Abstract
Placebo effects are beneficial effects that are attributable to the brain–mind responses to the context in which a treatment is delivered rather than to the specific actions of the drug. They are mediated by diverse processes — including learning, expectations and social cognition — and can influence various clinical and physiological outcomes related to health. Emerging neuroscience evidence implicates multiple brain systems and neurochemical mediators, including opioids and dopamine. We present an empirical review of the brain systems that are involved in placebo effects, focusing on placebo analgesia, and a conceptual framework linking these findings to the mind–brain processes that mediate them. This framework suggests that the neuropsychological processes that mediate placebo effects may be crucial for a wide array of therapeutic approaches, including many drugs.
I would rather know the person who has the disease than the disease the person has. Hippocrates
Modern medicine has been very successful at treating many forms of disease, particularly those for which the physiological mechanisms can be identified and the pathology objectively assessed. However, it has proved difficult to treat the pain and psychological distress that are integral to many diseases1 and to treat related disorders such as depression, chronic pain, anxiety and fatigue. Unlike diseases in which the pathology occurs primarily in peripheral organs, pain and distress are rooted in complex brain functions. They are influenced by brain pathology, internal thoughts and brain states, and conceptions of the social and environmental context. As a result, we lack objective physiological measures for disorders that are characterized by pain and distress, and a comprehensive understanding of the brain mechanisms underlying their generation and regulation.
New inroads are being made through the multidisciplinary study of placebo effects — that is, the effects of manipulating the informational context surrounding a medical treatment. Placebos are drugs, devices or other treatments that are physically and pharmacologically inert. Placebo interventions do not, by definition, have any direct therapeutic effects on the body. However, all treatments are delivered in a context that includes social and physical cues, verbal suggestions and treatment history (FIG. 1). This context is actively interpreted by the brain and can elicit expectations, memories and emotions, which in turn can influence health-related outcomes in the brain and body. Placebo effects are thus brain–body responses to context information that promote health and well-being. When brain responses to context information instead promote pain, distress and disease, they are termed nocebo effects.
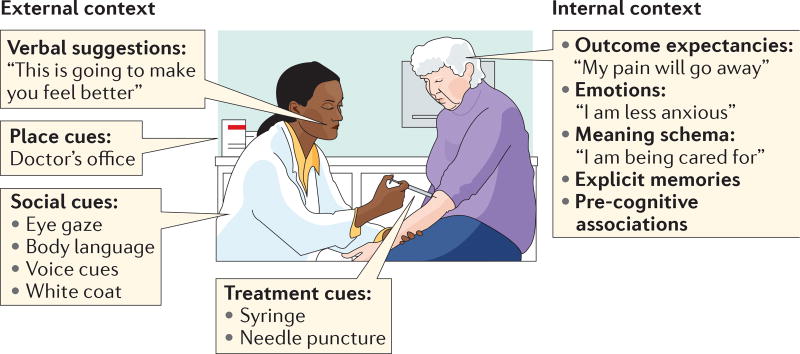
Figure 1. Elements of treatment context.
Whether treatment consists of an active drug or a placebo, the clinical setting that surrounds treatment includes multiple types of context information that are perceived and interpreted by the patient’s brain. The external context includes treatment, place and social cues, along with verbal suggestions. The internal context consists of memories, emotions, expectancies and appraisals of the meaning of the context for future survival and well-being. These features combine to make up the treatment context and are the ‘active ingredients’ of placebo effects.
Understanding placebo and nocebo effects is important for both clinicians and neuroscientists. Placebo responses are substantial across diverse clinical disorders2–4 and, in some cases, are related to objective pathology5 and survival6. A large part of the overall therapeutic response to drugs7–10, surgery11,12, psychotherapy13 and other treatments may be due to the treatment context — and thus mechanisms shared with placebo effects — rather than the specific treatment itself. Even when attempting to understand the effects of drugs or other treatments is the primary goal, considering placebo effects is crucial, as drug effects occur alongside or even interact with internal psychological and brain processes7,14–18. In some cases, individuals who show the largest drug effects also show the largest placebo effects19, which is one indicator that some drugs and placebos may share mechanisms. If so, obtaining reliable drug effects may require establishing a suitable treatment context (for example, the right type of psychological or social support), and screening to remove placebo responders in clinical trials may eliminate those who most benefit from active drug treatment.
For neuroscientists, placebo studies provide a way to investigate how the brain systems that process contextual information influence physiology and clinically relevant outcomes. Humans are endowed with uniquely powerful systems for representing context20, which help to tailor our responses to the needs of a given situation21. Clinical contexts in particular integrate diverse psychological elements (FIG. 1), including learned associations between cues (for example, a doctor’s white coat) and past positive and negative experiences, conceptual knowledge based on verbal suggestions that induce expectations about treatment outcomes, and social interactions (for example, the patient–care provider relationship). Placebo effects on health-related outcomes such as pain and affective physiology, which we focus on in this Review, share many similarities with context effects on visual perception22–24, memory25, decision making26–28, athletic29 and cognitive30 performance, and other processes. Together, these studies provide a foundation for an integrated science of context processing, and studies of placebo may shed light on mechanisms of context effects that do not involve placebo manipulations20,31.
Here, we present a brain systems-oriented view of the mechanisms underlying placebo effects. The neuroscience of placebo effects is a new and rapidly evolving field that integrates diverse areas of human and animal neuroscience, and complements studies of placebo effects on peripheral physiology5, clinical pharmacology2 and other outcomes20,31. We first briefly discuss the behavioural, clinical and physiological outcomes that are affected by placebo treatments. Then, we review neuroimaging evidence relating to the systems-level neurobiology that underlies placebo effects; we focus primarily on pain, which has been most extensively studied. Next, we relate the resulting consensus view on the neural architecture of placebo effects in pain to brain placebo effects in depression, emotion and Parkinson disease (PD). Finally, we present a framework for mapping the psychological processes underlying placebo effects onto brain systems and highlight several areas for further research.
Clinical and laboratory placebo effects
Placebos have been used throughout the history of medicine to soothe the emotions of troubled patients and are still used for this purpose today32. It is widely believed that placebos can make people ‘feel better’, but is that the extent of their clinical importance? What kinds of health-related outcomes can placebo treatments affect? For some, the presence of a placebo effect suggests that symptoms were not caused by ‘real’ or ‘organic’ disease. For example, patients who report pain relief after placebo treatment might be judged to be malingerers33. However, this inference is only valid if placebo treatments have no actual effects on pain pathophysiology or experience.
As we explain below, clinical studies have demonstrated meaningful placebo effects in multiple disorders, and laboratory studies have provided evidence for placebo effects on health-relevant behavioural, autonomic, endocrine and immune measures (Supplementary information S1 (table); see also REFS 5,34). These studies suggest that it is implausible — and perhaps unethical — to dismiss placebo responses as irrelevant to health and pathology.
Placebo effects in clinical studies
Most clinical trials are not suitable for estimating placebo effects because they lack natural history controls. However, a small subset of clinical studies with appropriate controls (FIG. 2) have demonstrated causal effects of placebo treatment on measures that are typically used as primary disease end points35,36 in multiple forms of chronic pain37–40, depression10,41–43, PD44,45,161 and asthma5,46,47 (but see REF. 48). Placebo effects can be as large as the effects of accepted drug treatments40,49 or larger42,43, and can reduce disability and increase quality of life over a period of months or longer38,43. In some cases, particularly in cardiovascular disease6,50,51, adherence to placebo medication is associated with reduced mortality.
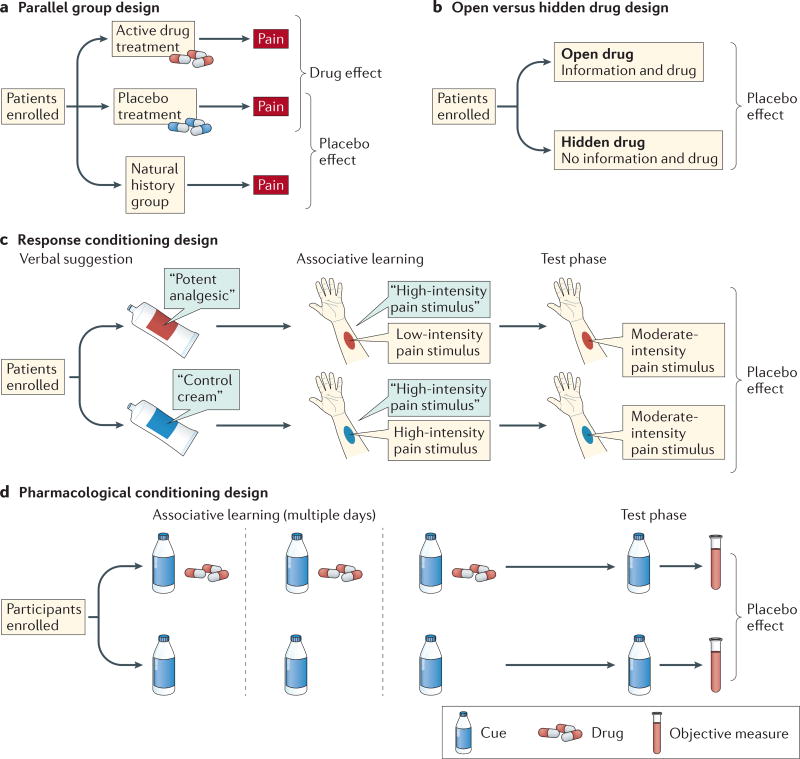
Figure 2. Paradigms for assessing placebo effects.
Most paradigms used to assess placebo treatments fall into one of four categories. a | In a parallel group design, placebo effects are measured by comparing outcomes in a placebo group with those in a no-treatment control group. This is the most common paradigm in clinical trials. b | In an open versus hidden design, drugs are delivered either with (open) or without (hidden) the knowledge of the patient. This design permits assessment of the effects of treatment context in clinical settings without withholding treatment. Extended designs such as the balanced placebo design9 cross open versus hidden administration with verum versus sham drugs, enabling researchers to assess placebo–drug interactions. c | Response conditioning designs use instructions combined with reinforcement to maximize the effectiveness of placebo treatments. In a common variant, initial verbal instructions are provided that one cream (the placebo) is an effective analgesic and another (the control) is not. Then, painful stimulation is given on both placebo-treated and control-treated skin sites. Participants are told that the stimulus intensity will be the same on both sites, but in fact it is surreptitiously reduced for the placebo-treated site, reinforcing belief in the placebo and associations with relief. During a final test phase, equivalent levels of painful stimulation are applied to both sites, and the effects of the placebo conditioning procedure are assessed. This is the most common paradigm used in neuroimaging studies; placebo and control treatments are often compared in a within-person crossover design. d | Pharmacological conditioning designs combine instructions and cues paired with active drugs during a conditioning phase, which often occurs over multiple days. Placebo effects are determined by presenting cues alone and comparing outcomes in drug-paired versus non-drug-paired groups. Response conditioning and pharmacological conditioning designs have been used in both humans and non-human animals.
Placebo-related factors are also an important component of standard clinical treatments that are administered in hospitals and clinics, which are typically provided ‘open-label,’ with full information about drug delivery and its expected benefits. In many cases, hidden drug administration, which eliminates patients’ treatment expectations, markedly reduces the effects of drugs8,17,40,52,53 and other treatments. These clinical results demonstrate the important functional improvements that are caused by the brain’s interpretation of the treatment context.
Autonomic responses
The autonomic and neuroendocrine systems are governed by the brain, including ‘higher’ brain regions such as the prefrontal cortex (PFC)54,55, and can be influenced by threatening psychological contexts56 and verbal instructions57,58. Several studies have found that placebo analgesia is associated with changes in autonomic activity59–61, and other studies have found evidence for diverse autonomic effects of placebo (see REFS 5,34 and Supplementary information S1 (table)). One such study assessed pain, autonomic responses and electroencephalography (EEG) across three levels of placebo ‘strength’ (REF. 60). Participants received three identical, inert (placebo) creams that they believed to vary in strength. A painful stimulus was then applied to the skin that was treated with the creams. During the training phase, the intensity of painful stimulation was reduced to differing degrees for the three placebo creams — not at all for the ‘control’ cream, slightly for the ‘weak placebo’ cream and markedly for the ‘strong placebo’ cream — creating differential expectations and associations of relief. In the subsequent test phase, stimulus intensity was identical across skin treated with each cream, but the authors observed a graded reduction in noxious stimulus-evoked skin conductance, pupil diameter and EEG N1–P2 amplitudes in proportion to the placebo ‘dose’. This paradigm, which we refer to as response conditioning (FIG. 2), experimentally manipulates associative learning and cognitive expectancy, and is the most popular experimental paradigm for studying placebo effects in the laboratory.
Neuroendocrine responses
Placebo treatments can also affect hormonal responses that are mediated via forebrain control of hypothalamus–pituitary–hormone systems (Supplementary information S1 (table)). Nocebo suggestions that a treatment will increase pain can increase peripheral cortisol levels in humans62,63, an effect that is blocked by the anxiolytic benzodiazepine diazepam62. Strikingly, this effect was induced with verbal instructions alone, without requiring conditioning, but in other cases associative learning might be crucial (Supplementary information S1 (table)). For example, the serotonin receptor agonist sumatriptan increases blood levels of cortisol and growth hormone. After repeated injections of sumatriptan, injections of saline alone can induce increases in the levels of both hormones, even when suggestions induce expectations for opposing responses65. These hormonal effects also have parallels in animal models, which use pharmacological conditioning to associate context cues with drug effects. In rats, after repeated injections of morphine, injections of saline alone reduce pain behaviours and the levels of adrenocorticotropic hormone and corticosterone64.
Placebos might also affect other hormone systems, including those that regulate appetite. In one study, participants who drank a milkshake labelled as ‘indulgent’ showed reduced levels of the pro-hunger hormone ghrelin compared with those who drank an identical milkshake labelled as ‘sensible’ (REF. 66). These findings complement animal work on anticipatory brain regulation of appetitive hormones such as insulin, which is in many cases mediated by autonomic output to the periphery67.
Immune responses
The autonomic and neuroendocrine systems interact with the immune system in multiple ways, providing a substrate for placebo effects on immune responses. The most compelling demonstrations of such interactions come from pharmacological conditioning studies (FIG. 2) in which taste cues, such as a uniquely flavoured drink, have been paired with immunosuppressive drugs (in particular, cyclosporin A). In both humans and rodents68–70, subsequent exposure to the taste cues alone suppresses peripheral immune responses, particularly T lymphocyte proliferation and the release of interleukin-2 and interferon-γ from peripheral lymphocytes. Relatively little is known about the brain mechanisms underlying such effects, although recent work suggests that they are mediated by noradrenergic sympathetic efferents71, require the insula and hypothalamus for their expression72 and may be correlated with anxiety73, implicating forebrain control of the response.
Other recent studies suggest that placebo manipulations may influence inflammatory responses, an aspect of immune function that is implicated in multiple aspects of health. In one study, exposure to pro-drug advertising materials coupled with administration of a placebo ‘antihistamine’ reduced the size of skin wheal responses to an allergen challenge74. In another study, verbal suggestions about altitude-induced headaches increased blood levels of prostaglandin, an important inflammatory mediator, which were reversed by administration of a placebo213. Notably, in this study, the suggestions were provided to one participant and were transmitted by social communication to others, demonstrating the power of social influences. Together, these studies suggest that psychological context may have more pervasive effects on physiology than is currently recognized.
Placebo effects and decision making
Despite the physiological effects reviewed above, most demonstrations of placebo effects depend primarily on patient self-reports, mainly because self-reports are the accepted ‘gold standard’ for measuring pain and distress. One still-common view is that these placebo effects amount to various forms of ‘decision bias’ — effects on decision making — in the absence of meaningful changes in pathology or function. For example, if patients report less pain after a placebo treatment, it may be because they evaluate their experience relative to a different reference point75,76, combine information about experience and prior expectations into their reporting decisions (a Bayesian response bias)21, judge that it is more costly to over-report pain than under-report it (a reporting bias), or decide to simply report less pain to please the experimenter (a demand characteristic)214. Thus, part of the effects of placebos on symptom reporting and behaviour undoubtedly arise from effects on decision making or other central processes that are involved in the construction of subjective experiences77,78.
Even without immediately affecting pathology, placebo effects on decision making can have profound impacts on health. They can strongly influence choices about what to eat and drink26,28, how to exercise and socialize, which medications are preferable and will continue to be taken, and whether others in our environment are seen as enemies or friends79. Such effects on decision making can compound over time to influence health in important ways.
Nonetheless, the complexity of self-report provides a compelling rationale for studying placebo effects on objective measures that are more directly linked to specific aspects of pathology, perception and function. Direct measures of brain function, in particular, can provide both objective measures related to pain, suffering and brain disorders, and clues about the mechanisms by which suggestions and cues are translated into relief.
Neuroimaging evidence
Modern neuroimaging techniques, including functional MRI (fMRI), molecular imaging of glucose, dopamine and opioid activity using positron emission tomography (PET), EEG and magnetoencephalography (MEG), offer new insights into the neural mechanisms of placebo effects. Over the past 12 years, nearly 40 PET and fMRI studies of placebo effects on pain have provided an emerging picture of the brain systems that are involved in placebo analgesia and hyperalgesia (FIG. 3; see Supplementary information S2 (box)). These are accompanied by a small but growing literature on the effects of placebo on emotion80–84, PD44,45,85 and depression86,87, which provides converging evidence on the functions of the brain systems affected by placebo.
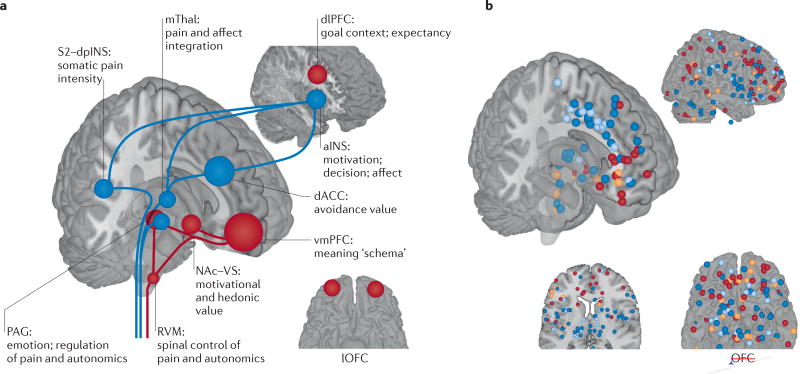
Figure 3. The neurophysiology of placebo analgesia.
a | An overview of the brain regions involved in the placebo effects on pain and their potential functions in this context. The areas shown in blue respond to painful stimuli and, on that basis, are expected to show reduced responses to pain after placebo treatment. These areas include the medial thalamus (mThal), anterior insula (aINS), dorsal anterior cingulate cortex (dACC), periaqueductal grey (PAG) and secondary somatosensory cortex–dorsal posterior insula (S2–dpINS). Areas shown in red are associated with increases in response to placebo treatment (either before or during painful stimulation), and activity in these regions is thought to be involved with the maintenance of context information and the generation of placebo-related expectations and appraisals. They include the ventromedial prefrontal cortex (vmPFC), dorsolateral PFC (dlPFC), lateral orbitofrontal cortex (lOFC), nucleus accumbens–ventral striatum (NAc–VS), PAG and rostroventral medulla (RVM). Some regions, including the PAG and dACC, show different effects depending on the study and timing relative to painful stimulation. b | Results from neuroimaging studies of placebo-induced analgesia. Each point represents a finding from an individual study, reported in standard Montreal Neurological Institute space (all studies are listed in Supplementary information S2 (box)). Red points show increases in activity under placebo versus control treatment (that is, the same cream without the belief that it is a painkiller), and blue points identify decreases in activity under placebo. These comparisons involved randomized assignment to placebo or control conditions, and so they can test the causal effects of placebo treatment on brain activity. Some studies also examined correlations between the magnitude of placebo analgesia and the magnitude of placebo-induced changes in brain responses. Orange points identify positive correlations between the magnitude of an individual’s activity increases under placebo versus control treatment and the magnitude of placebo analgesia. Light blue points identify negative correlations. These correlations do not necessarily reflect causal effects of placebo on brain activity but can provide important information on the nature of the individual differences that predispose a person towards showing a larger versus a smaller placebo response.
There are three major aims of these studies. One aim is to provide direct measures of the brain processes that give rise to pain and other clinical symptoms, providing objective targets for studies of placebo effects and other interventions. The second aim is to identify the functional systems that are engaged by placebo treatments and thus provide information on the mechanisms by which context can influence health and well-being. The third aim is to identify the factors that differentiate placebo responders from non-responders — or, equivalently, identify brain features that predict the magnitude of an individual’s placebo response.
Placebos reduce pain-related brain responses
Among the processes that show substantial placebo effects, pain is particularly amenable to study, because of its broad clinical relevance, experimental tractability and well-studied neural circuits and mechanisms. Established ‘pain-processing’ systems, which receive direct or indirect input from spinal nociceptive pathways (BOX 1) and encode the intensity of painful stimulation88, provide pain-related targets for tests of placebo interventions. These targets include the medial thalamus, the primary somatosensory cortex (S1) and the secondary somatosensory cortex (S2), as well as the dorsal posterior insula (dpINS), the mid- and anterior insula (aINS) and the dorsal anterior cingulate cortex (dACC) (FIG. 3).
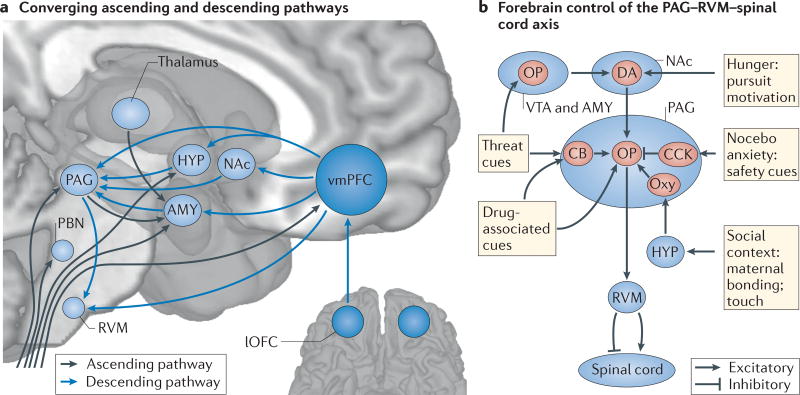
Box 1 | Converging circuitry and common mechanisms for analgesia and affective states.
Interactions among the prefrontal cortex (PFC), the forebrain and the periaqueductal grey (PAG)–rostroventral medulla (RVM)–spinal cord axis underlie multiple forms of analgesia, including placebo effects. These circuits are also integral to generating affective and motivational states. Likewise, the neurochemical systems implicated in placebo analgesia2 — including opioid (OP), dopamine (DA), serotonin, cholecystokinin (CCK) and oxytocin systems — have diverse roles in motivated behaviour beyond pain. Seen in this light, placebo-based modulation of pain is one example of a broader pattern of regulation of affect, perception and behaviour by cognitive and motivational context.
The context-based modulation of pain and motivation is supported by convergence between ‘bottom-up’ sensory processes and ‘top-down’ context at multiple levels of the neuraxis. Nociceptive afferents from the spinal cord project to brainstem regions (including the PAG and RVM), thalamic nuclei and forebrain regions (including the hypothalamus (HYP), amygdala (AMY) and ventromedial PFC (vmPFC))194,195 (see the figure, part a). These regions also receive monosynaptic inputs from the vmPFC55,196. Thus, brainstem and forebrain centres integrate input from the ‘lowest’ and ‘highest’ levels of the neuraxis, providing multiple convergence zones for sensory input and contextual information.
The PAG–RVM–spinal cord axis is important for many forms of pro- and anti-nociception in non-human animals, paralleling involvement in human placebo and nocebo effects, including forms of ‘stress’ analgesia present even in decerebrate animals197. This axis is, in turn, governed by evolutionarily newer forebrain neural and neurochemical systems, which interact with the PAG–RVM pathway to mediate diverse types of pain-modulatory effects (see the figure, part b). For example, in intact animals, both footshock-induced analgesia and morphine analgesia require OP–DA interactions in the ventral tegmental area (VTA) and nucleus accumbens (NAc)198,199, which influence ‘pain off’ spinal projection neurons in the RVM. Threat- or fear-conditioned analgesia relies on the release of OPs146,200 and cannabinoids (CBs)201 in the AMY and PAG, respectively, which also activates RVM ‘pain off’ neurons. Analgesia related to noxious stimulation202 and massage-like touch203 depends on oxytocin (labelled ‘Oxy’) release from hypothalamic projections to the PAG and subsequent OP release204. The PAG–RVM circuit also mediates some kinds of pro-nociceptive actions. CCK antagonizes OPs in this system205 and may underlie nocebo hyperalgesia62, OP hyperalgesia215 and safety signal-mediated hyperalgesia206. Other motivational states related to hypothalamic and forebrain circuits — including food pursuit207, micturition, and social conflict and defeat208,209 — can also influence nociception, providing additional clues that pain control circuits evolved as part of an integrated system governing adaptive behaviour. lOFC, lateral orbitofrontal cortex; PBN, parabrachial nucleus.
Placebo treatments can reduce pain-related activity in all of these regions, with the most consistent effects occurring in the dACC53,61,89–95, the thalamus90,94,96 and the aINS53,61,90,97,98 (FIG. 3). In many of these studies, large placebo analgesic responses were correlated with large decreases in brain responses to noxious stimulation in specific regions (the dACC53,90,94, the thalamus53,61,99–101 and the insula53,90,100,102). Several quantitative meta-analyses on a subset of the studies that are depicted in FIG. 3 indicate that these findings are reliable across paradigms and laboratories103–105. EEG and MEG studies have also shown that placebo treatments cause reductions in the amplitude of event-related potentials in response to painful laser stimuli60,77,107–110, indicating that such treatments have an effect on rapid (~150–300 ms) sensory and cognitive responses to painful events.
These placebo effects on pain-related responses are promising. However, pain is a complex sensory experience that also involves affect and decision making, and it remains unclear which aspects of the pain construction and evaluation process are affected by which types of placebo treatments. Many of the regions that normally generate pain and show the strongest placebo effects are involved in a range of other cognitive and affective processes that are distinct from pain, including basic perceptual and decision-making tasks111–114 and emotional responses that are independent of pain115. The regions most directly linked to nociceptive processing116 and most specific to pain112 are the dpINS and S2. Although placebo treatments have been shown to affect these regions61,101, such effects are not consistently identified in meta-analyses103, pointing to variability across studies and individuals. Some placebo paradigms — for example, those that involve extended conditioning or particularly powerful manipulations of belief — may have more profound effects on the sensory transmission of pain, whereas others may primarily affect emotion and decision making. Advances in neuroimaging methods, which are beginning to identify more precise signatures for pain and other affective processes (BOX 2), will permit stronger tests of which placebo paradigms influence pain-specific versus more general affective processes.
Box 2 | Brain-based biomarkers for pain and affect.
To study pain, depression and other conditions that affect the brain, we must first identify biomarkers — observable physiological measures — for the processes that give rise to them174. For example, functional MRI (fMRI) responses during pain in the dorsal anterior cingulate cortex (dACC) and anterior insula (aINS) are commonly used as markers for pain. When activity in these regions is affected by placebo, or other treatments, it is assumed that the treatment affects pain-related neural activity in these areas, and thus it is inferred that the placebo affects ‘pain processing’.
At first, this seems reasonable, as the dACC and aINS contain neurons that encode nociceptive information210. However, there are two central problems with this inference. First, each ‘voxel’ in a typical neuroimaging study contains approximately 5.5 million neurons211. Neurons in the dACC and other ‘pain-processing’ regions encode diverse forms of information, some unrelated to pain. The examination of fMRI activity across thousands of studies has revealed that the dACC and aINS are among the most frequently activated areas in the brain, regardless of the psychological task111–114. Second, pain is likely to be encoded in a distributed circuit, and it is unclear whether measures in individual brain regions — whether fMRI or cellular — are sufficient to capture the mechanisms underlying pain experience.
For a pattern of brain activity to be useful as a biomarker for pain, the pattern must be sensitive and specific to pain. These criteria, and other related metrics that can be derived from them (‘precision’ and ‘recall’, and positive and negative predictive value), are the bedrock of any diagnostic test. Sensitive measures of pain respond reliably, with large effect sizes that track the intensity of pain, and thus show a high probability of being present when pain is experienced. Specific measures respond only to pain and have a low probability of being present when pain is not present.
Recently, it has been demonstrated that distributed patterns of fMRI activity can be identified that are both sensitive and specific to pain. One pattern, termed the ‘neurologic pain signature’ (NPS)174, can be applied to individual participants to make accurate predictions about pain intensity and has been validated across several studies. Although it is activated by painful events and reduced by opioid treatment, it does not respond to other emotionally salient events115,174, demonstrating specificity to pain.
Markers such as the NPS provide more precise targets for a new generation of placebo studies. Thus far, there are few tests of placebo effects on such markers; but, in one study, a placebo manipulation that affected reported pain had no effect on responses in the NPS174. In another, cognitively ‘rethinking’ pain, a psychological intervention related to placebo, strongly affected pain reports but also had no effect on the NPS106. Rather, the effects of cognitive regulation were mediated by brain signals in an independent pathway connecting the ventromedial prefrontal cortex and nucleus accumbens. These findings suggest that the NPS was only influenced in a subset of studies in which pain reports were affected. Whereas placebos may affect nociception in fundamental ways in some cases, they may independently affect the evaluation and functional consequences of pain in others; such effects may be mediated by separate brain pathways.
Placebos engage endogenous pain modulation circuitry
A wealth of animal research has established numerous brain systems for the modulation of pain at multiple levels of the neuraxis, from the spinal cord up to the PFC117–120 (BOX 1). One important set of systems are descending pain modulation systems, which comprise projections from the brainstem to the spinal cord that can facilitate or reduce spinal nociceptive responses (for example, see REF. 120). These systems involve multiple pathways and neurochemical systems, including opioids, serotonin, dopamine, noradrenaline, oxytocin, cholecystokinin and neurokinin 1 (for a review, see REF. 2). Particularly important among them is a central opioidergic pathway from the midbrain periaqueductal grey (PAG) through the rostroventral medulla (RVM) to the spinal cord. The PAG receives direct projections from the ventromedial PFC (vmPFC), ventrolateral PFC (vlPFC), amygdala, nucleus accumbens (NAc) and hypothalamus55,121,122, permitting prefrontal cortical and limbic control over both afferent input and central pain circuitry. Beyond pain, PAG circuitry is critical for several other motivated behaviours122,123 and is activated during human emotional responses124–126.
Beginning with the work of Levine, Gordon and Fields127, multiple studies have shown that placebo analgesia can be blocked by the opioid antagonist naloxone (for example, see REF. 128), implicating opioidergic pathways in placebo effects. Neuroimaging studies that examine fMRI and opioid activity in brain areas rich in opioids, particularly the PAG and RVM, have complemented and expanded on this work. PET studies have found placebo-induced increases in µ-opioid activity (measured as decreases in binding of 11C-carfentanil) in the PAG129–131. Consistent placebo-induced increases in blood oxygenation level-dependent (BOLD) activity in the PAG during both the anticipation90,94 and experience61,103 of pain have been observed in fMRI studies, and several studies have shown that placebo-induced increases in PAG BOLD activity are correlated with the strength of analgesia61,90,132 (although not always; see REF. 53). Placebos have also been found to increase activity in the vicinity of the RVM61. Importantly, the placebo effects on PAG and RVM activation, as well as on pain-related brain activity, can be reversed by naloxone61. These findings directly implicate endogenous opioid responses in the brainstem as a mechanism of placebo analgesia.
In rodents and primates, activation of the endogenous opioid system can in some cases reduce the transmission of nociceptive signals in the dorsal horn of the spinal cord, which prevents them from reaching the brain. A small set of recent studies suggest that placebos can also engage this type of descending modulatory control. Placebo treatments can reduce spinal responses to painful stimuli133 and reduce secondary hyperalgesia around a site of painful stimulation134, which is thought to be spinally mediated in many cases. Conversely, nocebo suggestions of hyperalgesia can increase spinal fMRI responses to painful events59 and reverse the effects of normally analgesic procedures on spinal nociceptive reflexes135. Although later-stage modulation of pain in the cerebrum is still likely to contribute to many types of placebo effects77,78, these findings strongly suggest that placebos can influence spinal nociception and modulate ascending pain-related signals.
In addition to the evidence on descending modulatory systems, there is now substantial evidence that placebo treatments engage multiple systems in the PFC, NAc and amygdala, and influence the functional connectivity between them. Such engagement may include both changes in ‘mindset’, perhaps reflected in changes in tonic metabolic activity (for example, see REF. 136), and alterations in responses to distinct events.
Thus far, it has not been possible to identify direct correlates of tonic states. Most studies have focused on how responses during the anticipation and experience of pain are altered by placebo treatment by comparing pain responses under placebo with pain responses during a control condition. The most consistent placebo-related increases in response during pain occur in cortical regions: the dorsolateral PFC (dlPFC) and ventrolateral PFC (vlPFC)61,94,96,99,136–138; the vmPFC, including the rostral and pregenual cingulate and medial orbitofrontal cortex (OFC)61,94,99,138,139; and the mid-lateral OFC (mlOFC)17,61,95,96,98,102,136 (FIG. 3). In many cases, the increases in activity in these areas (lateral PFC17,53,94,99, vmPFC17,53,94,99 and mlOFC99) are correlated with the magnitude of reported analgesia. Many of these regions show anticipatory increases in activity before pain, and responses in these regions can predict the magnitude of placebo analgesia across individuals100.
Both lateral and medial prefrontal regions may have important roles in placebo analgesia, particularly through their connections with the brainstem. High-frequency stimulation (10 Hz) of the dlPFC in humans using transcranial magnetic stimulation (TMS) can reduce pain140, and low-frequency stimulation (1 Hz; which may have inhibitory effects) of this area may reverse placebo-induced analgesia141. Placebo-induced dlPFC activity is associated with increased activity in the PAG90, and a recent study using diffusion tensor imaging found that strong placebo responses were associated with greater integrity in the white matter tracts that connect both the dlPFC and the vmPFC with the PAG142. Placebo treatment also strengthens functional connectivity between the vmPFC and PAG, which can be measured in terms of correlations in fMRI time series61,139, and increases the correlation between the dACC and PAG129 in levels of opioid binding, which is consistent with central opioid release. Placebo-induced vmPFC– PAG connectivity is also reversible by naloxone61, further implicating opioid mechanisms. All of these findings are consistent with placebo-induced central opioid release under the control of the PFC.
These findings have parallels in animal models that corroborate the importance of PFC–PAG connectivity in pain control. Stimulation of the lateral orbital PFC in rats can reduce nociceptive responses, and these effects require opioid release in the PAG143. Other work in rodents indicates that lateral PFC–brainstem projections may mediate some of the analgesic effects of thalamic stimulation144. These findings imply that projections from the PFC to the PAG might be important for many forms of pain control beyond placebo analgesia (BOX 1).
The amygdala, NAc and ventral striatum (VS) are also closely connected to the medial PFC (for example, see REF. 122). Across studies, placebo analgesic treatments reliably reduce activity in the amygdala and increase activity in the NAc–VS103 (as these nearby regions are difficult to distinguish reliably with fMRI, we refer to them together), although they are discussed less frequently than the cortical regions discussed above.
In the amygdala, placebo treatments increase endogenous opioid responses (that is, they reduce opioid receptor binding), as observed by PET129, and reduce fMRI activity during pain16,17,61, an effect that can be blocked by naloxone61. Larger placebo-induced reductions in BOLD fMRI correlate with stronger placebo-induced analgesia53,100. The amygdala has a central role in encoding and maintaining sensory associations with potential threat (for example, see REF. 145), and placebo treatments may reduce the threat value and/or salience of pain cues. Opioid signalling in the amygdala, and projections to the PAG–RVM system, are also crucial for pain inhibition in animal models of threat-conditioned analgesia146. Together, these studies suggest that amygdala circuitry is important for both placebo and other analgesic effects that arise from competing motivational states. Further work is needed to determine whether placebo- and threat-related analgesia are mediated by opposing or similar influences on amygdala circuits.
A different pattern of placebo effects is found in the NAc–VS: placebo treatments cause increases in NAc–VS fMRI responses during pain53,96,103 and in opioid129–131 and dopamine130 activity as measured by PET. Dopamine and fMRI activity increases in the NAc–VS have been strongly linked with appetitive learning147, desire148, social rewards149 and motivational engagement150,151, as well as positive shifts in emotion152 and pain reduction induced by self-regulation106. The NAc may also have a particularly important role in the motivational and behavioural aspects of pain119,153,154. For example, in animal models, chronic inflammation and nerve injury induce signs of depression and fatigue (for example, reduced reward seeking) that are accompanied by structural changes in the NAc154 and vmPFC155. Strikingly, blocking the neuropeptide galanin in the NAc reversed both these structural and motivational effects154. In humans, fMRI-measured functional connectivity between the vmPFC and the NAc–VS predicts the development of chronic back pain 1 year later156, implicating this circuit in long-term pain-related behaviour. Thus, findings of placebo effects in the NAc–VS may have important consequences on pain-related behaviour and other motivational processes.
The NAc–VS may also be important for predicting individual differences in the strength of placebo effects (that is, identifying placebo responders). Strong placebo analgesic responses are predicted by NAc–VS structure and function, including stronger placebo-related opioid129,131 and fMRI activity17,53 responses during pain, increased grey matter volume157 and stronger fMRI responses in a reward-pursuit task unrelated to pain158. NAc–VS grey matter volume and placebo-induced opioid responses are also positively correlated with personality measures related to optimism, reward seeking and resilience132,157. These findings suggest that inter-individual differences in NAc–VS structure and function may provide clues as to why some individuals are placebo responders and others are not. In addition, activation of the NAc–VS during pain predicts the magnitude of opioid analgesia159, providing support for the notion that brain reward circuitry is implicated in both placebo effects and other forms of pain modulation.
Beyond pain: placebo effects on motivational systems
Although placebo effects on brain function have been most extensively investigated in the context of pain, a select group of studies has begun to show that many of the systems discussed above are involved in placebo effects in other areas too, including emotion80,82–84,160, motor performance45,161 and learning85 in PD, and depression86,87.
The earliest studies of the brain mechanisms underlying placebo effects in domains other than pain examined placebo effects in PD and depression. One landmark PET study of dopamine activity45 found that placebo administration increased striatal dopamine binding in patients with PD, particularly in those who perceived an improvement in clinical status with placebo treatment. Subsequent studies found that individuals with PD who showed placebo-induced improved motor performance also showed placebo-induced increases in subthalamic nucleus firing161, that PD patients’ therapeutic expectations are correlated with placebo-induced striatal dopamine release44 and that placebos mimic the effects of dopamine drugs on reward-learning signals in the striatum and vmPFC85. An adjacent region of the vmPFC, in the subgenual cingulate cortex, also showed placebo-related reductions in glucose metabolism in depression86. This region is thought to be a critical hub for depression162, and subgenual cingulate cortex stimulation has shown great promise as an intervention for treatment-resistant depression163. These studies provide promising links between the effects of placebo treatments on medial prefrontal–striatal circuitry and improvements in psychopathology.
Studies of placebo effects on emotion processing also dovetail with findings relating to placebo-mediated analgesia. In one study, a placebo ‘anxiolytic’ reduced both the unpleasantness of negative images and amygdalar and extrastriate cortical responses to the images80. These effects were also associated with increased activity in the lateral OFC and dACC, regions that are also implicated in placebo analgesia164. In another series of studies, treatment with a placebo ‘anti-nausea’ pill reduced ratings of disgust in response to negative images83,84. These effects were accompanied by reductions in insular and visual cortical activation and reduced functional connectivity between the insula and both the amygdala and visual cortices. Finally, a placebo nasal spray, paired with suggestions of increased touch pleasantness and reduced pain, produced similar increases in vmPFC, NAc–VS, amygdala and PAG activity during both pleasant and painful touch165. However, somatosensory cortical activity increased during pleasant touch and decreased during pain. Thus, placebo effects on pleasant touch may engage similar forebrain motivational circuitry but have opposite effects on somatosensory processes.
Together, these studies elucidate the neural circuitry underlying placebo effects. Converging evidence indicates that placebo treatments engage prefrontal–subcortical systems that are involved in valuation, emotion and expectation. These systems can affect both sensory aspects of pain, via descending brainstem and spinal modulation, and functional and affective aspects of pain as well as other emotional and motivational processes, via interactions with the striatum and amygdala. Placebo effects in cortical–brainstem systems depend in part on opioid involvement: placebos can cause central opioid release in the cortex, NAc–VS and PAG129, and placebo effects on both pain-related increases and decreases in fMRI activity are blocked by naloxone61. However, a range of other neurochemicals, including dopamine, cholecystokinin and oxytocin, are also likely to be involved in the placebo response2 (BOX 1); for example, one recent study found that intranasal oxytocin enhanced placebo analgesia166. Studies of placebo can also be viewed in conjunction with other forms of affective and perceptual regulation by context and expectancy, such as value-based modulation of hedonic responses28 and cue-based modulation of visual and auditory perception23,24,167,168, although full consideration of all of these studies is beyond the scope of this Review.
New frontiers in placebo research
The emerging neuroscience of placebo effects suggests a remarkable consistency in the brain systems engaged across studies and health-related domains, including pain, PD, depression and emotion. However, this apparent consistency belies a deep complexity, the untangling of which has barely begun. Placebo studies widely differ in both the outcomes that are assessed and the likely psychological processes involved. As Benedetti writes2: “There is not one placebo effect, but many.” To move forward, we must jointly consider the variety of psychological mechanisms that are involved in placebo effects and how they relate to brain systems.
In this section, we provide a framework that includes three types of psychological antecedents that give rise to placebo effects — pre-cognitive associations, conceptual processes (for example, expectancies) and affective or motivational states — with different brain substrates. These antecedents give rise to placebo effects on three kinds of outcomes: disease-related symptoms (for example, pain), physiological signs and other behaviours. Using this framework as a platform, we explore several frontiers and areas of new opportunity. The first frontier concerns differences across types of outcomes. We ask whether placebo effects on different outcomes really share common mechanisms, and what functional roles the regions consistently activated by placebos, such as the PFC, might have. The second frontier concerns the requisite antecedents for placebo effects. We explore the hypothesis that both conceptual and learning processes are required for many kinds of placebo effects and discuss how these may relate to brain systems. Finally, although in many cases placebo effects require learning — driven by experienced benefit after receiving the placebo — in some cases placebo effects persist despite contrary experiences. We ask what the mechanisms underlying such ‘self-reinforcing’ placebo effects might be.
Mapping psychological mechanisms onto brain systems
The principal building blocks of laboratory placebo paradigms include three elements: presentation of sensory cues associated with positive outcomes (for example, pain relief) or negative outcomes (for example, shock) through classical conditioning; verbal suggestions designed to induce expectations of therapeutic improvement or symptom exacerbation; and the delivery of placebo manipulations in a context that includes both rich associative cues (for example, a hospital setting) and information about the interpersonal relationship (for example, knowledge that treatment is provided by an expert caregiver). These ‘treatments’ can elicit a range of therapeutically relevant internal brain processes (FIG. 4a). One useful distinction is between processes that are pre-cognitive — that is, independent of what a person expects or believes — or conceptual — that is, dependent on thoughts, expectations and memories. Conditioned cues can elicit pre-cognitive associations, which are simple forms of memory that are supported by neuroplastic changes in specific circuits throughout the brain and the spinal cord. These associations can trigger multiple types of responses, depending on the nature of the circuit and its location in the brain, including autonomic and neuroendocrine responses, emotions and motivated behaviours. Verbal suggestions and background beliefs about the treatment context can engage multiple conceptual processes: expectations of specific outcomes, appraisals of the significance of both symptoms and treatment, and explicit memories of prior experiences. If they are sufficiently relevant for survival and well-being, both pre-cognitive associations and conceptual thought can induce emotional and motivational states, which may underlie some forms of placebo effects158,169. These mediating processes differentially influence different classes of observable outcomes, including reported experiences (for example, symptoms), behaviour and physiology.
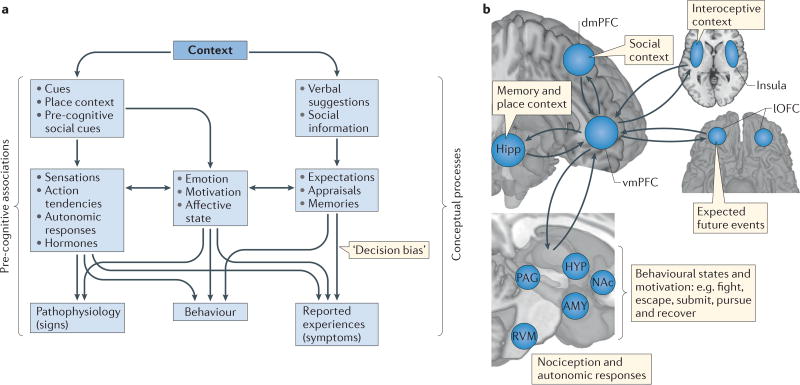
Figure 4. Concepts, associations and the representation of context.
a | Patient outcomes, and hence placebo effects, are measured as a function of pathophysiology (signs), reported experiences (symptoms) and behaviour. These outcomes are influenced in various ways by the two primary components of the treatment context: conceptual processes and pre-cognitive associations. Conceptual processes can influence expectations, appraisals and memories, which can directly influence emotional states, reported decisions and behaviour. Pre-cognitive associations influence physiological processes outside conscious control, which can in turn influence emotion, motivation and affective states as well as outcome measures. Thus, some types of placebo effects may be mediated by affective and motivational states, whereas others may be independent of such states, depending on the nature of the context and the outcome. b | Conceptual processes have been difficult to define and measure precisely in the brain, because they depend on the integration of information associated with multiple systems into an overall schema, or conceptualization of the situation and its implications for well-being, which guides the meaning or significance of events. The ingredients of such ‘meaning responses’, which are thought to be critical for placebo effects212, include inferences about social information (dorsomedial prefrontal cortex (dmPFC)), interoceptive assessments of one’s body state (insula), expectancies (lateral orbitofrontal cortex (lOFC)) and autobiographical memories and place context information (hippocampus (Hipp)). The ventromedial PFC (vmPFC) is positioned to integrate these elements into a coherent schema that informs and is informed by responses at other processing levels170, including brainstem and subcortical centres that regulate sensory, autonomic and neuroendocrine responses. AMY, amygdala; HYP, hypothalamus; NAc, nucleus accumbens; PAG, periaqueductal grey; RVM, rostroventral medulla.
Although placebo effects may engage similar classes of processes (for example, expectancies) across disorders and outcomes, resulting in substantial convergence in the neurobiological systems typically involved, no single process or system mediates all types of placebo effects. A challenge for the next generation of placebo studies will be to specify which psychological antecedents produce placebo effects on specific brain systems and outcomes.
Different mechanisms for different outcomes?
Although placebo effects can encompass symptom reports, physiological signs and other behaviours, it is far from clear which brain pathways are essential for the effects on each type of outcome. Most placebo studies have examined symptoms such as pain as a behavioural end point, and brain correlates of the strength of placebo effects104 are thus largely limited to the correlates of changes in symptoms. Direct comparisons of the brain mechanisms underlying placebo effects across outcomes (for example, pain, PD symptoms and emotion) and types of outcomes (for example, symptoms and physiology) have seldom been carried out.
It is thus still unclear whether brain regions such as the PFC and NAc, which seem to be consistently associated with placebo effects in pain, dopaminergic systems and beyond, are really engaged in the same way across disorders. The vmPFC and other prefrontal areas are critical hubs for conceptual meaning-making processes170 (FIG. 4b), making them natural candidates for common antecedents of placebo effects across outcomes. However, very few studies make direct comparisons across outcomes. A pair of studies82,160 have provided another kind of evidence on shared mechanisms: they examined whether placebo suggestions about one outcome (pain) influence another (emotion). A placebo ‘analgesic’ reduced the unpleasantness of negative images and the magnitude of the P2–N2 complex, an EEG marker of early visual processing82. Subsequent fMRI160 scans revealed that placebo administration reduced amygdala and insula responses to unpleasant images and increased activation in the subgenual ACC, a part of the vmPFC zone. Thus, this provides some initial evidence that placebos engage brain mechanisms in a way that transfers across outcomes.
A related question is which aspects of placebo-induced changes in fronto–striatal–brainstem systems are related to effects on pathophysiology, internal states that drive behaviour in meaningful ways or decision bias — effects of conceptual processes on symptoms, without concomitant effects on behaviour and pathophysiology. For some researchers, vmPFC activation might be taken as an indicator of decision bias. Responses of the vmPFC during the viewing of desirable items predict how much a participant is willing to pay for the item171 and are sensitive to beliefs about the object (for example, the price of a bottle of wine26) and one’s goals (for example, dieting goals when viewing food items172). However, vmPFC activation in placebo studies might also indicate effects of more enduring significance, in two ways. If conceptual processes affect health behaviours (for example, choices about food, exercise and social behaviour)150, they can have a lasting, long-term impact on disease. For example, smokers who showed larger vmPFC responses during viewing of anti-smoking advertisements were more likely to subsequently attempt to quit smoking, and vmPFC responses were more predictive than standard focus-group responses to the advertisments173. Despite their prominent role in decision making, vmPFC responses in placebo studies may also be related to influences on pathophysiology, including nociception and physiological responses (BOX 1). The vmPFC projects directly to the PAG, hypothalamus and other autonomic centres54, and its connections with the PAG mediate some kinds of conceptually driven autonomic responses, such as social evaluative threat56. Thus, one may ask, which potential outcomes is vmPFC (or lateral PFC, or NAc) activity related to in any given placebo study?
Two new research directions may provide important clues. The first is examination of PFC–brainstem connectivity. Enhanced vmPFC–PAG connectivity in placebo studies suggests descending regulation of pain physiology or autonomic or neuroendocrine responses. In future studies, it may be possible to separate patterns of prefrontal activity that differentially relate to brainstem or spinal cord responses and measures of pathophysiology, symptom reports independent of pathophysiology and long-term placebo-induced changes in behaviour.
The second direction is the development of brain measures that provide new markers of neuropathophysiology for mental health and neurological disorders (BOX 2). In pain, these include brain indices of early nociception59,133 and central pain-construction processes174. In PD, they include measures of dopamine activity45 and dopamine-linked brain processes85. The brain correlates of placebo effects on these new measures may be similar to or different from those related to self-reported outcomes. For example, in one recent study of placebo analgesia, the brain patterns predictive of the magnitude of placebo effects on pain reports versus pain-related brain responses were distinct, although frontal cortical systems were involved in both100.
Understanding the role of conceptual processes
Another frontier is understanding which types of placebo mechanisms — including pre-cognitive associations, conceptual thought and emotional states — are required to elicit changes in brain processes that are relevant to health and disease. Nearly all of the studies that produced convincing placebo-induced decreases in pain-related brain responses and increases in activity in pain- and emotion-modulatory circuitry (FIG. 3) utilized the response conditioning procedure, which involves both creating expectations via verbal suggestions and reinforcing those expectations through classical conditioning. Although conditioning is frequently thought of as creating ‘hard-wired’ associations in neural circuits, decades of empirical work suggests that in many cases conditioned responses in humans and rodents alike depend on the information value of the cue — that is, the expected outcome — rather than obligatory, pre-cognitive associations175–178. Thus, it is still unknown whether placebo-induced neuromodulation is created by the belief in the placebo, expectations of positive outcomes or specific associations learned through reinforcement.
Conditioning and expectancy have traditionally been offered as competing alternatives for placebo effects179–182. However, there is growing evidence that a combination of pre-cognitive associations and conceptual processes may be required. Placebo effects elicited by verbal suggestions alone have been reported, including some effects on physiology (Supplementary information S1 (table)), but on the whole these effects are weak and inconsistent across studies. The most compelling example — that is, the induction of cortisol release by suggestions of strong upcoming pain — may have worked by eliciting strong emotional responses. Perhaps surprisingly, reinforcement alone without verbal instructions does not often yield robust placebo effects either; adding verbal instructions to reinforcement alone typically produces much stronger effects109,183,184.
In addition, conditioned placebo effects can often be reversed by verbal suggestions. For example, autonomic responses can be conditioned in humans and animals by pairing previously neutral sensory cues (for example, lights) with shocks. Such responses can be reversed in a single trial by instructing participants that the light– shock contingency is no longer in effect58 (for a review of related paradigms, see REF. 178). In a minority of cases, placebo effects may be insensitive to beliefs: for example, in conditioned immunosuppression185 and some forms of pharmacological conditioning65,186. However, even in these cases, verbal suggestions may support conditioning during learning; thus, having the right belief may still support the formation of placebo effects in these systems.
One way in which conceptual processes may interact with experiences (that is, reinforcement) is by guiding attributions — beliefs about the nature of the events that caused pain relief or other therapeutic outcomes. For example, imagine that you take a pill to relieve a headache and an hour later the headache disappears. You must decide whether to attribute the relief you feel to the pill or the natural course of events. Attributions such as this probably guide what we learn from experiencing outcomes with multiple potential causes in many situations. Several placebo studies184,187,188 have found that response conditioning — pairing a cream with reductions in the intensity of painful stimuli — resulted in placebo analgesia (and reduced EEG potentials188) during a later test, but only as long as participants were not aware of the reduction and believed that the stimuli were just as intense on the placebo-treated site. When participants were informed that the stimulus intensity would be reduced, no placebo analgesia occurred, although the conditioning procedure was otherwise identical. Conditioned analgesia required both the experience of reduced symptoms and the attribution of efficacy to the cream. Thus, another potential function of prefrontal cortical activation after placebo treatment is to guide attributions of efficacy; if they favour the treatment, learned placebo may be strengthened in other systems, including the amygdala, the NAc–VS and the brainstem.
Attribution may be important in appetitive learning as well as in pain: a positive outcome following what one believes is a good choice reinforces the choice, but a positive outcome following a bad choice may be attributed to luck. Several recent studies suggest that this type of attribution shapes appetitive learning: participants learn the reward values of cues faster when reward feedback is compatible with prior beliefs induced by verbal suggestion189–191. These studies imply that dopaminergic reward learning is enhanced by prior beliefs in the reward value of the cues. All of these effects are consistent with the idea that suggestions influence the credit-assignment process during learning and suggest that conditioned placebo responses in the NAc–VS, and possibly other regions, may depend on attribution of benefit to the placebo.
Self-reinforcing placebo effects?
One of the mysteries surrounding placebo effects is that they can sometimes be stable or even increase in magnitude over time184,192,193. But if placebo effects are a conditioning phenomenon that is learned during a training phase, then they should extinguish during subsequent testing, when symptoms (such as experienced pain) are higher than expected in placebo conditions. Although it remains largely unexplored at the brain level, two of the mechanisms discussed here might be particularly important for creating self-reinforcing placebo effects that last through time. First, if experienced benefits are attributed to a treatment when they match prior beliefs (for example, when pain experience is low) but not otherwise (for example, when pain is high), then disconfirmatory experiences will be discarded and belief in the placebo will persist. Second, if placebo treatments have deep effects on the sensory processes that give rise to symptoms (for example, spinal responses to painful events), belief in the placebo will not be disconfirmed because the ascending noxious input will be dampened. These conditions allow placebo effects to become self-fulfilling prophecies. Much work remains to test these mechanisms and the brain processes that support them, but doing so could help us to understand and ultimately harness the power of belief for creating positive, long-term change.
Conclusions
A substantial part of the therapeutic benefit patients experience when undergoing medical treatment is caused by their brain’s response to the treatment context. Laboratory investigations of placebo effects provide a way of examining the brain mechanisms underlying these effects. Consistent findings across studies include reduced activity in brain areas associated with pain and negative emotion, and increased activity in fronto–striatal–brainstem circuits. In most cases, the creation of robust placebo effects across disorders and outcomes seems to require appropriate conceptual beliefs — maintained in prefrontal cortical networks — that are supported by experience-dependent learning in striatal and brainstem circuits. However, the critical ingredients for eliciting placebo effects, at both the psychological and brain level, are just beginning to be understood. These ingredients may differ substantially depending on whether the outcomes are symptoms, behaviours or changes in physiology. A better understanding of the neuroscience of placebo could yield rich benefits for both neuroscience and human health.
Supplementary Material
Key points.
Placebo effects are effects of the context surrounding medical treatment. They can have meaningfully large impacts on clinical, physiological and brain outcomes.
Effects of placebo treatments are consistent across studies from different laboratories. These effects include reduced activity in brain areas associated with pain and negative emotion, and increased activity in the lateral and medial prefrontal cortex, ventral striatum and brainstem.
Placebo effects in pain, Parkinson disease, depression and emotion are enabled by engagement of common prefrontal–subcortical motivational systems, but the similarity across domains in the way these systems are engaged has not been directly tested.
Meaningfully large placebo effects are likely to require a mixture of both conceptual belief in the placebo and prior experiences of treatment benefit, which engage brain learning processes.
In some cases, placebo effects are self-reinforcing, suggesting that they change symptoms in a way that precludes extinction. The mechanisms that drive these effects remain to be uncovered, but doing so could have profound translational implications.
Acknowledgments
The authors thank J. Sills and E. Hitchcock for research support, the members of the Cognitive and Affective Neuroscience Lab, S. Maier and L. Watkins for helpful discussions, and L. Ruzic for help with the summary in Figure 3. This work was funded by grants NIMH 2R01MH076136 and R01DA027794 (to T.D.W.). This work was also supported in part by the Intramural Research Program of the US National Institutes of Health’s National Center for Complementary and Integrative Health.
Glossary
- Context
The combination of all of the elements surrounding a given event that can be psychologically meaningful, including interpersonal dynamics, situational features owing to a place or location, memories, goals for the future and internal body or brain states.
- Cues
Stimuli that signify the occurrence, or evoke a representation, of another stimulus or internal experience.
- Emotions
Coordinated responses to biologically relevant events (such as threats and opportunities) that involve changes in multiple systems, including peripheral physiology.
- Nocebo effects
Deleterious outcomes (for example, an increase in pain or an increase in negative side effects) owing to beliefs about the treatment context.
- Placebo responders
Individuals who show an improvement in symptoms after receiving inert treatments (that is, placebos).
- Placebo analgesia
A reduction in pain that can be attributed to the treatment context.
- Response conditioning
The process of associating neutral stimuli with biologically meaningful outcomes, through which neutral stimuli may begin to induce anticipatory responses that are associated with the outcomes themselves.
- Expectancy
A conscious, conceptual belief about the future occurrence of an event. It is a subclass of predictive processes, which may be conscious or unconscious.
- Analgesia
Pain relief, which can be caused by many factors, including medical treatments (for example, opioid analgesia), features of the treatment context (placebo analgesia) and affective states (for example, stress-induced analgesia).
- Nociceptive
Receiving input from stimuli that can cause damage to tissues.
- Descending pain modulation systems
Endogenous, biological mechanisms for suppressing ascending nociceptive information at the level of the spinal cord.
- Pre-cognitive associations
Links between events and/or objects that exist outside conscious awareness. These links are generally created through conditioning procedures or innate (evolutionarily afforded) associations.
- Conceptual processes
Processes that depend on an interpretation of the situational context and its relationship to prior information (for example, memories and rules), including interoceptive cues from the body, and which can be updated in response to verbally presented or symbolic information.
- Schema
A conceptual, ‘situational’ pattern — inferred from a combination of sensory cues, internal motivation, interoceptive information and thoughts — that can activate scripts that guide behaviour based on the nature of the situation rather than any single cue.
- Attributions
Inferred causality; the process of assigning an observed effect (for example, a symptom) to an underlying cause or mechanism.
Biographies
Tor D. Wager is a professor of psychology and neuroscience, and a faculty member in the Institute for Cognitive Science at the University of Colorado, Boulder, USA. He received his Ph.D. from the University of Michigan, Ann Arbor, USA, in cognitive psychology in 2003, and served as an assistant and associate professor at Columbia University, New York, USA, from 2004 to 2009. Since 2010, he has directed Boulder’s Cognitive and Affective Neuroscience Laboratory. He has a deep interest in how thinking influences affective experiences, affective learning and brain–body communication. His laboratory also focuses on the development and deployment of analytical methods, and has developed several publicly available software toolboxes for functional MRI analysis. Tor D. Wager’s homepage. http://wagerlab.colorado.edu
Lauren Y. Atlas is an investigator and Section Chief at the National Institutes of Health, in the National Center for Complementary and Integrative Health, Bethesda, Maryland, USA. She completed her Ph.D. in psychology at Columbia University, New York, USA, followed by postdoctoral studies at New York University, USA. Her research integrates experimental psychology, neuroimaging, psychophysiology and computational approaches to understand how psychological and contextual factors influence pain, emotion and clinical outcomes. Lauren Y. Atlas’s homepage. https://nccih.nih.gov/research/intramural/atlas-lab
Footnotes
Competing interests statement
The authors declare no competing interests.
See online article: S1 (table) | S2 (box)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Institute of Medicine of the National Academies, editor. Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; 2011. pp. 1–350. [PubMed] [Google Scholar]
- 2.Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. 2014;84:623–637. doi: 10.1016/j.neuron.2014.10.023. This review discusses the pharmacological foundation of many types of placebo effects and addresses the translational and ethical implications of placebo studies. [DOI] [PubMed] [Google Scholar]
- 3.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Carlino E, Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meissner K. The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Phil. TransRSoc. B. 2011;366:1808–1817. doi: 10.1098/rstb.2010.0403. This review focuses on the evidence that placebos influence autonomic nervous system responses, including effects on gastrointestinal, cardiovascular and pulmonary functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pressman A, Avins AL, Neuhaus J, Ackerson L, Rudd P. Adherence to placebo and mortality in the Beta Blocker Evaluation of Survival Trial (BEST) Contemp. Clin. Trials. 2012;33:492–498. doi: 10.1016/j.cct.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenk LA, Sprenger C, Geuter S, Buchel C. Expectation requires treatment to boost pain relief: an fMRI study. Pain. 2014;155:150–157. doi: 10.1016/j.pain.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 2004;3:679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 9.Rohsenow DJ, Marlatt GA. The balanced placebo design: methodological considerations. Addict. Behav. 1981;6:107–122. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch I, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flood A, Lorence D, Ding J, McPherson K, Black NA. The role of expectations in patients’ reports of post-operative outcomes and improvement following therapy. Med. Care. 1993;31:1043–1056. doi: 10.1097/00005650-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Goetz CG, et al. Placebo response in Parkinson’s disease: comparisons among 11 trials covering medical and surgical interventions. Mov. Disord. 2008;23:690–699. doi: 10.1002/mds.21894. [DOI] [PubMed] [Google Scholar]
- 13.Wampold BE, et al. A meta-analysis of outcome studies comparing bona fide psychotherapies: empiricially, ” all must have prizes”. Psychol. Bull. 1997;122:203–215. [Google Scholar]
- 14.Kleijnen J, de Craen AJ, van Everdingen J, Krol L. Placebo effect in double-blind clinical trials: a review of interactions with medications. Lancet. 1994;344:1347–1349. doi: 10.1016/s0140-6736(94)90699-8. [DOI] [PubMed] [Google Scholar]
- 15.Flaten MA, Simonsen T, Olsen H. Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosomat. Med. 1999;61:250–255. doi: 10.1097/00006842-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Kong J, et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage. 2009;45:940–949. doi: 10.1016/j.neuroimage.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atlas LY, et al. Dissociable influences of opiates and expectations on pain. J. Neurosci. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. This paper used pharmacological fMRI of remifentanil, an opioid agonist, to examine how placebo effects combine with drug effects during open drug administration and found that placebo analgesia and opioid analgesia have additive, dissociable effects on pain and brain responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atlas LY, Wielgosz J, Whittington RA, Wager TD. Specifying the non-specific factors underlying opioid analgesia: expectancy, attention, and affect. Psychopharmacology. 2014;231:813–823. doi: 10.1007/s00213-013-3296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedetti F, et al. The specific effects of prior opioid exposure on placebo analgesia and placebo respiratory depression. Pain. 1998;75:313–319. doi: 10.1016/s0304-3959(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 20.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81:1223–1239. doi: 10.1016/j.neuron.2014.02.042. This review focuses on placebo analgesia from a Bayesian predictive-coding perspective and addresses the relationship between expectations, experience and decision making. [DOI] [PubMed] [Google Scholar]
- 22.Sterzer P, Frith C, Petrovic P. Believing is seeing: expectations alter visual awareness. Curr. Biol. 2008;18:R697–R698. doi: 10.1016/j.cub.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Summerfield C, de Lange FP. Expectation in perceptual decision making: neural and computational mechanisms. Nat. Rev. Neurosci. 2014;15:745–756. doi: 10.1038/nrn3838. [DOI] [PubMed] [Google Scholar]
- 24.Summerfield C, Egner T. Expectation (and attention) in visual cognition. Trends Cogn. Sci. 2009;13:403–409. doi: 10.1016/j.tics.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Edelson M, Sharot T, Dolan RJ, Dudai Y. Following the crowd: brain substrates of long-term memory conformity. Science. 2011;333:108–111. doi: 10.1126/science.1203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plassmann H, O’Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc. Natl Acad. Sci. USA. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J. Neurosci. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plassmann H, Wager TD. In: The Interdisciplinary Science of Consumption. Kringelbach M, Knutson B, Preston S, editors. 2014. pp. 219–240. [Google Scholar]
- 29.Beedie CJ, Foad AJ. The placebo effect in sports performance. Sports Med. 2009;39:313–329. doi: 10.2165/00007256-200939040-00004. [DOI] [PubMed] [Google Scholar]
- 30.Boot WR, Simons DJ, Stothart C, Stutts C. The pervasive problem with placebos in psychology why active control groups are not sufficient to rule out placebo effects. Persp. Psychol. Sci. 2013;8:445–454. doi: 10.1177/1745691613491271. [DOI] [PubMed] [Google Scholar]
- 31.Carlino E, Frisaldi E, Benedetti F. Pain and the context. Nat. Rev. Rheumatol. 2014;10:348–355. doi: 10.1038/nrrheum.2014.17. [DOI] [PubMed] [Google Scholar]
- 32.Sherman R, Hickner J. Academic physicians use placebos in clinical practice and believe in the mind– body connection. J. Gen. Intern. Med. 2008;23:7–10. doi: 10.1007/s11606-007-0332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochoa JL. Chronic pains associated with positive and negative sensory, motor, and vaso-motor manifestations: CPSMV (RSD; CRPS?). Heterogeneous somatic versus psychopathologic origins. Contemp. Neurol. 1997;2:1–20. [Google Scholar]
- 34.Barrett B, et al. Placebo, meaning, and health. Perspect. Biol. Med. 2006;49:178–198. doi: 10.1353/pbm.2006.0019. [DOI] [PubMed] [Google Scholar]
- 35.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59:195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Vase L, Petersen GL, Riley JL, Price DD. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145:36–44. doi: 10.1016/j.pain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Vase L, Riley JL, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 38.Kaptchuk TJ, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. This clinical trial of placebo acupuncture found that patients with irritable bowel syndrome improved most when clinicians delivered treatment in a warm, supportive manner, which provides evidence that the patient–care provider relationship can influence treatment outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaptchuk TJ, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kam-Hansen S, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci. Transl Med. 2014;6:218ra5. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutherford BR, Roose SP, Sneed J. Mind over medicine: the influence of expectations on antidepressant response. J. Am. Psychoanal. Assoc. 2009;57:456–460. doi: 10.1177/00030651090570020909. [DOI] [PubMed] [Google Scholar]
- 42.Kirsch I. Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prevent. Treat. 1998;1 Article 2A. [Google Scholar]
- 43.Leuchter AF, Hunter AM, Tartter M, Cook IA. Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. Br. J. Psychiatry. 2014;205:443–449. doi: 10.1192/bjp.bp.113.140343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lidstone SC, et al. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch. Gen. Psychiatry. 2010;67:857–865. doi: 10.1001/archgenpsychiatry.2010.88. This study examined placebo effects in patients with PD and found that the strength of expectations for treatment influenced both clinical symptom reduction and striatal dopamine binding. [DOI] [PubMed] [Google Scholar]
- 45.de la Fuente-Fernandez R, et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 46.Kemeny ME, et al. Placebo response in asthma: a robust and objective phenomenon. J. Allergy Clin. Immunol. 2007;119:1375–1381. doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Luparello T, Lyons HA, Bleecker ER, McFadden ER., Jr Influences of suggestion on airway reactivity in asthmatic subjects. Psychosomat. Med. 1968;30:819–825. doi: 10.1097/00006842-196811000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler ME, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N. Engl. J. Med. 2011;365:119–126. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vase L, Robinson M, Verne G, Price D. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 50.Avins AL, et al. Placebo adherence and its association with morbidity and mortality in the studies of left ventricular dysfunction. J. Gen. Intern. Med. 2010;25:1275–1281. doi: 10.1007/s11606-010-1477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollo A, Vighetti S, Rainero I, Benedetti F. Placebo analgesia and the heart. Pain. 2003;102:125–133. doi: 10.1016/s0304-3959(02)00345-7. [DOI] [PubMed] [Google Scholar]
- 52.Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J. Neurosci. 1999;19:3639–3648. doi: 10.1523/JNEUROSCI.19-09-03639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bingel U, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 54.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 55.Price J. Prefrontal cortical networks related to visceral function and mood. Ann. NY Acad. Sci. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 56.Wager TD, et al. Brain mediators of cardiovascular responses to social threat, part II: prefrontal– subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phelps EA, et al. Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 58.Grings WW, Schell AM, Carey CA. Verbal control of an autonomic response in a cue reversal situation. J. Exp. Psychol. 1973;99:215–221. [Google Scholar]
- 59.Geuter S, Büchel C. Facilitation of pain in the human spinal cord by nocebo treatment. J. Neurosci. 2013;33:13784–13790. doi: 10.1523/JNEUROSCI.2191-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura Y, et al. Investigating dose-dependent effects of placebo analgesia: a psychophysiological approach. Pain. 2012;153:227–237. doi: 10.1016/j.pain.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Eippert F, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. This study demonstrated, for the first time, that placebo-induced reductions in pain-related fMRI activity are reversible by naloxone. [DOI] [PubMed] [Google Scholar]
- 62.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J. Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansen O, Brox J, Flaten MA. Placebo and nocebo responses, cortisol, and circulating β-endorphin. Psychosomat. Med. 2003;65:786–790. doi: 10.1097/01.psy.0000082626.56217.cf. [DOI] [PubMed] [Google Scholar]
- 64.Guo JY, et al. Placebo analgesia affects the behavioral despair tests and hormonal secretions in mice. Psychopharmacology. 2011;217:83–90. doi: 10.1007/s00213-011-2259-7. [DOI] [PubMed] [Google Scholar]
- 65.Benedetti F, et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. This multiday study separated placebo effects that depend on conditioning from those that depend on instructions, and found that placebo effects on pain and motor performance in PD reverse immediately with instructions, whereas placebo effects on growth hormone and cortisol mimic pharmacological conditioning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crum AJ, Corbin WR, Brownell KD, Salovey P. Mind over milkshakes: mindsets, not just nutrients, determine ghrelin response. Health Psychol. 2011;30:424–429. doi: 10.1037/a0023467. [DOI] [PubMed] [Google Scholar]
- 67.Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav. Brain Res. 2000;110:175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 68.Ader R, Cohen N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science. 1982;215:1534–1536. doi: 10.1126/science.7063864. [DOI] [PubMed] [Google Scholar]
- 69.Goebel MU, et al. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16:1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 70.Schedlowski M, Pacheco-Lopez G. The learned immune response: Pavlov and beyond. Brain Behav. Immun. 2010;24:176–185. doi: 10.1016/j.bbi.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Exton MS, et al. Behaviorally conditioned immunosuppression in the rat is regulated via noradrenaline and β-adrenoceptors. J. Neuroimmunol. 2002;131:21–30. doi: 10.1016/s0165-5728(02)00249-7. [DOI] [PubMed] [Google Scholar]
- 72.Pacheco-Lopez G, et al. Neural substrates for behaviorally conditioned immunosuppression in the rat. J. Neurosci. 2005;25:2330–2337. doi: 10.1523/JNEUROSCI.4230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ober K, et al. Plasma noradrenaline and state anxiety levels predict placebo response in learned immunosuppression. Clin. Pharmacol. Ther. 2012;91:220–226. doi: 10.1038/clpt.2011.214. [DOI] [PubMed] [Google Scholar]
- 74.Kamenica E, Naclerio R, Malani A. Advertisements impact the physiological efficacy of a branded drug. Proc. Natl Acad. Sci. USA. 2013;110:12931–12935. doi: 10.1073/pnas.1012818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 76.Staudinger MR, Buchel C. How initial confirmatory experience potentiates the detrimental influence of bad advice. Neuroimage. 2013;76:125–133. doi: 10.1016/j.neuroimage.2013.02.074. [DOI] [PubMed] [Google Scholar]
- 77.Wager TD, Matre D, Casey KL. Placebo effects in laser-evoked pain potentials. Brain Behav. Immun. 2006;20:219–230. doi: 10.1016/j.bbi.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martini M, Lee M, Valentini E, Iannetti G. Intracortical modulation, and not spinal inhibition, mediates placebo analgesia. Eur. J. Neurosci. 2015;41:498–504. doi: 10.1111/ejn.12807. [DOI] [PubMed] [Google Scholar]
- 79.Halperin E, Russell AG, Trzesniewski KH, Gross JJ, Dweck CS. Promoting the Middle East peace process by changing beliefs about group malleability. Science. 2011;333:1767–1769. doi: 10.1126/science.1202925. [DOI] [PubMed] [Google Scholar]
- 80.Petrovic P, et al. Placebo in emotional processing — induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. This study demonstrated that placebo anxiolytics modulate BOLD responses to emotional images and that these modulations were paralleled by fMRI activation in some of the same brain regions as previously found in placebo analgesia. [DOI] [PubMed] [Google Scholar]
- 81.Zhang W, Guo J, Zhang J, Luo J. Neural mechanism of placebo effects and cognitive reappraisal in emotion regulation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:364–373. doi: 10.1016/j.pnpbp.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Zhang W, Luo J. The transferable placebo effect from pain to emotion: changes in behavior and EEG activity. Psychophysiology. 2009;46:626–634. doi: 10.1111/j.1469-8986.2009.00786.x. This study, which found that a placebo analgesic also modulated negative affect and EEG responses to unpleasant pictures, represents one of the few studies to formally examine placebo effects across domains. [DOI] [PubMed] [Google Scholar]
- 83.Schienle A, Übel S, Schöngaßner F, Ille R, Scharmüller W. Disgust regulation via placebo: an fMRI study. Soc. Cogn. Affect. Neurosci. 2013;9:985–990. doi: 10.1093/scan/nst072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schienle A, Übel S, Scharmüller W. Placebo treatment can alter primary visual cortex activity and connectivity. Neuroscience. 2014;263:125–129. doi: 10.1016/j.neuroscience.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt L, Braun EK, Wager TD, Shohamy D. Mind matters: placebo enhances reward learning in Parkinson’s disease. Nat. Neurosci. 2014;17:1793–1797. doi: 10.1038/nn.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayberg HS, et al. The functional neuroanatomy of the placebo effect. Am. J. Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 87.Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M. Changes in brain function of depressed subjects during treatment with placebo. Am. J. Psychiatry. 2002;159:122–129. doi: 10.1176/appi.ajp.159.1.122. [DOI] [PubMed] [Google Scholar]
- 88.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 89.Watson A, et al. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wager TD, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. This study used a heat pain model to examine the neural basis of placebo analgesia and was the first fMRI study of placebo analgesia. [DOI] [PubMed] [Google Scholar]
- 91.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Koyama T, McHaffie JG, Laurienti P, Coghill RC. The subjective experience of pain: where expectations become reality. Proc. Natl Acad. Sci. USA. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keltner J, et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J. Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geuter S, Eippert F, Attar CH, Büchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 2013;67:227–236. doi: 10.1016/j.neuroimage.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiech K, et al. Anterior insula integrates information about salience into perceptual decisions about pain. J. Neurosci. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee HF, et al. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153:1301–1310. doi: 10.1016/j.pain.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 97.Lu H-C, et al. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: a 3T-fMRI study. Pain. 2009;148:75–83. doi: 10.1016/j.pain.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J. Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kong J, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J. Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J. Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elsenbruch S, et al. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain. 2012;153:382–390. doi: 10.1016/j.pain.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 102.Kong J, et al. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154:459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atlas LY, Wager TD. In: Placebo. Benedetti F, Enck P, Frisaldi E, Schedlowski M, editors. Springer; 2014. pp. 37–69. [Google Scholar]
- 104.Koban L, Ruzic L, Wager TD. In: Placebo and Pain. Colloca L, Flaten MA, Meissner K, editors. Academic; 2013. pp. 89–102. [Google Scholar]
- 105.Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum. Brain Mapp. 2013;34:738–752. doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woo C-W, Roy M, Buhle JT, Wager T. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol. 2015;13:e1002036. doi: 10.1371/journal.pbio.1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorenz J, et al. Cortical correlates of false expectations during pain intensity judgments — a possible manifestation of placebo/nocebo cognitions. Brain Behav. Immun. 2005;19:283–295. doi: 10.1016/j.bbi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 108.Aslaksen PM, Bystad M, Vambheim SM, Flaten MA. Gender differences in placebo analgesia: event-related potentials and emotional modulation. Psychosomat. Med. 2011;73:193–199. doi: 10.1097/PSY.0b013e3182080d73. [DOI] [PubMed] [Google Scholar]
- 109.Colloca L, et al. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain. 2009;139:306–314. doi: 10.1016/j.pain.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 110.Watson A, El-Deredy W, Vogt BA, Jones AK. Placebo analgesia is not due to compliance or habituation: EEG and behavioural evidence. Neuroreport. 2007;18:771–775. doi: 10.1097/WNR.0b013e3280c1e2a8. [DOI] [PubMed] [Google Scholar]
- 111.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc. Natl Acad. Sci. USA. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J. Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- 114.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woo CW, et al. Separate neural representations for physical pain and social rejection. Nat. Commun. 2014;5:5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat. Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 117.Porreca F, Ossipov MH, Gebhart G. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 118.Gebhart G. Descending modulation of pain. Neurosci. Biobehav. Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Heinricher M, Fields H. In: Wall & Melzack’s Textbook of Pain. McMahon S, Koltzenburg M, Tracey I, Turk DC, editors. Elsevier Health Sciences; 2013. pp. 129–142. [Google Scholar]
- 120.Fields HL. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 121.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keay K, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci. Biobehav. Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 123.Wright JS, Panksepp J. Toward affective circuit-based preclinical models of depression: sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neurosci. Biobehav. Rev. 2011;35:1902–1915. doi: 10.1016/j.neubiorev.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 124.Satpute AB, et al. Identification of discrete functional subregions of the human periaqueductal gray. Proc. Natl Acad. Sci. USA. 2013;110:17101–17106. doi: 10.1073/pnas.1306095110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buhle JT, et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc. Cogn. Affect. Neurosci. 2013;8:609–616. doi: 10.1093/scan/nss038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. This study demonstrated that placebo analgesia can be blocked with the opioid antagonist naloxone and was the first to demonstrate a biological mechanism for placebo. [DOI] [PubMed] [Google Scholar]
- 128.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain. 1996;64:535–543. doi: 10.1016/0304-3959(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 129.Wager T, Scott D. Placebo effects on human μ-opioid activity during pain. Proc. Natl Acad. Sci. USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scott DJ, et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 131.Zubieta J, et al. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J. Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peciña M, et al. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. 2013;38:639–646. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. This study used fMRI to image the spinal cord and found that spinal responses to noxious stimuli are modulated with placebo, which implicates descending modulation. [DOI] [PubMed] [Google Scholar]
- 134.Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. J. Neurosci. 2006;26:559–563. doi: 10.1523/JNEUROSCI.4218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goffaux P, de Souza J, Potvin S, Marchand S. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients. Pain. 2009;145:18–23. doi: 10.1016/j.pain.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 136.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia — imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. This PET study was the first to use neuroimaging to investigate mechanisms of the placebo response and found that both placebo analgesia and opioid analgesia induce changes in blood flow in the rostral ACC. [DOI] [PubMed] [Google Scholar]
- 137.Craggs JG, Price DD, Perlstein WM, Verne GN, Robinson ME. The dynamic mechanisms of placebo induced analgesia: evidence of sustained and transient regional involvement. Pain. 2008;139:660–669. doi: 10.1016/j.pain.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lui F, et al. Neural bases of conditioned placebo analgesia. Pain. 2010;151:816–824. doi: 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 139.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 140.Borckardt JJ, et al. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology. 2006;105:557–562. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 141.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 142.Stein N, Sprenger C, Scholz J, Wiech K, Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012;153:2210–2217. doi: 10.1016/j.pain.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 143.Zhang YQ, Tang JS, Yuan B, Jia H. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain. 1997;72:127–135. doi: 10.1016/s0304-3959(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 144.Zhang S, Tang JS, Yuan B, Jia H. Electrically-evoked inhibitory effects of the nucleus submedius on the jaw-opening reflex are mediated by ventrolateral orbital cortex and periaqueductal gray matter in the rat. Neuroscience. 1999;92:867–875. doi: 10.1016/s0306-4522(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 145.Johansen JP, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl Acad. Sci. USA. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Helmstetter FJ, Tershner SA, Poore LH, Bellgowan PS. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Res. 1998;779:104–118. doi: 10.1016/s0006-8993(97)01104-9. [DOI] [PubMed] [Google Scholar]
- 147.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 148.Kober H, et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc. Natl Acad. Sci. USA. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychol. Sci. 2011;22:894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
- 150.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 152.Wager TD, Hughes B, Davidson M, Lindquist ML, Ochsner KN. Prefrontal– subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat. Neurosci. 2014;17:1304–1312. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwartz N, et al. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Metz AE, Yau H-J, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl Acad. Sci. USA. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baliki MN, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J. Neurosci. 2009;29:4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scott DJ, et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 159.Wanigasekera V, et al. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc. Natl Acad. Sci. USA. 2012;109:17705–17710. doi: 10.1073/pnas.1120201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang W, Qin S, Guo J, Luo J. A follow-up fMRI study of a transferable placebo anxiolytic effect. Psychophysiology. 2011;48:1119–1128. doi: 10.1111/j.1469-8986.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 161.Benedetti F, et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat. Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 162.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 163.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 164.Petrovic P, et al. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150:59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 165.Ellingsen D-M, et al. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc. Natl Acad. Sci. USA. 2013;110:17993–17998. doi: 10.1073/pnas.1305050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kessner S, Sprenger C, Wrobel N, Wiech K, Bingel U. Effect of oxytocin on placebo analgesia: a randomized study. JAMA. 2013;310:1733–1735. doi: 10.1001/jama.2013.277446. [DOI] [PubMed] [Google Scholar]
- 167.Rahnev D, Lau H, de Lange FP. Prior expectation modulates the interaction between sensory and prefrontal regions in the human brain. J. Neurosci. 2011;31:10741–10748. doi: 10.1523/JNEUROSCI.1478-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kok P, Brouwer GJ, van Gerven MA, de Lange FP. Prior expectations bias sensory representations in visual cortex. J. Neurosci. 2013;33:16275–16284. doi: 10.1523/JNEUROSCI.0742-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Flaten MA, Aslaksen PM, Lyby PS, Bjorkedal E. The relation of emotions to placebo responses. Phil. Trans. R. Soc. B. 2011;366:1818–1827. doi: 10.1098/rstb.2010.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal–subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chib VS, Rangel A, Shimojo S, O’Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J. Neurosci. 2009;29:12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 173.Falk EB, Berkman ET, Whalen D, Lieberman MD. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychol. 2011;30:177–185. doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wager T, et al. An fMRI-based neurologic signature of physical pain. N. Engl. J .Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rescorla RA. Pavlovian conditioning. It’s not what you think it is. Am. Psychol. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- 176.Gallistel CR, Matzel LD. The neuroscience of learning: beyond the Hebbian synapse. Annu. Rev. Psychol. 2013;64:169–200. doi: 10.1146/annurev-psych-113011-143807. [DOI] [PubMed] [Google Scholar]
- 177.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat. Rev. Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kirsch I, Lynn SJ, Vigorito M, Miller RR. The role of cognition in classical and operant conditioning. J. Clin. Psychol. 2004;60:369–392. doi: 10.1002/jclp.10251. [DOI] [PubMed] [Google Scholar]
- 179.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol. Bull. 2004;130:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 180.Kirsch I. Response expectancy as a determinant of experience and behavior. Am. Psychol. 1985;40:1189–1202. [Google Scholar]
- 181.Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. J. Pers. Soc. Psychol. 1985;48:47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 182.Wickramasekera I. A conditioned response model of the placebo effect; predictions from the model. Biofeedback Self Regul. 1980;5:5–18. doi: 10.1007/BF00999060. [DOI] [PubMed] [Google Scholar]
- 183.Carlino E, et al. Role of explicit verbal information in conditioned analgesia. Eur. J. Pain. 2015;19:546–553. doi: 10.1002/ejp.579. [DOI] [PubMed] [Google Scholar]
- 184.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 185.Wendt L, et al. Placebo-induced immunosuppression in humans: role of learning and expectation. Brain Behav. Immun. 2013;29:S17. [Google Scholar]
- 186.Benedetti F, Amanzio M, Baldi S, Casadio C, Maggi G. Inducing placebo respiratory depressant responses in humans via opioid receptors. Eur. J. Neurosci. 1999;11:625–631. doi: 10.1046/j.1460-9568.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- 187.Morton DL, Watson A, El-Deredy W, Jones AK. Reproducibility of placebo analgesia: effect of dispositional optimism. Pain. 2009;146:194–198. doi: 10.1016/j.pain.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 188.Morton DL, Brown CA, Watson A, El-Deredy W, Jones AK. Cognitive changes as a result of a single exposure to placebo. Neuropsychologia. 2010;48:1958–1964. doi: 10.1016/j.neuropsychologia.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 189.Doll BB, Jacobs WJ, Sanfey AG, Frank MJ. Instructional control of reinforcement learning: a behavioral and neurocomputational investigation. Brain Res. 2009;1299:74–94. doi: 10.1016/j.brainres.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Biele G, Rieskamp J, Krugel LK, Heekeren HR. The neural basis of following advice. PLoS Biol. 2011;9:e1001089. doi: 10.1371/journal.pbio.1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li J, Delgado MR, Phelps EA. How instructed knowledge modulates the neural systems of reward learning. Proc. Natl Acad. Sci. USA. 2011;108:55–60. doi: 10.1073/pnas.1014938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vase L, Robinson M, Verne G, Price D. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 193.Kirsch I, Henry D. Extinction versus credibility in the desensitization of speech anxiety. J. Consult. Clin. Psychol. 1977;45:1052–1059. doi: 10.1037//0022-006x.45.6.1052. [DOI] [PubMed] [Google Scholar]
- 194.Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J. Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active versus passive emotional coping. Brain Res. Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 197.Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216:1185–1192. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- 198.Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- 199.Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J. Neurosci. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Helmstetter FJ. Stress-induced hypoalgesia and defensive freezing are attenuated by application of diazepam to the amygdala. Pharmacol. Biochem. Behav. 1993;44:433–438. doi: 10.1016/0091-3057(93)90487-e. [DOI] [PubMed] [Google Scholar]
- 201.Butler RK, Finn DP. Stress-induced analgesia. Prog. Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 202.Yang J, et al. Central oxytocin enhances antinociception in the rat. Peptides. 2007;28:1113–1119. doi: 10.1016/j.peptides.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 203.Lund I, et al. Repeated massage-like stimulation induces long-term effects on nociception: contribution of oxytocinergic mechanisms. Eur. J. Neurosci. 2002;16:330–338. doi: 10.1046/j.1460-9568.2002.02087.x. [DOI] [PubMed] [Google Scholar]
- 204.Yang J, et al. Oxytocin in the periaqueductal gray participates in pain modulation in the rat by influencing endogenous opiate peptides. Peptides. 2011;32:1255–1261. doi: 10.1016/j.peptides.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 205.Watkins LR, Kinscheck IB, Mayer DJ. Potentiation of opiate analgesia and apparent reversal of morphine tolerance by proglumide. Science. 1984;224:395–396. doi: 10.1126/science.6546809. [DOI] [PubMed] [Google Scholar]
- 206.Wiertelak EP, Maier SF, Watkins LR. Cholecystokinin antianalgesia: safety cues abolish morphine analgesia. Science. 1992;256:830–833. doi: 10.1126/science.1589765. [DOI] [PubMed] [Google Scholar]
- 207.Dum J, Herz A. Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacol. Biochem. Behav. 1984;21:259–266. doi: 10.1016/0091-3057(84)90224-7. [DOI] [PubMed] [Google Scholar]
- 208.Rodgers RJ, Hendrie CA. Social conflict activates status-dependent endogenous analgesic or hyperalgesic mechanisms in male mice: effects of naloxone on nociception and behaviour. Physiol. Behav. 1983;30:775–780. doi: 10.1016/0031-9384(83)90177-4. [DOI] [PubMed] [Google Scholar]
- 209.Langford DJ, et al. Varying perceived social threat modulates pain behavior in male mice. J. Pain. 2011;12:125–132. doi: 10.1016/j.jpain.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 210.Shyu BC, Sikes RW, Vogt LJ, Vogt BA. Nociceptive processing by anterior cingulate pyramidal neurons. J. Neurophysiol. 2010;103:3287–3301. doi: 10.1152/jn.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 212.Moerman DE. Meaning, Medicine and the ‘Placebo Effect’. Cambridge Univ. Press; 2002. [Google Scholar]
- 213.Benedetti F, Durando J, Vighetti S. Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. Pain. 2014;155:921–928. doi: 10.1016/j.pain.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 214.Orne TM. On the social psychology of the psychological experiment: with particular reference to demand characteristics and their implications. Am. Psychol. 1962;17:776–783. [Google Scholar]
- 215.Xie JY, et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J. Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.