discussions of TB treatment providers (qualitative) regarding brief advice and cessation support provided to self-reported tobacco users from August 2015 to July 2017.
Results: All 27 centres initiated tobacco cessation. Of 2132 registered TB patients, 377 (18%) were tobacco users, 333 (88%) of whom used smokeless tobacco. There was a progressive drop in documentation of tobacco status at each visit, reaching respectively 36% and 30% at the end of treatment for new and retreatment TB patients. Seven-day point prevalence abstinence at 6 months was 32% among new and 15% among retreatment cases. Enablers for integration included NGO collaboration, supervision and capacity building. Challenges included providers spending 15–45 min per patient (10 min recommended), multiple addictions, documentation load, self-reporting and social normalisation of tobacco.
Conclusions: Integration of tobacco cessation into routine TB care in an urban NGO setting was feasible, although without continued support, rigour in documentation declined. This should be scaled up with special attention paid to tackling smokeless tobacco and related operational challenges.
Keywords: integration, operational research, SORT IT, mixed-methods study
Abstract
Contexte : La consommation de tabac compromet les résultats du traitement de la tuberculose (TB). L'arrêt du tabac est bénéfique aux patients TB au niveau individuel et dans la perspective plus large des maladies non transmissibles associées à la consommation de tabac.
Objectif : Evaluer la faisabilité, l'efficacité et les perceptions des prestataires de soins concernant l'intégration d'un bref conseil relatif à l'arrêt du tabac dans la prise en charge de routine de la TB par les prestataires de DOTS de 27 centres de traitement de la TB gérés par trois organisations non gouvernementales (ONG) dans des zones urbaines d'Inde.
Schéma : Une étude à méthodes mixtes (schéma de triangulation) impliquant l'analyse de données de programme et des entretiens semi structurés (quantitatifs) et une analyse thématique des discussions en groupe focal de prestataires de traitement de TB (qualitatifs) relatifs à un conseil bref et à un soutien à l'arrêt du tabac offert aux fumeurs auto déclarés d'août 2015 à juillet 2017.
Résultats : Les 27 centres ont mis en route l'arrêt du tabac. Sur 2132 patients TB enregistrés, 377 (18%) étaient des fumeurs, dont 333 (88%) recouraient à du tabac sans fumée. Il y a eu une diminution progressive de la documentation de la consommation de tabac lors de chaque consultation, atteignant 36% et 30% en fin de traitement pour les patients nouveaux et ceux en retraitement, respectivement. La prévalence ponctuelle de 7 jours d'abstinence à 6 mois a été de 32% parmi les nouveaux patients et de 15% parmi les cas en retraitement. Les facteurs favorables à cette intégration ont inclus la collaboration, la supervision et le renforcement des capacités des ONG. Les défis ont inclus les 15–45 min passées par les prestataires de soins auprès de chaque patient (10 min étaient recommandées), les addictions multiples, la charge administrative, l'auto déclaration et la normalisation sociale du tabac.
Conclusion : L'intégration de l'arrêt du tabac dans la prise en charge de routine de la TB dans un contexte d'ONG urbaine s'est avérée faisable, mais sans un soutien continu, la rigueur de la documentation a diminué. Cette stratégie devrait être étendue en portant une attention particulière vis-à-vis du tabac sans fumée et des défis opérationnels.
Abstract
Marco de referencia y objetivos: El consumo de tabaco pone en peligro el desenlace del tratamiento de la tuberculosis (TB). El abandono del tabaco es útil para los pacientes con TB desde el punto de vista individual y desde la perspectiva más amplia de las enfermedades no transmisibles que se asocian con el tabaquismo. En el presente estudio se evaluó la factibilidad, la eficacia y las percepciones de los proveedores de atención de salud con respecto a la integración de un asesoramiento breve sobre el abandono del tabaco en la atención corriente de la TB, practicado por quienes proveen el DOTS en 27 centros de tratamiento de la tuberculosis de tres organizaciones no gubernamentales (ONG) en una zona urbana de la India.
Métodos: Fue este un estudio de métodos mixtos (técnica de triangulación) que comportó un análisis de los datos del programa y entrevistas semiestructuradas (evaluación cuantitativa) y análisis temáticos en sesiones de grupos de opinión con los proveedores de tratamiento antituberculoso (evaluación cualitativa), sobre el asesoramiento breve y el apoyo al abandono del tabaquismo dirigidos a los pacientes que comunicaban su consumo de tabaco; el estudio tuvo lugar de agosto del 2015 a julio del 2017.
Resultados: Los 27 centros iniciaron el apoyo al abandono del tabaquismo. De los 2132 pacientes con TB registrados, 377 (18%) eran consumidores de tabaco y de ellos 333 (88%) utilizaban el tabaco sin humo. Se observó una disminución progresiva de la verificación del tabaquismo en cada consulta y al final del tratamiento, solo se practicaba en un 36% de los casos nuevos y un 30% de los pacientes en retratamiento. La prevalencia puntual de abstinencia durante 7 días a los 6 meses fue 32% en los casos nuevos y 15% en los casos de retratamiento. Entre los factores mencionados como facilitadores de la integración se destacaron la colaboración con una ONG, la supervisión y el mejoramiento de la capacidad. Las dificultades a la integración consistieron en que cada proveedor debe utilizar 15–45 min por paciente (10 min recomendados), las adicciones múltiples, la carga que representa verificar el tabaquismo, la validación del abandono autonotificado y la normalización social del consumo de tabaco.
Conclusiones: Se demostró que es factible integrar el apoyo al abandono del tabaco en la atención corriente de la TB en un centro urbano administrado por una ONG; no obstante, sin un apoyo continuo disminuye el rigor en la documentación del consumo. Se recomienda ampliar la escala de aplicación de estas iniciativas, con una atención especial en el tabaco sin humo y las dificultades operativas.
Tobacco consumption and tuberculosis (TB) are leading global public health problems.1 Tobacco use is responsible for approximately seven million deaths annually2 and is the second overall global cause of death.3 India ranks second in tobacco consumption worldwide, with an estimated 267 million tobacco users, 75% of whom use smokeless tobacco.4 An estimated 10.4 million cases of TB and 1.8 million deaths due to TB were reported globally in 2015.5 Again, India ranks first in the number of TB cases, with 2.1 million.6 India thus faces joint epidemics of tobacco consumption and TB.1,6
The global colliding epidemics of tobacco consumption and TB, as well as their lethal interaction,1 have been described in two systematic reviews.7,8 Tobacco smoking is associated with an increased risk of tuberculous infection, reduced adherence to anti-tuberculosis treatment, higher risk of recurrent TB and increased TB mortality.7 Both smoking and TB damage the lungs, and interact at immunological and cellular level to reduce the immune response to TB, which negatively influences treatment efficacy. However, most tobacco-related immunological abnormalities are reversible within 6 weeks of smoking cessation.7,8 Smokeless tobacco use, in particular, has been associated with a higher relative risk of death from all causes.9 Tobacco cessation could therefore afford substantial benefits to TB patients. Households and communities may also profit through reduced household expenditure on tobacco products and reduced risk of contracting TB.10
In 2007, the World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (The Union) published guidelines on tobacco cessation interventions for TB patients.11,12 Such interventions are particularly important in India, where 40% of the TB burden13 and 50% of TB deaths are attributed to smoking.14
Although previous studies from India,15 Pakistan,16 Nepal,17 Bangladesh,18 Indonesia,19 Brazil,20 China21 and South Africa22 have shown encouraging results from introducing tobacco cessation advice for TB patients, most of these were restricted to government-run health care facilities. Enhanced collaboration with non-governmental organisations (NGOs) in the public sector has been emphasised in the End TB strategy, and is important for scaling up and reaching vulnerable populations.23 Although it has been demonstrated that behavioural support provided by DOTS providers is effective,16 it cannot be assumed that tobacco cessation interventions can be easily added to DOTS providers' workload as a supplementary activity, given their busy schedule.20,24 Understanding provider perceptions of this aspect would be useful in guiding interventions.25
We thus aimed to assess and understand issues around feasibility, effectiveness and provider perceptions on integrating tobacco cessation for current tobacco users in urban India. In 27 NGO-run centres with trained DOTS providers, the specific objectives were to determine 1) the number of centres that started implementing brief tobacco cessation; 2) the characteristics of TB patients who were current tobacco users, stratified by type of tobacco; and 3) tobacco use status, stratified by TB category, following tobacco cessation advice.
We also explored NGO DOTS provider perspectives as regards training and enabling factors and challenges in integrating tobacco cessation advice as an added activity in routine TB care.
STUDY POPULATION DESIGN AND METHODS
Study design
This was a mixed-methods study including a triangulation design, i.e., the use of multiple methods or data sources to develop a comprehensive understanding.26 The quantitative element involved a retrospective analysis of TB patients registered under routine programme conditions from August 2015 to July 2017, and a semi-structured questionnaire filled out by DOTS providers. The qualitative aspect involved focus group discussions (FGDs) with DOTS providers.
Study setting
General: India and Maharashtra State
India is the second-most populous country in the world, with a population of over 1.25 billion.27 Maharashtra, the second largest state in India, has a population of over 112 million. Mumbai, the capital of Maharashtra State, has a population of approximately 12 million.28
Of all adult inhabitants in Maharashtra, 27% are tobacco users, 92% of whom use smokeless tobacco.29 Smokeless tobacco includes chewing tobacco items such as betel quid with tobacco, khaini, gutkha, paan masala and other products such as mishri, gul and snuff, which are applied to the teeth and gums or inhaled.
Specific setting: The National TB Programme and NGO-run DOTS centres
The study was implemented in 27 NGO-run DOTS centres in 10 urban reporting units based in Mumbai. These 27 DOTS centres, managed by three different national NGOs, received patients for anti-tuberculosis treatment who had been diagnosed at designated microscopy centres. Anti-tuberculosis treatment was provided by trained NGO DOTS providers as per Revised National Tuberculosis Control Programme (RNTCP) guidelines.30
Study population
All TB patients enrolled in the 27 NGO-run DOTS centres from August 2015 to July 2016 were included in the study and screened for tobacco use during the first week of presenting at any NGO DOTS centre. All DOTS providers trained in tobacco cessation at the centres were included in exploring provider perspectives.
Tobacco cessation intervention
The Narotam Sekhsaria Foundation (NSF), Mumbai, India, has initiated a tobacco cessation programme called ‘LifeFirst’, which is being implemented in hospitals, primary health care centres, workplaces and schools in Maharashtra (Figure). NSF has partnered with three local NGOs running 27 DOTS centres under the RNTCP, and provided training and technical support for implementing tobacco cessation activities at the DOTS centres. The training provided DOTS providers with the necessary skills to identify current tobacco users, to document the types and patterns of tobacco use and to provide brief advice and cessation support in line with WHO32 and Union guidelines.31
FIGURE.
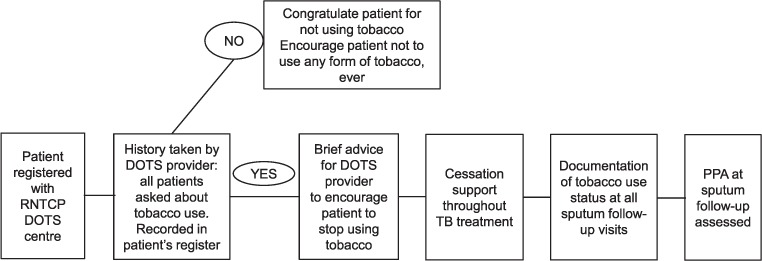
LifeFirst Tobacco cessation intervention in NGO DOTS centres under the RNTCP (adapted from ABC intervention from The Union Guide).31 RNTCP = Revised National Tuberculosis Control Programme; TB = tuberculosis; PPA = point prevalence of abstinence.
The DOTS providers obtained practical skills in a 1-day training course involving presentations, discussions and role plays. Training topics included details of tobacco use in India (prevalence, products, and patterns, health effects and benefits of quitting), national tobacco control policy, and pathways and basis of tobacco addiction. The ‘5As and 5Rs’32 were used to introduce cessation, and the ‘ABC approach’31 for the implementation of the intervention was covered in detail. It was expected that these skills would allow all trained DOTS providers to independently initiate the intervention in their own DOTS centres.10 No refresher sessions were conducted.
Adaptation of the Union guide
The Union guidelines31 for addressing smokers in a TB treatment setting were adapted to the study setting. Context-specific changes in the content of counselling, especially tailored to the sociocultural milieu of tobacco and alternative non-pharmacological products, were made to the content by a team of national and international experts in tobacco cessation and tuberculosis treatment, to make it relevant for the smokeless tobacco user population.
Given the very high prevalence of smokeless tobacco in India,4 the same was expected in the study setting. The component of smokefree homes as mentioned in the Union guide was not considered for implementation, as the objective of this component was to prevent exposure to secondhand smoke in the home, and this was not relevant to the study setting. Working towards a tobacco-free home for TB patients was also not considered, as it would have required the identification of tobacco users in the patients' homes, providing them with brief advice and cessation support and follow-up. This would have added a considerable load to the study design, and was not part of the initial study.
The time period in the definition of a current tobacco user was changed from 3 months, as suggested by the guidelines to include users who had stopped smoking due to illness, to 30 days, as patients who use smokeless tobacco do not tend to stop due to their TB symptoms before diagnosis.33
Monitoring of tobacco use status
Current tobacco users—any TB patient who had used any form of tobacco, even once, in the last 30 days—identified by verbal screening of all TB patients and registered with the DOTS centre were provided with brief cessation advice during the first week of treatment. This included tailored information about the harmful effects of tobacco use, the benefits of quitting, relevance to anti-tuberculosis treatment, tips to overcome cravings, and management of withdrawal symptoms. Each patient was followed up until the end of anti-tuberculosis treatment, and additional support was provided through face-to-face counselling at every possible opportunity during anti-tuberculosis treatment and tailored to the individual needs of the patients. The tobacco use status of all patients was documented by the DOTS providers at the scheduled sputum follow-up visits to the centres. The definitions of tobacco use status as part of the cessation intervention are shown in Table 1.
TABLE 1.
Definitions of tobacco use status as part of the cessation intervention, Mumbai, India

Data variables, sources and data collection
Quantitative data on the characteristics of TB patients (sex, age, weight, classification and type of TB, sputum reports, treatment category, treatment outcome) were sourced from the TB treatment cards and TB treatment registers. Details of tobacco use (type, products, frequency) were sourced from a dedicated tobacco cessation register. Instead of having an individual smoking cessation card, a smoking cessation register and quarterly reporting, as suggested in The Union guide,31 our study used only a cessation register, which included the name, age, treatment category and registration number for the identification of the patient and details regarding tobacco use, including type and name of product(s), frequency of use, and tobacco use status at all sputum follow-up examinations, with dates; the register was filled out by the DOTS providers.
A pre-tested, semi-structured, self-administered questionnaire was used to gather information on perceptions about training in tobacco cessation and knowledge, skills and level of confidence. This was assessed using an ordinal Likert (scored) scale.34 Qualitative data were collected 6 months after the 1-day training course for DOTS providers. Three FGDs with 8–10 participants in each group, involving all DOTS providers, were conducted to gather information on enabling factors and challenges. A topic guide was used to facilitate the discussions, which were held in local languages (Marathi and Hindi). Discussions were conducted at the NSF meeting room, and were moderated by a trained, experienced interviewer. Transport to and from the meeting venue was offered and refreshments were provided. A note taker took handwritten notes, and the discussions were audio-recorded after obtaining informed consent. At the end of each FGD, a summary of the discussions was shared with the participants for their validation. Audio recordings of the FGDs were transcribed and translated into English on the same day.
Analysis and statistics
The principal aspects used for assessing feasibility included the number of DOTS centres that managed to start and sustain the cessation intervention, completeness of documentation, and provider perspectives on enablers and challenges in the integration of tobacco cessation and TB treatment activities. The primary outcome measure for effectiveness was the 7-day self-reported point prevalence abstinence (PPA) from tobacco use at the end of anti-tuberculosis treatment. Enabling factors and challenges faced by providers focused on the integration of the tobacco cessation intervention into routine TB care.
EpiData software was used for quantitative data entry and analysis (v 3.1 for entry and v 2.2.2.183 for analysis; EpiData Association, Odense, Denmark). Data were double-entered and validated. Proportions were used to express results; the χ2 test for trend was used to assess linear trends and reported in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.35 There were no missing data.
For qualitative data, a thematic analysis approach was used and reported in line with COREQ (Consolidated criteria for reporting qualitative) guidelines.36 Notes from the FGDs were read and coded independently by the first two authors (HAG and RZ), and discussed with two independent persons for consensus. Clusters of linked codes were grouped into categories, emergent themes and verbatim quotes. Data coding and analysis were done manually.
Ethical considerations
Ethical approval of the study protocol was obtained from the NSF Joint Ethics Committee, Mumbai, India, and the Ethics Advisory Group of The Union, Paris, France. Written informed consent was provided by all DOTS providers who participated in filling out the semi-structured questionnaire and the FGDs. Consent was also obtained for audio recording.
RESULTS
Feasibility: DOTS centres implementing brief tobacco cessation intervention and patient characteristics
The tobacco cessation intervention was implemented in all the 27 NGO DOTS centres within 1 month of training, and was sustained thereafter. Tables 2 and 3 show the demographic and clinical characteristics of TB patients who were current tobacco users, stratified by type of tobacco.
TABLE 2.
Current tobacco use among TB patients, stratified by demographic and clinical characteristics, in 27 NGO-run TB centres in Mumbai, India, August 2015–July 2017
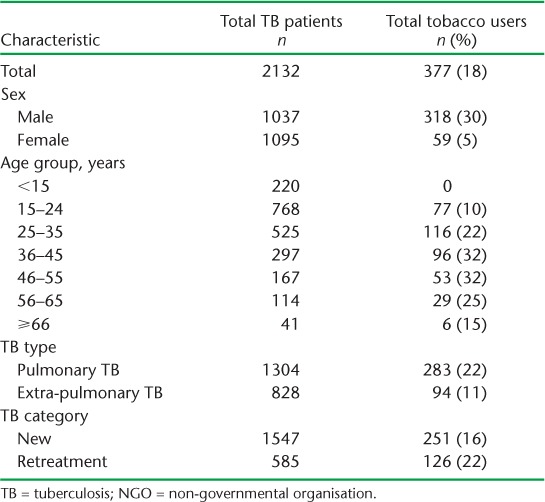
TABLE 3.
Demographic and clinical characteristics of TB patients who are current tobacco users, stratified by type of tobacco used, in 27 NGO-run TB centres in Mumbai, India, August 2015–July 2017
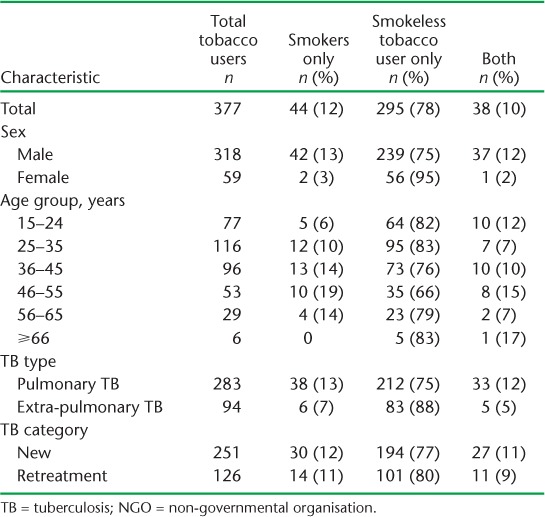
Of 2132 TB patients registered for treatment at the 27 DOTS centres, 377 (18%) were current tobacco users. Of these, 84% were males, with smokeless tobacco constituting 88% of overall tobacco use. The prevalence of tobacco use in the 15–24 years age group was 10%. Tobacco use was higher among pulmonary (22%) and retreatment (22%) TB patients. Smokeless tobacco use was equally predominant when stratified by TB type and category.
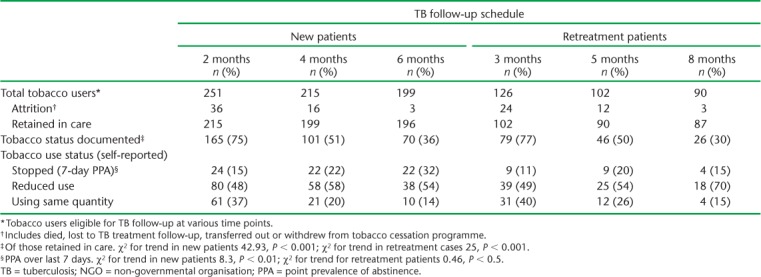
Table 4 shows tobacco use status stratified by category of TB and in accordance with the TB follow-up visit schedule. For new TB patients, there was a progressive drop in documentation of follow-up tobacco status, from 77% at 2 months to 36% at the end of anti-tuberculosis treatment (χ2 for trend 42.93, P < 0.001). For retreatment TB, the drop was similar, from 77% at 3 months to 30% at the end of treatment (χ2 for trend 25, P < 0.001).
TABLE 4.
Tobacco use status after brief advice on tobacco cessation among current tobacco users stratified by TB category in 27 NGO-run TB centres in Mumbai, India, August 2015–July 2017
Effectiveness: Tobacco use status after brief advice and cessation support for tobacco cessation
The 7-day PPA, i.e., the proportion of individuals who self-reported having stopped tobacco use during the last 7 days before assessment, increased from 15% at 2 months to 32% at 6 months in new TB patients (χ2 for trend 8.3, P < 0.01), and from 11% at 3 months to 15% at 8 months in retreatment cases (χ2 for trend 0.46, P < 0.5).
Of the 96 patients whose tobacco use status was documented at the end of treatment, 80 (83%) were smokeless tobacco users, in line with the proportion among all tobacco users. Of these, 26 reported not using tobacco at the end of treatment (PPA = 33%). Among smokers and dual tobacco users, no patients reported not using tobacco at the end of treatment. All 26 (100%) smokeless tobacco users who stopped using tobacco had a successful TB treatment outcome; this proportion was 89% among those who did not report stopping tobacco use.
Provider perceptions
Knowledge, skills and confidence of DOTS providers
All DOTS providers at the 27 centres (20 females and 5 males, mean age 38 years, range 20–61) provided information. In terms of educational status, 16% had completed primary school, 60% secondary school and 24% had studied beyond secondary school level.
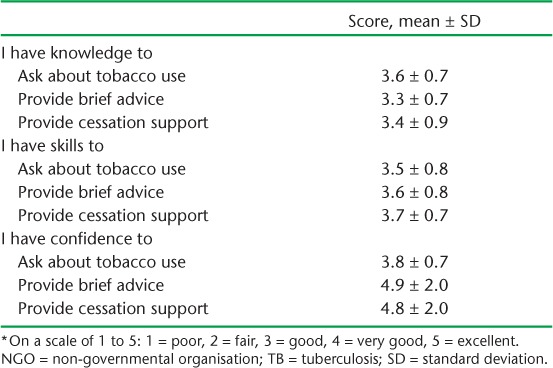
Table 5 shows the perceptions of the DOTS providers of their knowledge, skills and confidence in asking about tobacco use, providing brief advice and ensuring cessation support 6 months after the 1-day training course. Scores ranged between good (score = 3/5) and very good (score = 4/5) in all parameters.
TABLE 5.
Provider perceptions of their knowledge, skills and level of confidence (scale from 1 to 5) * 8 months after initial training in providing brief tobacco cessation advice at 27 NGO-run TB centres in Mumbai, India, July 2016
Enabling factors and challenges in integrating cessation advice into TB care
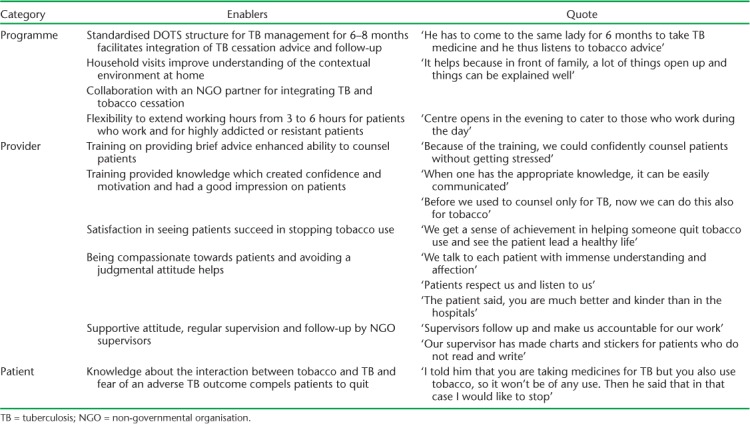
Factors enabling the integration of cessation advice into routine TB care as expressed by DOTS providers during the FGDs are given in Table 6. Enablers were categorised according to programme-, provider- and patient-level factors. The structured DOTS approach to TB management, collaboration with NGOs, capacity building and supervision support provided to DOTS providers were highlighted as important enabling factors.
TABLE 6.
Enabling factors according to providers in integrating tobacco cessation advice into routine TB care at 27 NGO-run TB centres in Mumbai, India, July 2016
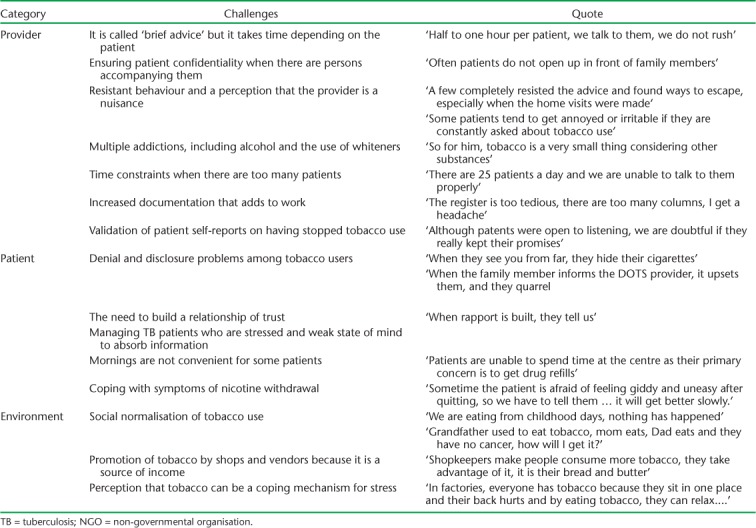
Challenges faced by DOTS providers in integrating tobacco cessation into their routine TB activity are given in Table 7. These included provider-, patient- and environmental-level factors. The need to provide more than the recommended 10 min per patient for giving advice, managing patients with multiple addictions, high documentation load and validation of self-reported tobacco cessation were highlighted. Social and cultural normalisation of tobacco use were also considered a challenge.
TABLE 7.
Challenges expressed by providers in integrating tobacco cessation advice into routine TB care at 27 NGO-run TB centres in Mumbai, India, July 2016
Suggestions by DOTS providers for improving the integration of tobacco cessation and TB care activities
The following suggestions were given by DOTS providers to improve the integration of tobacco cessation into routine TB care:
Refresher training courses and support on a bi-monthly basis to highlight and address new challenges and identify solutions
Capacity building on how to address support for individuals who also use alcohol, whiteners (correction fluid) and other drugs in a holistic manner
Provision of support tools for counselling that avoids stereotyped verbal advice at each encounter. Specific suggestions included use of informative charts, games and entertainment, including peer-led sessions for awareness and advocacy
Introduction of group information/counselling sessions led by physicians as a means of reinforcing the brief advice given by DOTS providers
Simplification of the documentation/registers to avoid time-consuming paper-based monitoring
The possibility of referral to experts for difficult patients and/or people with multiple addictions.
DISCUSSION
The integration of tobacco cessation interventions at the 27 NGO-run DOTS centres following a 1-day training course yielded encouraging results. All DOTS centres were able to start the intervention and sustain it thereafter. A progressive trend in quit rates was observed during the treatment period, although the quality of documentation declined. DOTS providers also felt that they had acquired the necessary skills needed to implement the intervention.
The study highlights the important role NGOs can play in integrating tobacco cessation interventions within the framework of routine TB care. In a country such as India, which is struggling to cope with an overwhelming TB burden, this is a welcome inclusive approach. Such involvement also embraces the new vision of the End TB Strategy, which promotes greater NGO collaboration.23
A universal revelation by DOTS providers was that the 5–10 min recommended for brief advice and cessation support31 for tobacco cessation was insufficient in this setting. The time actually spent ranged between 15 and 45 min, and had to be tailored to individual patient needs, problems of disclosure and the presence of multiple addictions. In reality, the intended ‘brief advice’ may thus not be very ‘brief’. This finding may have implications for busy TB centres, and merits further evaluation.
Our study had several strengths: we included three NGOs, all their centres and all their DOTS providers; our study is thus likely to be representative of the NGO context on the ground. We also assessed the cessation intervention in relation to all forms of tobacco use, which is unique. Furthermore, data from registers were cross-checked against patient cards, double-entered and validated, and tobacco use status was standardised. We therefore believe the study data to be robust. In addition, due to the simple documentation formats and Excel-based calculations, it is possible for any programme supervisor to conduct basic analysis for monitoring and reporting if the intervention is replicated. Finally, we used a triangulation design to link quantitative and qualitative information; all reporting was in accordance with STROBE35 and COREQ36 guidelines.
The main study limitations are that we may have underestimated the prevalence of tobacco use due to the unwillingness of patients to disclose their true status; our reliance on self-reported tobacco use status may therefore have resulted in responder bias, which may have influenced the true status of declared outcomes. Unfortunately, biochemical validation methods, such as the cotinine test,11 were not available. The study was conducted as operational research under programme conditions, and was not designed as a randomised control trial; it was therefore prone to confounding by factors outside of the intervention that could potentially have accounted for observed changes. Despite these limitations, the study findings have a number of implications for policy and practice.
First, the prevalence of tobacco use in our study was 18%, lower than that reported in the general population,29 and lower than that of TB patients in Gujarat (46.3%)15 and Kerala (71.2%).37 It is therefore possible that we underestimated the prevalence in our setting. It is possible that the patients in our NGO setting were different in terms of their willingness to fully disclose their level of tobacco use and would require specific counselling before receiving brief advice. DOTS providers emphasised the need for additional time to establish a relationship with their patients and gain their trust. As the additional time spent equates to an increase in workload for DOTS providers, we would require dedicated counsellors to respond to this need. This aspect merits specific evaluation.
Second, almost nine in ten tobacco users preferred smokeless tobacco only. This highlights the need to address tobacco consumption from a wider perspective than the traditional one, which focuses on smoking cessation. The association between the use of smokeless tobacco and its implications for TB and TB treatment outcomes needs to be studied further. The problem of smokeless tobacco consumption, which is accentuated by its sociocultural acceptance in India, needs to be addressed. In addition to enhanced awareness campaigns, there is an urgent need to conceive specific cessation strategies for smokeless tobacco which do not exist as yet. Such strategies will need feasibility assessment through operational research, and may bear fruit both within and beyond the framework of TB.
Third, we observed a progressive increase in the proportion of individuals who stopped tobacco use during TB treatment: 32% among new patients and 15% among those on retreatment. The cessation intervention was thus effective and beneficial to individual patients. These quit rates are lower than those reported from Bangladesh,21 China,18 Sudan,38 Indonesia19 (only smokers) and Gujarat, India15 (including smokeless tobacco users also). In addition, there was a steady decline in the quality of documentation related to the brief advice and cessation support provided by DOTS providers. This may be attributed to the observational nature of the study, as there were no refresher training courses during the implementation phase. Providing brief advice and tobacco cessation support to TB patients thus not only affects TB treatment outcomes, it can also have a protective effect against the entire range of non-communicable diseases associated with tobacco use.
A shortcoming of this study is that our figures were derived only from those patients whose tobacco status was documented. As completeness of data documentation declined during TB follow-up visits, there is a possibility of ascertainment bias. Only one other study, from China, has highlighted similar lapses in documentation as an operational problem.21 The quantification of tobacco use and its documentation was added to support the counselling effort and to monitor patient progress. This led to an additional outcome, ‘reduced tobacco use’, at the end of treatment, which was used to motivate patients to further reduce and stop tobacco use. However, it might be difficult to continue using this parameter given the limited availability of time and the need to maintain simplicity in recording in a programme setting. The issue of time-consuming documentation adding to routine workload was highlighted by providers and may be the reason for this finding. Ways forward to simplify documentation and improving continued supervision are needed.
Finally, DOTS providers made a number of important recommendations to cope with ongoing operational challenges, including continued capacity building, management of patients with multiple addictions, the possibility of referring patients and the need to simplify documentation. These operational aspects will need serious attention.
CONCLUSION
In an urban NGO setting in India, the integration of tobacco cessation interventions into a framework of routine TB care was feasible, brought individual patient benefits and was well accepted by providers. The approach merits further scale-up, especially among smokeless tobacco users, with due attention being paid to operational issues such as flexible timing, ongoing training and support and dealing with other addictions. From a broader health systems perspective, similar initiatives for the integration of tobacco cessation into other relevant national programmes should be considered.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and Médecins Sans Frontières (MSF/Doctors Without Borders), Luxembourg. The specific SORT IT programme that resulted in this publication was jointly developed and implemented by The Union South-East Asia Office (New Delhi, India); the Centre for Operational Research, The Union (Paris, France); the Operational Research Unit (LUXOR), MSF Brussels Operational Center (Luxembourg); the Department of Preventive and Social Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research (Puducherry); the Department of Community Medicine, Sri Manakula Vinayagar Medical College and Hospital (Puducherry); the Department of Preventive and Social Medicine, Medical College Baroda (Vadodara); and the National Institute for Research in Tuberculosis (Chennai, India).
The authors express their sincere thanks to all the patients, all the DOTS providers and staff of the three non-governmental organisations (Maharashtra Janavikas Kendra, Lok Seva Sangam and Peoples Association for Training and Health, Mumbai, India) for their participation in the study, and M D'Costa for her help in data collection. The training programme and open access publication costs were funded by the Department for International Development (London, UK), The Union, MSF and La Fondation Veuve Emile Metz-Tesch (Luxembourg). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The training of DOTS providers and qualitative data collection among the DOTS providers was supported by Narotam Sekhsaria Foundation, Mumbai, India.
Footnotes
Conflicts of interest: none declared.
References
- 1. Pai M, Mohan A, Dheda K, . et al. Lethal interaction: the colliding epidemics of tobacco and tuberculosis. Expert Rev Anti Infect Ther 2007; 5: 385– 391. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. . WHO report on the global tobacco epidemic, 2017. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 3. Eriksen M, Mackay J, Schluger N, Islami G, Farhad H, Drope J.. The tobacco atlas. Atlanta, GA, USA: American Cancer Society, 2015. [Google Scholar]
- 4. Ministry of Health and Family Welfare. . Government of India. Global Adult Tobacco Survey–2. Fact sheet India 2016–17. New Delhi, India: MHFW, 2017. [Google Scholar]
- 5. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 6. World Health Organization. . Global tuberculosis report, 2015. WHO/HTM/TB/2015.22 Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 7. Schneider N K, Novotny T E.. Addressing smoking cessation in tuberculosis control. Bull World Health Organ 2007; 85: 820– 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slama K, Chiang C-Y, Enarson D.. Helping patients to stop smoking. Int J Tuberc Lung Dis 2007; 11: 733– 738. [PubMed] [Google Scholar]
- 9. Gupta P C, Pednekar M S, Parkin D M, Sankaranarayanan R.. Tobacco-associated mortality in Mumbai (Bombay) India. Results of the Bombay Cohort Study. Int J Epidemiol 2005; 34: 1395– 1402. [DOI] [PubMed] [Google Scholar]
- 10. Slama K, Chiang C-Y, Enarson D.. Tobacco cessation and brief advice. Int J Tuberc Lung Dis 2007; 11: 612– 616. [PubMed] [Google Scholar]
- 11. WHO & International Union Against Tuberculosis and Lung Disease. . A WHO/The Union monograph on TB and tobacco control: joining efforts to control two related global epidemics. WHO/HTM/TB/2007.390 Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 12. Slama K, Chiang C-Y, Enarson D.. Introducing brief advice in tuberculosis services. Int J Tuberc Lung Dis 2007; 11: 496– 499. [PubMed] [Google Scholar]
- 13. World Health Organization. . WHO Factsheet. Tuberculosis and tobacco. Geneva, Switzerland: WHO, 2009. [Google Scholar]
- 14. Gajalakshmi V, Peto R, Kanaka T S, Jha P.. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43 000 adult male deaths and 35 000 controls. Lancet 2003; 362: 507– 515. [DOI] [PubMed] [Google Scholar]
- 15. Kaur J, Modi B, Sachdeva K, . et al. Promoting tobacco cessation by integrating ‘brief advice’ in tuberculosis control programme. World Health Organ South-East Asia J Public Health 2013; 2: 28– 33. [DOI] [PubMed] [Google Scholar]
- 16. Siddiqi K, Khan A, Ahmad M. et al. Action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trial. Ann Intern Med 2013; 158: 667– 675. [DOI] [PubMed] [Google Scholar]
- 17. Campbell I A, Chaudhary R D, Holdsworth G M C, Lyne O D.. Brief advice to tuberculosis patients in Nepal to stop smoking: a pilot study by the Britain Nepal Medical Trust. Int J Tuberc Lung Dis 2014; 18: 1438– 1442. [DOI] [PubMed] [Google Scholar]
- 18. Siddiquea B N, Islam M A, Bam T S, . et al. High quit rate among smokers with tuberculosis in a modified smoking cessation programme in Dhaka, Bangladesh. Public Health Action 2013; 3: 243– 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bam T, Aditama T, Chiang C-Y, Rubaeah R, Suhaemi A.. Smoking cessation and smokefree environments for tuberculosis patients in Indonesia-a cohort study. BMC Public Health 2015; 15: 604– 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sereno A B, Soares E C C, Lapa E Silva J R, . et al. Feasibility study of a smoking cessation intervention in directly observed therapy short-course tuberculosis treatment clinics in Rio de Janeiro, Brazil. Rev Panam Salud Publica 2012; 32: 451– 456. [PubMed] [Google Scholar]
- 21. Lin Y, Wang L-X, Qiu L-X, . et al. A smoking cessation intervention among tuberculosis patients in rural China. Public Health Action 2015; 5: 183– 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louwagie G M C, Okuyemi K S, Ayo-Yusuf O A.. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: a randomized controlled trial. Addiction 2014; 109: 1942– 1952. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization. . The End TB Strategy. WHO/HTM/TB/2015.19 Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 24. Dogar O, Elsey H, Khanal S, Siddiqi K.. Challenges of integrating tobacco cessation interventions in TB programmes: case studies from Nepal and Pakistan. J Smok Cessat 2017; 11: 108– 115. [Google Scholar]
- 25. Siddiqi K, Lee C K.. An integrated approach to treat tobacco addiction in countries with high tuberculosis incidence. Trop Med Int Health 2009; 14: 420– 428. [DOI] [PubMed] [Google Scholar]
- 26. Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville A J.. The use of triangulation in qualitative research. Oncol Nurs Forum 2014; 41: 545– 547. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization. . Global tuberculosis report, 2014. WHO/HTM/TB/2014.08 Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 28. Office of the Registrar General & Census Commissioner, Ministry of Home Affairs, Government of India. . List of districts of Maharashtra. New Delhi, India: Ministry of Home Affairs, 2011. http://www.census2011.co.in/census/state/districtlist/maharashtra.html Accessed May 2018. [Google Scholar]
- 29. Ministry of Health and Family Welfare, Government of India. . Global Adult Tobacco Survey–2: India 2016–17. Maharashtra Factsheet. New Delhi, India: Ministry of Health and Family Welfare, 2017. [Google Scholar]
- 30. Central TB Division, Directorate of Health Services, Ministry of Health & Family Welfare, Government of India. . Technical and operational guidelines for tuberculosis control. New Delhi, India: Ministry of Health and Family Welfare, 2005. [Google Scholar]
- 31. Bissell K, Fraser T, Chiang C-Y, Enarson D A.. Smoking cessation and smoke-free environments for tuberculosis patients. 2nd ed Paris, France: International Union Against Tuberculosis and Lung Disease, 2010. [Google Scholar]
- 32. World Health Organization. . Toolkit for delivering the 5As and 5Rs brief tobacco interventions to TB patients in primary care. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 33. Deepak K G, Daivadanam M, Pradeepkumar A S, Mini G K, Thankappan K R, Nichter M.. Smokeless tobacco use among patients with tuberculosis in Karnataka: the need for cessation services. Natl Med J India 2012; 25: 142– 145. [PubMed] [Google Scholar]
- 34. Martin B A, Bruskiewitz R H, Chewning Betty A.. Effect of a tobacco cessation continuing professional education program on pharmacists' confidence, skills, and practice-change behaviors. J Am Pharm Assoc 2010; 50: 9– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P C, Vandenbroucke J P.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495– 1499. [DOI] [PubMed] [Google Scholar]
- 36. Tong A, Sainsbury P, Craig J.. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007; 19: 349– 357. [DOI] [PubMed] [Google Scholar]
- 37. Pradeepkumar A S, Thankappan K R, Nichter M.. Smoking among tuberculosis patients in Kerala, India: proactive cessation efforts are urgently needed. Int J Tuberc Lung Dis 2008; 12: 1139– 1145. [PubMed] [Google Scholar]
- 38. El Sony A, Slama K, Salieh M, . et al. Feasibility of brief tobacco cessation advice for tuberculosis patients: a study from Sudan. Int J Tuberc Lung Dis 2007; 11: 150– 155. [PubMed] [Google Scholar]


