Abstract
A patient with neurogenic thoracic outlet syndrome was initially treated with scalenectomy, first rib resection, and wrapping of the brachial plexus (BP) with amnion membrane (AM) to prevent postoperative adhesions. Twelve months later, at reoperation for recurrent symptoms, the AM was observed to be intact. The BP had no scar tissue around it. Recurrence was due to scarring around the nerve roots superior to the portion of the plexus that had been wrapped with AM. It was concluded that the AM had successfully protected the portion of the BP that had been wrapped. Longer term studies are in progress.
Surgery for neurogenic thoracic outlet syndrome (NTOS) decompression has a failure rate of 25% to 30%, primarily due to postoperative scar tissue compressing the brachial plexus (BP).1 Whereas scarring can be prevented by wrapping the BP with physical barriers, successful wrapping materials have not been found to date.
Unpublished anecdotal reports of successful wrapping of peripheral nerve repairs with amnion membrane (AM) have recently appeared. With this in mind, we began employing AM (AlloWrap DS; AlloSource, Centennial, Colo) after thoracic outlet decompression operations.
In June 2017, a patient already in the AM treatment protocol required reoperation 1 year postoperatively for recurrence of NTOS symptoms. The surprising finding of no scarring over the AM at this operation is the subject of this report. The patient has read and given consent to publication of this case report.
Case report
In June 2016, a 34-year-old neurology technician presented with constant pain in her neck, right trapezius, shoulder, arm, and supraclavicular area plus tenderness in her right anterior chest wall and axilla. She also had constant paresthesia in her right fourth and fifth fingers, daily occipital headaches, and right arm weakness. The symptoms, having developed spontaneously several weeks earlier, had not improved with physical therapy for NTOS and neurogenic pectoralis minor syndrome (NPMS).
Physical examination demonstrated mild tenderness over the right anterior scalene muscle, pectoralis minor muscle, and axilla, with no tenderness on the left side in these areas. Tinel sign was present on the right over the BP in the neck and over the radial tunnel and carpal tunnel. Phelan's sign was present on the right and absent on the left. The result of the upper limb tension test of Elvey was strongly positive on the right and minimally positive on the left. The elevated arm stress test elicited positive symptoms on the right in 10 seconds, whereas there were no symptoms on the left side at 60 seconds.
A diagnostic right pectoralis minor muscle block produced a good response, and an anterior scalene muscle block gave a good to excellent response. A diagnosis of right NTOS and right NPMS was made.
Treatment
In June 2016, BP decompression was performed by transaxillary pectoralis minor tenotomy, partial myomectomy, and first rib resection. Anterior and middle scalenectomy and BP neurolysis were also performed through a supraclavicular incision. When neurolysis was completed, a 4- × 8-cm piece of glutaraldehyde-treated AM (AlloWrap DS) was wrapped around the dissected BP nerves. The wound was then closed in standard fashion.
Initial follow-up
For the first 2 to 3 months postoperatively, she experienced good relief of symptoms. However, after 3 months, her preoperative symptoms returned. Her examination 12 months postoperatively revealed several physical findings of recurrent NTOS and NPMS. Pectoralis minor and scalene area muscle blocks each gave good responses.
Reoperation
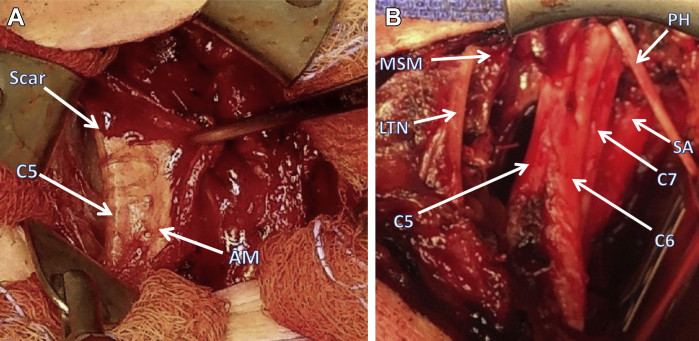
One year after her initial BP procedure, reoperation revealed the amnion wrap to be completely intact. The nerves beneath the wrap were clean and glistening (Fig). However, the proximal portion of the BP, superior to the amnion wrap, was totally surrounded by dense scar tissue.
Fig.
A, Operating room photograph showing supraclavicular exposure of amnion membrane (AM; AlloWrap DS) surrounding brachial plexus (BP) 1 year after scalenectomy and first rib resection. Orientation is the same in both (A) and (B), with the clavicle at the bottom. No scar tissue is seen over the AM, and the C5 nerve root is seen just under it. In this photograph, the middle scalene muscle, long thoracic nerve, and phrenic nerve as shown in (B) are all covered with blood. The other four nerve roots lay within the AM wrap and were free of adhesions when the wrap was opened. C5, C5 nerve root and upper trunk of BP; Scar, scar around C5, lying above the AM and covered with blood. B, Operating room photograph showing BP anatomy after complete anterior scalenectomy and partial middle scalenectomy plus BP neurolysis. The upper end is cephalad. Not seen are the C8 and T1 nerve roots, which lie deep to C5, C6, and C7. C5, C5 nerve root; C6, C6 nerve root; C7, C7 nerve root; LTN, long thoracic nerve; MSM, remaining portion of middle scalene muscle; PH, phrenic nerve; SA, subclavian artery.
Through the previous transaxillary incision, adhesive scar tissue was excised, and a piece of AM was placed over the cords of the BP to lessen rescarring. This incision was closed, the neck now prepared, and the previous supraclavicular incision reopened.
The previously placed AM was observed (as noted before) and left in place. Neurolysis was unnecessary here. Medial to this old AM, at the nerve roots, scarred residual anterior scalene muscle fibers, causing the recurrence and lying beneath the phrenic nerve, were dissected and excised at the base of each of the five nerve roots of the BP. These roots were then wrapped with AM, and the wound was closed.
Follow-up
Three months postoperatively, she was working full-time as a postpartum nurse. She had increased arm and hand strength and noted improved right hand dexterity. Pain was greatly reduced in her arm and neck. Paresthesia occurred only occasionally in the ulnar fingers. She estimated her early improvement at 80%. She had hyperesthesia of the neck incision, a change after the procedure. She was sleeping well.
Discussion
The recurrent symptoms for which this patient was reoperated on were not due to a failure of the AM; rather, failure was due to scar formation in the area of an unwrapped portion of the BP at the level of the nerve roots. It pointed out the importance of removing as much of the anterior scalene muscle as is safely possible and wrapping every portion of exposed nerve.
The history of using AM is >100 years old. It began with its use to cover stasis ulcers and burns, then to cover dural defects after brain trauma.2, 3, 4, 5 AM is immune privileged, meaning that it is not rejected as a foreign body when it is implanted in humans.6
Amnionic epithelial cells can act like stem cells. They can be converted into any of the three basic germ layers7 and can perform as ocular stem cells.8 In addition, AM is anti-inflammatory, antibacterial, antiviral, antiangiogenic, and proapoptotic. It also is a promotor of epithelialization and is nontumorigenic tissue.9, 10 Another unusual property is AM's ability to act as a bridge in promoting axonal regeneration in damaged adult brain.11
AM has been used in three general types of applications: to prevent adherence of scar tissue to nerve12 and tendon repairs13; as a dressing to reduce pain and infection and to stimulate epithelialization after burns14, 15 or trauma while an area recovers from injury; and as a cover to assist in closing defects. AM has been used as a dressing to repair corneas and conjunctiva16, 17 and to cover injury to the oral or nasal mucosa, pharynx, or tympanic membrane.18, 19
In this report, processed AM (treated with glutaraldehyde) was used as a barrier to prevent normal scar tissue formations from reaching the BP after thoracic outlet decompression. During the past 2 years, AM has been employed for this purpose in 97 NTOS operations. The first 40 patients have now been observed for 1 year. Their recurrence rate is 5% to date. They must be observed for 24 months before an acceptable long-term result can be established.
From previous studies, it is known that 80% of recurrences will be evident in the first 18 months after thoracic outlet decompression. In these previous studies, the failure rate for thoracic outlet decompression at the 2-year follow-up level was 25% to 30%. This was true for all three surgical approaches: transaxillary first rib resection, supraclavicular scalenectomy, and supraclavicular scalenectomy with first rib resection.1 Most recurrences were due to scar tissue adherence to the BP regardless of the operative approach; a few were from other associated diagnoses.
The AM used in this patient was treated with glutaraldehyde in its preparation by AlloSource. Although glutaraldehyde inactivates cellular viability, this is not a concern, as the function of the amnion in this application is one of a protective barrier against scar tissue's reaching the BP after implantation. When the AM has finally been degraded after several months, the healing period is over and scar tissue has matured so that it no longer adheres to nerves. This is the explanation for almost all failures of NTOS surgery occurring within the first 24 months.
This is the first NTOS patient with AM to undergo reoperation and our first opportunity to examine AM 1 year after surgery. It is impressive that the amnion layer survived the 1-year time interval without obvious degradation. This was the fate of other materials used previously. We assume they were absorbed too quickly to protect the nerves throughout the normal healing time of 12 to 15 months.
Previous antiadhesive materials, synthetic polymer sheets (SurgiWrap [MAST Biosurgery, San Diego, Calif] and Seprafilm [Sanofi-Aventis, Bridgewater, NJ]) that had successfully reduced adhesions in the abdomen, failed to reduce the failure rate after thoracic outlet operations.20 Glutaraldehyde-treated, double-sided AM (AlloWrap DS) is the first material used to protect the BP from scar tissue that appears to be successful. Although it is too early to know the long-term results, the 1-year results are encouraging. The operating room observations of the AM in this case report give the authors hope that this material will prove helpful to future surgical patients.
Yet to be reported are other successful clinical uses of AM, including wrapping Achilles tendon repairs, covering the ends of amputated nerves to help prevent chronic phantom sensation, and wrapping nerves damaged by extremity tumors (R.M. Wilkins, MD, personal communication). Separately, placing AM over the dura in closing laminectomy sites has reduced postoperative scarring, making reoperations significantly easier and safer (T. Birney, MD, personal communication).
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Sanders R.J., Pearce W.H. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10:626–634. doi: 10.1067/mva.1989.15575. [DOI] [PubMed] [Google Scholar]
- 2.Davis J.W. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307–395. [Google Scholar]
- 3.Stern M. The grafting of preserved amniotic membrane to burned and ulcering skin grafts: a preliminary report. JAMA. 1913;60:973–974. [Google Scholar]
- 4.Sabella N. Use of fetal membranes in skin grafting. Med Records N Y. 1913;83:478–480. [Google Scholar]
- 5.Choa Y., Storer H., Penfield W. A new method of preventing adhesions: the use of amnioplastin after craniotomy. Br Med J. 1940;1:517–520. doi: 10.1136/bmj.1.4134.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akle C.A., Adinolfi M., Welsh K.I. Immunogenicity of human amniotic endothelial cells after transplantation into volunteers. Lancet. 1981;2:1002–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 7.Toda A., Motonori M., Yoshida T., Nikaido T. The potential of amnionic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–228. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- 8.Mason S.L., Stewart R.M., Kearns V.R., Williams R.L., Sheridan C.M. Ocular epithelial transplantation: current uses and future potential. Regen Med. 2011;6:767–782. doi: 10.2217/rme.11.94. [DOI] [PubMed] [Google Scholar]
- 9.Mamede A.C., Carvalho M.J., Abrantes A.M., Laranjo M., Maia C.J., Botelho M.F. Amnionic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349:447–458. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 10.Fetterolf D.E., Snyder R.J. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24:299–307. [PubMed] [Google Scholar]
- 11.Davis G.E., Blaker S.N., Engvall E., Varon S., Manthorpe M., Gage F.H. Human amnion membrane serves as a substratum for growing axons in vitro and in vivo. Science. 2017;236:1106–1109. doi: 10.1126/science.3576223. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.S., Sohn S.K., Lee M.J., Roh M.S., Kim C.H. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur Vol. 2010;35:214–219. doi: 10.1177/1753193409352410. [DOI] [PubMed] [Google Scholar]
- 13.Demirkon F., Colakoglu N., Herek O., Gurkan E. The use of amnionic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122:396–399. doi: 10.1007/s00402-002-0418-3. [DOI] [PubMed] [Google Scholar]
- 14.Andonovska D., Dzokic G., Spasevska L., Trajkovska T., Popovska K., Todorov I. The advantages of application of amnion membrane in the treatment of burns. Prizoli. 2008;29:183–198. [PubMed] [Google Scholar]
- 15.Mohammadi A.A., Johari H.G., Eskandari S. Effect of amniotic membrane on graft take in extremity burns. Burns. 2013;39:1137–1141. doi: 10.1016/j.burns.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 16.DeRotth A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23:522–525. [Google Scholar]
- 17.Dua H.S., Gomes J.A., King A.J., Maharajan V.S. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Zohar Y., Talmi Y.P., Finkelstein Y., Shvili Y., Sadov R., Laurian N. Use of human amniotic membrane in otolaryngologic practice. Laryngoscope. 1987;97:978–980. [PubMed] [Google Scholar]
- 19.Khademi B., Bahranifard H., Azarpira N., Behboodi E. Clinical application of amniotic membrane as a biologic dressing in oral cavity and pharyngeal defects after tumor resection. Arch Iran Med. 2013;16:503–506. [PubMed] [Google Scholar]
- 20.Sanders R.J., Hammond S.L., Rao N.M. Observations on the use of Seprafilm on the brachial plexus in 249 operations for neurogenic thoracic outlet syndrome. Hand. 2007;2:179–183. doi: 10.1007/s11552-007-9044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]