Abstract
Background
Lumbar spine TBS, a texture index derived from lumbar spine dual-energy x-ray absorptiometry (DXA) images, enhances fracture prediction. No studies to date have studied a broad range of clinical variables to determine which patients might experience the greatest benefit from the use of TBS.
Methods
Using the Manitoba BMD Registry, we identified 37,176 subjects with baseline DXA, FRAX®-based fracture probability, lumbar spine TBS, and minimum 5 years of observation. Subgroups considered were based on sex, age, body mass index (BMI), prior fracture, chronic obstructive lung disease (COPD), high alcohol use, rheumatoid arthritis (RA), high glucocorticoid use, osteoporotic femoral neck T-score, number of comorbidities, diabetes, secondary osteoporosis, and prior osteoporosis treatment. Non-traumatic major osteoporotic fractures (MOF, n=3741) and hip fractures (HF, n=1008) were identified using population-based health services data. We analyzed baseline TBS using analysis of covariance (ANCOVA). FRAX-adjusted hazard ratios (HR) per SD reduction in TBS were estimated and tested for interactions. Categorical net reclassification improvement (NRI) was estimated using fixed FRAX-based intervention cut-offs.
Results
Adjusted baseline TBS was significantly lower (p≤0.001) for women (-4.2%), osteoporotic hip T-score (-4.0%), COPD (-2.8%), diabetes (-2.6%), high alcohol use (-2.3%), prior fracture (-2.2%), glucocorticoid use (-1.5%), RA (-0.9%) and secondary osteoporosis (-0.8%), whereas recent osteoporosis therapy was associated with greater TBS (+1.5%). HRs per SD reduction in TBS for fracture prediction were larger for age <65 vs 65+ (MOF p-interaction=0.004, HF p-interaction<0.001), without vs with prior fracture (MOF p-interaction=0.003, HF p-interaction=0.048), without vs with glucocorticoid use (HF p-interaction=0.029), lower vs higher comorbidity score (HF p-interaction<0.001), and without vs with osteoporosis treatment (MOF p-interaction=0.005). NRI for using the TBS adjustment to FRAX in all subjects was 1.2% for MOF (p=0.002) and 1.7% for HF (p=0.016). NRI was greater in subjects age <65 y (MOF: 1.7%, HF: 5.6%), no prior fracture (HF: 2.4%), non-osteoporotic T-score (HF: 3.0%), and high glucocorticoid use (MOF: 3.9%).
Conclusion
TBS is sensitive to the effects of multiple risk factors for fracture. TBS-adjusted fracture risk assessment resulted in significant improvements for multiple subgroups.
Keywords: Osteoporosis, fracture prediction, FRAX, trabecular bone score
1. Introduction
Trabecular bone score (TBS) is a bone texture index derived from lumbar spine dual-energy x-ray absorptiometry (DXA) images. Multiple studies and a mixture of study designs have demonstrated an association between reduced TBS and increased fracture risk [1]. More recently, a technique to incorporate TBS in FRAX for improved fracture-risk assessment has been developed [2] with TBS-adjusted FRAX predictions shown to result in small but significant improvements in risk classification over conventional FRAX risk estimates [3–6].
Studies involving older subjects [7–9], patients exposed to glucocorticoids [10–13], hyperparathyroidism [14–16], diabetes mellitus [17–20], and renal disease [21,22] have shown that these groups typically have decreased TBS values and increased fracture risk compared to controls. With the exception of [9,12,16,17,22], all of these studies were retrospective. A recent retrospective study by Martineau et al [5] showed that fracture risk reclassification from the use of TBS-adjusted FRAX was greatest in women close to an intervention threshold and in women under 65 years of age; however, that study was not designed to identify other clinical factors for which the use of TBS might significantly impact on management.
The clinical utility of TBS, as with any risk factor, is largely determined by its impact on management decisions. TBS can potentially alter the fracture risk assessment and, in those patients close to an intervention threshold, management decisions in two major ways: if a given risk factor is associated with significantly lower TBS, and/or if a given TBS reduction associated with a particular risk factor is in turn associated with a larger fracture risk hazard ratio (HR). The former assumes that TBS has the same effect in the presence/absence of the risk factor under consideration (i.e., no interaction) whereas the latter indicates a larger effect on fracture risk (i.e., significant interaction). Either or both together can result in TBS having an impact on management.
To date, no studies have simultaneously explored the relationship between multiple clinical variables and their effect on baseline TBS, HR for TBS to predict fracture, and risk reclassification using the TBS-adjusted FRAX. The purpose of the current study was to examine the relative impact of commonly encountered clinical variables on TBS in routine clinical practice in order to determine those subgroups in which TBS is most likely to be beneficial.
2. Materials and Methods
2.1. Patient population
In the Province of Manitoba, Canada, health services are provided to virtually all residents through a single public health care system. Manitoba Health maintains computerized databases of physician billing claims and hospital separations for all residents of the province eligible to receive health services. The Manitoba Bone Density Program is a targeted case-finding clinical program with the associated database validated and described elsewhere [23,24]. This database has been shown to exceed 99% in terms of completeness and accuracy. We performed a historical cohort study in men and women, age 40 years or older, who had undergone baseline BMD measurement of the spine and hip by DXA using a narrow, fan-beam scanner configuration (Prodigy, GE Healthcare, Madison, WI, USA) with at least 5 years of follow up for assessing incident fractures. We excluded individuals with BMI <15 or >37 kg/m2 since TBS is not recommended in extremes of body size [1]. All participants had medical coverage during the observation period ending March 31, 2013. In cases of multiple eligible data sets, only the first record was included in the analysis. The study was approved by the Health Research Ethics Board for the University of Manitoba.
2.2. Measurement of BMD and TBS
All DXA scans were performed and analyzed in accordance with the manufacturer's recommendations. BMD measurements were recorded for the lumbar spine BMD for L1 through L4 (L1–L4, excluding obvious artifacts) and the femoral neck. The resulting data approximated a normal distribution. Instruments were cross-calibrated using anthropomorphic phantoms. No clinically significant differences were identified; therefore, all analyses are based on unadjusted numerical results generated by the instrument.
All TBS measurements were performed in the Bone Disease Unit at the University of Lausanne, Lausanne, Switzerland (TBS iNsight Software, Version 2.1, Med-Imaps, Pessac, France), using anonymized spine DXA files from the Manitoba database to ensure blinding of the Swiss investigators to all clinical parameters and outcomes. No significant differences in mean TBS measurements were seen for the three DXA scanners used. All three instruments used for this study exhibited stable long-term performance (coefficient of variation [CV] < 0.5%) and satisfactory in vivo precision. Short-term reproducibility (CV) for TBS was 2.1% and for lumbar spine BMD was 1.7% in 92 individuals with repeat spine DXA scans performed within 28 days.
2.3. Fracture Outcomes
Each health system contact includes information on a patient's demographics, date and type of service, and diagnoses from (1) physician billing claims (inpatient, outpatient, and private office) coded using the International Classification of Disease, 9th edition, Clinical Modification (ICD-9-CM) system and (2) hospital discharge abstracts, for which the diagnoses and procedures have been coded using the ICD-9-CM system prior to 2005 and the ICD-10-CA system thereafter. Anonymous linkage of these databases to the BMD database was possible via a unique scrambled health identification number, thereby allowing for the creation of a longitudinal record of health services and outcomes. Longitudinal health service records were examined for the presence of fracture codes before and after BMD testing that were not associated with trauma codes using previously validated algorithms [25]. Hip fracture (HF) and major osteoporotic fracture (MOF) (hip, clinical spine, forearm, and humerus fractures) were studied as these are the basis for the 10-year absolute fracture risk estimates generated by FRAX. We required that hip and forearm fractures be accompanied by a site-specific fracture reduction, fixation, or casting code, which enhances the diagnostic and temporal specificity of an acute fracture.
2.4. Fracture Probability and Other Covariates
The ten-year probabilities for MOF and for HF were calculated using FRAX, fracture risk assessment tool, developed by the World Health Organization Collaborating Centre at Sheffield, UK, (Canadian version (FRAX® Desktop Multi-Patient Entry, version 3.7). The Canadian FRAX tool was calibrated using nationwide hip fracture data [26], and its predictions agreed closely with observed fracture rates in Manitoba and the general Canadian population [27,28]. Data required for calculating fracture probability with FRAX were assessed through a combination of data from the BMD registry, self-reported information at the time of BMD testing, hospital discharge abstracts, physician claims and a province-wide retail pharmacy database as previously described [29]. Anthropomorphic data (height and weight) were measured at the time of DXA, and BMI was calculated. In addition to prior osteoporotic fractures, we identified prior diagnoses of diabetes, rheumatoid arthritis, chronic obstructive pulmonary disease (COPD, a proxy for smoking), alcohol/substance abuse (a proxy for high alcohol intake), prolonged (>3 months) systemic corticosteroid use in the last year, and osteoporosis medication use (>6 months) in the year before BMD testing. Secondary osteoporosis was defined from the following previously diagnosed conditions: hyperthyroidism, ankylosing spondylitis, inflammatory bowel disease, cerebrovascular disease, Parkinson’s disease, muscular dystrophy, celiac disease or other disorders of malabsorption, chronic liver disease, organ transplantation, gastrectomy or small bowel resection. To define burden of comorbidity in the 1-year prior to their baseline DXA test for each subject we used the Johns Hopkins Adjusted Clinical Group® (ACG®) Case-Mix System (version 9) [30,31]. Aggregated Diagnosis Groups (ADGs) represent 32 comorbidity clusters of every ICD diagnostic codes.
We then derived TBS-adjusted FRAX fracture probability using a method previously described in detail by McCloskey et al [2]. This procedure incorporates both competing mortality and an age-TBS interaction in the calculation. This resulted in both FRAX and TBS-adjusted FRAX fracture probabilities of MOF and HF being available for all subjects. For all participants, the FRAX probabilities for MOFs and HFs were calculated initially using the femoral neck BMD and other covariates, and then recalculated including the TBS in the FRAX assessment.
2.5. Clinical variable subgroups
Our analysis examined subgroups based on multiple clinical variables: age (above or below 65 years of age), sex, BMI (greater or less than 30 kg/m2), history of prior osteoporotic fracture, COPD, prior diagnosis of rheumatoid arthritis, high alcohol intake, prolonged (>3 months) systemic corticosteroid use in the last year, secondary osteoporosis, osteoporotic femoral neck T-score, ADG comorbidity score (low <3, moderate 3-5, or high >5), prior diagnosis of diabetes, and osteoporosis treatment. These subgroups were chosen due to their association with increased MOF and HF risk.
2.6. Statistical Analysis
All statistical analyses were performed using Statistica (Version 12.0, StatSoft, Inc., Tulsa, OK, USA). The criterion for statistical significance was set at a p value of 0.05. Descriptive statistics for demographic and baseline characteristics are presented as mean ± SD for continuous variables or count (percent) for categorical variables. All models were age- and BMI-adjusted.
Analysis of covariance (ANCOVA) was used to assess independent effects of the clinical variables on TBS considered simultaneously. Results were reported as the percent change with 95% confidence intervals (CI). Hazard ratios (HR) per SD reduction in TBS for both MOF and HF were determined along with 95% CI using Cox proportional hazards regression. Stratified models were constructed to assess the effect of TBS within subgroups defined from the clinical variables. Two-way interaction terms between the clinical variables and TBS (e.g., sex*TBS, diabetes*TBS) were examined and tested for significance.
Using fixed FRAX-based intervention criteria - MOF ≥ 20% or HF ≥ 3% - we computed the percentage of patients reclassified within each subgroup defined from the clinical variables. These intervention cutoffs are the basis of the US National Osteoporosis Foundation (NOF) guidelines, and we have previously shown that the NRI results from the use of TBS-adjusted FRAX are comparable when using different guidelines [5]. Using logistic regression, we also estimated the odds ratio (OR) for reclassification with 95% CI for reclassification using the TBS adjustment to FRAX where all clinical variables were considered simultaneously. Impact on reclassification was studied using the Net Reclassification Improvement (NRI). NRI [32] is a technique which measures the impact of including an additional clinical variable on the classification of predicted risk. NRI was used to examine the clinical impact of applying the TBS adjustment to FRAX for fixed MOF risk of 20% or HF risk of 3% within subgroups defined by various clinical variables, as detailed above. The NRI was calculated as per the method detailed by Pencina et al [32] and reported as recommended by Leening et al [33]. For individuals that sustain a fracture in follow up, NRI cases is the probability of moving 'up' to a higher FRAX risk category minus the probability of moving 'down' to a lower FRAX risk category. Conversely, for individuals who remain fracture-free in follow up, NRI non-cases is the probability of moving into a lower FRAX risk category minus the probability of moving into a higher FRAX risk category. Values of NRI cases and NRI non-cases greater than zero indicate an improvement in risk classification, whereas negative values indicate worse risk classification. An asymptotic test of significance for the null hypothesis of NRI=0 based upon the multinomial distribution was performed [32].
3. Results
3.1. Population characteristics
A total of 37,176 subjects, with a mean age of 64 years, satisfied the eligibility criteria. Table 1 summarizes the baseline characteristics. On average, TBS was significantly lower (p <0.001) in subjects with incident fractures (MOF and HF) than in those without.
Table 1.
Baseline characteristics stratified by incident major osteoporotic fracture (MOF) and incident hip fracture (HF).
| All Cases | MOF | No MOF | p-value | HF | No HF | p-value | |
|---|---|---|---|---|---|---|---|
| N | 37,176 | 3741 | 33,435 | 1008 | 36,168 | ||
| Age (years) | 63.6 ± 10.9 | 68.5 ± 11.0 | 63.0 ± 10.7 | <0.001 | 73.8 ± 9.3 | 63.3 ± 10.8 | <0.001 |
| Sex (women) | 34,316 (92.3%) | 3503 (93.6%) | 30,813 (92.2%) | 0.001 | 945 (93.8%) | 33,371 (92.3%) | 0.081 |
| BMI (kg/m2) | 26.1 ± 4.3 | 25.6 ± 4.3 | 26.1 ± 4.3 | <0.001 | 25.0 ± 4.2 | 26.1 ± 4.3 | <0.001 |
| Prior fracture | 5070 (13.6%) | 955 (25.5%) | 4115 (12.3%) | <0.001 | 274 (27.2%) | 4796 (13.3%) | <0.001 |
| Femoral neck T-score | -1.4 ± 1.0 | -1.9 ± 0.9 | -1.3 ± 1.0 | <0.001 | -2.3 ± 0.8 | -1.4 ± 1.0 | <0.001 |
| FRAX 10 year MOF risk without TBS (%) | 10.7 ± 7.8 | 16.0 ± 10.3 | 10.1 ± 7.3 | <0.001 | 20.6 ± 10.7 | 10.5 ± 7.6 | <0.001 |
| FRAX 10 year HF risk without TBS (%) | 2.6 ± 4.3 | 5.1 ± 6.2 | 2.3 ± 4.0 | <0.001 | 7.7 ± 7.0 | 2.4 ± 4.1 | <0.001 |
| Lumbar spine TBS | 1.32 ± 0.12 | 1.27 ± 0.12 | 1.33 ± 0.12 | <0.001 | 1.25 ± 0.12 | 1.32 ± 0.12 | <0.001 |
BMI=Body mass index
3.2. Baseline TBS
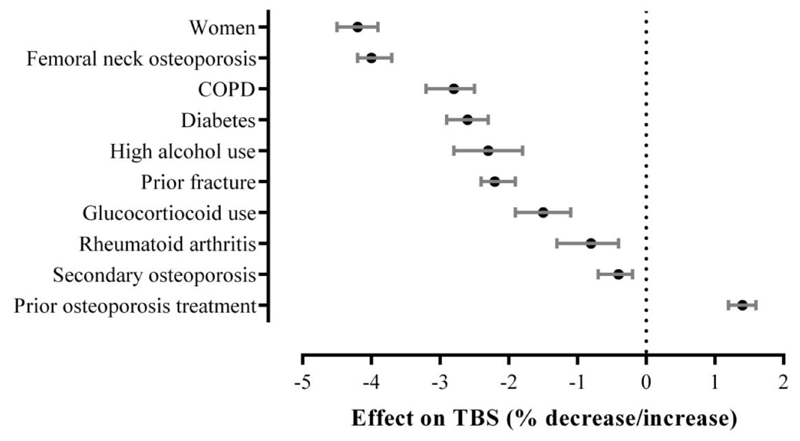
Factors affecting baseline TBS are shown in Figure 1. Significant reductions in age- and BMI-adjusted TBS (rank ordered) were associated with women (-4.2%), osteoporotic hip T-score (-4.0%), COPD (-2.8%), diabetes (-2.6%), high alcohol use (-2.3%), prior fracture (-2.2%), glucocorticoid use (-1.5%), RA (-0.9%) and secondary osteoporosis (-0.8%). Recent osteoporosis therapy was associated with greater TBS (+1.5%). No statistically significant differences in TBS were noted for subjects with different levels of comorbidity scores. Supplementary Table 1 summarizes all factors and their independent effects on baseline least-squares mean TBS.
Figure 1.
Rank-ordered relative change in TBS with 95% CI bars attributable to the presence (versus absence) of clinical variables from multivariable ANCOVA models (age and BMI-adjusted).
3.3. Fracture prediction
Over a mean follow-up period of 8.7 (±2.7) years, 3741 (10.1%) of these subjects suffered incident MOFs, with a total of 1008 (2.7%) incident HFs recorded. Numbers of MOFs and HFs within each subgroup are shown in Table 2. The calculated HRs associated with the various clinical subgroups for both MOF and HF are shown in Table 3, with significant values plotted in Figure 2. Each SD reduction in TBS was significantly associated with greater risk of MOFs for each subgroup considered (with the exception of men, RA, and high alcohol consumption), with the largest effects in subjects aged < 65 years and those with low comorbidity. For HFs, each SD reduction in TBS was significantly associated with greater risk for women, subjects less than 65 years of age, low or moderate comorbidity, and those with BMIs less than 30 kg/m2, whereas associations with other clinical variables were non-significant.
Table 2.
Net reclassification improvement (NRI) for incident major osteoporotic fracture (MOF) and hip fracture (HF) cases using the TBS-adjustment to FRAX assuming MOF ≥ 20% and HF ≥ 3% intervention cut-offs, respectively.
| Clinical variables | Subgroups | N (overall %) | MOF |
HF |
||||
|---|---|---|---|---|---|---|---|---|
| Number of fractures (subgroup %) | NRI | p-value | Number of fractures (subgroup %) | NRI | p-value | |||
| All subjects | 37,176 | 3,741 (10.1%) | 1.2% | 0.002 | 1,008 (2.7%) | 1.7% | 0.016 | |
| Sex | Women | 34,316 (92.3%) | 3,503 (10.2%) | 1.1% | 0.006 | 945 (2.8%) | 1.8% | 0.010 |
| Men | 2,860 (7.7%) | 238 (8.3%) | 2.3% | 0.054 | 63 (2.2%) | -0.9% | 0.78 | |
| Age (years) | < 65 | 20,431 (55.0%) | 1,353 (6.6%) | 1.7% | <0.001 | 170 (0.8%) | 5.6% | 0.017 |
| ≥ 65 | 16,745 (45.0%) | 2,388 (14.3%) | 0.8% | 0.14 | 838 (5.0%) | -0.1% | 0.92 | |
| BMI (kg/m2) | < 30 | 29,952 (80.6%) | 3,090 (10.3%) | 1.4% | 0.001 | 871 (2.9%) | 1.9% | 0.012 |
| ≥ 30 | 7,224 (19.4%) | 651 (9.0%) | 0.2% | 0.80 | 137 (1.9%) | 0.7% | 0.74 | |
| Diabetes | Yes | 3704 (10.0%) | 437 (11.8%) | 2.5% | 0.030 | 155 (4.2%) | 3.1% | 0.15 |
| No | 33,472 (90.0%) | 3304 (9.9%) | 1.0% | 0.016 | 853 (2.5%) | 1.3% | 0.074 | |
| Prior fracture | Yes | 5,070 (13.6%) | 955 (18.8%) | 1.0% | 0.32 | 274 (5.4%) | -0.5% | 0.68 |
| No | 32,106 (86.4%) | 2,786 (8.7%) | 1.0% | 0.011 | 734 (2.3%) | 2.4% | 0.006 | |
| COPD | Yes | 3,214 (8.6%) | 429 (13.3%) | 3.2% | 0.019 | 126 (3.9%) | 1.1% | 0.63 |
| No | 33,962 (91.4%) | 3,312 (9.8%) | 0.8% | 0.030 | 882 (2.6%) | 1.7% | 0.019 | |
| High alcohol use | Yes | 1,030 (2.8%) | 132 (12.8%) | 4.0% | 0.094 | 30 (2.9%) | -4.7% | 0.42 |
| No | 36,146 (97.2%) | 3,609 (10.0%) | 1.0% | 0.006 | 978 (2.7%) | 1.9% | 0.007 | |
| Rheumatoid arthritis | Yes | 1,335 (3.6%) | 181 (13.6%) | -0.6% | 0.69 | 50 (3.7%) | 0.6% | 0.77 |
| No | 35,841 (96.4%) | 3,560 (9.9%) | 1.2% | 0.001 | 958 (2.7%) | 1.7% | 0.017 | |
| Glucocorticoid use | Yes | 2,164 (5.8%) | 254 (11.7%) | 3.9% | 0.039 | 83 (3.8%) | -0.3% | 0.87 |
| No | 35,012 (94.2%) | 3,487 (10.0%) | 1.0% | 0.012 | 925 (2.6%) | 1.9% | 0.012 | |
| Secondary osteoporosis | Yes | 2,690 (7.2%) | 357 (13.3%) | 0.2% | 0.90 | 114 (4.2%) | -0.2% | 0.91 |
| No | 34,486 (92.8%) | 3,384 (9.8%) | 1.2% | 0.002 | 894 (2.6%) | 1.9% | 0.012 | |
| Femoral neck osteoporosis | Yes | 4,713 (12.7%) | 1,051 (22.3%) | 1.5% | 0.15 | 430 (9.1%) | 2.3% | <0.001 |
| No | 32,463 (87.3%) | 2,690 (8.3%) | 1.0% | 0.007 | 578 (1.8%) | 3.0% | 0.008 | |
| Osteoporosis treatment* | Yes | 9,698 (26.1%) | 1,354 (14.0%) | 1.6% | 0.020 | 352 (3.6%) | 0.9% | 0.35 |
| No | 27,478 (73.9%) | 2,387 (8.7%) | 0.7% | 0.10 | 656 (2.4%) | 2.0% | 0.029 | |
| Comorbidity | High | 12,330 (33.2%) | 1639 (13.3%) | 1.2% | 0.064 | 488 (4.0%) | 1.6% | 0.10 |
| Moderate | 16,808 (45.2%) | 1546 (9.2%) | 1.1% | 0.032 | 409 (2.4%) | 1.1% | 0.27 | |
| Low | 8,038 (21.6%) | 556 (6.9%) | 0.9% | 0.28 | 111 (13.8%) | 2.9% | 0.26 | |
Significant effects are in bold. BMI body mass index, COPD chronic obstructive pulmonary disease.
Treatment in the year before BMD testing.
Table 3.
Hazard ratios (HR) per 1 SD reduction in TBS with 95% confidence intervals (CI) for incident major osteoporotic fracture (MOF) and hip fracture (HF).
| Clinical variables | Subgroups | MOF |
HF |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | p-interaction | HR (95% CI) | p-value | p-interaction | ||
| All Cases | 1.19 (1.15-1.23) | <0.001 | 1.13 (1.05-1.20) | <0.001 | |||
| Sex | Women | 1.20 (1.16-1.25) | <0.001 | 0.25 | 1.13 (1.05-1.21) | <0.001 | 0.84 |
| Men | 1.10 (0.97-1.24) | 0.13 | 1.14 (0.90-1.44) | 0.27 | |||
| Age (years) | < 65 | 1.33 (1.26-1.41) | <0.001 | 0.004 | 1.53 (1.31-1.78) | <0.001 | <0.001 |
| ≥ 65 | 1.12 (1.07-1.17) | <0.001 | 1.05 (0.98-1.13) | 0.18 | |||
| BMI (kg/m2) | < 30 | 1.19 (1.15-1.24) | <0.001 | 0.86 | 1.15 (1.06-1.23) | <0.001 | 0.26 |
| ≥ 30 | 1.19 (1.10-1.28) | <0.001 | 1.03 (0.88-1.21) | 0.69 | |||
| Diabetes | Yes | 1.18 (1.08-1.30) | <0.001 | 0.73 | 1.17 (1.00-1.37) | 0.053 | 0.36 |
| No | 1.19 (1.14-1.23) | <0.001 | 1.09 (1.02-1.18) | 0.017 | |||
| Prior fracture | Yes | 1.11 (1.03-1.18) | 0.004 | 0.003 | 1.02 (0.89-1.16) | 0.81 | 0.048 |
| No | 1.19 (1.15-1.23) | <0.001 | 1.13 (1.05-1.20) | <0.001 | |||
| COPD | Yes | 1.18 (1.07-1.30) | <0.001 | 0.44 | 1.08 (0.90-1.29) | 0.41 | 0.59 |
| No | 1.19 (1.15-1.23) | <0.001 | 1.14 (1.06-1.23) | <0.001 | |||
| High alcohol use | Yes | 1.16 (0.97-1.37) | 0.096 | 0.55 | 0.99 (0.69-1.43) | 0.96 | 0.21 |
| No | 1.19 (1.15-1.23) | <0.001 | 1.13 (1.06-1.21) | <0.001 | |||
| Rheumatoid arthritis | Yes | 1.15 (0.99-1.34) | 0.072 | 0.61 | 0.89 (0.66-1.21) | 0.47 | 0.083 |
| No | 1.19 (1.15-1.24) | <0.001 | 1.14 (1.06-1.22) | <0.001 | |||
| Glucocorticoid use | Yes | 1.15 (1.02-1.31) | 0.027 | 0.51 | 0.93 (0.74-1.17) | 0.53 | 0.029 |
| No | 1.19 (1.15-1.24) | <0.001 | 1.14 (1.07-1.23) | <0.001 | |||
| Secondary osteoporosis | Yes | 1.17 (1.04-1.31) | 0.007 | 0.94 | 1.14 (0.93-1.39) | 0.21 | 0.92 |
| No | 1.19 (1.15-1.24) | <0.001 | 1.13 (1.05-1.21) | 0.001 | |||
| Femoral neck osteoporosis | Yes | 1.15 (1.07-1.22) | <0.001 | 0.19 | 1.08 (0.98-1.20) | 0.13 | 0.36 |
| No | 1.21 (1.16-1.26) | <0.001 | 1.15 (1.06-1.26) | 0.001 | |||
| Osteoporosis treatment* | Yes | 1.15 (1.08-1.22) | <0.001 | 0.005 | 1.12 (1.00-1.25) | 0.057 | 0.58 |
| No | 1.22 (1.16-1.27) | <0.001 | 1.15 (1.06-1.25) | <0.001 | |||
| High | 1.15 (1.10-1.21) | <0.001 | 0.091 | 1.06 (0.97-1.17) | 0.21 | ||
| Comorbidity | Moderate | 1.18 (1.12-1.25) | <0.001 | 1.17 (1.05-1.30) | 0.003 | <0.001 | |
| Low | 1.31 (1.19-1.43) | <0.001 | 1.23 (1.01-1.51) | 0.044 | |||
Significant effects are in bold. BMI body mass index, COPD chronic obstructive pulmonary disease.
Treatment in the year before BMD testing.
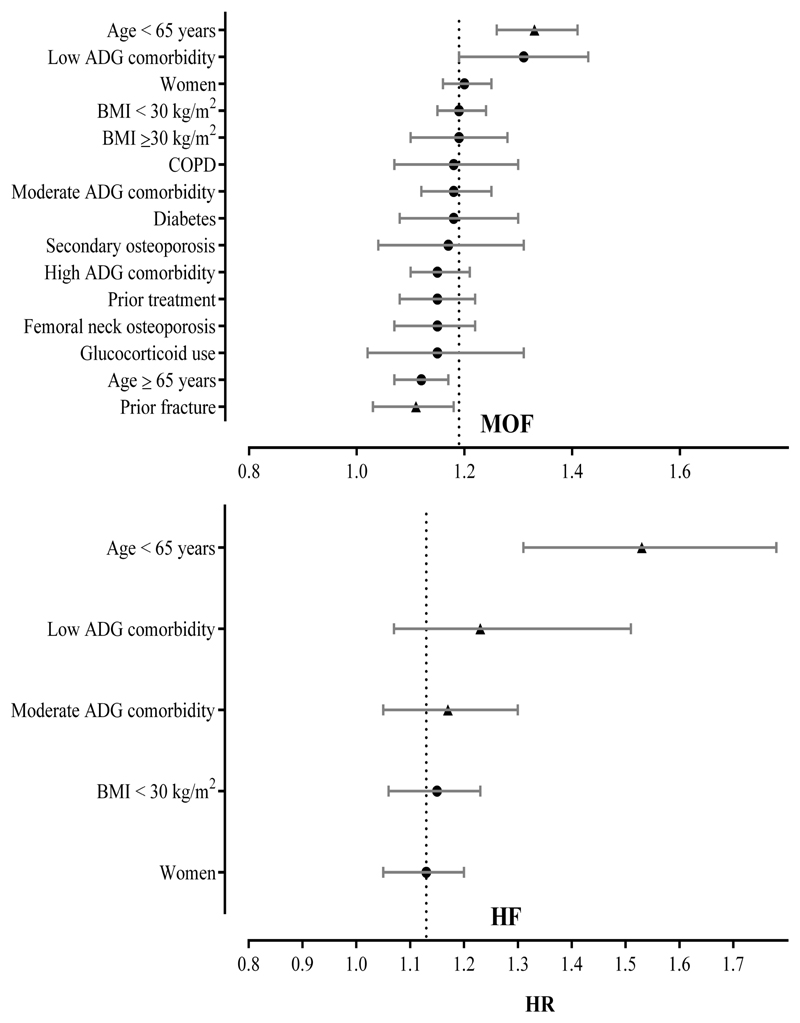
Figure 2.
Rank-ordered HRs with 95% CI bars for each SD reduction in TBS to predict MOF (panel above) and HF (panel below) stratified by clinical variables. Only statistically significant HRs are included. The dashed line indicates the HR for all cases. Variables with statistically significant interactions are denoted by a triangle.
No significant interactions were detected for sex (men vs women), BMI (< 30 kg/m2 vs ≥ 30 kg/m2), COPD (with vs without), RA (with vs without), diabetes (with vs without) or secondary osteoporosis (with vs without). In contrast, significant interactions were noted for some clinical variables. Our analysis revealed significant interactions with larger effects for age <65 vs ≥65 (MOF p=0.004, HF p<0.001), without vs with prior fracture (MOF p=0.003, HF p=0.048), without vs with glucocorticoid use (HF p=0.029), lower vs higher comorbidity score (HF p <0.001), and without vs with osteoporosis treatment (MOF p=0.005).
3.4. Risk reclassification
Percentages of patients reclassified using the TBS-adjustment to FRAX and ORs for reclassification according to the subgroups defined by the clinical variables are presented in Table 4. Overall reclassifications using the MOF criterion were similar to using the HF criterion with a total of 2.5% (1.0% into a lower risk and 1.5% into a higher risk category) and 3.0% (0.9% lower and 2.1% higher), respectively. For most clinical subgroups, reclassification 'up' into higher risk categories exceeded reclassification 'down' into lower risk categories; however, for the HF intervention criterion in patients with femoral neck osteoporosis, downwards reclassification at 3.4% was more common than upwards reclassification at 1.8%. For the MOF criterion, upwards reclassifications ranged from 0.6% in older patients (≥65 years) to 5.0% in patients with femoral neck osteoporosis, while downwards reclassifications ranged from 0.2% in older patients to 4.2% in those with femoral neck osteoporosis. For the HF criterion, upwards reclassifications ranged from 1.1% in older patients to 3.8% in those with diabetes, while downwards reclassifications varied between 0.6% in those without femoral neck osteoporosis to 3.4% in those with femoral neck osteoporosis. ORs for MOF reclassification (OR: 3.45, 95% CI 2.89-4.11) and HF reclassification (1.92, 95% CI1.68-2.19) were greater for younger compared to older patients. Of note, TBS had opposing effects on reclassification in men compared to women, lower for MOF (OR: 0.37, 95% CI 0.26-0.51) but greater for HF (1.62, 95% CI1.35-1.94). Prior osteoporosis treatment vs no treatment was associated with lower MOF reclassification (OR: 0.79, 95% CI0.67-0.95) and HF reclassification (0.80, 95% CI 0.68-0.94).
Table 4.
Percentage reclassified and odds ratios (OR) for reclassification from multivariable logistic regression using the TBS-adjustment to FRAX assuming fixed MOF ≥ 20% and HF ≥ 3% intervention cut-offs.
| Clinical variables | Subgroups | MOF |
HF |
||||
|---|---|---|---|---|---|---|---|
| % Reclassified Overall (Down : Up)* | OR (95% CI) | p-value | % Reclassified Overall (Down : Up)* | OR (95% CI) | p-value | ||
| All subjects | 2.5 (1.0 : 1.5) | 3.0 (0.9 : 2.1) | |||||
| Sex | Women | 2.6 (1.1 : 1.5) | 1.00 (ref) | 2.8 (0.8 : 2.0) | 1.00 (ref) | ||
| Men | 1.4 (0.5 : 0.9) | 0.37 (0.26-0.51) | <0.001 | 5.6 (2.3 : 3.2) | 1.62 (1.35-1.94) | <0.001 | |
| Age (years) | < 65 | 4.5 (2.0 : 2.5) | 3.45 (2.89-4.11) | <0.001 | 4.2 (1.0 : 3.2) | 1.92 (1.68-2.19) | <0.001 |
| ≥ 65 | 0.9 (0.2 : 0.6) | 1.00 (ref) | 2.0 (0.8 : 1.1) | 1.00 (ref) | |||
| BMI (kg/m2) | < 30 | 2.0 (0.7 : 1.3) | 0.91 (0.75-1.10) | 0.31 | 2.9 (0.7 : 2.2) | 0.93 (0.80-1.09) | 0.38 |
| ≥ 30 | 2.6 (1.1 : 1.5) | 1.00 (ref) | 3.0 (1.0 : 2.0) | 1.00 (ref) | |||
| Diabetes | Yes | 3.4 (1.0 : 2.4) | 1.22 (1.00-1.49) | 0.049 | 4.7 (0.9 : 3.8) | 1.45 (1.22-1.72) | <0.001 |
| No | 2.4 (1.0 : 1.4) | 1.00 (ref) | 2.8 (0.9 : 1.9) | 1.00 (ref) | |||
| Prior fracture | Yes | 7.3 (2.8 : 4.4) | 2.94 (2.55-3.39) | <0.001 | 5.4 (1.9 : 3.5) | 1.80 (1.56-2.08) | <0.001 |
| No | 1.7 (0.7 : 1.0) | 1.00 (ref) | 2.6 (0.8 : 1.9) | 1.00 (ref) | |||
| COPD | Yes | 4.5 (1.2 : 3.3) | 1.37 (1.13-1.67) | 0.001 | 5.2 (1.5 : 3.6) | 1.51 (1.26-1.80) | <0.001 |
| No | 2.3 (1.0 : 1.3) | 1.00 (ref) | 2.8 (0.9 : 1.9) | 1.00 (ref) | |||
| High alcohol use | Yes | 4.4 (1.3 : 3.1) | 2.01 (1.44-2.79) | <0.001 | 4.8 (1.7 : 3.0) | 1.57 (1.16-2.12) | 0.003 |
| No | 2.4 (1.0 : 1.4) | 1.00 (ref) | 2.9 (0.9 : 2.0) | 1.00 (ref) | |||
| Rheumatoid arthritis | Yes | 4.9 (2.2 : 2.7) | 1.66 (1.24-2.22) | <0.001 | 4.9 (1.7 : 3.1) | 1.46 (1.11-1.92) | 0.006 |
| No | 2.4 (1.0 : 1.4) | 1.00 (ref) | 2.9 (0.9 : 2.0) | 1.00 (ref) | |||
| Glucocorticoid use | Yes | 4.9 (2.2 : 2.7) | 2.21 (1.74-2.81) | <0.001 | 5.3 (2.5 : 2.8) | 1.50 (1.20-1.87) | <0.001 |
| No | 2.3 (0.9 : 1.4) | 1.00 (ref) | 2.8 (0.8 : 2.0) | 1.00 (ref) | |||
| Secondary osteoporosis | Yes | 2.6 (0.9 : 1.6) | 1.01 (0.81-1.24) | 0.96 | 3.5 (1.4 : 2.1) | 1.09 (0.91-1.31) | 0.36 |
| No | 2.5 (1.0 : 1.5) | 1.00 | 2.9 (0.9 : 2.1) | 1.00 | |||
| Femoral neck osteoporosis | Yes | 9.2 (4.2 : 5.0) | 3.65 (3.16-4.22) | <0.001 | 5.2 (3.4 : 1.8) | 1.34 (1.15-1.57) | <0.001 |
| No | 1.5 (0.6 : 1.0) | 1.00 (ref) | 2.7 (0.6 : 2.1) | 1.00 (ref) | |||
| Osteoporosis treatment | Yes | 2.1 (1.0 : 1.1) | 0.79 (0.67-0.95) | 0.010 | 2.4 (0.9 : 1.5) | 0.80 (0.68-0.94) | 0.007 |
| No | 2.6 (1.0 : 1.6) | 1.00 (ref) | 3.2 (0.9 : 2.2) | 1.00 (ref) | |||
| Comorbidity | High | 3.4 (1.3 : 2.1) | 1.26 (1.01-1.57) | 0.105 | 3.5 (1 : 2.5) | 1.04 (0.86-1.25) | |
| Moderate | 2.3 (1.1 : 1.2) | 1.17 (0.95-1.45) | 2.9 (0.9 : 2.0) | 1.07 (0.90-1.27) | 0.73 | ||
| Low | 1.5 (0.4 : 1.1) | 1.00 (ref) | 2.3 (0.8 : 1.5) | 1.00 (ref) | |||
Significant effects are in bold.
Down: Reclassification from treatment to non-treatment; Up: Reclassification from non-treatment to treatment, BMI body mass index, COPD chronic obstructive pulmonary disease.
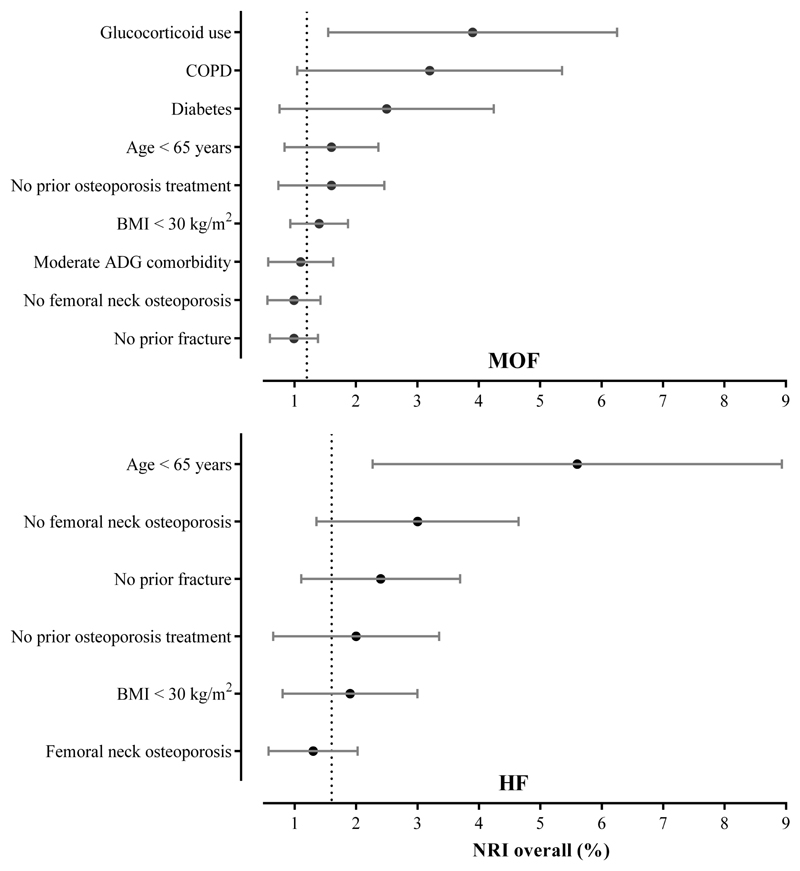
Table 2 and Figure 3 present the NRI analysis overall and for each subgroup using the TBS-adjustment to FRAX. The detailed breakdown of NRI for cases and non-cases separately is shown in Supplementary Table 2. Overall correct reclassification from including TBS in the fracture risk assessment was modest with an NRI of 1.2% for MOFs and 1.7% for HFs (both p<0.001). For MOFs, a greater NRI was seen for subjects with a history of glucocorticoid use (3.9%, p=0.039), COPD (3.2%, p=0.019), diabetes (2.5%, p=0.030), and age < 65 years (1.7%, p<0.001). In the case of HFs, a greater NRI was seen for age < 65 years (5.6%, p=0.017), non-osteoporotic femoral neck T scores (3.0%, p=0.008), and subjects with no prior fracture (2.4%, p=0.006). Compared to MOFs, fewer subgroups demonstrated significant NRIs for HF.
Figure 3.
Rank-ordered overall NRI with 95% CI bars for TBS-adjustment to FRAX for predicting incident MOF (panel above) and HF (panel below) stratified by clinical variables. The dashed line indicates the HR for all cases.
4. Discussion
We identified various clinical variables which are associated with baseline TBS, and determined that female sex, femoral neck osteoporosis, COPD and diabetes are associated with the greatest decreases in age- and BMI-adjusted TBS, whilst recent osteoporosis treatment is associated with increased TBS. Several other studies have reported clinical factors that correlate with lower TBS (e.g., increasing age [7], glucocorticoid use [10–13], and diabetes [17–20]) but this is the first study to examine the relative and independent effect of multiple clinical variables on TBS within a single population. Importantly, we have been able to show that, with the exception of comorbidity score and prior osteoporosis treatment, all clinical variables considered were associated with a significantly lower age- and BMI-adjusted TBS. Previous studies that have explored the utility of TBS in specific subgroups have examined effects on baseline TBS, TBS for fracture prediction or NRI. The strength of the current paper is that it looks at all three simultaneously across a wide range of conditions/subgroups. Supplementary Table 3 provides a comprehensive summary of the salient results.
Another strength of this work is that we were able to assess interactions between clinical risk factors. Statistically significant interactions were noted between TBS and age for both MOF and HF prediction, with HRs in older patients attenuated compared to younger patients. TBS interactions were also significant according to prior fracture and previously osteoporosis treatment (at least for MOF). The latter may reflect the fact that TBS is relatively unresponsive to anti-resorptive therapies [34–36]. Although the effect of TBS in assessing MOF risk is slightly weaker in patients previously treated for osteoporosis, this population still demonstrated a small but statistically significant increase in NRI. These results can be contrasted to those of the conventional FRAX tool which has been shown to provide accurate fracture risk prediction in patients treated for osteoporosis [37]
Our findings are in keeping with previously published results. In particular, our results can be compared to those of McCloskey et al [3] which concluded that TBS-adjusted FRAX performed similarly in men and women. Likewise, we found that the interaction term for sex*TBS was not significant. Our results are also compatible with those of Schousboe et al [38] who found that an increasing BMI attenuated the predictive effect of TBS in men with incident vertebral fractures. In our study, obese patients (BMI ≥30 kg/m2) showed no significant improvement in NRI. Previous studies have suggested that TBS may be particularly useful in patients with diabetes [17,18,20]. Our results support this conclusion with NRI being higher in patients with versus without diabetes for both MOF (2.5% vs 1.0%, respectively) and HF (3.1% vs 1.3%, respectively), suggesting that TBS may help guide management in this patient population. Likewise, our results are also consistent with reports that TBS was effective in improving fracture risk assessment and influencing therapeutic choices in patients undergoing glucocorticoid therapy [10–12].
Our results show that the use of TBS-adjusted FRAX scores resulted in the larger HR/SD for younger subjects (<65 years of age), and in those with low comorbidity, and women, compared with older subjects, those with comorbidity and men, respectively. It is interesting to note that, despite not demonstrating corresponding statistically significant differences in TBS, a low or moderate comorbidity score was still associated with a greater HR for HF per SD TBS-adjusted FRAX score than in those with high comorbidity.
Certain limitations of this study should be acknowledged. In particular, the population studied was from a clinical registry and may be prone to referral bias. Conversely, this suggests that our results are applicable to patients encountered in routine clinical practice. Our study population was almost exclusively Caucasian, which may limit applicability to other ethnic populations, although no significant population heterogeneity was seen in the meta-analysis by McCloskey et al [3]. In addition, our analysis was limited to examining the impact of TBS-adjusted FRAX on reclassification of patients in the context of fixed FRAX-based intervention cut-offs (MOF ≥ 20%, HF ≥ 3%); however, we have previously shown that the significant improvement in overall NRI was independent of the specific intervention thresholds used [5]. Another limitation of this exploratory study is that we did not correct for multiple comparisons. Some of our results could have occurred by chance. Subsequent studies will be important in confirming our findings. Finally, the TBS adjustment for FRAX was developed using the Manitoba cohort, the same cohort used for the current analysis.
5. Conclusion
The use of TBS-adjusted fracture risk assessment resulted in significant improvements overall. We found that TBS is sensitive to the effects of multiple risk factors for fracture. TBS was beneficial for most subgroups considered, either in terms of improved fracture-risk prediction or fracture-risk reclassification.
Supplementary Material
Highlights.
Baseline TBS was sensitive to most of the clinical variables tested.
TBS predicted major osteoporotic fractures and/or hip fracture overall, and several of the clinical variables considered showed significant interactions with TBS.
TBS-adjusted FRAX resulted in significant risk reclassification and/or improved fracture risk classification for most of the clinical variables considered.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC 2012/2013 -18). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Healthy Living, and Seniors, or other data providers is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
Funding
This study had no external funding body.
Footnotes
Disclosures
John A. Kanis: Grants from Amgen, grants from Lilly, non-financial support from Medimaps, grants from Unigene, non-financial support from Asahi, grants from Radius Health, outside the submitted work; and Dr Kanis is the architect of FRAX but has no financial interest. Governmental and NGOs: National Institute for health and clinical Excellence (NICE), UK; International Osteoporosis Foundation; INSERM, France; Ministry of Public Health, China; Ministry of Health, Australia; Ministry of Health, Abu Dhabi; National Osteoporosis Guideline Group,UK; WHO.
Didier Hans: Co-ownership in the TBS patent. Stock options or royalties: Med-Imaps. Research grants: Amgen, Eli Lilly, Servier, Nycomed-Takeda.
Eugene McCloskey: Nothing to declare for FRAX and the context of this paper, but numerous ad hoc consultancies/ speaking honoraria and/or research funding from Amgen, Bayer, General Electric, GSK, Hologic, Lilly, Merck Research Labs, Novartis, Novo Nordisk, Nycomed, Ono, Pfizer, ProStrakan, Roche, Sanofi-Aventis, Servier, Tethys, UBS and Warner-Chilcott
Nicholas Harvey: Nothing to declare for FRAX and the context of this paper, but has received consultancy/ lecture fees/ honoraria/ grant funding from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharma.
P Martineau, W. Leslie, H. Johansson, A. Oden declare that they have no conflict of interest.
Roles
Authors' roles: conception, design and analysis (WDL), interpretation of data (All Authors); drafting the article (PM); critically revising the article for important intellectual content (All Authors); final approval of the version to be published (All Authors); and agreement to be accountable for all aspects of the work (All Authors). WDL had full access to all the data in the study and takes the responsibility for the integrity of the data and the accuracy of the data analysis.
References
- [1].Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: The 2015 ISCD official positions Part 2: Trabecular bone score. J Clin Densitom. 2015;18:309–330. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- [2].McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Kanis JA. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int. 2015;96:500–509. doi: 10.1007/s00223-015-9980-x. [DOI] [PubMed] [Google Scholar]
- [3].McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJ, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31:940–948. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- [4].Su Y, Leung J, Hans D, Lamy O, Kwok T. The added value of trabecular bone score to FRAX® to predict major osteoporotic fractures for clinical use in Chinese older people: The Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporos Int. 2017;28:111–117. doi: 10.1007/s00198-016-3741-1. [DOI] [PubMed] [Google Scholar]
- [5].Martineau P, Leslie W, Johansson H, Oden A, McCloskey E, Hans D, Kanis J. Clinical utility of using lumbar spine trabecular bone score to adjust fracture probability: The Manitoba BMD Cohort. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3124. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- [6].Couraud G, Souffir C, Gaigneux E, Kolta S, Roux C, Briot K. Adjusting FRAX® on TBS for identification of subjects at high risk of fractures. Bone. 2017;101:214–218. doi: 10.1016/j.bone.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [7].Dufour R, Winzenrieth R, Heraud A, Hans D, Mehsen N. Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women. Osteoporos Int. 2013;24:2837–2846. doi: 10.1007/s00198-013-2384-8. [DOI] [PubMed] [Google Scholar]
- [8].Simonelli C, Leib E, Mossman N, Winzenrieth R, Hans D, McClung M. Creation of an age-adjusted, dual-energy x-ray absorptiometry–derived trabecular bone score curve for the lumbar spine in non-hispanic US white women. J Clin Densitom. 2014;17:314–319. doi: 10.1016/j.jocd.2013.09.002. [DOI] [PubMed] [Google Scholar]
- [9].Schousboe JT, Vo T, Taylor BC, Cawthon PM, Schwartz AV, Bauer DC, Orwoll ES, Lane NE, Barrett-Connor E, Ensrud KE, for the Osteoporotic Fractures in Men (MrOS) Study Research Group Prediction of incident major osteoporotic and hip fractures by trabecular bone score (TBS) and prevalent radiographic vertebral fracture in older men. J Bone Miner Res. 2016;31:690–697. doi: 10.1002/jbmr.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leib ES, Winzenrieth R. Bone status in glucocorticoid-treated men and women. Osteoporos Int. 2016;27:39–48. doi: 10.1007/s00198-015-3211-1. [DOI] [PubMed] [Google Scholar]
- [11].Paggiosi MA, Peel NFA, Eastell R. The impact of glucocorticoid therapy on trabecular bone score in older women. Osteoporos Int. 2015;26:1773–1780. doi: 10.1007/s00198-015-3078-1. [DOI] [PubMed] [Google Scholar]
- [12].Eller-Vainicher C, Morelli V, Ulivieri FM, Palmieri S, Zhukouskaya VV, Cairoli E, Pino R, Naccarato A, Scillitani A, Beck-Peccoz P, Chiodini I. Bone quality, as measured by trabecular bone score in patients with adrenal incidentalomas with and without subclinical hypercortisolism. J Bone Miner Res. 2012;27:2223–2230. doi: 10.1002/jbmr.1648. [DOI] [PubMed] [Google Scholar]
- [13].Bréban S, Briot K, Kolta S, Paternotte S, Ghazi M, Fechtenbaum J, Roux C. Identification of rheumatoid arthritis patients with vertebral fractures using bone mineral density and trabecular bone score. J Clin Densitom. 2012;15:260–266. doi: 10.1016/j.jocd.2012.01.007. [DOI] [PubMed] [Google Scholar]
- [14].Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, Udesky J, Cremers S, Sarquis M, Guo X-DE, Hans D, et al. Trabecular bone score (TBS)—A novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2013;98:1963–1970. doi: 10.1210/jc.2012-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, Pepe J, Diacinti D, Piemonte S, Carnevale V, Minisola S. “Trabecular Bone Score” (TBS): An indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone. 2013;53:154–159. doi: 10.1016/j.bone.2012.11.041. [DOI] [PubMed] [Google Scholar]
- [16].Eller-Vainicher C, Filopanti M, Palmieri S, Ulivieri FM, Morelli V, Zhukouskaya VV, Cairoli E, Pino R, Naccarato A, Verga U, Scillitani A, et al. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur J Endocrinol. 2013;169:155–162. doi: 10.1530/EJE-13-0305. [DOI] [PubMed] [Google Scholar]
- [17].Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, Shin CS. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100:475–482. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- [18].Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014;25:1969–1973. doi: 10.1007/s00198-014-2704-7. [DOI] [PubMed] [Google Scholar]
- [19].Zhukouskaya VV, Ellen-Vainicher C, Gaudio A, Privitera F, Cairoli E, Ulivieri FM, Palmieri S, Morelli V, Grancini V, Orsi E, Masserini B, et al. The utility of lumbar spine trabecular bone score and femoral neck bone mineral density for identifying asymptomatic vertebral fractures in well-compensated type 2 diabetic patients. Osteoporos Int. 2016;27:49–56. doi: 10.1007/s00198-015-3212-0. [DOI] [PubMed] [Google Scholar]
- [20].Leslie WD, Aubry-Rozier B, Lamy O, Hans D. TBS (Trabecular Bone Score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- [21].Naylor KL, Lix LM, Hans D, Garg AX, Rush DN, Hodsman AB, Leslie WD. Trabecular bone score in kidney transplant recipients. Osteoporos Int. 2016;27:1115–1121. doi: 10.1007/s00198-015-3424-3. [DOI] [PubMed] [Google Scholar]
- [22].Naylor KL, Prior J, Garg AX, Berger C, Langsetmo L, Adachi JD, Goltzman D, Kovacs CS, Josse RG, Leslie WD. Trabecular bone score and incident fragility fracture risk in adults with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:2032–2040. doi: 10.2215/CJN.00720116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leslie WD, Metge C. Establishing a regional bone density program. J Clin Densitom. 2003;6:275–282. doi: 10.1385/JCD:6:3:275. [DOI] [PubMed] [Google Scholar]
- [24].Leslie WD, Caetano PA, MacWilliam LR, Finlayson GS. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8:25–30. doi: 10.1385/JCD:8:1:025. [DOI] [PubMed] [Google Scholar]
- [25].Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C, Goltzman D, Kreiger N, Prior J, Leslie WD. Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health. 2012;12:301. doi: 10.1186/1471-2458-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leslie WD, Lix LM, Langsetmo L, Berger C, Goltzman D, Hanley DA, Adachi JD, Johansson H, Oden A, McCloskey E, Kanis JA. Construction of a FRAX® model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int. 2011;22:817–827. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- [27].Leslie WD, O’Donnell S, Lagacé C, Walsh P, Bancej C, Jean S, Siminoski K, Kaiser S, Kendler DL, Jaglal S, F. the O.S.E.W. Group Population-based Canadian hip fracture rates with international comparisons. Osteoporos Int. 2010;21:1317–1322. doi: 10.1007/s00198-009-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fraser L-A, Langsetmo L, Berger C, Ioannidis G, Goltzman D, Adachi JD, Papaioannou A, Josse R, Kovacs CS, Olszynski WP, Towheed T, et al. Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int. 2011;22:829–837. doi: 10.1007/s00198-010-1465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Independent clinical validation of a Canadian FRAX tool: Fracture prediction and model calibration. J Bone Miner Res. 2010;25:2350–2358. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- [30].Ns S, Jp W. Applying population-based case mix adjustment in managed care: the Johns Hopkins Ambulatory Care Group system. Manag Care Q. 1994;2:21–34. [PubMed] [Google Scholar]
- [31].Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452–472. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- [32].Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-212. [DOI] [PubMed] [Google Scholar]
- [33].Leening MJG, Vedder MM, Witteman JCM, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;160:122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- [34].Popp AW, Guler S, Lamy O, Senn C, Buffat H, Perrelet R, Hans D, Lippuner K. Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: A three-year study. J Bone Miner Res. 2013;28:449–454. doi: 10.1002/jbmr.1775. [DOI] [PubMed] [Google Scholar]
- [35].Kalder M, Kyvernitakis I, Albert US, Baier-Ebert M, Hadji P. Effects of zoledronic acid versus placebo on bone mineral density and bone texture analysis assessed by the trabecular bone score in premenopausal women with breast cancer treatment-induced bone loss: Results of the ProBONE II substudy. Osteoporos Int. 2015;26:353–360. doi: 10.1007/s00198-014-2955-3. [DOI] [PubMed] [Google Scholar]
- [36].Petranova T, Sheytanov I, Monov S, Nestorova R, Rashkov R. Denosumab improves bone mineral density and microarchitecture and reduces bone pain in women with osteoporosis with and without glucocorticoid treatment. Biotechnol Biotechnol Equip. 2014;28:1127–1137. doi: 10.1080/13102818.2014.967827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, for the Manitoba Bone Density Program Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012;27:1243–1251. doi: 10.1002/jbmr.1582. [DOI] [PubMed] [Google Scholar]
- [38].Schousboe JT, Vo TN, Langsetmo L, Taylor BC, Cawthon PM, Schwartz AV, Bauer DC, Orwoll ES, Lane NE, Barrett-Connor E, Ensrud KE, et al. Association of trabecular bone score (TBS) with incident clinical and radiographic vertebral fractures adjusted for lumbar spine BMD in older men: A prospective cohort study. J Bone Miner Res. 2017;32:1554–1558. doi: 10.1002/jbmr.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.