Abstract
Zika virus (ZIKV) has emerged since 2013 as a significant global human health threat following outbreaks in the Pacific Islands and rapid spread throughout South and Central America. Severe congenital and neurological sequelae have been linked to ZIKV infections. Assessing the ability of common mosquito species to transmit ZIKV and characterizing variation in mosquito transmission of different ZIKV strains is important for estimating regional outbreak potential and for prioritizing local mosquito control strategies for Aedes and Culex species. In this study, we evaluated the laboratory vector competence of Aedes aegypti, Culex quinquefasciatus, and Culex tarsalis that originated in areas of California where ZIKV cases in travelers since 2015 were frequent. We compared infection, dissemination, and transmission rates by measuring ZIKV RNA levels in cohorts of mosquitoes that ingested blood meals from type I interferon-deficient mice infected with either a Puerto Rican ZIKV strain from 2015 (PR15), a Brazilian ZIKV strain from 2015 (BR15), or an ancestral Asian-lineage Malaysian ZIKV strain from 1966 (MA66). With PR15, Cx. quinquefasciatus was refractory to infection (0%, N = 42) and Cx. tarsalis was infected at 4% (N = 46). No ZIKV RNA was detected in saliva from either Culex species 14 or 21 days post feeding (dpf). In contrast, Ae. aegypti developed infection rates of 85% (PR15; N = 46), 90% (BR15; N = 20), and 81% (MA66; N = 85) 14 or 15 dpf. Although MA66-infected Ae. aegypti showed higher levels of ZIKV RNA in mosquito bodies and legs, transmission rates were not significantly different across virus strains (P = 0.13, Fisher’s exact test). To confirm infectivity and measure the transmitted ZIKV dose, we enumerated infectious ZIKV in Ae. aegypti saliva using Vero cell plaque assays. The expectorated plaque forming units PFU varied by viral strain: MA66-infected expectorated 13±4 PFU (mean±SE, N = 13) compared to 29±6 PFU for PR15-infected (N = 13) and 35±8 PFU for BR15-infected (N = 6; ANOVA, df = 2, F = 3.8, P = 0.035). These laboratory vector competence results support an emerging consensus that Cx. tarsalis and Cx. quinquefasciatus are not vectors of ZIKV. These results also indicate that Ae. aegypti from California are efficient laboratory vectors of ancestral and contemporary Asian lineage ZIKV.
Author summary
Assessing the ability of common mosquito species to transmit Zika virus (ZIKV) and characterizing variation in mosquito transmission of different ZIKV strains is important for estimating regional outbreak potential and for prioritizing local mosquito control strategies for Aedes and Culex species. In this study, we evaluated the laboratory vector competence of Aedes aegypti, Culex quinquefasciatus, and Culex tarsalis that originated in areas of California where ZIKV cases in travelers since 2015 were frequent. We observed variation in infection loads between ZIKV strains in Ae. aegypti, but transmission rates were not different. In addition, there was a positive relationship between ZIKV RNA levels in infected mosquitoes ascertained from bodies and ZIKV RNA transmission rates. Our data add to the growing body of evidence supporting the role of Aedes aegypti as a ZIKV vector and refute Cx. quinquefasciatus and Cx. tarsalis as vectors.
Introduction
Zika virus (ZIKV) is a mosquito-transmitted flavivirus that was first isolated in 1947 in the Zika forest of Uganda from a sentinel rhesus macaque [1]. Since its discovery, human ZIKV cases have been reported across Africa and Asia, but until 2007 the virus received little attention from researchers as it was thought to cause only mild disease. Following epidemics in Micronesia in 2007, French Polynesia in 2013, and Brazil in 2015 [2], ZIKV has now been confirmed as a cause of the neurological disease Guillain-Barre syndrome and congenital disorders, including microcephaly in infants [3]. Despite a dramatic decline in Brazilian cases since 2016, ZIKV remains a significant global human health threat [4], as other countries including Argentina, Bolivia, Peru, and Ecuador reported an increase in cases in 2017 [5].
Reducing mosquito vector populations is an effective way to mitigate mosquito-borne disease transmission [6]. Therefore, identifying ZIKV vector species is crucial for accurate risk assessments for mosquito transmission and to target vector control measures to mitigate ZIKV disease. Several Aedes species have been identified as competent vectors in laboratory studies, including the primary vector Aedes (Ae.) aegypti [7–21], Ae. albopictus [7,8,10,11,15,17,19,20,22,23], Ae. notoscriptus [10], Ae. camptorhynchus [10], Ae. luteocephalus [24], Ae. vexans [25], and Ae. vittatus [24]. Culex species generally do not become infected with ZIKV and are incapable of transmitting [7,9,10,12,14,17,23,26–29]. Exceptions include a study from Guadalajara, Mexico, where infectious ZIKV was detected in pooled mosquito tissue samples from field-collected Cx. tarsalis, Cx. coronator, and Cx. quinquefasciatus [30]. ZIKV RNA has also been detected in pooled field samples of Cx. quinquefasciatus from China [31]. Evidence for ZIKV transmission by Culex species is limited to Cx. quinquefasciatus and includes ZIKV RNA detected in saliva on Flinders Technology Associates (FTA) cards provided to a cohort of laboratory-infected mosquitoes from Brazil [32] and transmission to 1-day-old mice from mosquitoes from China, although inconsistently with other murine studies [33–35], no murine fatality was noted [36].
Previous studies demonstrate that ZIKV vector competence is more complex than simple mosquito species-level designations, and thus region-specific mosquito genotypes and multiple ZIKV strains must be evaluated to assess region-specific vector competence. For example, Ae. aegypti from the Dominican Republic transmit ZIKV isolated from Cambodia in 2010 (FSS 13025) and Mexico in 2015 (MEX1-7) more effectively than Ae. aegypti from Salvador, Brazil [21]. Furthermore, ZIKV from Brazil in 2015 (BeH815744) has higher infectivity than a French Polynesian strain from 2013 (H/PF13) in Ae. aegypti from Singapore [8]. The source of virus also matters; fresh ZIKV was more infectious in comparative studies than freeze-thawed virus [12].
California (CA) vector control districts have been combating stable Ae. aegypti populations in the state since 2013 [37], including in many counties in Southern CA. In addition, between 2015 and March 2018, 640 travel-associated ZIKV infections were reported in CA [38], 137 (21% of cases in state) of which were in Los Angeles County where the Ae. aegypti used for vector competence experiments here were collected. Due to the presence of Ae. aegypti and numerous travel-associated ZIKV infections, there is a risk of the establishment of local mosquito-borne ZIKV transmission. Additionally, genetic variation between Central Valley and Southern CA Ae. aegypti populations has been observed [39], even between populations in neighboring cities, such as Fresno and Clovis [40]. These findings indicate that gene flow is limited between Ae. aegypti populations and leave open the possibility that important traits, such as vector competence, may also vary among Ae. aegypti throughout the state. To better assess local ZIKV transmission risk, we evaluated the laboratory vector competence of Ae. aegypti from Los Angeles, CA, for ZIKV isolates from Puerto Rico (2015), Brazil (2015), and Malaysia (1966). We also evaluated the laboratory vector competence of two highly abundant Culex species, Cx. quinquefasciatus from Orange County, CA, and Cx. tarsalis from Kern County, CA, with a Puerto Rico (2015) ZIKV strain.
Materials and methods
Sources of ZIKV strains, mosquitoes, and mice
Three Asian-lineage strains of ZIKV were used in our experiments. A 2015 Puerto Rican strain was isolated from human serum in 2015 (PR15, PRVABC59), passaged 4 times in Vero cells, and sequenced. The coding sequence for the complete genome of the passaged we used was identical to GenBank accession number KX601168. An Asian-lineage Malaysian ZIKV strain isolated from Ae. aegypti mosquitoes in 1966 (MA66, P6-740 [41]) that had been passaged in suckling mouse brains 6 times and once in Vero cells before it was received from the Centers for Disease Control was passaged once more in Vero cells. The complete coding genome sequence of our passage of MA66 was 100% identical to GenBank accession number KX601167.1. A Brazilian strain isolated from human serum in 2015 (BR15, SPH2015) was passaged 3 times in Vero cells and sequenced. The complete genome coding sequence of BR15 was identical to GenBank accession number KU321639. Strains MA66 and PR15 were obtained from Dr. Aaron Brault at the U.S. Centers for Disease Control and Prevention in Fort Collins, Colorado. Dr. Mike Busch at Blood Systems Research Institute, San Francisco, CA, provided the BR15 strain. All ZIKV strains and their source Vero cells were confirmed mycoplasma negative by PCR according to the manufacturer’s instructions (Agilent Mycoplasma Plus PCR Primer Kit, Santa Rosa, CA.)
The Ae. aegypti mosquitoes used in this study were field-collected as larvae in Los Angeles, CA, in 2016 and morphologically identified. The F6 generation was used for this study. Adult Cx. quinquefasciatus mosquitoes were field-collected as adults in Orange County, California in 2016 and morphologically identified. The F5 generation was used for this study. The Cx. tarsalis mosquitoes were field-collected in the Kern National Wildlife Refuge, Kern County, CA in 2002, morphologically identified, and have been maintained continuously in colony since.
Female interferon-deficient (IFN-α/βR−/−; C57BL/6) mice aged 4–8 weeks (B6.129S2-Ifnar1tm1Agt/Mmjax, The Jackson Laboratory, Sacramento, CA) were used for all experiments. Differences in ZIKV viremia levels and kinetics in male versus female mice have not been observed [33].
ZIKV vector competence experiments
Mice were inoculated with 5 log10 Vero plaque forming units (PFU) of ZIKV via subcutaneous injection. ZIKV-infected mice were presented to mosquitoes 2 days post-inoculation, at peak viremia [33]. Mice were anesthetized prior to mosquito exposure with a ketamine (VETone Zetamine CIII, 75 mg/kg), xylazine (AnaSed, 10 mg/kg), and acepromazine (AceproJect, 1 mg/kg) solution administered intraperitoneally. The ZIKV viremia in each mouse was determined by Vero cell plaque assay from 30 μL of whole blood collected immediately prior to the mosquito feed. Viremic mice were presented for two cohorts of adult female mosquitoes 30–60 minutes on one of three arrangements depending on species: (1) 25 Cx. tarsalis in pint cartons (amazon.com), (2) 50 Ae. aegypti in pint cartons, or (3) >100 Cx. quinquefasciatus in a 1 ft3 mesh cage (BugDorm, MegaView Science, Taiwan). Engorged females were sorted from non-fed individuals by vacuum aspiration. Mosquito ages at the time of blood-feeding were 4–14 days post eclosion (dpe) for Cx. tarsalis, 14–21 dpe for Cx. quinquefasciatus, and 4–12 dpe for Ae. aegypti. Cx. tarsalis and Ae. aegypti were held at 26°C, 80% relative humidity, and 12:12 h light:dark cycle. Cx. quinquefasciatus were maintained at room temperature (22°C and 33% relative humidity) to ensure survival. All mosquitoes had constant access to 10% sucrose before and after blood-feeding, except during a 24-hour starvation period prior to presentation of the viremic mice. At days 14 and 21 post bloodfeed, mosquitoes were cold-anesthetized at -20°C for 5 minutes and then legs and wings were removed with forceps while immobilized on ice. Saliva was collected by inserting the proboscis into a capillary tube containing fetal bovine serum (FBS, GenClone) for 20 minutes. Individual bodies, legs+wings, and the saliva sample from each mosquito were stored separately in 2 mL tubes containing a 5 mm glass bead and 250 μL Dulbecco’s modified eagle medium (DMEM, Gibco) supplemented with 50μg/mL of penicillin/streptomycin and 20% FBS. All samples were stored at -80°C until further processing.
ZIKV RNA extraction
Mosquito tissues and glass capillary tubes containing saliva samples were homogenized in DMEM by shaking for 2 minutes at 30 shakes/second using a Tissuelyser (Qiagen, Hilden, Germany). Viral RNA was extracted using the MaxMax Viral RNA Extraction Kit (ThermoFisher, Waltham, MA). A total of 50 μL of homogenate for mosquito tissue and 100 μL of saliva samples were extracted. All RNA extracts were eluted in 50 μL of elution buffer (Buffer EB, Qiagen) and stored at -80°C until further testing.
ZIKV RT-qPCR
ZIKV RNA titers were determined for each body, legs+wings, and saliva sample using the Taqman Fast Virus One-Step Master Mix (ThermoFisher) reverse transcription RT-qPCR kit with a previously described ZIKV-specific assay (primers: ZIKV 1086, ZIKV 1162c, and ZIKV 1107-FAM; [42]). At least two technical replicates were performed for all samples. Samples with a mean cycle threshold (Ct) value of 38 or below were considered positive for ZIKV RNA. This limit of detection was determined from prior testing of serially diluted samples of known ZIKV RNA concentrations with the same extraction and RT-qPCR reagents and protocols and equipment [43].
Infectivity of mosquito saliva
To estimate infectious ZIKV in expectorated Ae. aegypti saliva, viral titrations were performed on a random sample of RT-qPCR-positive saliva samples at the second or third thaw in Vero cell culture by plaque assay. In brief, cell monolayers were inoculated with 110 μL of undilute saliva from individual mosquitoes mixed with DMEM containing 2% (vol/vol) FBS, and 100 U/mL penicillin/streptomycin. After a one hour incubation period to allow for viral infection of cells, 0.8% agarose/DMEM was added to cover the cells. The plates were incubated at 37°C in 5% CO2 for 8 days. The cells were then fixed with 4% formaldehyde and stained with 0.05% crystal violet. Plaques were visualized as holes in the Vero cell monolayer and counted to determine PFU values. The limit of detection of the assay was 2.3 PFU where 110 μL of the total saliva sample (250 μL) was inoculated directly onto the cells. Since the volume of saliva was limited, each sample was tested in just 1 replicate.
Infection definitions, cohort grouping, and statistical analyses
In this study, we calculated infection rates as the number of RT-qPCR positive individual bodies divided by the number of individuals that ingested blood and were tested, dissemination rates as the number of RT-qPCR positive pooled leg & wing sets from each individual divided by the number of individuals that ingested blood and were tested, and transmission rates as the number of RT-qPCR positive saliva samples divided by the total number of individuals that ingested blood and were tested. For Ae. aegypti and Cx. tarsalis, multiple cohorts of the same species fed on different mice infected with the same ZIKV strain with slight (≤1 log10) variations in viremias. Preliminary analysis across same-species cohorts that fed on different mice infected with the same ZIKV strain revealed no significant differences (Fisher’s exact test, P>0.05) in infection, dissemination and transmission rates. We therefore combined the data presented for each ZIKV strain for Ae. aegypti and Cx. tarsalis, while also reporting the magnitudes of viremia in all mice (Table 1). Comparisons of ZIKV RNA levels and PFU in saliva samples between ZIKV strains was performed using a one-way ANOVA with Tukey’s correction for pairwise comparisons (reported as Padj) and ZIKV RNA detection rates were compared using two-tailed Fisher’s exact tests (scipy.stats). Data were plotted using matplotlib (Python).
Table 1. ZIKV infection, dissemination, and transmission rates in California Aedes and Culex mosquitoes 14 and 21 days post ingestion of blood from viremic mice.
| Mosquito Species | Source in California | Generation | ZIKV strain | Blood titer log10 PFU/mL | Infected (%) | Disseminated (%) | Transmitted (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 14 | Day 21 | Day 14 | Day 21 | Day 14 | Day 21 | |||||
| Cx. tarsalis | Kern County | Colony | PR15 | 5.7, 6.4, 5.4 | 2/46 (4) | 6/20 (30) | 2/46 (4) | 1/20 (5) | 0/46 (0) | 0/20 (0) |
| Cx. quinquefasciatus | Los Angeles | F5 | PR15 | 4.6 | 0/42 (0) | 0/37 (0) | 0/42 (0) | 0/37 (0) | 0/42 (0) | 0/37 (0) |
| Ae. aegypti | Los Angeles | F6 | MA66 | 4.3, 4.8 | 73/85 (86) | 22/23 (96) | 69/85 (79) | 21/23 (91) | 45/85 (53) | 20/23 (87) |
| Ae. aegypti | Los Angeles | F6 | PR15 | 5.7, 6.4, 5.4 | 39/46 (85) | 22/23 (96) | 36/46 (78) | 18/23 (78) | 30/46 (65) | 17/23 (74) |
| Ae. aegypti | Los Angeles | F6 | BR15 | 4.7 | 18/20* (90) | n.c. | 18/20*(90) | n.c. | 15/20 *(75) | n.c. |
Infection, dissemination, and transmission rates in mosquitoes that ingested ZIKV from viremic mice, determined by ZIKV RNA detection in bodies, legs+wings, and saliva, respectively. Denominators in rates represent all mosquitoes in cohorts. Multiple values in the mouse blood titer column show viremias for individual mice just before mosquitoes were presented to feed; cohorts of mosquitoes that fed on different mice within this range of viremias were combined since preliminary analysis of each cohort showed no differences in infection, dissemination and transmission rates (data not shown). n.c. indicates samples were not collected at that time point.
*Ae. aegypti that ingested BR15 were harvested 15 dpf.
Ethics statement
All procedures involving mice were performed in accordance with IACUC protocol #19404 that was reviewed and approved by the UC Davis IACUC on June 29, 2017. The UC Davis IACUC adheres to the Office of Laboratory Animal Welfare Health Research Extension Act of 1985 (Public Law 99–158) as well as the United State Department of Agriculture’s Animal Welfare Act. UC Davis is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC) and has an Animal Welfare Assurance (number A3433-01) on file with the Office of Laboratory Animal Welfare (OLAW).
Results
Cx. tarsalis and Cx. quinquefasciatus from California were incapable of transmitting Puerto Rican ZIKV in laboratory vector competence experiments
Cx. tarsalis and Cx. quinquefasciatus mosquitoes were tested 14 or 21 days after ingesting blood from ZIKV-infected interferon receptor deficient mice. Two Cx. tarsalis bodies out of the 46 individuals tested (4%) had low levels of ZIKV RNA at 14 dpf (Ct < 38; 48 ZIKV genomes/body). Both infected individuals also had detectable ZIKV in their legs and wings, indicating disseminated infections. Neither of the ZIKV-infected Cx. tarsalis contained detectable ZIKV RNA in their saliva samples (Table 1). The Cx. tarsalis infection rate significantly increased from 4% to 30% (2/46 to 6/20, P<0.01 Fisher exact test) from 14 to 21 dpf. Among the 6 infected Cx. tarsalis at 21 dpf, ZIKV RNA was detected in 1 leg and wing sample but, consistent with a lack of transmission 14 dpf, no ZIKV RNA was detected in the saliva (Table 1). We did not detect ZIKV RNA in any Cx. quinquefasciatus mosquito tissues 14 (N = 42) or 21 dpf (N = 37; Table 1).
Ae. aegypti from Los Angeles, CA, were highly competent laboratory ZIKV vectors
At 14 dpf, ZIKV infection, dissemination, and transmission rates measured by the presence of ZIKV RNA in Ae. aegypti that ingested MA66 were 86%, 79%, and 53%, respectively (Table 1). For Ae. aegypti that ingested ZIKV PR15, the infection, dissemination, and transmission rates on 14 dpf were 85%, 78%, and 65%, respectively (Table 1). ZIKV BR15-exposed individuals harvested 15 dpf had infection, dissemination, and transmission rates of 90%, 90%, and 75%, respectively (Table 1). ZIKV RNA infection, dissemination, and transmission rates in Ae. aegypti that ingested MA66 or PR15 at 21 dpf were equal or higher than 14 dpf rates. The transmission rate between 14 and 21 dpf increased significantly in Ae. aegypti infected with MA66 (53% vs. 87%, P<0.01, Fisher’s exact), but not PR15 (65% vs. 74%, P = 0.59, Fisher’s exact; Table 1). Transmission rates were not significantly different across viruses (P = 0.13, Fisher’s exact).
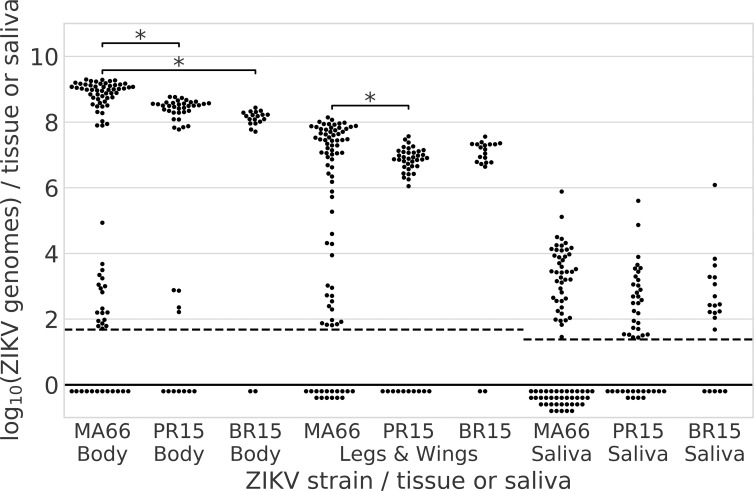
The mean ZIKV RNA level (8.9 log10) in MA66-infected bodies was significantly higher than the mean for BR15 (8.2 log10, ANOVA, degrees of freedom (df) = 2, F-statistic (F) = 16.3, Padj<0.01) and PR15-infected individuals (8.4 log10, ANOVA, df = 2, F = 16.3, Padj<0.01; Fig 1). The mean ZIKV RNA level in MA66-infected leg+wing tissue (7.5 log10) was also significantly higher than PR15-infected leg+wing samples (7.0 log10, ANOVA, df = 2, F = 8.4, Padj<0.01). Higher ZIKV RNA levels in MA66-infected Ae. aegypti likely do not reflect the dose ingested, where flavivirus infections of mosquitoes typically show a strong dose response, since viremias in both ZIKV MA66-infected mice were lower than those for PR15. ZIKV RNA levels in saliva were not significantly different among strains (ANOVA, df = 2, F = 0.96, P = 0.39).
Fig 1. Infecting (bodies), disseminating (legs+wings) and transmitted (saliva) ZIKV RNA levels 14 or 15 days after Ae. aegypti orally ingested one of three Asian lineage ZIKV strains.
Each dot represents the mean log10 ZIKV genome copies per tissue or saliva sample from an individual Ae. aegypti. Ae. aegypti from Los Angeles, California, USA, were fed on viremic mice infected with ZIKV from Malaysia 1966 (MA66), Puerto Rico 2015 (PR15) or Brazil 2015 (BR15). Mosquitoes exposed to BR15 were assayed 15 dpf, MA66 and PR15 cohorts were assayed 14 dpf. Each dot represents the mean of two or more RT-qPCR technical replicates. The dashed lines represent the limits of detection (LOD). Dots below dashed line represent tested samples with an undetectable Ct or a Ct value of >38. The LOD for saliva was lower than for tissues because RNA was extracted from a higher proportion of the saliva sample volume. Asterisks show significant differences in means across groups of the same tissue type, P<0.01, ANOVA, Tukey post-hoc test. No asterisk indicates no significant difference across groups.
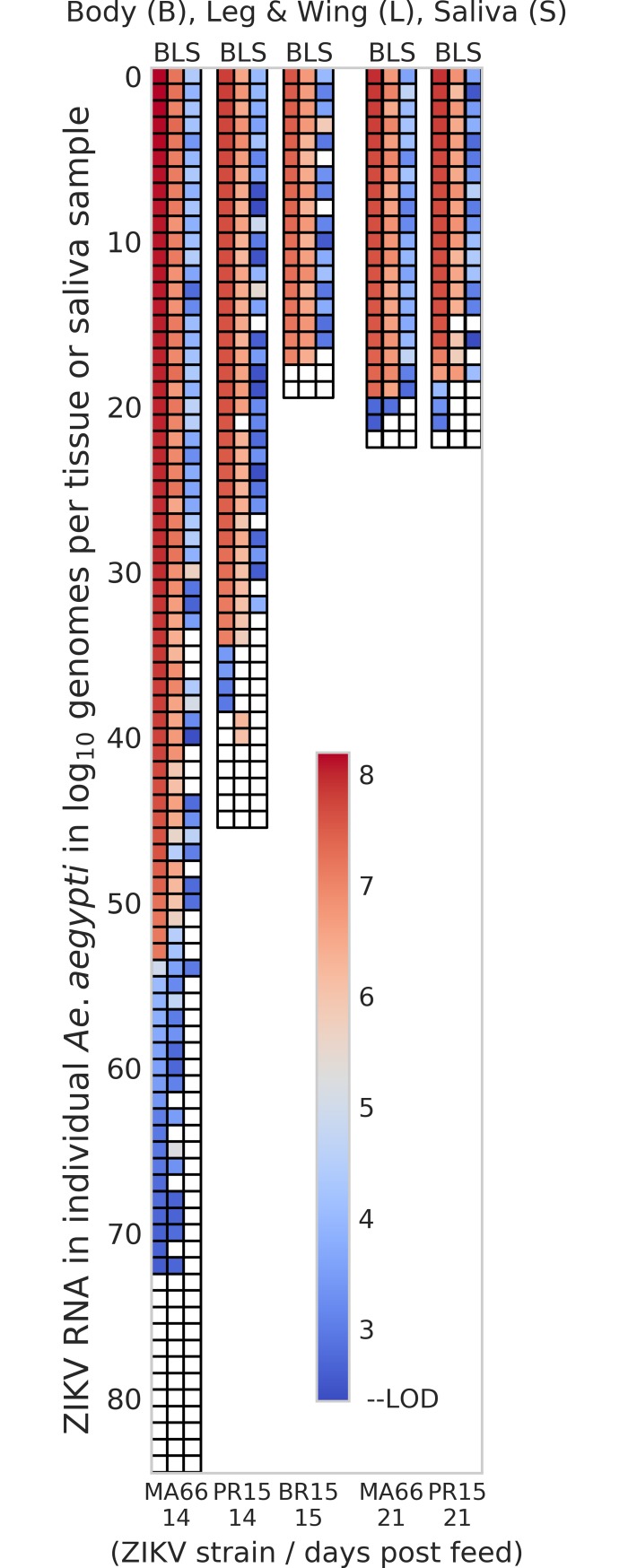
A bimodal distribution of ZIKV RNA levels was observed across cohorts of ZIKV PR15- or MA66-infected bodies, with high (>6 log10 genomes/body) and low (<6 log10 genomes/body) clusters of individuals (Fig 1). MA66-infected Ae. aegypti that were highly infected (>6 log10 genomes/body) had higher transmission rates (81%, N = 54) compared to low titer (<6 log10 genomes/body) individuals (5%, N = 19; P<0.0001, Fisher’s exact). We also examined the relationship between infection, dissemination and transmission at an individual mosquito level for Ae. aegypti (Fig 2). Most Ae. aegypti that became infected developed disseminated infections. Individuals with higher (red/pink in figure) ZIKV RNA levels in legs+wings were more likely to transmit ZIKV RNA than mosquitoes with low (blue in figure) RNA levels in legs+wings. None of the PR15-infected Ae. aegypti with <6 log10 genomes/body transmitted ZIKV RNA (N = 4).
Fig 2. Individual mosquito ZIKV RNA levels in body, legs+wings, and saliva of Aedes aegypti from Los Angeles, CA, USA.
Ae. aegypti from Los Angeles ingested blood from viremic ZIKV-infected interferon receptor deficient mice that had been inoculated with different ZIKV strains. Each colored box represents an individual mosquito sample showing the magnitude of ZIKV RNA detected in each body (B), legs+wings (LW), and saliva (S). The red to blue color scale shows high (red) to low (blue) ZIKV RNA levels. Samples with no detectable ZIKV RNA are colored white. ZIKV BR15-infected mosquitoes were not tested 21 dpf.
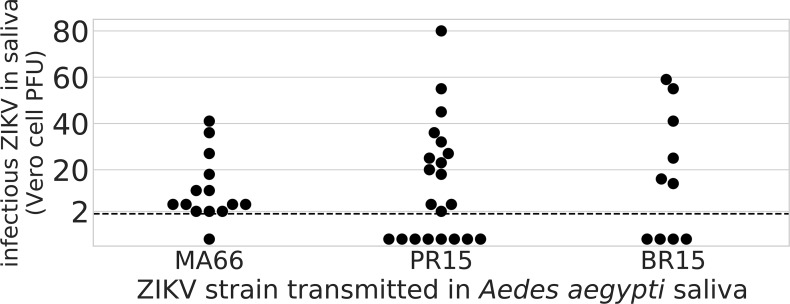
To confirm infectivity and measure the transmitted ZIKV dose, plaque assays were performed on Ae. aegypti saliva collected 14 or 15 dpf to enumerate infectious ZIKV in Vero cell plaque forming units (PFU). Out of 45 RTq-PCR positive saliva samples that were tested by plaque assay, 32 (71%) yielded at least 1 detectable plaque. The expectorated PFU varied by viral strain: the MA66-infected individuals transmitted 13±4 PFU (mean±SE, N = 13) compared to 29±6 for PR15 (N = 13) and 35±8 for BR15 (N = 6; ANOVA, df = 2, F = 3.8, P = 0.035; Fig 3).
Fig 3. Infectious ZIKV levels in expectorated Ae. aegypti saliva.
Saliva from Ae. aegypti mosquitoes from Los Angeles, CA, USA, infected with either MA66, PR15, or BR15 ZIKV was collected in capillary tubes 14 (MA66 and PR15) or 15 (BR15) dpf. Vero cell plaque assays were performed on RT-qPCR positive saliva expectorants to quantify infectious viruses. The limit of detection (LOD) of the assay was 2.3 PFU and is shown as a dashed line. Dots below the dashed line represent saliva samples with no detectable plaques.
Discussion
Understanding the mosquito species that vector ZIKV is important for estimating regional outbreak potential and for informing local mosquito control strategies, especially since Aedes and Culex species differ in life history traits and host-seeking behaviors that could impact control efforts. For example, oviposition traps bias towards Ae. aegypti that lay in artificial containers [44] while Culex typically prefer natural pools [45]. For Cx. tarsalis, we detected an overall ZIKV infection rate of 12% (8/66) in mosquitoes tested 14 and 21 dpf. Disseminated infections in Cx. tarsalis were detected at <5% on both 14 and 21 dpf, with high Ct values indicating low ZIKV RNA levels. We postulate that the disseminated infections detected in Cx. tarsalis may reflect false positives given that mosquitoes with true disseminated infections typically achieve very high viral RNA titers due to prolonged infection of multiple tissues. The absence of detectable ZIKV RNA in saliva at 14 or 21 dpf is evidence that Cx. tarsalis from CA is not capable of transmitting ZIKV in laboratory experiments. Furthermore, Cx. tarsalis feeds less often on human hosts compared to the highly anthropophilic Ae. aegypti [45–47], making human-mosquito-human ZIKV transmission by Cx. tarsalis unlikely. We also found no evidence for ZIKV infection of Cx. quinquefasciatus from California, with no ZIKV RNA detected in bodies, legs/wings or saliva from nearly 80 individuals. This is the first data showing ZIKV vector competence for California mosquitoes, and it supports results from many other studies which demonstrate that Cx. quinquefasciatus is not a competent laboratory vector of ZIKV. By contrast, Ae. aegypti mosquitoes exhibited infection rates of 85–90% and transmission rates of 53–80% at 14 dpf. The transmitted dose of infectious ZIKV by Californian Ae. aegypti is consistent with the range of doses observed in similar studies with Brazilian Ae. aegypti [48,49].
Ae. aegypti that ingested ZIKV MA66 in our laboratory vector competence studies developed higher ZIKV RNA levels than PR15- or BR15-infected mosquitoes. This pattern contrasted with the lower transmission rate and lower expectorated PFU of MA66-infected Ae. aegypti at 14 dpf.
A possible explanation for the lower transmissibility of MA66 at 14 dpf is that it lacks a A188V mutation in the NS1 gene that both PR15 and BR15 possess, which has been linked to higher infectivity (where infectivity can influence transmissibility) in mosquitoes when interferon-deficient mice are used for blood-feeding [7]. ZIKV strains from recent American outbreaks have also been shown to exhibit higher infection and transmission rates than historic Asian-lineage strains [8]. Additional vector competence studies involving region-specific Ae. aegypti and Ae. albopictus mosquito populations with sequenced genomes and multiple distinct ZIKV isolates will improve our understanding of the both mosquito and virus genetics involved in ZIKV vector competence, which could inform our ability to accurately estimate regional outbreak potential.
Among ZIKV MA66-infected Ae. aegypti, we observed that mosquitoes with low RNA copy numbers in bodies were less likely to transmit than those with infections that exceeded 6 log10 genomes per body. This pattern is consistent with the presence of a midgut barrier to infection [50]. In that case, the mosquitoes with low body RNA levels represent infections that have not escaped the midgut while mosquitoes with high body RNA levels correspond to individuals with ZIKV that has disseminated to secondary amplification tissues.
This laboratory vector competence study confirmed that Ae. aegypti from Los Angeles, California, USA, can transmit Asian lineage ZIKV and that Cx. tarsalis and Cx. quinquefasciatus are inefficient ZIKV vectors. Given that Culex mosquitoes are poor ZIKV vectors and seek primarily non-human hosts, they are unlikely to facilitate a ZIKV outbreak. Thus, vector control efforts targeting ZIKV should remain focused on reducing urban Aedes populations.
Acknowledgments
We thank Dr. Aaron Brault at the U.S. Centers for Disease Control and Prevention in Fort Collins, Colorado, and Dr. Mike Busch at Blood Systems Research Institute, San Francisco, CA, for providing the ZIKV strains used in these studies. We thank the Orange County and Greater Los Angeles County Vector Control Districts for providing the field-collected Cx. quinquefasciatus and Ae. aegypti used in these studies.
Data Availability
All relevant data are within the paper.
Funding Statement
Primary funding for this work was provided by Abt Associates and a consortium of vector control districts in California: Coachella Valley, Orange County, Greater Los Angeles County, San Gabriel Valley, West Valley, Kern, Butte County, Tulare, Sacramento-Yolo, Placer, and Turlock. OCW and CMB also acknowledge financial support from NASA Health and Air Quality grant NNX15AF36G, and CMB and LLC acknowledge funding support from the Pacific Southwest Regional Center of Excellence for Vector-Borne Diseases funded by the U.S. Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000516). KKR was supported by a National Institutes of Health Ruth L. Kirschstein National Research Service Award T32 OD O11147. Part of this work was supported by start-up funds provided to LLC by the Pathology, Microbiology and Immunology Department in the School of Veterinary Medicine at UC Davis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. One funder, Abt Associates, provided support in the form of salaries for authors [MK and RT], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Dick GWA, Kitchen SF, Haddow AJ. Zika Virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. Oxford University Press; 1952;46: 509–520. [DOI] [PubMed] [Google Scholar]
- 2.Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. who.int; 2016;94: 675–686C. doi: 10.2471/BLT.16.171082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krauer F, Riesen M, Reveiz L, Oladapo OT, Martínez-Vega R, Porgo TV, et al. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review. PLoS Med. Public Library of Science; 2017;14: e1002203 doi: 10.1371/journal.pmed.1002203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO | Zika situation report. World Health Organization; 2017; Available: http://who.int/entity/emergencies/zika-virus/situation-report/10-march-2017/en/index.html
- 5.Sanchez JD. PAHO WHO | Regional Zika Epidemiological Update (Americas) July 26, 2017. In: Pan American Health Organization / World Health Organization [Internet]. 15 Aug 2017 [cited 23 Aug 2017]. Available: http://www.paho.org/hq/index.php?option=com_content&id=11599&Itemid=41691
- 6.Curtis CF, Davies CR. Present use of pesticides for vector and allergen control and future requirements. Med Vet Entomol. Blackwell Science, Ltd; 2001;15: 231–235. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Liu J, Du S, Shan C, Nie K, Zhang R, et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature. nature.com; 2017;545: 482–486. doi: 10.1038/nature22365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pompon J, Morales-Vargas R, Manuel M, Huat Tan C, Vial T, Hao Tan J, et al. A Zika virus from America is more efficiently transmitted than an Asian virus by Aedes aegypti mosquitoes from Asia. Sci Rep. 2017;7: 1215 doi: 10.1038/s41598-017-01282-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes RS, Campos SS, Ferreira-de-Brito A, de Miranda RM, da Silva KAB, de Castro MG, et al. Culex quinquefasciatus from Rio de Janeiro Is Not Competent to Transmit the Local Zika Virus. PLoS Negl Trop Dis. Public Library of Science; 2016;10: e0004993 doi: 10.1371/journal.pntd.0004993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchemin J-B, Mee PT, Lynch SE, Vedururu R, Trinidad L, Paradkar P. Zika vector transmission risk in temperate Australia: a vector competence study. Virol J. BioMed Central; 2017;14: 108 doi: 10.1186/s12985-017-0772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciota AT, Bialosuknia SM, Zink SD, Brecher M, Ehrbar DJ, Morrissette MN, et al. Effects of Zika Virus Strain and Aedes Mosquito Species on Vector Competence. Emerg Infect Dis. 2017;23: 1110–1117. doi: 10.3201/eid2307.161633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weger-Lucarelli J, Rückert C, Chotiwan N, Nguyen C, Garcia Luna SM, Fauver JR, et al. Vector Competence of American Mosquitoes for Three Strains of Zika Virus. PLoS Negl Trop Dis. journals.plos.org; 2016;10: e0005101 doi: 10.1371/journal.pntd.0005101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa-da-Silva AL, Ioshino RS, Araújo HRC de, Kojin BB, Zanotto PM de A, Oliveira DBL, et al. Laboratory strains of Aedes aegypti are competent to Brazilian Zika virus. PLoS One. 2017;12: e0171951 doi: 10.1371/journal.pone.0171951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall-Mendelin S, Pyke AT, Moore PR, Mackay IM, McMahon JL, Ritchie SA, et al. Assessment of Local Mosquito Species Incriminates Aedes aegypti as the Potential Vector of Zika Virus in Australia. PLoS Negl Trop Dis. Public Library of Science; 2016;10: e0004959 doi: 10.1371/journal.pntd.0004959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep. The Author(s); 2016;6: 28792 doi: 10.1038/srep28792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richard V, Paoaafaite T, Cao-Lormeau V-M. Vector Competence of French Polynesian Aedes aegypti and Aedes polynesiensis for Zika Virus. PLoS Negl Trop Dis. 2016;10: e0005024 doi: 10.1371/journal.pntd.0005024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heitmann A, Jansen S, Lühken R, Leggewie M, Badusche M, Pluskota B, et al. Experimental transmission of Zika virus by mosquitoes from central Europe. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.2.30437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boccolini D, Toma L, Di Luca M, Severini F, Romi R, Remoli ME, et al. Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Euro Surveill. ncbi.nlm.nih.gov; 2016;21 doi: 10.2807/1560-7917.ES.2016.21.35.30328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl Trop Dis. journals.plos.org; 2016;10: e0004543 doi: 10.1371/journal.pntd.0004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. e-sciencecentral.org; 2016;21 doi: 10.2807/1560-7917.ES.2016.21.18.30223 [DOI] [PubMed] [Google Scholar]
- 21.Roundy CM, Azar SR, Rossi SL, Huang JH, Leal G, Yun R, et al. Variation in Aedes aegypti Mosquito Competence for Zika Virus Transmission. Emerg Infect Dis. 2017;23: 625–632. doi: 10.3201/eid2304.161484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong P-SJ, Li M-ZI, Chong C-S, Ng L-C, Tan C-H. Aedes (Stegomyia) albopictus (Skuse): A Potential Vector of Zika Virus in Singapore. PLoS Negl Trop Dis. Public Library of Science; 2013;7: e2348 doi: 10.1371/journal.pntd.0002348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azar SR, Roundy CM, Rossi SL, Huang JH, Leal G, Yun R, et al. Differential Vector Competency of Aedes albopictus Populations from the Americas for Zika Virus. Am J Trop Med Hyg. The American Society of Tropical Medicine and Hygiene; 2017;97: 330–339. doi: 10.4269/ajtmh.16-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis. 2015;15: 492 doi: 10.1186/s12879-015-1231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gendernalik A, Weger-Lucarelli J, Garcia Luna SM, Fauver JR, Rückert C, Murrieta RA, et al. American Aedes vexans Mosquitoes are Competent Vectors of Zika Virus. Am J Trop Med Hyg. ASTMH; 2017;96: 1338–1340. doi: 10.4269/ajtmh.16-0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amraoui F, Atyame-Nten C, Vega-Rúa A, Lourenço-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.35.30333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodson BL, Rasgon JL. Vector competence of Anopheles and Culex mosquitoes for Zika virus. PeerJ. 2017;5: e3096 doi: 10.7717/peerj.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney JL, Romo H, Duggal NK, Tzeng W-P, Burkhalter KL, Brault AC, et al. Transmission Incompetence of Culex quinquefasciatus and Culex pipiens pipiens from North America for Zika Virus. Am J Trop Med Hyg. 2017;96: 1235–1240. doi: 10.4269/ajtmh.16-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y-JS, Ayers VB, Lyons AC, Unlu I, Alto BW, Cohnstaedt LW, et al. Culex Species Mosquitoes and Zika Virus. Vector Borne Zoonotic Dis. 2016;16: 673–676. doi: 10.1089/vbz.2016.2058 [DOI] [PubMed] [Google Scholar]
- 30.Elizondo-Quiroga D, Medina-Sánchez A, Sánchez-González JM, Eckert KA, Villalobos-Sánchez E, Navarro-Zúñiga AR, et al. Zika Virus in Salivary Glands of Five Different Species of Wild-Caught Mosquitoes from Mexico. Sci Rep. 2018;8: 809 doi: 10.1038/s41598-017-18682-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu S, Song S, Liu H, Li Y, Li X, Gao X, et al. ZIKA virus isolated from mosquitoes: a field and laboratory investigation in China, 2016. Sci China Life Sci. 2017; doi: 10.1007/s11427-017-9196-8 [DOI] [PubMed] [Google Scholar]
- 32.Guedes DR, Paiva MH, Donato MM, Barbosa PP, Krokovsky L, Rocha SWDS, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6: e69 doi: 10.1038/emi.2017.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, et al. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016;94: 1362–1369. doi: 10.4269/ajtmh.16-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19: 720–730. doi: 10.1016/j.chom.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. Elsevier; 2016;16: 3208–3218. doi: 10.1016/j.celrep.2016.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo X-X, Li C-X, Deng Y-Q, Xing D, Liu Q-M, Wu Q, et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect. 2016;5: e102 doi: 10.1038/emi.2016.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gloria-Soria A, Brown JE, Kramer V, Hardstone Yoshimizu M, Powell JR. Origin of the dengue fever mosquito, Aedes aegypti, in California. PLoS Negl Trop Dis. 2014;8: e3029 doi: 10.1371/journal.pntd.0003029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDPH [Internet]. 5 Jan 2018. Available: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Zika.aspx
- 39.Pless E, Gloria-Soria A, Evans BR, Kramer V, Bolling BG, Tabachnick WJ, et al. Multiple introductions of the dengue vector, Aedes aegypti, into California. PLoS Negl Trop Dis. Public Library of Science; 2017;11: e0005718 doi: 10.1371/journal.pntd.0005718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y, Collier TC, Conner WR, Hanemaaijer MJ. Spatial and temporal distribution of genome divergence among California populations of Aedes aegypti. bioRxiv. biorxiv.org; 2017; Available: http://www.biorxiv.org/content/early/2017/07/20/166629.abstract
- 41.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. journals.plos.org; 2012;6: e1477 doi: 10.1371/journal.pntd.0001477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14: 1232–1239. doi: 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone M, Lanteri MC, Bakkour S, Deng X, Galel SA, Linnen JM, et al. Relative analytical sensitivity of donor nucleic acid amplification technology screening and diagnostic real-time polymerase chain reaction assays for detection of Zika virus RNA. Transfusion. 2017;57: 734–747. doi: 10.1111/trf.14031 [DOI] [PubMed] [Google Scholar]
- 44.Ritchie SA, Long S, Hart A, Webb CE, Russell RC. An adulticidal sticky ovitrap for sampling container-breeding mosquitoes. J Am Mosq Control Assoc. 2003;19: 235–242. [PubMed] [Google Scholar]
- 45.Reiter P, Others. A portable battery-powered trap for collecting gravid Culex mosquitoes. Mosq News. 1983;43: 496–498. [Google Scholar]
- 46.Thiemann TC, Wheeler SS, Barker CM, Reisen WK. Mosquito host selection varies seasonally with host availability and mosquito density. PLoS Negl Trop Dis. 2011;5: e1452 doi: 10.1371/journal.pntd.0001452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. Annual Reviews; 2013;58: 433–453. doi: 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- 48.Roundy Christopher M., Azar Sasha R., Rossi Shannan L., Huang Jing H., Leal Grace, Yun Ruimei, et al. Variation in Aedes aegypti Mosquito Competence for Zika Virus Transmission. Emerging Infectious Diseases. 2017;23: 625–632. doi: 10.3201/eid2304.161484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandes RS, Campos SS, Ribeiro PS, Raphael LM, Bonaldo MC, Lourenço-de-Oliveira R. Culex quinquefasciatus from areas with the highest incidence of microcephaly associated with Zika virus infections in the Northeast Region of Brazil are refractory to the virus. Mem Inst Oswaldo Cruz. 2017;112: 577–579. doi: 10.1590/0074-02760170145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turell MJ, Gargan TP 2nd, Bailey CL. Replication and dissemination of Rift Valley fever virus in Culex pipiens. Am J Trop Med Hyg. 1984;33: 176–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.