Abstract
Background
Although healthcare organizations have decreased hospital-acquired pressure injury (HAPI) rates, HAPIs are not eliminated, driving further examination in both nursing and health services research.
Objective
The objective was to describe HAPI incidence, risk factors, and risk-adjusted hospital variation within a California integrated healthcare system.
Methods
Inpatient episodes were included in this retrospective cohort if patients were hospitalized between January 1, 2013, and June 30, 2015. The primary outcome was development of a HAPI over time. Predictors included cited HAPI risk factors in addition to incorporation of a longitudinal comorbidity burden (Comorbidity Point Score, Version 2 [COPS2]), a severity-of-illness score (Laboratory-Based Acute Physiology Score, Version 2 [LAPS2]), and the Braden Scale for Predicting Pressure Ulcer Risk.
Results
Analyses included HAPI inpatient episodes (n = 1661) and non-HAPI episodes (n = 726,605). HAPI incidence was 0.57 per 1,000 patient days (95% CI [0.019, 3.805]) and 0.2% of episodes. A multivariate Cox proportional hazards model showed significant (p < .001) hazard ratios (HRs) for the change from the 25th to the 75th percentile for age (HR = 1.36, 95% CI [1.25, 1.45]), higher COPS2 scores (HR = 1.10, 95% CI [1.04, 1.16]), and higher LAPS2 scores (HR = 1.38, 95% CI [1.28, 1.50]). Female gender, an emergency room admission for a medical reason, and higher Braden scores showed significant protective HRs (HR < 1.00, p < .001). After risk adjustment, significant variation remained among the 35 hospitals.
Discussion
Results prompt the consideration of age, severity of illness (LAPS2), comorbidity indexes (COPS2), and the Braden score as important predictors for HAPI risk. HAPI rates may be low; however, because of significant individual site variation, HAPIs remain an area to explore through both research and quality improvement initiatives.
Keywords: California, hospitalization, pressure ulcer, risk adjustment
A hospital-acquired pressure injury (HAPI; formerly known as a pressure ulcer) is a localized injury to the skin and/or underlying tissue during an inpatient hospital stay. The result of pressure, shear, or both, HAPI development is additionally associated with other factors (e.g., advanced age, immobility, perfusion, nutritional status, hematological measures, illness severity, and presence of diabetes; Coleman et al., 2013; National Pressure Ulcer Advisory Panel [NPUAP], European Pressure Ulcer Advisory Panel, & Pan Pacific Pressure Injury Alliance, 2014). Generally considered preventable, HAPIs are accepted nursing quality indicators (Baharestani et al., 2009; Bergquist-Beringer, Davidson, & Cuddigan, 2017). If a HAPI reaches Stage 3 (full-thickness skin loss) or Stage 4 (full-thickness skin loss and tissue loss), it is a “never event” that is reported to Centers for Medicare and Medicaid Services and that results in subsequent limited reimbursement (Levinson, 2010). Most importantly, HAPIs can be associated with a longer hospital stay, pain, infection, and even death (Lyder et al., 2012).
HAPI rates have decreased across the United States; national HAPI rates fell from 40.3 to 30.9 per 1,000 discharges between 2010 and 2014 after Centers for Medicare and Medicaid Services and Agency for Healthcare Research and Quality (2016) efforts. For 210 U.S. academic medical centers, HAPI Stage 3 and 4 incidence rates decreased from 11.8 cases per 1,000 patients in 2008 to 0.8 cases per 1,000 patients in 2012 (Padula et al., 2015). These decreases in HAPI rates reflect concerted efforts by healthcare organizations and support the use of current risk assessment and preventive efforts. However, despite advances in prevention and treatment, HAPIs are not eliminated, driving further examination in both nursing and health services research.
The purpose of this study was to describe HAPI incidence, risk factors, and risk-adjusted variation in HAPI incidence within a contemporary 35-hospital inpatient cohort. The setting for our work was Kaiser Permanente (KP), an integrated healthcare delivery system, serving approximately 11.3 million members across the United States. The 35 hospitals in this study are within the state of California, which includes approximately 8 million of the aforementioned KP members.
The conceptual underpinnings for this study were based on seminal work on the etiology, and thus factors, of risk for pressure injury: immobility, decreased activity, change in sensation, and tissue tolerance (Bergstrom, Braden, Laguzza, & Holman, 1987). Tissue tolerance includes extrinsic factors (moisture, sheer) and intrinsic factors such as age, perfusion, and nutrition. Domains from this model influenced the composition of the Braden Scale for Predicting Pressure Ulcer Risk (Bergstrom, Braden, Kemp, Champagne, & Ruby, 1998; Bergstrom et al., 1987). The model was later expanded to include biomechanical, physiological, and epidemiological evidence in addition to expert consensus for additional factors such as acute illness and chronic conditions such as diabetes (Coleman et al., 2014).
Our work complements and expands on previous work by taking advantage of granular electronic medical record (EMR) data, a common medical record number system, and standardized nursing documentation. In addition to including previously cited HAPI risk factors, we incorporated data elements that have not been previously available in a cohort of this size that explored HAPI incidence, risk, and variation: longitudinal comorbidity burden scores, severity-of-illness scores, and a tool unique to nursing, the Braden Scale for Predicting Pressure Ulcer Risk (Bergstrom et al., 1998, 1987). Last, we addressed the problem of censoring explicitly; this is an important consideration given that HAPI risk factors are also associated with mortality (Lyder et al., 2012).
METHODS
Data Source
Under a mutual exclusivity agreement, physicians of The Permanente Medical Group, Inc., and The Southern California Permanente Medical Group care for patients insured by Kaiser Foundation Health Plan, Inc., at facilities owned by Kaiser Foundation Hospitals (KFH), Inc. Inpatient registered nurses are employees of KFH. Deployment of the Epic EMR (www.epicsystems.com), known internally as KP HealthConnect, was started in 2006 and completed in all facilities by 2010. KP HealthConnect data were used for this study. This project was approved by the KP Northern California and Southern California Institutional Review Boards for the Protection of Human Subjects, which have jurisdiction over all the hospitals included in this report.
Episode Selection Criteria
Patient hospital episodes meeting the following inclusion criteria were eligible for this retrospective cohort study: (a) age ≥ 18 years at admission; (b) admission date from January 1, 2013, to June 30, 2015; (c) overnight inpatient hospitalization that occurred at one of 35 KP Northern or Southern California hospitals; and (d) hospitalization that was not for childbirth, psychiatric reasons, or rehabilitation. Patients admitted for observation were excluded. Hospitalizations that began at a non-KP hospital with subsequent transfer to one of the study sites were also excluded. However, hospitalizations that began at a KP hospital and experienced subsequent intersystem hospital transport were included, and we concatenated hospital stays into hospitalization episodes, as described previously (Escobar, Gardner, Greene, Draper, & Kipnis, 2013; Escobar et al., 2008).
Settings
The 35 community hospitals in this cohort ranged in size from 50 to 460 licensed beds, with seven hospitals having specialty services (e.g., cardiovascular and neurosurgery). Additional hospital details are provided (see Table, Supplemental Digital Content 1, https://www.hcup‐us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp). Registered nurses at all 35 hospitals were expected to implement similar HAPI preventive measures when patients had a Braden Scale score ≤ 18, signifying HAPI risk (Bergstrom et al., 1998). EMR fields for documentation were identical across sites. The expected standard of care included use of appropriate bed and chair surfaces such as mattress overlays, regular turning, repositioning, offloading pressure, and common clinical pathways for the management of nutrition and incontinence. Further description of this standard of care is in previous publications (Crawford, Corbett, & Zuniga, 2014; Omery et al., 2014).
Data Collection
HAPI
The dependent variable for the analyses was the time in days from admission to the development of a HAPI during an inpatient hospitalization. Observation continued until the first HAPI event was documented, the patient was transferred to a non-KFH hospital, or the patient died. Because HAPIs are quality indicators, an organizational system for continuous quality outcome tracking and performance improvement exists, and tracking of HAPIs is given careful attention. HAPIs are tracked within a risk management database that employs common definitions across all 35 study hospitals. When a HAPI is recorded in the database, dedicated staff (wound ostomy, continence or wound care certified nurses, and/or HAPI in-service frontline staff nurses) verify their presence and stage and whether the HAPI is related to the use of a medical device. Stage classifications for this cohort were based on NPUAP 2007 definitions (NPUAP, European Pressure Ulcer Advisory Panel, & Pan Pacific Pressure Injury Alliance, 2014). Patients may develop multiple HAPIs at different times within a hospitalization episode. Therefore, for the purposes of these analyses, only time to the first HAPI event was used.
Predictors and Risk Factors
Using previously described linkage methods and audit strategies (Escobar et al., 2013, 2011, 2008; Selby, 1997), the following independent variables, which are commonly employed in health services research, were captured: age, gender, admission dates/times, discharge dates/times, admission diagnoses, and present-on-admission comorbidities. We also linked the above data to the quality assurance database that tracks HAPIs. Three additional risk factor variables were used to measure pressure injury risk (the Braden Scale), acute severity of illness (Laboratory-Based Acute Physiology Score, Version 2 [LAPS2]), and a longitudinal comorbidity score (Comorbidity Point Score, Version 2 [COPS2]).
The Braden Scale (Bergstrom et al., 1998, 1987) is a part of the KP daily nursing assessment, and its EMR documentation is a practice expectation. The total Braden Scale score is composed of six subscales that reflect a patient’s state of sensation/ perception, moisture, activity, mobility, nutrition, and friction/ shear. A total Braden score can range from 6 to 23, with lower scores indicating a higher risk. Braden Scale scores may be entered multiple times during a hospitalization; the lowest total Braden score entered within the first 24 hours after hospital admission was used in the analysis.
Severity of illness at hospital admission was measured using the comprehensive LAPS2 (Escobar et al., 2013). This score is based on the most extreme laboratory results in the 72 hours preceding hospital entry time and employs vital signs, neurological status, pulse oximetry, and 16 laboratory tests. Higher scores indicate increasing physiologic derangement: The univariate 30-day mortality risk for the LAPS2 is 1.0% for scores < 60, 5.0% for scores between 60 and 109, and 13.7% for scores ≥ 110. The LAPS2 is now generated in real time in selected KP hospitals (Escobar et al., 2013, 2015, 2016).
The COPS2 is based on all diagnoses incurred by a patient in the preceding 12 months. The COPS2 is assigned monthly to all adults in KP in California. Higher scores indicate increasing mortality burden: The univariate 30-day mortality risk is 1.7% for scores < 40, 5.2% for scores of 40–64, and 9.0% for scores ≥ 65 (Escobar et al., 2013, 2015, 2016). For comparison, the Charlson Comorbidity Index, previously used in HAPI research for scoring the potential impact of multiple comorbidities (Gardiner, Reed, Bonner, Haggerty, & Hale, 2016; Omery et al., 2014), was also examined. In addition, presence of diabetes and stroke at admission were included.
Statistical Analysis
Unadjusted summary statistics were used to compare the characteristics of hospitalization episodes with and without HAPI. To account for nonindependent episodes, bootstrap sampling was used to calculate 95% confidence limits and assess statistical significance based on the t and χ2 statistics as appropriate (Good, 2005; Hesterberg, Monaghan, Moore, Clipson, & Epstein, 2003).
The primary study outcome—time to HAPI—is subject to censoring because of in-hospital death or transfer to a non-KFH hospital. The most commonly encountered form of a censored observation is right censoring, where an observation begins at the defined time but terminates before the outcome of interest is observed because occurrence of a competing outcome precluded continued observation. In this study, death and transfer to a non-KFH hospital were competing outcomes that created censoring the event of interest—time to HAPI. To calculate the most accurate estimates, we accounted for right censoring. If a patient died or was transferred, it is not possible to observe whether a HAPI developed or not. Moreover, the censoring is informative because, once a patient died or was transferred, the patient does not have the same future risk of HAPI as a noncensored hospitalization. Although standard survival analysis is commonly used to account for censoring, it assumes that the censoring is noninformative. In this study, we used a causespecific hazards model—a variation of the Cox proportional hazards regression—to measure the association of the independent variables and account for the competing risk nature of the outcome (Austin, Lee, & Fine, 2016). The cause-specific hazard regression represents the instantaneous rate of the outcome in patients who have not experienced a HAPI. Thus, a regression coefficient from this model can be interpreted as the effect of the predictor on a related degree of impact on the rate of a HAPI in patients who have not yet experienced one (Lau, Cole, & Gange, 2009).
Although the cause-specific Cox model can handle competing risks, it requires independent observations. However, the data for this study consists of nonindependent episodes (the outcomes for patients in the same hospital are more likely to be similar than those in a different hospital, and patients may experience multiple hospitalizations during the study period). We first fitted a cause-specific Cox proportional hazard fixed effects model, ignoring facility effects. We then fitted a mixed effects version of the model by adding facility as a random effect with a Gaussian distribution, a mean of 0, and a covariance matrix that is estimated from the data (Therneau & Grambsch, 2000). The facility effects were defined as the exponentiated estimates of the normal random effects from the model. Episodes were ascribed to the admitting facility (prognostic approach). Last, we evaluated the effect of having multiple episodes per patient by refitting the model to a data set containing one randomly selected episode per patient and comparing the results. The results described in this article are based on the full data set.
Model covariates were selected using a redundancy analysis approach (Harrell, 2015). A redundancy analysis is a rigorous approach to data reduction that involves removing predictors that are easily predicted from other predictors by using flexible parametric additive regression models. We evaluated possible nonlinear (splines and polynomial) effects and two-factor interactions by comparing the log likelihood of more complex models than a (linear) main-effects-only model. The proportional hazard assumption was evaluated via Schoenfeld residuals (Grambsch & Therneau, 1994). Performance of the mixed effects model based on the full data set was evaluated using the c-statistic and Cox and Snell pseudo R2 measures. In addition, the c-statistic, R2, and calibration of the fixed effects version of the model were evaluated using 1,000 bootstrap samples (Harrell, 2015).
RESULTS
Hospital Episode Characteristics
In this study, 466,609 patients who experienced 769,434 inpatient hospital stays at the 35 hospitals during the study period were identified. During the concatenation process, episodes where the initial hospital stay occurred at a non-KP hospital were excluded. The final study cohort consisted of 453,050 patients who experienced 728,266 inpatient episodes. Of these episodes, 6,331 involved interhospital transport to a nonsystem hospital. Table 1 summarizes key episode characteristics. There were 30.9% (n = 140,025) of patients who had more than one episode within the cohort period. Individual patient characteristics are available (see Table, Supplemental Digital Content 2, http://links.lww.com/NRES/A281).
TABLE 1.
Inpatient Episode Characteristics
| Characteristic | Mdn | M | (SD) |
|---|---|---|---|
| Age at admission | 67.0 | 65.4 | (17.3) |
| LOS (days) | 2.0 | 3.9 | (5.7) |
| LAPS2a (at admission) | 54.0 | 59.6 | (39.7) |
| COPS2b | 30.0 | 48.6 | (47.6) |
| Charlson Index (prior) | 4.0 | 5.0 | (4.4) |
| Braden score (lowest, first 24 hours) | 19.0 | 18.7 | (2.9) |
|
|
|||
| n | (%) | ||
|
|
|||
| Admission type | |||
| ED surgical | 71,125 | (9.8) | |
| Non-ED surgical | 163,066 | (22.4) | |
| ED medical | 437,642 | (60.1) | |
| Non-ED medical | 56,433 | (7.7) | |
| Mortality in-hospital (yes) | 20,120 | (2.8) | |
| Full code designation (yes) | 635,894 | (87.3) | |
| ICU admission during episode (yes) | 97,787 | (13.4) | |
| Diabetes history (yes) | 270,707 | (37.1) | |
| Stroke history (yes) | 89,505 | (12.2) | |
| Pressure injury history (yes) | 2,820 | (0.4) | |
Note. N = 728,266. COPS2 = Comorbidity Point Score, Version 2; ED = emergency department; ICU = intensive care unit; LAPS2 = Laboratory-Based Acute Physiology Score, Version 2; LOS = length of stay; Mdn = median.
LAPS2 measures acute physiologic instability during the 72 hours preceding admission; the higher the score, the greater the mortality risk due to acute physiologic derangement.
COPS2 is a longitudinal score based on 12 months of patient data; the higher the score, the greater the mortality risk due to comorbid illness.
HAPI Episode Characteristics
HAPI episode characteristics are summarized in Table 2. There were 1,661 HAPI episodes within this cohort, resulting in a rate of 0.57 (95% CI [0.019, 3.805]) per 1,000 patient days, 2.2 cases per 1,000 episodes, and 3.7 cases per 1,000 patients. The average length of stay until the first HAPI development was 11.4 days (median = 7.0 days, SD = 14.1 days). A visual display of the cumulative incidence graph for a HAPI over 30 days of hospitalization is seen in Figure 1. The two most common HAPI categories were Stage 2 (n = 697, 42.0%) and deep tissue injury (n = 626, 37.7%), with 20% classified as related to medical device use. Examples of medical devices associated with HAPI formation were bilevel noninvasive positive pressure breathing masks, endotracheal tubes, nasogastric tubes, and nasal cannula oxygen tubing. HAPI episodes resulting in Stages 3 and 4, deep tissue injuries, and unstageable HAPIs had the highest LAPS2 and COPS2 scores. However, Braden Scale scores remained low (approximately at 15) yet relatively stable across all stage categories (Tables 2 and 3).
TABLE 2.
Hospital-Acquired Pressure Injury Episode Characteristics
| Characteristic | Quantity | Value |
|---|---|---|
| Incidence/1,000 patient days | Estimate 95% CI |
0.57/1,000 patient days [0.019, 3.805] |
| Cases/1,000 episodes | Estimate | 2.2/1,000 episodes |
| Cases/1,000 patients | Estimate | 3.7/1,000 patients |
| Time to HAPI (days) | Mdn | 7.0 |
|
M (SD) |
11.4 (14.1) |
|
| HAPI, device related (yes) |
n (%) |
333 (20.0) |
Note. N = 1,661. Mdn = median.
FIGURE 1.
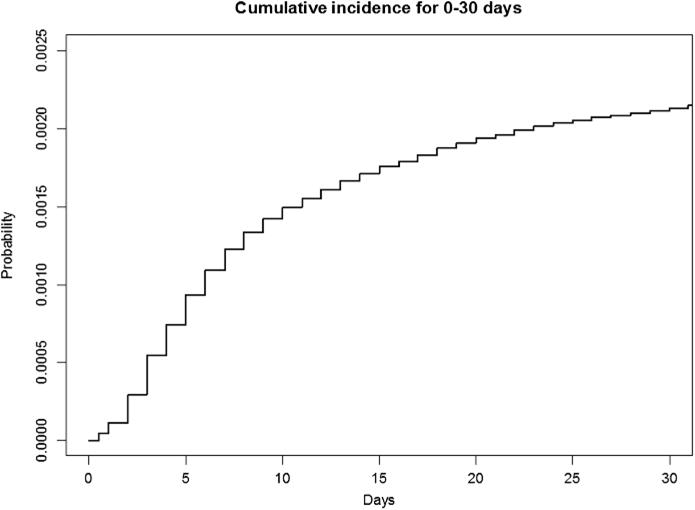
Cumulative incidence plot for hospital-acquired pressure injury hazard over 0–30 days of hospitalization.
TABLE 3.
Risk Factors by Hospital-Acquired Pressure Injury Stage
| Risk factor | Stage 1 (n = 164)
|
Stage 2 (n = 697)
|
Stage 3 or 4 (n = 45)
|
DTI (n = 626)
|
Unstageable (n = 129)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | |
| Braden scorea | 15.5 | (3.2) | 15.6 | (3.1) | 15.6 | (3.8) | 15.2 | (3.3) | 15.0 | (3.2) |
| LAPS2b | 88.9 | (50.1) | 91.3 | (48.6) | 105.5 | (54.2) | 94.3 | (50.7) | 100.3 | (53.7) |
| COPS2c | 76.2 | (58.5) | 79.1 | (56.6) | 82.9 | (46.8) | 78.6 | (58.2) | 79.4 | (59.4) |
Note. N = 1,661. HAPI stages are based on the 2007 National Pressure Ulcer Advisory Panel definitions. COPS2 = Comorbidity Point Score, Version 2; DTI = deep tissue injury; LAPS2 = Laboratory-Based Acute Physiology Score, Version 2.
The lowest score in the first 24 hours.
LAPS2 is a measure of acute physiologic instability during the 72 hours preceding admission; the higher the score, the greater the mortality risk due to acute physiologic derangement.
COPS2 is a longitudinal score based on 12 months of patient data; the higher the score, the greater the mortality risk due to comorbid illness.
HAPI Risk Factors
The average lowest total Braden Scale score within the first 24 hours was 18.7 (SD = 2.9) for the entire cohort of episodes, with the HAPI group average at 15.4. The average LAPS2 score was 93.3 for the HAPI group and 59.6 for the entire cohort, whereas the mean COPS2 score was 48.5 for the entire cohort and 78.8 for the HAPI group. Comparison of HAPI and nonHAPI episode risk factors is shown in Table 4.
TABLE 4.
Hospital-Acquired Pressure Injury: Multivariate Cox Proportional Hazards Model
| Risk factor | HAPI | M | (SD) | Q1 | Q3 | HR | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Age (years) | Yes | 71.3 | (13.9) | 63 | 82 | 1.36 | [1.25, 1.45] | <.001 |
| No | 65.4 | (17.3) | 55 | 79 | ||||
| COPS2 (score)a | Yes | 78.8 | (57.5) | 30 | 115 | 1.10 | [1.04, 1.16] | <.001 |
| No | 48.5 | (47.6) | 10 | 73 | ||||
| LAPS2 (score)b | Yes | 93.3 | (50.2) | 55 | 129 | 1.38 | [1.28, 1.50] | <.001 |
| No | 59.5 | (39.6) | 23 | 86 | ||||
| Braden Scale (score)c | Yes | 15.4 | (3.2) | 13 | 18 | 0.63 | [0.59, 0.67] | <.001 |
| No | 18.7 | (2.9) | 17 | 21 | ||||
|
|
||||||||
| n | (%) | |||||||
|
|
||||||||
| Full code (yes) | Yes | 1369 | (82.4) | 0.89 | [0.78, 1.03] | .11 | ||
| No | 634,525 | (87.3) | ||||||
| Diabetes (yes) | Yes | 840 | (50.6) | 1.09 | [0.98, 1.20] | .11 | ||
| No | 269,867 | (37.1) | ||||||
| Stroke (yes) | Yes | 366 | (22.0) | 1.03 | [0.90, 1.18] | .65 | ||
| No | 89,139 | (12.3) | ||||||
| Gender (female) | Yes | 723 | (43.5) | 0.77 | [0.70, 0.85] | <.001 | ||
| No | 387,785 | (53.4) | ||||||
| Admission type | ||||||||
| ED/surgical (yes) | Yes | 807 | (48.6) | 1.07 | [0.90, 1.29] | .44 | ||
| No | 436,835 | (60.1) | ||||||
| Non-ED/surgical (yes) | Yes | 480 | (28.9) | 1.00d | − | |||
| No | 70,645 | (9.7) | ||||||
| ED/medical (yes) | Yes | 132 | (7.9) | 0.62 | [0.52, 0.74] | <.001 | ||
| No | 56,301 | (7.7) | ||||||
| Non-ED/medical (yes) | Yes | 242 | (14.6) | 0.88 | [0.71, 1.10] | .27 | ||
| No | 162,824 | (22.4) | ||||||
Note. n = 1,661 (HAPI = yes); n = 726,605 (HAPI = no). Five thousand iterations of bootstrapped permutated tests were applied to all χ2 and t HAPI and non-HAPI values, resulting in p < .001 for univariate comparisons. Model c-statistic = 0.76, R2 = .02, calibrated slope = 0.99. Hazard ratio reflects the impact of the respective variable on the risk for HAPI over time when there is change from Q1 to Q3. COPS2 = Comorbidity Point Score, Version 2; ED = emergency department; HAPI = hospital-acquired pressure injury; HR = hazard ratio; LAPS2 = Laboratory-Based Acute Physiology Score, Version 2; Q1 = 25th percentile; Q3 = 75th percentile.
COPS2 is a longitudinal score based on 12 months of patient data; the higher the score, the greater the mortality risk due to comorbid illness.
LAPS2 measures acute physiologic instability during the 72 hours preceding admission; the higher the score, the greater the mortality risk due to acute physiologic derangement.
The lowest score in the first 24 hours after admission was used; lower scores represent a higher risk for pressure injury.
Reference group for admission type.
Prediction Model
The final model included age, gender, LAPS2, COPS2, lowest total Braden Scale score upon admission, diabetes and stroke indicators, admission category, care directive (full code or not), and admitting facility as random effects (Table 4; complete statistical results are available as Supplemental Digital Content 3, http://links.lww.com/NRES/A282). The causespecific Cox proportional hazards model had significant (p < .001) hazard ratios (HRs) for age (HR = 1.36, 95% CI [1.25, 1.45]), COPS2 (HR = 1.10, 95% CI [1.04, 1.16]), and LAPS2 (HR = 1.38, 95% CI [1.28, 1.50]). For continuous variables, model coefficients were multiplied by the difference between the 25th percentile/first quartile (Q1) score and the 75th percentile/third quartile (Q3) score. Therefore, HR results in Table 4 and Figure 2 reflect the impact of a continuous variable on the increased rate of HAPI over time when changed from Q1 (25th percentile) to Q3 (75th percentile; Harrell, 2015). Female gender (HR = 0.77, 95% CI [0.70, 0.85]), an emergency room admission for a medical reason (HR = 0.62, 95% CI [0.52, 0.74]), and higher Braden Scale scores (HR = 0.63, 95% CI [0.59, 0.67]) showed significant (p < .001) protective HRs (<1.0; see Hosmer, Lemeshow, & May, 2008). After risk adjustment, significant variation remained among the 35 hospitals.
FIGURE 2.
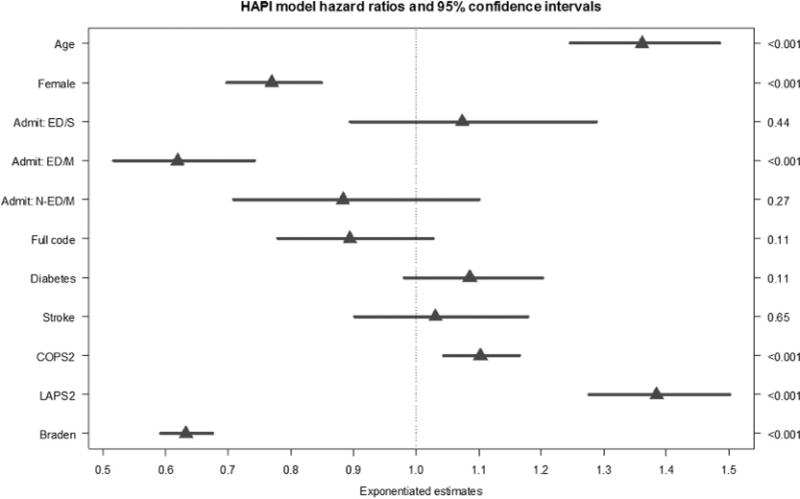
Hazard ratio graph with 95% confidence intervals based on the multivariate Cox proportional hazards model (Table 4). The admission category of non-ED/surgical (N-ED/S) is the reference group and is not displayed. Admit ED/S = admission from the emergency department and a surgery during the episode; Admit ED/M = admission from the emergency room for a medical reason and did not have surgery during the episode; Admit N-ED/M = admitted directly to the hospital (not through the emergency department) for a medical reason; Full code = designation as a full code for the episode; LAPS2 = the Laboratory-based Acute Physiology Score, Version 2 (This is a score measuring acute physiologic instability during the 72 hours preceding admission—the higher the score, the greater the mortality risk due to acute physiologic derangement.); COPS2 = Comorbidity Point Score, Version 2 (This is a longitudinal score based on 12 months of patient data—the higher the score, the greater the mortality risk due to comorbid illness.); Braden = the lowest Braden score in the first 24 hours since admission (lower Braden scores indicate a greater risk for pressure injury development).
The COPS2 and Charlson scores were highly correlated, and including the COPS2 showed slightly better performance. The final fixed effects model contained only linear main effects (c-statistic = 0.76, R2 = .02, calibration slope = 0.99). The main effects model was not substantially different from the more elaborate models. The facility random effect variance (0.50) was significant (likelihood ratio test, χ2 = 550.04, p < .001) and was kept in the mixed effects model. The mixed effects model had c-statistic = 0.78 and R2 = .04. The analysis based on the data set containing one randomly selected episode per patient yielded similar results. After controlling for all other variables in the random effects model, variation in HAPI risk across hospitals was significant (SD = 0.71, p < .001) indicating that the HAPI risk increases by >90% for hospitals with effects above 1 SD (Figure 3).
FIGURE 3.
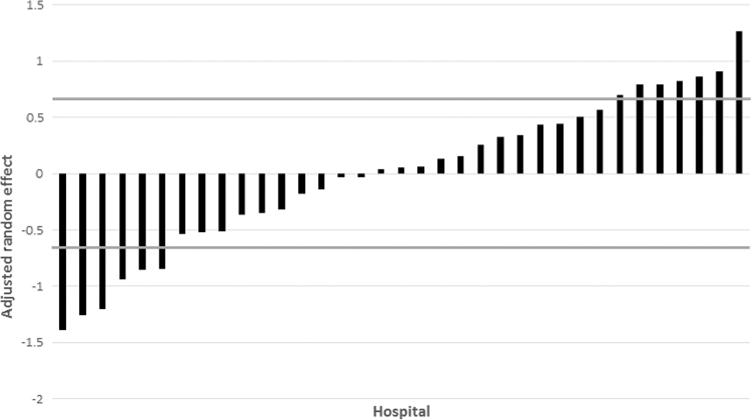
Risk-adjusted hospital effect for hospital-acquired pressure injury (HAPI). The x-axis shows individual hospitals, and the y-axis shows the risk-adjusted hospital random effect on the probability of experiencing a HAPI for each hospital (vertical bars). The horizontal lines denote 1 SD marks. The HAPI risk increases by >90% for hospitals with effects above 1 SD.
DISCUSSION
We have described risk-adjusted variation in HAPI incidence and hazard risk for HAPI over time within a 35-hospital inpatient cohort, resulting in one of the largest studies (both with the number of episodes and hospitals) to date in the era of comprehensive EMRs. Although comprehensive EMRs have become more common, the fact remains that many hospitals still cannot extract and format granular clinical data. Thus, our study is valuable because it includes multiple individual HAPI risk factors in conjunction with severity of illness and longitudinal comorbidity scores as well as HAPI risk-specific Braden Scale scores.
Another valuable aspect of this study was the utilization of a cause-specific Cox proportional hazard model. This model emphasizes the effect of the variable on a specific outcome through censoring competing events. Not censoring can lead to overestimation of cumulative incidence (Austin et al., 2016), and additional event data (such as death) are now included in the outcome. Although a cause-specific model is not best suited for estimation of individual risk (predicting a given outcome at a given time), it does promote etiology—where HRs can be used to estimate an effect size (Lau et al., 2009; Noordzij, Leffondré, van Stralen, Dekker, & Jager, 2013, p. 2).
The overall HAPI incidence rate (0.57 per 1,000 patient days, 3.7 cases per 1,000 patients, and 2.2 per 1,000 episodes) in the cohort was very low and is consistent with the previous 2014 report of 0.66 per 1,000 patient days for 21 Northern California KP hospitals (Crawford et al., 2014). The rate of 2.2 HAPI per 1,000 episodes is much lower than reported from the Agency for Healthcare Research and Quality. The HAPI rate of 3.7 per 1,000 patients is higher than the 0.8 rate per 1,000 patients reported by Padula et al. (2015); however, our study rate includes all HAPI stages—even Stage 1.
Despite the integrated nature of this hospital system and a low rate for HAPI, the analyses showed substantial residual interhospital variation in HAPI incidence. This variation is remarkable given the set of variables included in the model (e.g., severity of illness, comorbidities, age, Braden Scale score). Although Figure 2 shows that 63% of hospitals fell within 1 SD of the sample mean, six hospitals were below this boundary (i.e., showing a type of protective effect) and seven were above (i.e., suggesting a higher risk). This suggests that further research is still needed to understand between-hospital variation. For example, examination of variation in HAPI stages and types (device related or not), specific comorbidities, and hospital setting (intensive care unit vs. ward) could prove fruitful. Administrative characteristics such as staffing, staff skill mix, and competencies on HAPI prevention could be considered as part of future research and/or performance improvement. Such work could include examining why, given identical risk factors, some patients develop HAPIs in one hospital but not in another.
For this study, age and gender were significant in the model, with results aligning with previous studies. Increased age was associated with risk for HAPI in previous multivariate models (Alderden, Whitney, Taylor, & Zaratkiewicz, 2011; Gardiner et al., 2016; Lyder et al., 2012; Raju, Su, Patrician, Loan, & McCarthy, 2015). Three studies showed increased HAPI risk for men when compared with women (Coleman et al., 2013). In this study, being female had significantly less HAPI risk over time than being male, with an HR < 1.0 conceived as a protective ratio (Hosmer et al., 2008). Age and gender are immutable variables, yet knowledge of the association with HAPI assists clinicians in recognizing patients at risk within the first hours of admission.
Importantly, the Braden Scale—in use for 30 years— remains a predictor of HAPI risk even after controlling for multiple other factors. In this study, the lowest Braden Scale total score in the first 24 hours of admission—when changed from 13 (Q1) to 18 (Q3)—was a significant protective factor of HAPI over time. These results align with previous HAPI studies in which lower scores equate to a higher risk (Bergstrom et al., 1998; Coleman et al., 2013). Although only Braden Scale total scores were used in our study, other investigators have reported similar results with specific subscales; for example, immobility scores have been associated with HAPI (Cox, 2011; Raju et al., 2015; Slowikowski & Funk, 2010). In a 2006 systematic review and meta-analysis of 20 studies, Braden Scale scores showed sensitivity (57.1%) and specificity (67.5%) and a pooled risk for pressure ulcer development (OR = 4.08, 95% CI [2.56, 6.48]; Pancorbo-Hidalgo, Garcia-Fernandez, LopezMedina, & Alvarez-Nieto, 2006). The Braden Scale is an embedded part of nursing practice for many healthcare organizations in the United States, with total and subscale scoring directing preventive interventions. The Braden Scale captures multiple domains (such as activity, sensation, and mobility) that are not captured by other predictors in our model. Thus, additional research on the predictive capacity of Braden Scale subscales in large data sets may yield evidence for enhanced customization of preventive interventions.
The LAPS2 severity-of-illness score—in our study, change from 55 (Q1) to 129 (Q3)—was also a independently significant predictor. Therefore, a LAPS2 score of ≥60 to ≥110 delineates not only risk for 30-day mortality (Escobar et al., 2015, 2016) but also HAPI hazard risk over time. In a recent systematic review, laboratory values such as albumin (n = 7 studies) and hemoglobin (n = 6 studies), both of which are LAPS2 components, had strong associations with HAPI risk (Coleman et al., 2013). Hatanka et al. (2008) developed a laboratory data-based predictive model for HAPI risk that included significant results for albumin, hemoglobin, and C-reactive protein. Vital signs, which are included in the LAPS2, have also been associated with HAPI. These include low blood pressure (Bly, Schallom, Sona, & Klinkenberg, 2016), low blood pressure and use of vasopressors (Alderden et al., 2011; Bly et al., 2016; Cox, 2011), and high temperature (Nijs et al., 2009). Associations have also been shown using composite severity-of-illness scores (Manzano et al., 2010; Theaker, Mannan, Ives, & Soni, 2000). One notable example is the Acute Physiology and Chronic Health Evaluation II (Knaus, Draper, Wagner, & Zimmerman, 1985). However, the Acute Physiology and Chronic Health Evaluation II score has been calibrated for only patients in the intensive care unit. In contrast, the LAPS2 is calibrated for all hospitalizations and captures the combined effects of 16 laboratory test results, vital signs, neurological status, and pulse oximetry.
Preexisting comorbidities must be considered (Schneeweiss & Maclure, 2000). For this study, a COPS2 score change from 30 (Q1) to 115 (Q3) was associated with an increased risk for HAPI over time. Just as with LAPS2, COPS2 scores in this range not only delineate risk for 30-day mortality as scores increase (Escobar et al., 2015, 2016) but also appear to now be associated with HAPI risk over time. An example of a comorbidity index employed in past HAPI research is the Charlson Comorbidity Index. One recent large sample cohort study covering 15 hospitals did show the combination of age, body mass index, and Charlson Comorbidity Index as associated with HAPI risk (Gardiner et al., 2016). In our work, the COPS2, which correlates well with the Charlson Comorbidity Index, performed better. The mean absolute error (observed outcome − predicted outcome) for the model with the COPS2 and the Charlson Comorbidity Index was 0.089 and 0.104, respectively. There were smaller prediction errors when using the COPS2. In addition, the COPS2 had several other advantages in this study: It is based on 12 months of data, it is more granular, and it is now generated monthly on all KP adults in California.
An unexpected finding in this study was that nonsurgical admissions through the emergency department had significantly less HAPI risk over time when compared with other admission categories. This category includes example diagnoses of pneumonia, chest pain, congestive heart failure, and unspecified septicemia. We can speculate that surgical patients may have a higher risk because of immobility before and after surgery or from the length of time in the operating room for the surgical procedure. Further research is needed.
For the application of study findings at the clinical level, results could lead to EMR-based systems that prompt healthcare providers to consider the combined effects of age, type of admission, severity-of-illness scores (LAPS2), and comorbidity burden (COPS2)—in addition to low Braden Scale scores at the time of admission—to identify patients at a high HAPI risk. Many nurses understand the nursing care expectations related to different Braden Scale scores; however, scores such as LAPS2 and COPS2 may be new and not widely used by nurses. Future nursing education could include understanding LAPS2 and COPS2 risk scores and predictive models.
One important implication of our work is that our approach could be extended to other areas. For example, it would be possible to examine the association of LAPS2 and COPS2 with other nursing quality indicators. Another recommendation is to explore the predictive capability of the Braden Scale total score and subscales with respect to outcomes such as mortality and length of stay. Collaborative studies regarding careful examination of the process of care at hospitals with high and low adjusted HAPI rates could yield new insights.
Limitations
There are limitations to the analyses. Patients in this cohort received their care within the same healthcare system, which has a high degree of integration as well as a relatively low baseline HAPI incidence. To be used in a different setting, the model would need recalibration. Although it could be extrapolated from the low HAPI incidence that preventive care was provided, the authors were not able to capture the exact quality of implemented HAPI preventive nursing efforts across our study hospitals. It was also not possible to obtain HAPI location or, for device-related injuries, the percentages for specific types of medical devices.
This study also has some limitations related to specific data elements. Because the COPS2 includes diabetes, this resulted in a nonsignificant HR for a predictor known to be strongly associated with HAPI (Lyder et al., 2012; Slowikowski & Funk, 2010). It was also not possible to obtain data on other known risk factors, such as body mass index (Gardiner et al., 2016; Lyder et al., 2012) and the individual contribution of chronic renal failure (Nijs et al., 2009).
Conclusions
Even within an integrated system with low HAPI incidence, significant residual variation remained across 35 hospitals. Future work will need to include examination of hospital level, administrative, and staffing variables, in addition to quantifying the degree to which evidence-based interventions impact variation in HAPI incidence. Nursing researchers and clinicians are encouraged to explore large data sets, predictive analytics, and the potential of EMR-generated composite scoring. For such future work, we have indicated that inclusion of three quantitative tools—Braden Scale scores, a severity-of-illness score, and a comorbidity burden score—will need to play a continuing and critical role.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Helena Gurascier and Rebecca Gambatese, both senior analysts from the Utility for Care Data Analysis Department, Kaiser Permanente, for providing data coding. They would also like to acknowledge Regina M. Valdez, senior research analyst, and Marla Gardner, project manager, who both assisted with project management. Last, the authors would like to acknowledge Jason Jones, Vice President for Information Support for Care Transformation, Kaiser Permanente, for his consultations on predictive analytics.
The research was supported by funding from the Sidney Garfield Memorial Fund, Kaiser Permanente.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.nursingresearchonline.com).
The authors have no conflicts of interest to disclose.
Contributor Information
June Rondinelli, Nurse Scientist and Interim Director, Regional Nursing Research Program, Kaiser Permanente, Southern California, Pasadena.
Stephen Zuniga, Biostatistician II, Clinical Intelligence and Decision Support, Kaiser Foundation Hospitals, Pasadena, California.
Patricia Kipnis, Principal Statistician/Associate Director, Decision Support, Kaiser Foundation Hospitals, Oakland, California.
Lina Najib Kawar, Nurse Scientist, Regional Nursing Research Program, Kaiser Permanente, Southern California, Pasadena.
Vincent Liu, Research Scientist, Division of Research, Kaiser Permanente, Oakland, California.
Gabriel J. Escobar, Research Scientist III, Regional Director for Hospital Operations Research, Division of Research, Kaiser Permanente, Oakland, California.
References
- Agency for Healthcare Research and Quality. 2015 National healthcare quality and disparities report and 5th anniversary update on the national quality strategy. Rockville, MD: Author; 2016. (AHRQ Publication No. 16-0015). Retrieved from https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/nhqdr15/2015nhqdr.pdf. [Google Scholar]
- Alderden J, Whitney JD, Taylor SM, Zaratkiewicz S. Risk profile characteristics associated with outcomes of hospitalacquired pressure ulcers: A retrospective review. Critical Care Nurse. 2011;31:30–43. doi: 10.4037/ccn2011806. [DOI] [PubMed] [Google Scholar]
- Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/circulationaha.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharestani MM, Black JM, Carville K, Clark M, Cuddigan JE, Dealey C, Sanada H. Dilemmas in measuring and using pressure ulcer prevalence and incidence: An international consensus. International Wound Journal. 2009;6:97–104. doi: 10.1111/j.1742-481X.2009.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist-Beringer SD, Davidson J, Cuddigan J. Module IV: Community vs. hospital/unit acquired pressure ulcers. 2017 Retrieved from https://members.nursingquality.org/NDNQIPressureUlcerTraining/
- Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: A multisite study of the predictive validity of the Braden Scale. Nursing Research. 1998;47:261–269. doi: 10.1097/00006199-199809000-00005. [DOI] [PubMed] [Google Scholar]
- Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nursing Research. 1987;36:205–210. [PubMed] [Google Scholar]
- Bly D, Schallom M, Sona C, Klinkenberg D. A model of pressure, oxygenation, and perfusion risk factors for pressure ulcers in the intensive care unit. American Journal of Critical Care. 2016;25:156–164. doi: 10.4037/ajcc2016840. [DOI] [PubMed] [Google Scholar]
- Coleman S, Gorecki C, Nelson EA, Closs SJ, Defloor T, Halfens R, Nixon J. Patient risk factors for pressure ulcer development: Systematic review. International Journal of Nursing Studies. 2013;50:974–1003. doi: 10.1016/j.ijnurstu.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, Nelson EA. A new pressure ulcer conceptual framework. Journal of Advanced Nursing. 2014;70:2222–2234. doi: 10.1111/jan.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. Predictors of pressure ulcers in adult critical care patients. American Journal of Critical Care. 2011;20:364–375. doi: 10.4037/ajcc2011934. [DOI] [PubMed] [Google Scholar]
- Crawford B, Corbett N, Zuniga A. Reducing hospitalacquired pressure ulcers: A quality improvement project across 21 hospitals. Journal of Nursing Care Quality. 2014;29:303–310. doi: 10.1097/ncq.0000000000000060. [DOI] [PubMed] [Google Scholar]
- Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Medical Care. 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: Contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS) Journal of Hospital Medicine. 2011;6:74–80. doi: 10.1002/jhm.817. [DOI] [PubMed] [Google Scholar]
- Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Medical Care. 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- Escobar GJ, Ragins A, Scheirer P, Liu V, Robles J, Kipnis P. Nonelective rehospitalizations and postdischarge mortality: Predictive models suitable for use in real time. Medical Care. 2015;53:916–923. doi: 10.1097/mlr.0000000000000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar GJ, Turk BJ, Ragins A, Ha J, Hoberman B, LeVine SM, Kipnis P. Piloting electronic medical record-based early detection of inpatient deterioration in community hospitals. Journal of Hospital Medicine. 2016;11(Suppl. 1):S18–S24. doi: 10.1002/jhm.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JC, Reed PL, Bonner JD, Haggerty DK, Hale DG. Incidence of hospital-acquired pressure ulcers—A population-based cohort study. International Wound Journal. 2016;13:809–820. doi: 10.1111/iwj.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good PI. Permutation, parametric and bootstrap tests of hypotheses. 3rd. New York, NY: Springer; 2005. [Google Scholar]
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biomedtrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- Harrell FE., Jr . Regression modeling strategies With applications to linear models, logistic and ordinal regression, and survival analysis. 2nd. New York, NY: Springer; 2015. [Google Scholar]
- Hatanka N, Yamamoto Y, Ichihara K, Mastuo S, Nakamura Y, Watanabe M, Iwatani Y. A new predictive indicator for development of pressure ulcers in bedridden patients based on common laboratory tests results. Journal of Clinical Pathology. 2008;61:514–518. doi: 10.1136/jcp.2007.050195. [DOI] [PubMed] [Google Scholar]
- Hesterberg T, Monaghan S, Moore DS, Clipson A, Epstein R. Bookstrap methods and permutation tests: Companion chapter 18 to The Practice of Business Statistics. New York, NY: W. H. Freeman; 2003. [Google Scholar]
- Hosmer DW, Lemeshow S, May S. Applied survival analysis: Regression modeling of time-to-event data. 2nd. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Critical Care Medicine. 1985;13:818–829. [PubMed] [Google Scholar]
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. American Journal of Epidemiology. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DR. (Publication OEI-06-09-00090).Adverse events in hospitals: National incidence among Medicare beneficiaries. 2010 Retrieved from https://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf.
- Lyder CH, Wang Y, Metersky M, Curry M, Kliman R, Verzier NR, Hunt DR. Hospital-acquired pressure ulcers: Results from the National Medicare Patient Safety Monitoring System Study. Journal of the American Geriatrics Society. 2012;60:1603–1608. doi: 10.1111/j.1532-5415.2012.04106.x. [DOI] [PubMed] [Google Scholar]
- Manzano F, Navarro MJ, Roldán D, Moral MA, Leyva I, Guerrero C, Granada GPP Group Pressure ulcer incidence and risk factors in ventilated intensive care patients. Journal of Critical Care. 2010;25:469–476. doi: 10.1016/j.jcrc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: Clinical practice guideline. 2nd. Osborne Park, Australia: Cambridge Media; 2014. [Google Scholar]
- Nijs N, Toppets A, Defloor T, Bernaerts K, Milisen K, Van Den Berghe G. Incidence and risk factors for pressure ulcers in the intensive care unit. Journal of Clinical Nursing. 2009;18:1258–1266. doi: 10.1111/j.1365-2702.2008.02554.x. [DOI] [PubMed] [Google Scholar]
- Noordzij M, Leffondré K, van Stralen KJ, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrology, Dialysis, Transplantation. 2013;28:2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- Omery A, Mussell D, Rondinelli J, Ecker M, Baker J, Shanks H, Kleinhelter P. Under pressure: Nursing interventions help prevent HAPUs. Nursing Management. 2014;45:36–43. doi: 10.1097/01.numa.0000444876.62569.51. [DOI] [PubMed] [Google Scholar]
- Padula WV, Makic MB, Wald HL, Campbell JD, Nair KV, Mishra MK, Valuck RJ. Hospital-acquired pressure ulcers at academic medical centers in the United States, 2008–2012: Tracking changes since the CMS nonpayment policy. Joint Commission Journal on Quality and Patient Safety. 2015;41:257–263. doi: 10.1016/S1553-7250(15)41035-9. [DOI] [PubMed] [Google Scholar]
- Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Lopez-Medina IM, Alvarez-Nieto C. Risk assessment scales for pressure ulcer prevention: A systematic review. Journal of Advanced Nursing. 2006;54:94–110. doi: 10.1111/j.1365-2648.2006.03794.x. [DOI] [PubMed] [Google Scholar]
- Raju D, Su X, Patrician PA, Loan LA, McCarthy MS. Exploring factors associated with pressure ulcers: A data mining approach. International Journal of Nursing Studies. 2015;52:102–111. doi: 10.1016/j.ijnurstu.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Maclure M. Use of comorbidity scores for control of confounding in studies using administrative databases. International Journal of Epidemiology. 2000;29:891–898. doi: 10.1093/ije/29.5.891. [DOI] [PubMed] [Google Scholar]
- Selby JV. Linking automated databases for research in managed care settings. Annals of Internal Medicine. 1997;127:719–724. doi: 10.7326/0003-4819-127-8_Part_2-199710151-00056. [DOI] [PubMed] [Google Scholar]
- Slowikowski GC, Funk M. Factors associated with pressure ulcers in patients in a surgical intensive care unit. Journal of Wound, Ostomy, and Continence Nursing. 2010;37:619–626. doi: 10.1097/WON.0b013e3181f90a34. [DOI] [PubMed] [Google Scholar]
- Theaker C, Mannan M, Ives N, Soni N. Risk factors for pressure sores in the critically ill. Anaesthesia. 2000;55:221–224. doi: 10.1046/j.1365-2044.2000.01216.x. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox model. New York, NY: Springer; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


