Abstract
A floating thrombus in the ascending aorta was incidentally discovered in a patient with a descending thoracic aortic aneurysm and a history of alcoholism. The patient developed deep venous thrombosis and pulmonary embolism. However, he refused to undergo surgical excision of the thrombus in the ascending aorta. Therefore, treatment with rivaroxaban was administered for 3 months, and it completely dissolved the thrombus. Anticoagulant therapy may be an alternative treatment when surgery cannot be performed.
Thrombi originating from the ascending aorta are rarely discovered without the manifestation of symptoms.1 Aortic thrombosis is reportedly caused by atherosclerosis and hypercoagulability.2 However, in some cases, the cause can be difficult to determine.3 We describe the case of a patient with a floating thrombus in the ascending aorta, pulmonary embolism, and slightly elevated homocysteine level. The ascending aortic thrombus dissolved completely after 3-month treatment with rivaroxaban. Informed consent was obtained from the patient for the publication of his case.
Case report
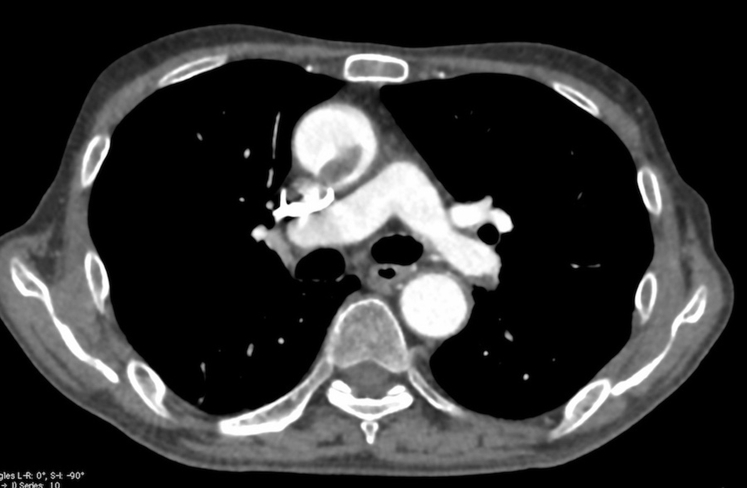
A 72-year-old man was admitted to our hospital for examination of an aneurysm in the descending thoracic aorta. He had a history of alcoholism and was a current smoker. On an enhanced computed tomography (CT) scan, a large thrombus in the ascending aorta, deep venous thrombosis, and pulmonary embolism were incidentally revealed in addition to the aneurysm in the descending thoracic aorta (Fig 1). A screening test for hypercoagulability revealed no specific findings.
Fig 1.
Floating thrombus attached at 7-o'clock position of ascending aortic wall.
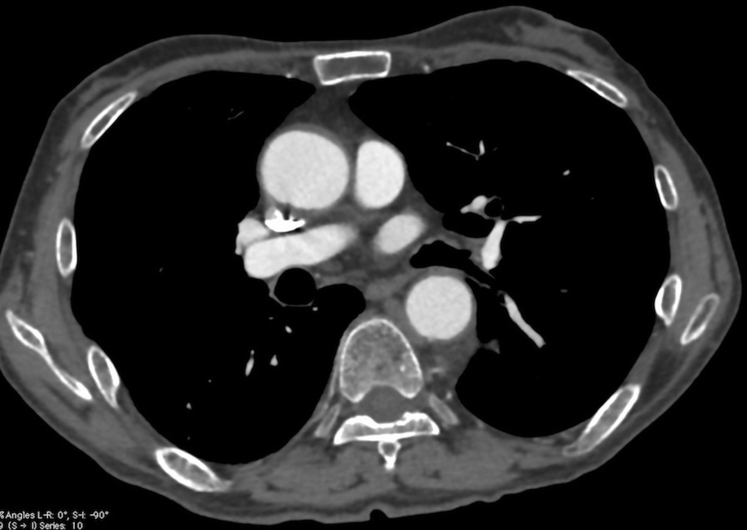
Despite being at risk for embolism, he refused to undergo surgical treatment for the ascending aortic thrombus. Accordingly, he was prescribed rivaroxaban, 15 mg twice daily for 3 months, followed by 15 mg once daily. Heparin and other oral anticoagulants were not administered. At the 1-month follow-up after initiation of rivaroxaban, the CT scan showed no emboli in the pulmonary artery in addition to a reduction in size of the thrombi in the ascending aorta and descending thoracic aortic aneurysm. At the 3-month follow-up, the CT scan revealed an irregular aortic intimal surface, and the floating thrombus in the ascending aorta was no longer detected (Fig 2). No embolic events were observed while the patient was receiving anticoagulation therapy. In addition, the volume of the intraluminal thrombus in the descending thoracic aorta decreased; however, the descending thoracic aortic aneurysm slightly increased from 5.82 × 5.09 cm to 6.23 × 5.6 cm.
Fig 2.
The ascending aortic thrombus is completely dissolved, and an irregular aortic intimal surface is shown from the 6- to 7-o'clock position.
After treatment, the patient agreed to undergo endovascular repair of the aneurysm. Thoracic endovascular stent grafting was successfully performed using a stent graft (GORE TAG; W. L. Gore & Associates, Flagstaff, Ariz) at 5 months after initiation of rivaroxaban. Subsequently, the CT scan revealed the irregular intimal surface of the ascending aorta despite continuing anticoagulant therapy.
After resolution of the ascending aortic thrombus, results of the blood examination for hypercoagulability performed by a hematologist revealed a slightly increased homocysteine level (16.4 nmol/mL).
Discussion
Rivaroxaban completely dissolved the thrombi in this patient, without causing embolic events during therapy.
Although the incidence and distribution of aortic thrombi remain unclear, an aortic thrombus not associated with an aortic aneurysm is rare. Machleder et al4 reported 48 cases (0.45%) of nonaneurysmal aortic thrombi of 10,671 consecutive autopsies. Moreover, a meta-analysis conducted by Fayad et al5 showed that the incidence of aortic thrombus was the lowest in the ascending aorta, at 11.6%, whereas it was >30% in the aortic arch and descending aorta.
Catastrophic embolic events involving the central nervous system, heart, mesentery, and extremities can develop from ascending aortic thrombi.1, 3 Predictive factors for embolic events include mobility and the quality of the peduncle.6 Surgical excision of the aortic thrombus is the treatment of choice because of the risk of an embolic event; however, because this patient refused to undergo surgery, we treated him with anticoagulation therapy.
The development of a thrombus is greatly dependent on Virchow's triad. In our patient, because blood velocity in the ascending aorta was high, hypercoagulability or abnormalities of the aortic wall were believed to have caused the development of the thrombus.
Hypercoagulability caused by blood disorders, malignant neoplasms, anticancer therapy, hormonal therapy, collagen diseases, and pregnancy is associated with the development of thrombi.1, 2 In this patient, the level of homocysteine, which has atherogenic and prothrombotic properties, was high. Furthermore, because nutritional deficiencies including vitamin B deficiency are highly prevalent in patients with sustained heavy alcohol consumption,7 an elevated blood homocysteine level can reflect a deficiency in folate, vitamin B6, or vitamin B12.8 Although modest alcohol consumption may be beneficial in reducing the risk of cardiovascular and cerebrovascular diseases, heavy alcohol consumption may be associated with thrombogenicity and hyperhomocysteinemia.
In addition, atherosclerotic changes in the aortic wall have been reported to cause aortic thrombi.1, 9 In our patient, the CT scan revealed the aortic thrombus attached to the irregular intimal surface of the aorta, which suggested atherosclerosis.
Wall shear stress was reported to be proportional to the blood flow velocity in certain conditions. von Knobelsdorff-Brenkenhoff et al10 reported heterogeneity in the ascending aortic blood flow. Low blood velocities in the ascending aorta near the posterior wall suggested a low wall shear stress, whereas high blood velocities near the right anterior wall suggested a high wall shear stress. Furthermore, low wall shear stress was associated with the development of high-risk plaques due to inflammation,11 whereas high wall shear stress was linked to atherosclerotic plaque erosion.12 Because morphologic changes in the aorta occur with advancing age, changes in aortic wall shear stress are more likely to occur in the right posterior wall, which is believed to be the most vulnerable to thrombus formation on the basis of biomechanics. Endo et al9 reported thrombi developing at the site of plaque rupture. In our patient, the thrombus originated in the right posterior wall of the ascending aorta, which was different from that described in other reports. This indicates that causes of ascending aortic thrombus other than biomechanics can exist.
Surgical excision of an ascending aortic thrombus is the treatment of choice because of the risk of embolism.1, 3, 5, 9 Thrombus exclusion using a stent graft is considered an alternative to surgery,13, 14 whereas medications, including anticoagulants and thrombolytics, are considered for high-risk patients. Ito et al15 reported the rapid dissolution of a thrombus after the administration of a tissue plasminogen activator, suggesting that the risk of embolic events can be increased by not dissolving but breaking up thrombi using thrombolytic therapy. Moreover, hemorrhagic complications remain a concern with the use of this medication.
Several authors have reported successful results using anticoagulants, such as warfarin, for the treatment of aortic thrombi.1, 5, 16 Marin-Acevedo et al2 reported complete resolution of an ascending aortic thrombus using rivaroxaban. Although the optimal drug, dose, and duration of anticoagulation therapy are crucial, they are not established. Our patient refused surgery; thus, we prescribed him anticoagulation therapy. The floating thrombus originating from the ascending aorta decreased in size 1 month after the initiation of therapy and was completely dissolved after 3 months of therapy, without the development of embolic events. However, Weiss et al1 reported that an embolic event occurred 7 months following the cessation of anticoagulation therapy after thrombus resolution.
The size of the descending aortic aneurysm in this patient increased, whereas the amount of intra-aneurysmal thrombi decreased. Li et al17 reported that the amount of intraluminal thrombi was negatively correlated with the aortic wall stress, using finite element analysis of the abdominal aortic aneurysm, suggesting that a decreasing amount of intra-aneurysmal thrombi in the descending thoracic aortic aneurysm may increase the risk of rupture. We obtained informed consent from our patient to perform endovascular aortic repair, which was successful.
Conclusions
Patients with floating thrombi in the ascending aorta are at risk of catastrophic embolic events. Although surgical excision is generally recommended, anticoagulants can be an alternative treatment when surgery cannot be performed.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Weiss S., Bühlmann R., von Allmen R.S., Makaloski V., Carrel T.P., Schmidli J. Management of floating thrombus in the aortic arch. J Thorac Cardiovasc Surg. 2016;152:810–817. doi: 10.1016/j.jtcvs.2016.03.078. [DOI] [PubMed] [Google Scholar]
- 2.Marin-Acevedo J.A., Koop A.H., Diaz-Gomez J.L., Guru P.K. Non-atherosclerotic aortic mural thrombus: a rare source of embolism. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220592. bcr-2017-220592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima M., Tsuchiya K., Honda Y., Koshiyama H., Kobayashi T. Acute pulmonary embolism after cerebral infarction associated with a mobile thrombus in the ascending aorta. Gen Thorac Cardiovasc Surg. 2009;57:654–656. doi: 10.1007/s11748-009-0444-y. [DOI] [PubMed] [Google Scholar]
- 4.Machleder H.I., Takiff H., Lois J.F., Holburt E. Aortic mural thrombus: an occult source of arterial thromboembolism. J Vasc Surg. 1986;4:473–478. [PubMed] [Google Scholar]
- 5.Fayad Z.Y., Semaan E., Fahoum B., Briggs M., Tortolani A., D'Ayala M. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. 2013;27:282–290. doi: 10.1016/j.avsg.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Karalis D.G., Chandrasekaran K., Victor M.F., Ross J.J., Jr., Mintz G.S. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 1991;17:73–78. doi: 10.1016/0735-1097(91)90706-f. [DOI] [PubMed] [Google Scholar]
- 7.Bode C., Bode J.C. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 8.Selhub J., Jacques P.F., Wilson P.W., Rush D., Rosenberg I.H. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 9.Endo H., Ishii H., Tsuchiya H., Takahashi Y., Shimoyamada H., Isomura A. Pathologic features of lone aortic mobile thrombus in the ascending aorta. Ann Thorac Surg. 2016;102:e313–e315. doi: 10.1016/j.athoracsur.2016.03.107. [DOI] [PubMed] [Google Scholar]
- 10.von Knobelsdorff-Brenkenhoff F., Karunaharamoorthy A., Trauzeddel R.F., Barker A.J., Blaszczyk E., Markl M. Evaluation of aortic blood flow and wall shear stress in aortic stenosis and its association with left ventricular remodeling. Circ Cardiovasc Imaging. 2016;9:e004038. doi: 10.1161/CIRCIMAGING.115.004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitsioudis G., Chatzizisis Y.S., Wolf P., Missiou A., Antoniadis A.P., Mitsouras D. Combined non-invasive assessment of endothelial shear stress and molecular imaging of inflammation for the prediction of inflamed plaque in hyperlipidaemic rabbit aortas. Eur Heart J Cardiovasc Imaging. 2017;18:19–30. doi: 10.1093/ehjci/jew048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sameshima N., Yamashita A., Sato S., Matsuda S., Matsuura Y., Asada Y. The values of wall shear stress, turbulence kinetic energy and blood pressure gradient are associated with atherosclerotic plaque erosion in rabbits. J Atheroscler Thromb. 2014;21:831–838. doi: 10.5551/jat.23093. [DOI] [PubMed] [Google Scholar]
- 13.Rancic Z., Pfammatter T., Lachat M., Frauenfelder T., Veith F.J., Mayer D. Floating aortic arch thrombus involving the supraaortic trunks: successful treatment with supra-aortic debranching and antegrade endograft implantation. J Vasc Surg. 2009;50:1177–1180. doi: 10.1016/j.jvs.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 14.Kahlberg A., Montorfano M., Cambiaghi T., Bertoglio L., Melissano G., Chiesa R. Endovascular stent-grafting of the ascending aorta for symptomatic parietal thrombus. J Endovasc Ther. 2016;23:969–972. doi: 10.1177/1526602816664877. [DOI] [PubMed] [Google Scholar]
- 15.Ito H., Takahashi K., Sasaki H., Akiho H., Katahira Y., Saito H. Large thrombus in the ascending aorta successfully treated by thrombolysis—an unusual cause of acute massive myocardial infarction. Jpn Circ J. 2001;65:572–574. doi: 10.1253/jcj.65.572. [DOI] [PubMed] [Google Scholar]
- 16.Bowdish M.E., Weaver F.A., Liebman H.A., Rowe V.L., Hood D.B. Anticoagulation is an effective treatment for aortic mural thrombi. J Vasc Surg. 2002;36:713–719. [PubMed] [Google Scholar]
- 17.Li Z.Y., U-King-Im J., Tang T.Y., Soh E., See T.C., Gillard J.H. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J Vasc Surg. 2008;47:928–935. doi: 10.1016/j.jvs.2008.01.006. [DOI] [PubMed] [Google Scholar]