Abstract
Aberrant right subclavian artery is the most common brachiocephalic artery congenital abnormality and may result in dysphagia from external compression by the aberrant artery on the esophagus. Repair of this anatomic variant can be performed by both open and hybrid endovascular techniques. This case illustrates a complication of a hybrid repair resulting in proximal migration of a vascular occlusion plug, presenting as recurrent dysphagia and need for open surgical extraction of the plug.
Aberrant right subclavian artery (ARSA) is the most common brachiocephalic congenital abnormality. Most patients are asymptomatic, but 5% of patients will develop dysphagia as the ARSA runs posterior to the esophagus, creating a mass effect as the artery grows.1 This anatomic variant was first described in 1761 by London surgeon David Bayford, and the syndrome, coined dysphagia lusoria, was described in 1979.2 The incidence of ARSA is 0.4% to 2.3%, and 60% of patients with ARSA will have a Kommerell diverticulum at the origin of the aberrant subclavian artery.3, 4 This diverticulum is a remnant of the dorsal aorta from embryonic development; enlargement of the artery may cause an aneurysm, resulting in thoracic aorta dissection or rupture. Open, endovascular, and hybrid approaches to repair have been described.3 The Amplatzer vascular plug (St. Jude Medical, St. Paul, Minn) was first used to occlude an aberrant subclavian artery in 2006.5 This case illustrates proximal migration of an Amplatzer plug in an ARSA, resulting in recurrent dysphagia. The patient provided informed consent for publication of this case and all associated images.
Case report
A 54-year-old woman presented to her primary care physician in 2014 with a yearlong complaint of dysphagia and dyspnea. During the preceding year, she had difficulty in swallowing solid food, pills, and liquids. She had an unremarkable medical and surgical history and no significant family history. Her body mass index was 27, with otherwise normal findings on physical examination. She had no significant weight loss. During workup for her dyspnea, a computed tomography (CT) scan of the chest was ordered by her pulmonologist; this demonstrated an ARSA passing posterior to the esophagus, resulting in compression. Furthermore, CT revealed a large (2.5 cm) Kommerell diverticulum (Fig 1). A subsequent Gastrografin contrast study revealed normal esophageal motility, no evidence of Schatzki ring, and a prominent extrinsic mass effect on the cervical esophagus at the level of the aortic arch. She was referred for operative management with the diagnosis of dysphagia lusoria. Before surgery, standard laboratory test results, coronary angiography findings, and pulmonary function test results were unremarkable. The proposed surgery was a staged endovascular repair, with left carotid-subclavian bypass and left subclavian artery proximal occlusion followed by a second operation with right carotid-subclavian bypass, right proximal subclavian artery occlusion, and thoracic endovascular aortic repair (TEVAR).
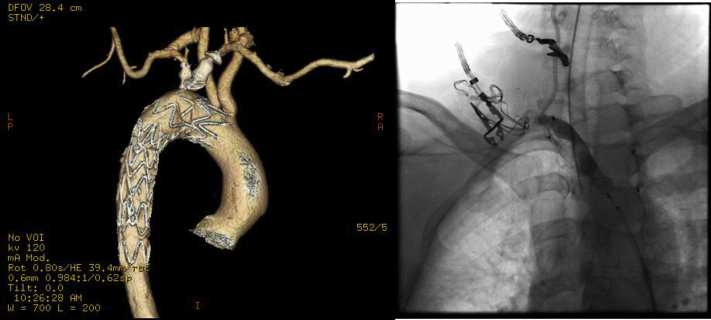
Fig 1.
Computed tomography (CT) reconstruction of posterior view of the aortic arch demonstrating aberrant right subclavian artery (ARSA) and Kommerell diverticulum.
The patient underwent a left carotid-subclavian artery bypass with 8-mm heparin-bonded externally supported polytetrafluoroethylene. The following day, the patient was taken to the endovascular suite, where a 16-mm vascular occlusion plug was placed to occlude the left subclavian artery through a transradial approach; this was staged because of unavailability of the hybrid suite on the day of her first operation. She was discharged the following day without complication.
The patient was brought back for the second stage of surgery 2 months later. She first underwent a right carotid-subclavian artery bypass. A 14-mm vascular occlusion plug was deployed in the right subclavian artery just proximal to the vertebral artery (Fig 2). Through a right femoral cutdown, TEVAR was performed with a 28- × 150-mm Bolton Relay (Bolton Medical, Sunrise, Fla) thoracic graft. Completion arteriography revealed no evidence of endoleak or other complication. The patient was discharged 2 days later without complication.
Fig 2.
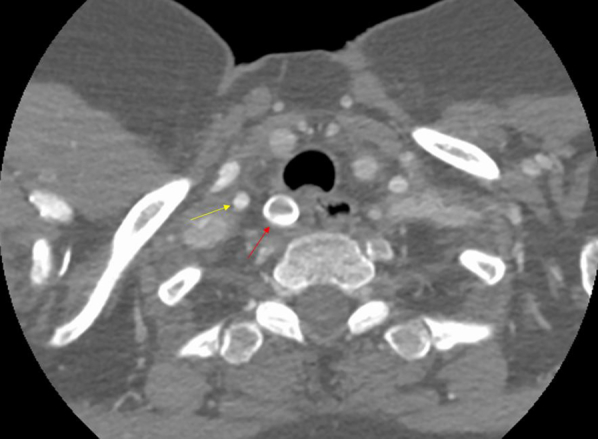
Immediate postoperative computed tomography (CT) showing the vascular occlusion plug (red arrow) just proximal to the origin of the right vertebral artery (yellow arrow).
At 1-month follow-up, the patient was recovering normally with complete resolution of her dysphagia. CT angiography revealed the aortic graft in appropriate position with exclusion of the aneurysm and widely patent bilateral carotid-subclavian bypass grafts.
The patient continued to do well until 13 months after surgery, when she complained of recurrent dysphagia. CT arteriography revealed proximal migration of the right subclavian vascular plug in the ARSA to a position causing external esophageal compression (Fig 3). This was confirmed with an upper gastrointestinal contrast study (Fig 4).
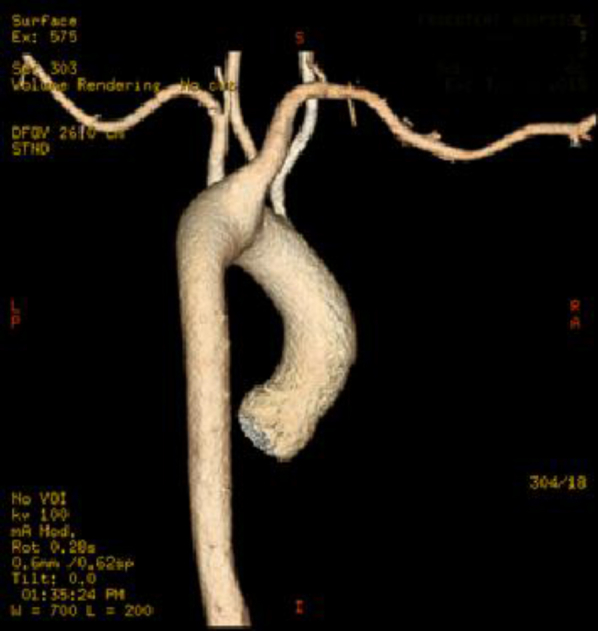
Fig 3.
Computed tomography (CT) arteriogram (left) demonstrates proximal migration of the vascular occlusion plug in the aberrant right subclavian artery (ARSA) compared with intraoperative angiogram (right) with tip at right vertebral artery origin.
Fig 4.
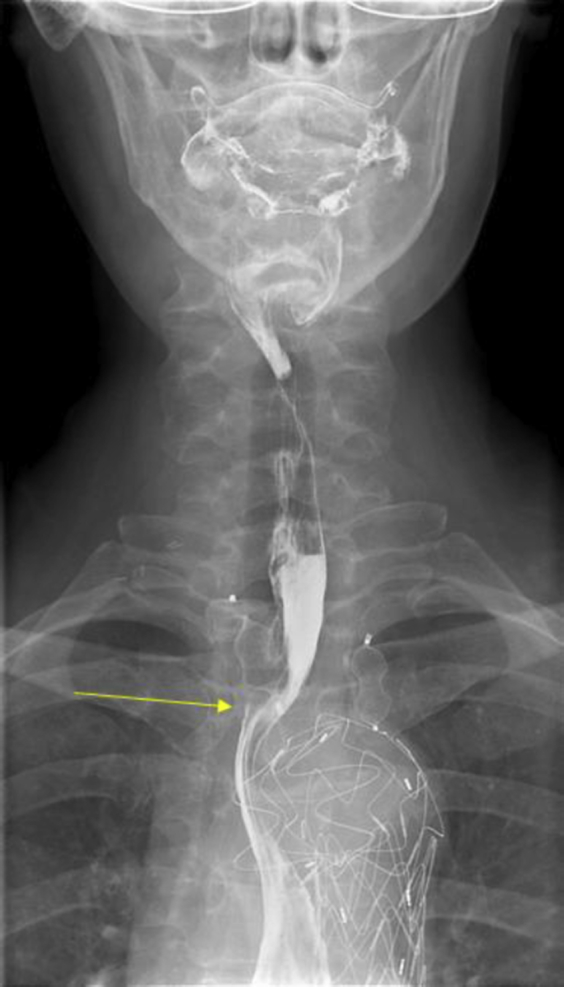
Upper gastrointestinal contrast study demonstrating external esophageal compression (arrow) by vascular occlusion plug.
The patient was taken to the operating room. A right posterolateral thoracotomy through the fourth intercostal space was performed, and after mobilization of the esophagus, the ARSA was identified and isolated to the origin of the Kommerell diverticulum. The vascular plug was palpated within the artery and removed through a transverse arteriotomy after proximal and distal control. The artery was then ligated and divided. Postoperatively, the patient developed a chylothorax, which was successfully treated with octreotide and a no-fat diet; she was discharged home on postoperative day 6. She recovered uneventfully and has had resolution of her dysphagia.
Discussion
Women represent 75% of patients with a left-sided aortic arch and ARSA.6 Whereas the majority of ARSAs will course posterior to the esophagus (80%), the ARSA may course in between the trachea and esophagus or anterior to the esophagus in 15% and 5% of cases, respectively.7 Although most remain asymptomatic, an aberrant subclavian artery can produce dysphagia, chest pain, dyspnea, or blood pressure differences in the upper extremity.8 The prevalence of ARSA is low, but the incidence may be increasing as advanced imaging for other medical issues becomes more common. Whereas there is no consensus, it has been proposed that patients who are symptomatic, have a diverticulum orifice >30 mm, or have a diameter of the descending aorta adjacent to the diverticulum >50 mm should undergo surgical repair.9, 10
Tanaka et al10 reviewed 179 interventions for diverticula of Kommerell from 2004 to 2014. Open repair represented the majority (75%), followed by hybrid TEVAR (21%) and total TEVAR (4%). They concluded that the choice of treatment strategy should be based on anatomy, the patient's comorbidities, and the surgeon's experience. In our patient, with an ARSA and left-sided aortic arch, a two-stage hybrid approach was used that has previously been described by Idrees et al.11 In a series of 10 patients, 3 patients underwent a two-stage approach in a similar fashion to our patient and had no deaths after a mean follow-up of almost 2 years. Prior successful intentional endovascular occlusion of both subclavian arteries has been reported as long as a carotid-subclavian bypass is performed to reduce complications of upper limb ischemia or subclavian steal.12
The use of a vascular plug in a hybrid operation for ARSA with a Kommerell diverticulum was published more than a decade ago and has been used by other centers.5, 13 Because of the short distance of the origin of the vertebral artery and the proximal anastomosis of the right carotid-subclavian bypass, the vascular plug was thought to be ideal as an occlusive device. This device has been reported as a user-friendly and accurate occlusive device for medium- and large-diameter vessels with high flow.14 Its application spans from splenic artery embolization to arteriovenous fistula closure and treatment of pelvic congestive syndrome.15 Very few complications, including migration, are reported in the literature.
Migration of an Amplatzer plug was reported by Maleux et al16 after it was used to inhibit backflow from the left subclavian artery following extra-anatomic bypass for a thoracic aortic coarctation. However, unlike in our patient, these authors were able to treat their patient conservatively without the increased morbidity of an open operation. It seems likely that retropulsion occurred because of repetitive force from inflow from the carotid-subclavian bypass, coupled with the relatively large size of the proximal artery. To our knowledge, this is the first case report of migration of an Amplatzer plug in the treatment of ARSA. Device migration resulted in recurrence of dysphagia, with need for thoracotomy and plug extraction. Whereas migration of the Amplatzer plug does not appear to be a common event, we present this complication to warn future surgeons of possible deleterious effects after its use. Vascular plugs should be used in these hybrid operations only with caution. Strong consideration should be given to coil embolization of the artery proximal to the plug either as an alternative technique or to prevent retropulsion of the plug if one is used.
From the Midwestern Vascular Surgical Society
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Upchurch G.R. Thoracic and thoracoabdominal aortic aneurysms: evaluation and decision making. In: Cronenwett J.L., Johnston K.W., editors. Rutherford’s vascular surgery. 8th ed. Saunders; Philadelphia: 2014. pp. 2084–2101. [Google Scholar]
- 2.Asherson N., Bayford D. His syndrome and sign of dysphagia lusoria. Ann R Coll Surg Engl. 1979;61:63–67. [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C., Shu C., Li M., Li Q., Kopp R. Aberrant subclavian artery pathologies and Kommerell’s diverticulum: a review and analysis of published endovascular/hybrid treatment options. J Endovasc Ther. 2012;19:373–382. doi: 10.1583/11-3673MR.1. [DOI] [PubMed] [Google Scholar]
- 4.Kadir S. Regional anatomy of the thoracic aorta. In: Kadir S., editor. Atlas of normal and variant angiographic anatomy. Saunders; Philadelphia: 1991. pp. 19–54. [Google Scholar]
- 5.Hoppe H., Hohenwalter E.J., Kaufman J.A., Petersen B. Percutaneous treatment of aberrant right subclavian artery aneurysm with use of the Amplatzer septal occluder. J Vasc Interv Radiol. 2006;17:889–894. doi: 10.1097/01.RVI.0000217940.38669.A1. [DOI] [PubMed] [Google Scholar]
- 6.Molz G., Burri B. Aberrant subclavian artery (arteria lusoria): sex differences in the prevalence of various forms of the malformation. Evaluation of 1378 observations. Virchows Arch A Pathol Anat Histol. 1978;380:303–315. doi: 10.1007/BF00431315. [DOI] [PubMed] [Google Scholar]
- 7.Stone W.M., Ricotta J.J., Fowl R.J., Garg N., Bower T.C., Money S.R. Contemporary management of aberrant right subclavian arteries. Ann Vasc Surg. 2011;25:508–514. doi: 10.1016/j.avsg.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Brown D.L., Chapman W.C., Edwards W.H., Coltharp W.H., Stoney W.S. Dyphagia lusoria: aberrant right subclavian artery with a Kommerell’s diverticulum. Am Surg. 1993;59:582–586. [PubMed] [Google Scholar]
- 9.Ota T., Okada K., Takanashi S., Yamamoto S., Okita Y. Surgical treatment for Kommerell’s diverticulum. J Thorac Cardiovasc Surg. 2006;131:574–578. doi: 10.1016/j.jtcvs.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A., Milner R., Ota T. Kommerell’s diverticulum in the current era: a comprehensive review. Gen Thorac Cardiovasc Surg. 2015;63:245–259. doi: 10.1007/s11748-015-0521-3. [DOI] [PubMed] [Google Scholar]
- 11.Idrees J., Keshavamurthy S., Subramanian S., Clair D.G., Svensson L.G., Roselli E.E. Hybrid repair of Kommerell diverticulum. J Thorac Cardiovasc Surg. 2014;147:973–976. doi: 10.1016/j.jtcvs.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 12.Attmann T., Brandt M., Muller-Hulsbeck S., Cremer J. Two-staged surgical and endovascular treatment of an aneurysmal aberrant right subclavian (lusoria) artery. Eur J Cardiothorac Surg. 2005;27:1125–1127. doi: 10.1016/j.ejcts.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Ferrero E., Viazzo A., Carbonatto P., Pecchio A., Casabona R., Robaldo A. Aneurysm of the aberrant right subclavian artery: surgical and hybrid repair of two cases in a single center. Ann Vasc Surg. 2011;25:839.e5–839.e9. doi: 10.1016/j.avsg.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang W., Li H., Tam M.D., Zhou D., Wang D.X., Spain J. The Amplatzer vascular plug: a review of the device and its clinical applications. Cardiovasc Intervent Radiol. 2012;35:725–740. doi: 10.1007/s00270-012-0387-z. [DOI] [PubMed] [Google Scholar]
- 15.Tresley J., Bhatia S., Kably I., Mohan P.P., Salsamendi J., Narayanan G. Amplatzer vascular plug as an embolic agent in different vascular pathologies: a pictorial essay. Indian J Radiol Imaging. 2016;26:254–261. doi: 10.4103/0971-3026.184422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleux G., Rega F., Heye S., Troost E., Budts W. Asymptomatic migration of a first-generation Amplatzer vascular plug into the abdominal aorta: conservative management may be an option. J Vasc Interv Radiol. 2011;22:569–570. doi: 10.1016/j.jvir.2010.11.033. [DOI] [PubMed] [Google Scholar]