Abstract
Aims
Optimal blood pressure for prevention of cardiovascular (CV) events in patients with Type 2 diabetes mellitus (T2DM) remains uncertain and there is concern for increased risk with low diastolic blood pressure (DBP). This study analysed the association between blood pressure and CV outcomes in high-risk patients with T2DM.
Methods and results
Patients with T2DM and elevated CV risk were enrolled in the Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus—Thrombolysis in Myocardial Infarction 53 trial. Cardiovascular outcomes were compared in the biomarker subgroup (n = 12 175) after stratification by baseline systolic blood pressure (SBP) and DBP. Adjusted risk was calculated by blood pressure stratum using clinical covariates plus N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin-T (hsTnT). Trends were tested using linear and quadratic models. Adjusted risk of the composite endpoint of CV death, myocardial infarction (MI), or ischaemic stroke showed U-shaped relationships with baseline SBP and DBP (Pquadratic ≤ 0.01) with nadirs at SBP 130–140 or DBP 80–90 mmHg. Diastolic blood pressure <60 mmHg was associated with increased risk of MI (adjusted hazard ratio 2.30; 95% confidence interval 1.50–3.53) relative to DBP 80–90 mmHg. Adjusted odds of hsTnT concentration ≥14 ng/L showed U-shaped relationships with SBP and DBP (Pquadratic ≤ 0.01). The relationships between low DBP, elevated hsTnT, and increased MI remained after exclusion of patients with prior heart failure or NT-proBNP >median, suggesting that the relationship was not due to confounding from diagnosed or undiagnosed heart failure.
Conclusions
In patients with diabetes and elevated CV risk, even after extensive adjustment for underlying disease burden, there was a persistent association for low DBP with subclinical myocardial injury and risk of MI.
Keywords: Hypertension, Diabetes, Blood pressure, Biomarkers
Introduction
Type 2 diabetes mellitus (T2DM) and hypertension are two of the most powerful risk factors for adverse cardiovascular (CV) events.1–3 Control of blood pressure has been a central focus of national and international guidelines for the prevention of myocardial infarction (MI), stroke, and other CV morbidities.3–8 Controversy persists, however, regarding appropriate systolic blood pressure (SBP) and diastolic blood pressure (DBP) goals, with specific recommendations for patients with diabetes, atherosclerotic CV disease, advanced age, and renal insufficiency.3–7,9
While epidemiologic observations support a linear relationship between SBP and adverse CV events beginning at the pre-hypertension stage,3,10,11 there have been only limited randomized trial data to support aggressive blood pressure lowering, particularly in patients with T2DM.3,4 The optimal blood pressure for prevention of CV events in patients with T2DM remains uncertain.
There is additional concern that aggressive blood pressure reduction may result in harmfully low DBP.12–14 A U-shaped relationship between DBP and CV outcomes has been shown previously, though with potential for residual confounding as an important driver of this association.12–19 Elevated pulse pressure (PP), calculated as SBP minus DBP, is similarly associated with adverse CV outcomes by several potential mechanisms.13
High-sensitivity troponin-T (hsTnT), as a marker of subclinical myocardial injury, and N-terminal pro-B-type natriuretic peptide (NT-proBNP), as a marker of hemodynamic stress, are strong predictors of adverse CV events.20,21 Measurement of these biomarkers can add further nuance to the associations between SBP, DBP, PP, and clinical outcomes.12
The relationships between blood pressure, CV outcomes, and hsTnT/NT-proBNP have not been explored in a contemporary population of patients with T2DM. The Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus (SAVOR)—Thrombolysis in Myocardial Infarction (TIMI) 53 trial22,23 provides an opportunity to do so in a large cohort of diabetic patients with biomarker samples and prospectively recorded and adjudicated outcomes.
Methods
Study design and participants
The design, baseline patient characteristics, and primary results of the SAVOR-TIMI 53 trial (NCT01107886) have been reported previously.22–24 The SAVOR-TIMI 53 trial was a randomized, controlled, double-blind, event-driven trial enrolling patients with T2DM (hemoglobin A1c 6.5–12.0%), and either established CV disease or multiple CV risk factors. Major exclusion criteria included the use of an incretin-based antihyperglycemic therapy within the past 6 months, end-stage renal disease on haemodialysis, serum creatinine concentration greater than 6.0 mg/dL, uncontrolled CV risk factors, and significant non-CV comorbidities which would limit a participant’s ability to complete the study as designed.22,23 Patients were not excluded for prior heart failure. Written informed consent was provided by all study participants.
Endpoints
The primary major adverse cardiovascular event (MACE) endpoint of the trial was the composite of CV death, MI, or ischaemic stroke. An independent, blinded clinical events committee adjudicated each component of the primary endpoint as well as hospitalization for heart failure.22,23
Blood pressure measurement and management
Blood pressure was measured for each subject at the first study visit. Two measurements were taken with the patient seated, 5 min apart. Neither the type of sphygmomanometer nor the presence/absence of study staff in the room with the patient during blood pressure measurement was specified. The two systolic and DBPs recorded for each patient were averaged to create a single SBP and DBP per patient. Blood pressure was managed throughout the trial by treating physicians according to local practice guidelines.
Biomarker measurement
A subset of 12 180 patients had biomarker samples drawn at study entry. Twelve thousand, one hundred and seventy-five of these patients had SBP and DBP values available at baseline. Venous blood samples were stored at the participating site at −20° to −80°C prior to shipment to the TIMI Clinical Trials Laboratory (Boston, MA), where they were stored at −80°C or colder. High-sensitivity troponin-T concentration was measured with an electrochemiluminescent immunoassay (Roche Diagnostics) which is now the first high-sensitivity troponin assay approved for clinical use in the USA.25 N-terminal pro-B-type natriuretic peptide was measured using a sandwich immunoassay (proBNP II; Roche Diagnostics). All measurements were performed by laboratory personnel blinded to treatment allocation and clinical outcome.
Statistical analysis
Analyses were performed in the 12 175 patients included in the biomarker subgroup with baseline blood pressure values recorded. Baseline patient characteristics were recorded by bin of SBP (≤110, >110–120, >120–130, >130–140, >140–150, and >150 mmHg) and DBP (<60, 60–<70, 70–<80, 80– <90, and ≥90 mmHg), and PP quartile. Continuous variables were described by median and interquartile range and categorical variables were described by percentage. Baseline variables were compared between blood pressure bins with the Kruskal–Wallis test for continuous variables or the χ2 test for categorical variables.
Using Cox models, adjusted hazard ratio (HRadj) was calculated by SBP and DBP bins and PP quartile for MACE and its components. These multivariable models were adjusted for age, sex, race, history of heart failure, prior MI, history of hypertension, history of dyslipidaemia, current smoker, duration of T2DM (< 5 years, 5–9 years, 10–14 years, 15–19 years and ≥20 years), hemoglobin A1c (continuous), estimated glomerular filtration rate (continuous), treatment arm, NT-proBNP (continuous), and hsTnT (continuous), and stratified by established CV disease vs. multiple risk factors alone.
Median hsTnT concentration was calculated by baseline SBP, DBP, and PP. Additionally, the percent of patients with hsTnT concentration >14 ng/L was calculated for each blood pressure stratum. Adjusting for the clinical covariates described above, multivariable logistic regression was employed to estimate odds of hsTnT concentration >14 ng/L.
As sensitivity analyses, these calculations were repeated excluding patients with prior heart failure and with stratification by NT-proBNP concentration ≤ or > median. Adjusted and unadjusted trend tests were used to identify any linear or quadratic relationships across categories of blood or PP, assuming the categories were equally spaced. Likelihood ratio tests were employed to compare the quadratic and linear trend models. Finally, as a quality measure, the fractions of SBP and DBP recordings that were multiples of 5 or 10 were recorded.
All analyses were performed with a statistical software package (SAS version 9.4, SAS institute, Cary, NC, USA). A two-sided P-value of 0.05 was considered significant for all tests. All analyses were performed by the TIMI Study Group and the authors take full responsibility for the integrity of the database and the analyses.
Results
Baseline patient characteristics
Among the 12 175 patients included, 938 primary endpoint events, including 407 CV deaths, 426 MI, and 224 ischaemic strokes, occurred. Supplementary material online, Table S1 provides baseline patient characteristics by bin of SBP (≤110, >110–120, >120–130, >130–140, >140–150, and >150 mmHg). Compared with patients with low baseline SBP, patients with the highest SBP tended to be older (66 vs. 63 years) with a greater proportion having a pre-existing diagnosis of hypertension (92.2% vs. 64.5%), female sex (38.2% vs. 29.2%), and prior ischaemic stroke (13.5% vs. 9.6%). Supplementary material online, Table S2 provides baseline patient characteristics by DBP bin (<60, 60–<70, 70–<80, 80–<90, and ≥90 mmHg), and see Supplementary material online, Table S3 by PP quartile.
Cardiovascular outcomes by systolic blood pressure, diastolic blood pressure and pulse pressure
Systolic blood pressure
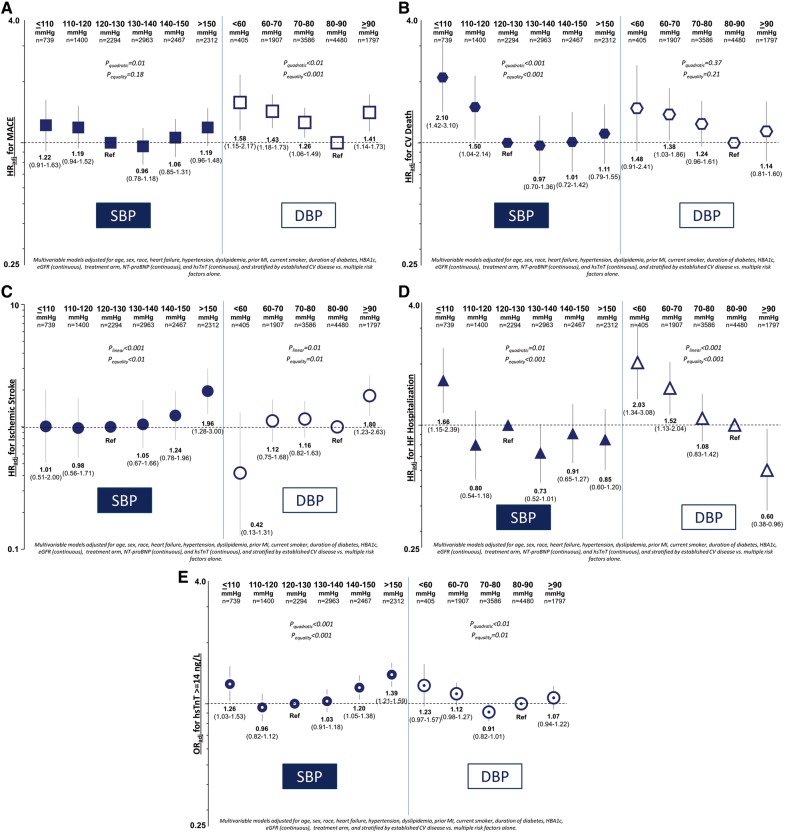
Baseline SBP showed U-shaped relationships with MACE and CV death after multivariable adjustment with a nadir at >130–140 mmHg (Figure 1A and B). The risk of MACE for SBP ≤110 mmHg was HRadj 1.22 [95% confidence interval (CI) 0.91–1.63] relative to SBP >120–130 mmHg and was HRadj 1.19 (95% CI 0.96–1.48) for SBP >150 mmHg (Pquadratic = 0.01; Figure 1A). Relative to SBP >120–130, the risk of CV death was HRadj 2.10 (95% CI 1.42–3.10) for SBP ≤110 mmHg and HRadj 1.11 (95% CI 0.79–1.55) for SBP >150 mmHg (Pquadratic < 0.001; Figure 1B). There was no statistically significant relationship between SBP and MI (Plinear = 0.20 and Pquadratic = 0.58; Take home figure), whereas there was a direct linear relationship for ischaemic stroke, with HRadj 1.96 (95% CI 1.28–3.00) for SBP >150 relative to >120–130 mmHg (Plinear < 0.001; Figure 1C). There was a U-shaped relationship between SBP and hospitalization for heart failure (Pquadratic = 0.01; Figure 1D).
Figure 1.
Adjusted risk for major adverse cardiovascular event (A), cardiovascular death (B), ischaemic stroke (C), hospitalization for heart failure (D), and high-sensitivity troponin-T ≥14 ng/L (E) by systolic blood pressure and diastolic blood pressure strata. CV, cardiovascular; DBP, diastolic blood pressure; hsTnT, high-sensitivity troponin-T; MACE, major adverse cardiovascular events; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure; HR, hazard ratio.
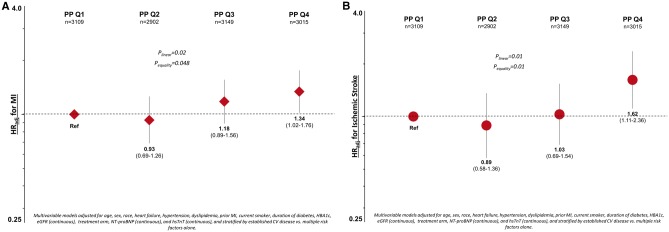
Figure 2.
Adjusted risk for myocardial infarction (A) and ischaemic stroke (B) by pulse pressure quartile. CV, cardiovascular; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PP, pulse pressure; hsTnT, high-sensitivity troponin-T; HR, hazard ratio.
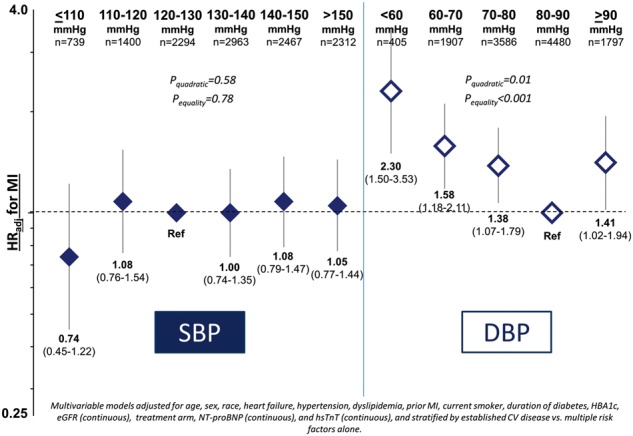
Take home figure.

Adjusted risk for myocardial infarction by systolic and diastolic blood pressure strata. CV, cardiovascular; DBP, diastolic blood pressure; hsTnT, high-sensitivity troponin-T; MACE, major adverse cardiovascular events; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure; HR, hazard ratio.
Diastolic blood pressure
Baseline DBP showed U-shaped relationships with MACE and MI after multivariable adjustment with a nadir at DBP 80–<90 mmHg (Figure 1A and Take home figure). Relative to DBP 80–<90 mmHg, the risk of MACE was HRadj 1.58 (95% CI 1.15–2.17) for DBP <60 mmHg and HRadj 1.41 (95% CI 1.14–1.73) for DBP ≥90 mmHg (Pquadratic < 0.01; Figure 1A). The risk of CV death was HRadj 1.48 (95% CI 0.91–2.41) for DBP <60 mmHg, HRadj 1.38 (95% CI 1.03–1.86) for DBP 60–<70 mmHg, and HRadj 1.14 (95% CI 0.81–1.60) for DBP ≥90 mmHg (Pquadratic = 0.37; Figure 1B). For MI, the adjusted risk was HRadj 2.30 (95% CI 1.50–3.53) for DBP <60 mmHg and HRadj 1.41 (95% CI 1.02–1.94) for DBP ≥90 mmHg (Pquadratic = 0.01; Take home figure). There was a direct linear relationship for ischaemic stroke, with HRadj 1.80 (95% CI 1.23–2.63) for DBP ≥90 relative to 80–<90 mmHg (Plinear = 0.01; Figure 1C). Risk for hospitalization for heart failure was highest with DBP <60 mmHg and decreased linearly with increasing DBP (Plinear < 0.001; Figure 1D).
Pulse pressure
Increasing PP was associated with a higher risk of MI (Plinear = 0.02) and ischaemic stroke (Plinear = 0.01; Figure 2). There was no statistically significant relationship between PP and MACE (see Supplementary material online, Table S4).
Subclinical myocardial injury
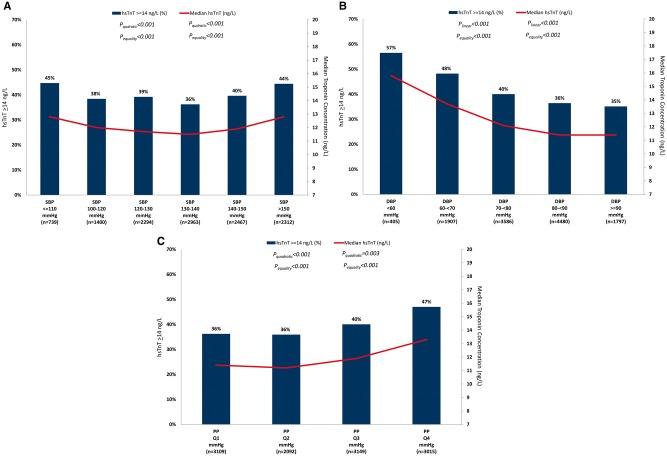
Median hsTnT concentration and the percent of patients with hsTnT concentration ≥14 ng/L showed U-shaped relationships with SBP and inverse linear relationships with DBP (Figure 3A and B). After multivariable adjustment, the odds of hsTnT concentration ≥14 ng/L showed U-shaped relationships with both SBP (Pquadratic < 0.001) and DBP (Pquadratic < 0.01; Figure 1E). There was a linear increase in the odds of elevated hsTnT with increasing PP (Plinear < 0.001; see Supplementary material online, Table S4).
Figure 3.
Median high-sensitivity troponin-T concentration and percent of patients with high-sensitivity troponin-T ≥14 ng/L by systolic blood pressure (A), diastolic blood pressure; (B), and pulse pressure (C) strata. DBP, diastolic blood pressure; hsTnT, high-sensitivity troponin-T; PP, pulse pressure; SBP, systolic blood pressure.
Further adjustment for heart failure
One thousand, six hundred and thirteen (13%) patients had a prior diagnosis of heart failure at study entry. In order to address residual confounding in the relationship between low blood pressure and adverse CV outcomes, these patients were excluded as a sensitivity analysis. Associations remained for low SBP with CV death and for low DBP with MACE and MI (see Supplementary material online, Tables S5 and S6). We then further examined only those patients with NT-proBNP ≤ median (median 141 ng/L). Among these 6091 patients, SBP continued to be associated positively with risk of ischaemic stroke (Plinear = 0.01; see Supplementary material online, Table S7). Low DBP remained strongly associated with MI, with an adjusted risk of HRadj 3.28 (95% CI 1.3–8.28; P = 0.01) for DBP <60 mmHg relative to DBP 80–<90 mmHg (see Supplementary material online, Table S8).
Stratification by hypertension at baseline
Nine thousand, nine hundred fifty-nine (82%) patients had hypertension at baseline. Among these patients, SBP showed a U-shaped relationship with CV death (Pquadratic = 0.02) and a direct linear relationship with ischaemic stroke after multivariable adjustment (P < 0.001; see Supplementary material online, Table S10). Diastolic blood pressure showed U-shaped relationships with MACE (Pquadratic = 0.01), MI (Pquadratic = 0.02), and all-cause mortality (Pquadratic = 0.02) and a linear relationship with ischaemic stroke (direct, Plinear = 0.01; see Supplementary material online, Table S11). These relationships were not as robust in patients without hypertension at baseline (see Supplementary material online, Tables S12 and S13), though SBP did show a U-shaped relationship with MACE (Pquadratic = 0.03) and CV death (Pquadratic = 0.04), and a direct linear relationship with MI (Plinear = 0.02).
Time-varying model
As a sensitivity analysis, we calculated adjusted risk for the above outcomes based on SBP and DBP using a time-varying model. The mean SBP was 136.6 (±16.6) at baseline, 134.3 (±16.8) at year one, and 135.44 (±17.3) at year 2. Using the time-varying model of adjusted risk, U-shaped relationships remained for both SBP and DBP with MACE, MI, and CV death and direct linear relationships remained with ischaemic stroke.
Quality assessment
Twenty-one percent of SBP measures were multiples of 10 and 32% were multiples of 5. These proportions were 24% and 34%, respectively, for DBP recordings.
Discussion
The SAVOR-TIMI 53 trial provides a large, well-characterized population of patients with T2DM at elevated CV risk. In an analysis of the relationship between baseline blood pressure, baseline troponin level, and CV outcomes, we found low SBP to be associated with increased risk of MACE, CV death, and heart failure hospitalization, and low DBP to be associated with MACE, MI, and heart failure hospitalization. These findings remained after extensive adjustment for baseline characteristics and CV biomarkers. The present results build upon recent work from the ARIC registry12 in a low-risk population and the CLARIFY registry14 in patients with stable coronary artery disease by demonstrating persistent associations between low blood pressure and adverse CV events specifically in a high-risk diabetic population.
Current evidence
Management of blood pressure has undergone significant evolution since the early recognition of hypertension as a risk factor for adverse CV events nearly one century ago.26 Despite an historical abundance of clinical trial data, there had been no widely endorsed American guidelines for management of hypertension since 2003 until the recently published 2017 guidelines from the American Heart Association, American College of Cardiology, and other expert groups.1,8,27 Recently, the Systolic Blood Pressure Intervention (SPRINT) trial reported reductions in CV death, all-cause death, and heart failure with an aggressive SBP goal of <120 mmHg compared with <140 mmHg.28 This study enrolled patients with elevated CV risk, but without T2DM.28–31 The Action to Control Cardiovascular Risk in Diabetes—Blood Pressure (ACCORD BP) trial, conversely, found that targeting a SBP of <120 mmHg as opposed to <140 mmHg in diabetic patients did not impact the rate of the composite CV outcome.32 And in the Heart Outcomes Prevention Evaluation (HOPE-3) trial of fixed-dose antihypertensive therapy in intermediate risk patients, the median achieved systolic/DBP lowering of 6/3 mmHg did not result in reduced rates of CV events. Further, there was a trend toward harm among patients with starting SBP ≤131.5 mmHg.33
In this evolving landscape, recent observational data have provided further perspective, including some concern for increased risk associated with low blood pressure.12,14,34,35 In a report from the Atherosclerosis Risk in Communities (ARIC) registry which analysed data from over 11 000 patients without established CV disease, very low DBP was associated with higher levels of cardiac troponin and increased rates of coronary heart disease events, hospitalization for heart failure, and all-cause mortality.12 Additionally, in analyses from the Prospective Comparison of ARNI with ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF)34,36 and Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS)35 trials, low SBP was associated with adverse CV outcomes. Heart failure severity remains an important potential modifier of these relationships. Finally, in a recently published report on the relationship between achieved blood pressure and CV outcomes in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trials, a mean achieved SBP <120 mmHg or DBP <70 mmHg was associated with an increase rate of adverse events.37
Blood pressure and cardiovascular outcomes
In this context, we show here in a population of diabetic patients with established CV disease or multiple CV risk factors U-shaped relationships between blood pressure and CV events. These relationships build upon those observed in previous lower risk populations and further control for confounding by elimination of patients with a prior diagnosis of heart failure and adjustment for NT-proBNP and hsTnT. This type of adjustment for biomarkers has not, to our knowledge, been performed in other patient cohorts.
U-shaped relationships were seen here for SBP with MACE, CV death, and heart failure hospitalization, and for DBP with MACE, MI, and heart failure hospitalization. These associations were robust across multiple strategies to address residual confounding. Additionally, the association between low blood pressure and reduced risk for ischaemic stroke provides assurance that low blood pressure was not simply a marker of overall frailty in this population.
In this context, the persistent association between low DBP, elevated troponin, and MI is notable. There is a compelling physiologic explanation for this finding with potential clinical importance. Coronary filling is dependent on central aortic pressure, largely during diastole, and this observation may support concern for low DBP as a cause of insufficient coronary perfusion, subclinical myocardial injury, and MI, as has been hypothesized by others.12,38
It is important to keep in mind, however, that these data are observational and do not support inference of a causal relationship. Another possible explanation for these findings incorporates PP. Patients with stiffened, calcified arteries are likely to have elevated PP due to both high SBP and low DBP. From this perspective, low DBP may for many patients be a marker of arterial stiffness, a risk factor for atherothrombotic events.13 In fact, elevated PP in this cohort was associated with increased risk of both ischaemic stroke and MI.
Further context is provided by Mendelian randomization observations of a linear relationship between SBP and adverse CV events in primary prevention patients exposed to a lifetime of genetic polymorphisms associated with higher blood pressure.39,40 These studies supply evidence of a long-term relationship between elevated SBP and CV events. The present analysis relies on a comparatively shorter follow-up of 2.1 years, which should be sufficient for high-risk patients with established CV disease to manifest CV events at low blood pressures through the mechanisms described above. As noted, the strongest association was between elevated troponin and incident MI in patients with low DBP, even after controlling for biomarkers and pre-existing heart failure, which may reflect a short-term effect of blood pressure lowering therapies despite a long-term generally protective effect of low blood pressure as suggested by Mendelian randomization.
We cannot causally link lower blood pressure to increased risk of MI in this observational study, but we rigorously demonstrate the association between low DBP and risk, which may be due to underlying comorbidities (e.g. hypertension, coronary disease) or blood pressure lowering therapy. Consistent with this latter possibility is the finding of increased MI with low DBP in the subset of patients with baseline hypertension, which is not found in the subset of patients without hypertension at study entry. It should be noted that because hypertension was a qualifying condition for SAVOR-TIMI 53 and is common, patients without hypertension in this trial are a select group who, by definition, may have other significant risk factors or established CV disease.
While the underlying mechanisms of the associations reported here ultimately cannot be resolved in this observational analysis, the findings do show a robust association between low DBP and MI that deserves further attention in randomized trials.
Quality comparison
Twenty-one percent of SBP measures and 24% of DBP values were multiples of 10 while 32% of SBP values and 34% of DBP values were multiples of 5. These numbers, while demonstrating some digit preference (goodness-of-fit P < 0.001 for SBP and DBP), compare favourably to other recent reports.13
Limitations
There are a number of limitations to this analysis. Most importantly, this analysis is observational and hypothesis-generating without the ability to infer causality. Second, only baseline blood pressure and troponin levels were analysed. Third, over 80% of patients enrolled in SAVOR-TIMI 53 had a pre-existing diagnosis of hypertension and an even greater proportion were taking a medication that lowers blood pressure, limiting the ability to compare these associations in non-hypertensive patients. Finally, only first events were included, potentially limiting the analysis of more severe events, such as CV death, owing to competing risk. Importantly, the quality metric of values that are multiples of five or ten suggests accurate recording of measured blood pressures. This analysis also benefits from a large cohort of at-risk patients with prospectively collected and adjudicated outcomes.
Conclusion
In conclusion, after extensive adjustment for underlying disease state, there was a persistent association between low DBP, subclinical myocardial injury, and risk of MI in diabetic patients at elevated cardiac risk.
Executive Committee: Eugene Braunwald (Study Chair), Deepak L. Bhatt (Co-Principal Investigator), Itamar Raz (Co-Principal Investigator), Jaime A. Davidson, Robert Frederich (non-voting), Boaz Hirshberg (non-voting), Ph. Gabriel Steg.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The SAVOR-TIMI 53 trial was sponsored by AstraZeneca (Cambridge, UK) and Bristol-Myers Squibb (NY, USA).
Conflict of interest: B.A.B. was sponsored by NIH grant T32HL007604, Training Grant in Cardiovascular Research at the time of this research. Consultant fees: Janssen Pharmaceuticals and Daiichi-Sankyo. B.M.S. discloses the following relationships: Consultant fees/honoraria- AstraZeneca Pharmaceuticals, Biogen Idec, Boehringer Ingelheim Pharmaceuticals, Inc, Dr Reddy's Laboratories Inc., Forest Laboratories, GE Healthcare, GlaxoSmithKline, Health@Scale, Lexicon, Merck&Co., Inc., St. Jude Medical; Research/Research Grants - AstraZeneca, Daiichi-Sankyo, Eisai, Merck, Poxel. Ph.G.S. discloses the following relationships: research grant from Merck, Sanofi, and Servier, speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, CSL-Behring, Daiichi-Sankyo, GlaxoSmithKline, Janssen, Lilly, Merck Novartis, Pfizer, Regeneron, Sanofi, Servier, The Medicines Company; C.L.F., Y.G. and O.M. have no conflicts of interest to disclose. A.C. discloses the following relationships: Advisory board: AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Glucome, Novo Nordisk, and Sanofi; is on the speaker's bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; and is shareholder of Glucome. I.R. discloses no relationships. D.L.B. discloses the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda.
Supplementary Material
References
- 1. Kannel WB, Gordon T, Schwartz MJ.. Systolic versus diastolic blood pressure and risk of coronary heart disease: the Framingham Study. Am J Cardiol 1971;27:335–346. [DOI] [PubMed] [Google Scholar]
- 2. Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, Cannon CP, de Lemos JA, Elliott WJ, Findeiss L, Gersh BJ, Gore JM, Levy D, Long JB, O’Connor CM, O’Gara PT, Ogedegbe O, Oparil S, White WB; American Society of Hypertension. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Coll Cardiol 2015;65:1998–2038. [DOI] [PubMed] [Google Scholar]
- 3. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P; Atlas Writing Group. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur Heart J 2017;doi: 10.1093/eurheartj/ehx628. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association. 8. Cardiovascular disease and risk management. Diabetes Care 2015;38:S49–S57. [DOI] [PubMed] [Google Scholar]
- 5. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E.. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 6. Kovell LC, Ahmed HM, Misra S, Whelton SP, Prokopowicz GP, Blumenthal RS, McEvoy JW.. US hypertension management guidelines: a review of the recent past and recommendations for the future. J Am Heart Assoc 2015;4:e002315.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2013;22:193–278.23777479 [Google Scholar]
- 8. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017; doi: 10.1161/HYP.0000000000000066. [Google Scholar]
- 9. Bhatt DL, James G, Pickering T, Devereux R.. Relation of arterial pressure level and variability to left ventricular geometry in normotensive and hypertensive adults. Blood Press Monit 1996;1:415–424. [PubMed] [Google Scholar]
- 10. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 11. Stamler J, Vaccaro O, Neaton JD, Wentworth D; and the MRFIT Research Group. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434–444. [DOI] [PubMed] [Google Scholar]
- 12. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E.. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016;68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selvaraj S, Steg PG, Elbez Y, Sorbets E, Feldman LJ, Eagle KA, Ohman EM, Blacher J, Bhatt DL; REACH Registry Investigators. Pulse pressure and risk for cardiovascular events in patients with atherothrombosis: from the REACH registry. J Am Coll Cardiol 2016;67:392–403. [DOI] [PubMed] [Google Scholar]
- 14. Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif J-C, Tendera M, Tavazzi L, Bhatt DL, Steg PG.. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 15. Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ.. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dorresteijn JA, van der Graaf Y, Spiering W, Grobbee DE, Bots ML, Visseren FL.. Relation between blood pressure and vascular events and mortality in patients with manifest vascular disease J-curve revisited. Hypertension 2012;59:14–21. [DOI] [PubMed] [Google Scholar]
- 17. Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW.. A calcium antagonist vs a non–calcium antagonist hypertension treatment strategy for patients with coronary artery disease: the International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290:2805–2816. [DOI] [PubMed] [Google Scholar]
- 18. Ravindrarajah R, Hazra NC, Hamada S, Charlton J, Jackson SHD, Dregan A, Gulliford MC.. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age: cohort study using electronic health records. Circulation 2017;135:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bangalore S, Schwamm L, Smith EE, Hellkamp AS, Suter RE, Xian Y, Schulte PJ, Fonarow GC, Bhatt DL.. Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur Heart J 2017;38:2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scirica BM, Bhatt DL, Braunwald E, Raz I, Cavender MA, Im KA, Mosenzon O, Udell J, Hirshberg B, Pollack PS, Steg PG, Jarolim P.. Prognostic implications of biomarker assessments in patients with type 2 diabetes at high cardiovascular risk. JAMA Cardiol 2016;1:989–998. [DOI] [PubMed] [Google Scholar]
- 21. Everett BM, Brooks MM, Vlachos HE, Chaitman BR, Frye RL, Bhatt DL.. Troponin and cardiac events in stable ischemic heart disease and diabetes. N Engl J Med 2015;373:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Price DL, Chen R, Udell J, Raz I.. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J 2011;162:818–825.e6. [DOI] [PubMed] [Google Scholar]
- 23. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 24. Mosenzon O, Raz I, Scirica BM, Hirshberg B, Stahre CI, Steg PG, Davidson J, Ohman P, Price DL, Frederich B, Udell JA, Braunwald E, Bhatt DL.. Baseline characteristics of the patient population in the Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus (SAVOR)‐TIMI 53 trial. Diabetes Metab Res Rev 2013;29:417–426. [DOI] [PubMed] [Google Scholar]
- 25. United States Food and Drug Administration. US FDA 510(k) Premarket Notification, 2017. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K162895 (1 September 2017).
- 26. Hay J. A British Medical Association Lecture on the significance of a raised blood pressure. Br Med J 1931;2:43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
- 28. The SPRINT Trial Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT.. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parati G, Ochoa JE, Bilo G, Zanchetti A.. SPRINT blood pressure: sprinting back to smirk's basal blood pressure? Hypertension 2017;69:15–19. [DOI] [PubMed] [Google Scholar]
- 30. Yusuf S, Lonn E.. The SPRINT and the HOPE-3 trial in the context of other blood pressure-lowering trials. JAMA Cardiology 2016;1:857–858. [DOI] [PubMed] [Google Scholar]
- 31. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016;134:904–905. [DOI] [PubMed] [Google Scholar]
- 32. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;2010:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lonn EM, Bosch J, Lopez-Jaramillo P, Zhu J, Liu L, Pais P, Diaz R, Xavier D, Sliwa K, Dans A, Avezum A, Piegas LS, Keltai K, Keltai M, Chazova I, Peters RJ, Held C, Yusoff K, Lewis BS, Jansky P, Parkhomenko A, Khunti K, Toff WD, Reid CM, Varigos J, Leiter LA, Molina DI, McKelvie R, Pogue J, Wilkinson J, Jung H, Dagenais G, Yusuf S; and the HOPE Investigators. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2009–2020. [DOI] [PubMed] [Google Scholar]
- 34. Böhm M, Young R, Jhund P, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K.. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: Results from PARADIGM-HF. Eur Heart J 2017;38:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Navar AM, Gallup DS, Lokhnygina Y, Green JB, McGuire DK, Armstrong PW, Buse JB, Engel SS, Lachin JM, Standl E, Van de Werf F, Holman RR, Peterson ED.. Hypertension control in adults with diabetes mellitus and recurrent cardiovascular events: global results from the trial evaluating cardiovascular outcomes with sitagliptin. Hypertension 2017;70:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR.. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 37. Bohm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder RE, Sliwa K, Weber MA, Williams B, Yusuf S.. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet 2017;389:2226–2237. [DOI] [PubMed] [Google Scholar]
- 38. Bhatt DL. Troponin and the J-curve of diastolic blood pressure: when lower is not better. J Am Coll Cardiol 2016;68:1723–1726. [DOI] [PubMed] [Google Scholar]
- 39. Ference BA, Julius S, Mahajan N, Levy PD, Williams KA Sr, Flack JM.. Clinical effect of naturally random allocation to lower systolic blood pressure beginning before the development of hypertension. Hypertension 2014;63:1182–1188. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL. Yes, hyperglycaemia is indeed a modifiable cardiac risk factor: so says Mendel. Eur Heart J 2015;36:1424–1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.