Abstract
Objective
To appraise the currency, completeness and quality of evidence from systematic reviews (SRs) of acute management of moderate to severe traumatic brain injury (TBI).
Methods
We conducted comprehensive searches to March 2016 for published, English-language SRs and RCTs of acute management of moderate to severe TBI. Systematic reviews and RCTs were grouped under 12 broad intervention categories. For each review, we mapped the included and non-included RCTs, noting the reasons why RCTs were omitted. An SR was judged as ‘current’ when it included the most recently published RCT we found on their topic, and ‘complete’ when it included every RCT we found that met its inclusion criteria, taking account of when the review was conducted. Quality was assessed using the AMSTAR checklist (trichotomised into low, moderate and high quality).
Findings
We included 85 SRs and 213 RCTs examining the effectiveness of treatments for acute management of moderate to severe TBI. The most frequently reviewed interventions were hypothermia (n = 17, 14.2%), hypertonic saline and/or mannitol (n = 9, 7.5%) and surgery (n = 8, 6.7%). Of the 80 single-intervention SRs, approximately half (n = 44, 55%) were judged as current and two-thirds (n = 52, 65.0%) as complete. When considering only the most recently published review on each intervention (n = 25), currency increased to 72.0% (n = 18). Less than half of the 85 SRs were judged as high quality (n = 38, 44.7%), and nearly 20% were low quality (n = 16, 18.8%). Only 16 (20.0%) of the single-intervention reviews (and none of the five multi-intervention reviews) were judged as current, complete and high-quality. These included reviews of red blood cell transfusion, hypothermia, management guided by intracranial pressure, pharmacological agents (various) and prehospital intubation. Over three-quarters (n = 167, 78.4%) of the 213 RCTs were included in one or more SR. Of the remainder, 17 (8.0%) RCTs post-dated or were out of scope of existing SRs, and 29 (13.6%) were on interventions that have not been assessed in SRs.
Conclusion
A substantial number of SRs in acute management of moderate to severe TBI lack currency, completeness and quality. We have identified both potential evidence gaps and also substantial research waste. Novel review methods, such as Living Systematic Reviews, may ameliorate these shortcomings and enhance utility and reliability of the evidence underpinning clinical care.
Introduction
Systematic reviews, as rigorous and replicable summaries of the existing research, have long been considered a cornerstone of evidence based medicine [1, 2]. Systematic reviews inform clinical care by underpinning clinical practice guidelines [3, 4] and guide future research by summarising what is known and highlighting what is unknown on a topic [5].
As part of growing interest in increasing value and reducing waste in research [6, 7], there have been renewed calls for well-conducted systematic reviews to underpin all proposals for new primary research [8]. Yet, making sense of what is likely to be numerous evidence syntheses on a specific topic is increasingly challenging [9]. Systematic reviews are growing exponentially: current estimates suggest that over 8,000 systematic reviews are published annually [10]. A further complication is that many systematic reviews are poorly conducted and reported [10], with unnecessary duplication of topics, and conflicting or misleading results common [9, 11].
The need for well-conducted and up-to-date evidence syntheses to inform clinical care and future research is particularly pertinent within the context of traumatic brain injury (TBI). TBI is a global health concern [12], with often devastating and ongoing physical and cognitive impairments, and substantial financial and social costs to individuals, families and communities [13]. In the area of acute management of moderate to severe TBI, approximately 200 randomised controlled trials (RCTs) have been conducted, exploring a myriad of pharmacological, surgical and other treatments [14]. To date, however, TBI trials have largely shown disappointing results, with relatively few interventions underpinned by convincing evidence to support their use [14–16]. Strategic TBI research planning is therefore critical, such that research resources can be directed to areas of need and duplication of effort is avoided.
Broad overviews of the existing and emerging evidence can facilitate such planning [5]. Previous authors have conducted overviews of a select systematic reviews of acute management of moderate to severe TBI (Cochrane Reviews only [17]; pharmacological treatments only [18]). Others have reviewed the findings, quality and reporting of RCTs in this area [14, 19, 20]. Bragge et al [21] mapped the primary and secondary research in TBI against stakeholder-prioritised research questions. To our knowledge, however, no-one has comprehensively examined systematic reviews across the entire field of acute management of moderate to severe TBI to determine their trustworthiness to inform clinical care and research.
As such, the aim of this research was to appraise the currency, completeness and quality of evidence from systematic reviews of acute management of moderate to severe TBI.
Methods
We applied an evidence mapping approach to primary and secondary research for the acute management of moderate to severe TBI. Evidence maps describe the quantity, design and characteristics of research in broad topic areas; providing a snapshot of what it is known and where evidence is lacking [21, 22]. Given the absence of reporting checklists for evidence maps [22], we followed the applicable sections of the PRISMA checklist for reporting systematic reviews [23].
Eligibility criteria
We included published, English-language systematic reviews and RCTs of interventions for the acute management of moderate to severe TBI across all participant age groups.
We used the PRISMA definition of a systematic review [23], applying the following minimum standards for inclusion: explicit inclusion criteria and search strategy reported, and provided a complete account of their included studies. Overviews of reviews were excluded as these are redundant within this project; narrative reviews were excluded, as were systematic reviews that did not seek to include RCTs (as stated in their inclusion criteria). Where a systematic review had been updated (for example, a Cochrane Review), we included the most recent version only.
We used the Cochrane definition of an RCT, in that participants were definitely or probably assigned prospectively to one of two (or more) groups using random allocation [24]. We excluded quasi-random RCTs (whereby the method of allocation was not truly random, such as day of the week).
Moderate to severe TBI was defined as a Glasgow Coma Scale (GCS) score ≤ 12, however we did not exclude reviews and RCTs if they only referred to moderate to severe TBI without providing a GCS-based definition. Acute management was defined as any intervention delivered in the pre-hospital or acute setting. Interventions delivered in the rehabilitation setting were excluded. Where systematic reviews included mixed populations (i.e. mild TBI or non-TBI, such as stroke) we included the review, but excluded these specific RCTs from our analysis. RCTs with mixed populations were excluded.
Searching
Initial searches were conducted in March 2015, with update searches in March 2016. For the 2015 search, we utilised an existing neurotrauma evidence synthesis repository, the Neurotrauma EvidenceMap [25], previously managed by some members of the author team, to search for systematic reviews. Comprehensive searches of Medline, Embase, CINAHL Plus and the Cochrane Library underpin the repository, with two screeners independently deciding on included reviews [18]. To search for RCTs, we utilised an overview of RCTs by Bragge et al [14], given their comprehensive searching of Medline, All Evidence based Medicine Reviews (OVID), EMBASE and CENTRAL in March 2015. Further RCTs were identified from the included study lists of the included systematic reviews.
For the 2016 search, we searched for systematic reviews in Epistemonikos [26], an evidence synthesis repository that employs continual searches of 26 health databases. For RCTs, we searched Cochrane’s CENTRAL, a composite database of (predominantly) RCTs found in Medline, EMBASE, and hand searching of journals. For both sources, we tailored a search string with TBI keywords and MESH terms (as appropriate), searching for articles published between January 2015 to March 2016 (see S1 File).
Screening
For the 2015 search, given two independent screeners had already determined the inclusion of systematic reviews into the Neurotrauma Evidence Map repository, one reviewer (AS) downloaded into Microsoft Word the systematic review titles grouped together under management of TBI and screened them on title and then full-text to determine eligibility. Any uncertainties were discussed and resolved with another reviewer (PB, LP). Given we used identical eligibility criteria as Bragge et al [14] (and screening for that review was conducted by PB and AS) we did not rescreen their included RCTs, instead including them all directly as included RCTs in this paper (with the exception of any that were still ongoing).
For the 2016 yield, two reviewers (two of MC, SM, VV or ED) independently screened citations for both systematic review and RCT searches on title and abstract using the online software program, Covidence. One reviewer (ED) then screened citations on full-text, which were checked by a second reviewer (AS). Any uncertainties were resolved by discussion.
Data extraction
One reviewer (AS) extracted the following characteristics for each systematic review: (1) year of publication and year of search, (2) participants (adult or paediatric, eligibility criteria), (3) intervention(s) and comparisons (type, dose and dose regimen, if relevant), and (4) the list of included RCTs. The same reviewer extracted the following RCT characteristics: (1) year or publication, (2) participants (adult or paediatric), (3) intervention(s) and comparisons (type, dose and timing, if relevant), and (4) outcomes (if relevant to specific systematic review inclusion criteria).
Quality assessment
We assessed the methodological quality of systematic reviews using the 11-item AMSTAR checklist [27], a valid and reliable quality assessment tool [28]. Systematic reviews retrieved from Neurotrauma EvidenceMap [25] were independently assessed by two authors (LP, OC or AS). For the more recent systematic reviews found in Epistemonikos [26], one reviewer (AS) assessed quality with the AMSTAR tool. To facilitate comparisons between systematic reviews, we grouped AMSTAR scores into the following quality categories: low (0 to 3), moderate (4 to 7) and high (8 to 11), according to the categories used in a Cochrane overview of systematic reviews [29].
Mapping approach
The mapping process was performed by one author (AS) in Microsoft Excel (2007), and involved the following steps:
Systematic reviews and RCTs were grouped by topic into 12 intervention categories (and then further, into ‘like’ interventions), based on those used by Bragge et al [14] and in discussion with the clinical authors. The 12 intervention categories included, (1) Airway, ventilation and oxygenation strategies, (2) Fluid management, (3) Hypothermia, (4) Intracranial, cerebral and blood pressure management, (5) Nutrition and glucose management, (6) Pharmacological therapies not elsewhere defined, (7) Glutamate receptor antagonists, (8) Prehospital and systems of care, (9) Sedation, pain management, anaesthesia and arousal, (10) Seizure prophylaxis, (11) Corticosteroids, and (12) Surgery. Systematic reviews that included multiple interventions (‘multi-intervention’ reviews) were ‘split’, so that each intervention included in the review was considered within its appropriate intervention category.
Systematic reviews and RCTs within each of the 12 intervention categories were plotted against each other. This involved cataloguing which RCTs were included /not included in each of the systematic reviews. In some instances, the systematic reviews included RCTs that did not meet our inclusion criteria (e.g. non-English language, not truly random allocation, mixed population, duplicate publication referring to an already included study). In these instances those RCTs were removed from the analysis, and not counted as one of the included studies in that systematic review.
- The non-included trials were then classified by comparing the PICO (participants, intervention, comparison, outcomes) information of each RCT with the systematic review inclusion criteria. One of three classifications was assigned to each RCT:
- Post-date the review: The trial appeared to meet the systematic review inclusion criteria but was published during or after the year the review’s search was conducted.
- Out of Scope: The trial did not appear to meet the systematic review inclusion criteria (irrespective of when it was published).
- True Missing: The trial was missing from the review despite meeting the systematic review inclusion criteria and being published within the review search dates. Where it was not possible to definitively classify an RCT as ‘post-date’ or ‘out of scope’ due to lack of information reported, it was classified as ‘true missing’.
Where there was uncertainty regarding classification, it was discussed with another member of the author team (PB, OC, VV, CL or ED) until a decision was reached.
Assessment of currency, completeness and quality
This mapping process allowed us to assess the currency, completeness, and quality of each systematic review. These terms were defined in the following ways:
Currency: When the systematic review included the most recently published trials. A review was considered ‘current’ when it had no RCTs classified as ‘post-date the review’, and not ‘current’ when it had one or more RCTs classified as ‘post-date the review’.
Completeness: Whether the systematic review captured all known RCTs that met its inclusion criteria, relative to when it was conducted. A review was considered ‘complete’ when it had no RCTs classified as ‘true missing’, and ‘incomplete’ when it had one or more RCT classified as ‘true missing’.
Quality was defined as the methodological quality of the review, as measured by the AMSTAR checklist [27]. Reviews were classified as high (score of 8 to 11), moderate (score of 4 to 7) or low (score 0 to 3) quality using an approach that has previously been applied to an overview of systematic reviews [29].
Visual presentation of currency, completeness and quality
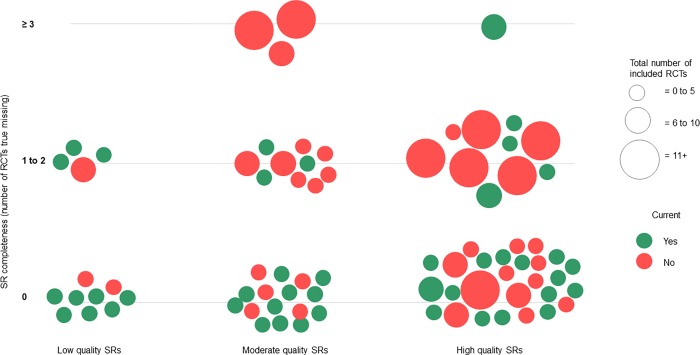
The findings relating to currency, completeness and quality of systematic reviews were presented visually, using the bubble plot format [22]. Bubble plots use four dimensions to display information: the size and colour of the bubble, and the x- and y- axes. In our bubble plot, each bubble represents a single systematic review. To facilitate ease of display, we grouped the data together in the following ways:
Size of the bubble: represents the number of included RCTs in the systematic review, from 0 to 5 (small), 6 to 10 (medium) and 11 or more (large).
Colour: represents currency, with current (green) or not current (red)
X-axis: represents systematic review quality, as low, moderate or high
Y-axis: represents completeness, with the number of RCTs defined as true missing grouped into three categories (0, 1 to 2 and 3 or more)
Results
Search results
We identified 67 systematic reviews from existing resources in the March 2015 search (see S1 Fig). In the 2016 update, we screened 1,092 systematic review citations on title and abstract, obtaining 91 of these in full-text. We included 19 systematic reviews, bringing the total number of included systematic reviews to 85. A list of key systematic reviews (meaning those a reader may reasonably expect to find in the review[30]) excluded on full-text is provided in S1 Table.
For RCTs, we included 194 RCTs in March 2015 from existing resources, and screened a further 672 citations on title and abstract in March 2016. Of these, 47 were screened in full-text and 19 RCTs were included to the original yield, bringing the total number of included RCTs to 213 (see S1 Fig).
Included systematic reviews and randomised controlled trials
We included 85 systematic reviews and 213 RCTs, examining the effectiveness of a range of interventions for the acute management of moderate to severe TBI. Eighty systematic reviews assessed a single intervention (‘single-intervention reviews’) and the remaining five reviews each assessed multiple interventions (‘multi-intervention reviews’ [19, 31, 32]). Given the five multi-intervention reviews were effectively split into 40 single-intervention reviews to facilitate the mapping process, we considered the currency and completeness of these reviews separately.
The interventions featured in the most systematic reviews included hypothermia (n = 17, 14.2%), hypertonic saline and/or mannitol (n = 9, 7.5%) and surgery (n = 8, 6.7%). Progesterone, monoaminergic agonists and nutrition (timing, delivery route and elements) were the topic of five (4.2%) systematic reviews each, with barbiturates, corticosteroids, antifibrinolytic agents, hyperventilation and hyperbaric hyperoxia each featuring in four (3.3%) systematic reviews.
Of the 85 systematic reviews, the majority (n = 56, 65.9%) included participants of any age, while approximately one third (n = 25, 29.4%) included only adults. Only four (4.7%) systematic reviews focussed solely on paediatric populations. Single-intervention reviews included a median of two RCTs (range 0 to 20 RCTs), whereas the multi-intervention reviews included a mean of 22 RCTs (range 3 to 47 RCTs).
Currency, completeness and quality across systematic reviews
Key systematic review characteristics and quality scores, the number of included RCTs, and the number of non-included RCTs (classified by reason for non-inclusion) for each systematic review are presented in Table 1. In the table, the reasons for non-inclusion of RCTs have been shortened to PD (post-dates the systematic review), S (out of scope) and T (true missing).
Table 1. Systematic review characteristics and quality, with number of included and non-included RCTs.
| Systematic review | Pop. | Intervention (vs comparison) | Search | RCTs | Qual. | Non-includ. RCTs | ||
|---|---|---|---|---|---|---|---|---|
| PD* | S^ | T# | ||||||
| 1. Airway, ventilation and oxygenation strategies | ||||||||
| Hyperbaric hyperoxia | ||||||||
| McDonough 2004[33] | All | Hyperbaric oxygen therapy** | 2003 | 2 | Mod | 3 | 1 | 1 |
| §Meyer 2010[31] | All | Hyperbaric oxygen therapy | 1980–2008 | 2 | Mod | 3 | 1 | 1 |
| §Lu 2012[19] | Adult | Hyperbaric oxygen therapy | 2011 | 2 | Low | 1 | 2 | 2 |
| Bennett 2012[34] | All | Hyperbaric oxygen therapy | 2012 | 5 | High | 1 | 1 | 0 |
| Hyperventilation | ||||||||
| §Roberts 1998[32] | All | Hyperventilation vs. normovent. | 1996 | 1 | Low | 0 | 0 | 0 |
| Roberts 1997[35] | All | Hyperventilation | 2008 | 1 | Mod | 0 | 0 | 0 |
| §Meyer 2010[31] | All | Hyperventilation | 1980–2008 | 1 | Mod | 0 | 0 | 0 |
| §Lu 2012[19] | Adult | Hyperventilation | 2011 | 1 | Low | 0 | 0 | 0 |
| Management guided by brain tissue oxygen | ||||||||
| Nangunoori 2012[36] | All | PbtO2-based vs ICP/CPP-based** | 1993–2010 | 0 | Low | 0 | 5 | 0 |
| Lazaridis 2014[37] | Adult | Monitoring (≥ 2: PbtO2, PRx, LPR)** | 2013 | 4 | Mod | 0 | 1 | 0 |
| 2. Fluid management | ||||||||
| Blood or blood product transfusion | ||||||||
| Nishijima 2012[38] | Adult | Platelet transfusion | 2011 | 0 | Mod | 0 | 3 | 0 |
| †Boutin 2015[39] | Adult | RBC transfusion | 2015 | 2 | High | 0 | 1 | 0 |
| 3. Hypothermia | ||||||||
| Hypothermia | ||||||||
| Harris 2002[40] | Adult | Hypothermia vs. normo.** | ?2001 | 7 | Mod | 14 | 11 | 4 |
| McIntyre 2003[41] | Adult | Hypothermia vs. normo.** | 2002 | 11 | High | 13 | 11 | 1 |
| Henderson 2003[42] | All | Hypothermia** | 2002 | 8 | Mod | 21 | 5 | 2 |
| Peterson 2008[43] | Adult | Hypothermia vs. SC | 2007 | 12 | Mod | 10 | 6 | 8 |
| †Saxena 2008[44] | All | Hypothermia min. 35◦ C | 2008 | 0 | High | 0 | 36 | 0 |
| Sydenham 2009[45] | All | Hypothermia max. 35◦ C | 2009 | 20 | High | 10 | 5 | 1 |
| §Meyer 2010[31] | All | Hypothermia | 1980–2008 | 9 | Mod | 12 | 5 | 10 |
| Fox 2010[46] | Adult | Early hypothermia vs normo.** | ?2008 | 11 | High | 5 | 18 | 2 |
| Sadaka 2012[47] | Adult | Hypothermia** | 2010 | 8 | Low | 7 | 19 | 2 |
| Georgiou 2013[48] | All | Systemic hypothermia** | 2011 | 17 | High | 5 | 13 | 1 |
| †Harris 2012[49] | Adult | Non-invasive head cooling | 2011 | 1 | High | 0 | 35 | 0 |
| §Lu 2012[19] | Adult | Hypothermia | 2011 | 8 | Low | 5 | 5 | 18 |
| Ma 2013[48] | Paed. | Hypothermia vs normo.** | ?2012 | 3 | Mod | 2 | 31 | 0 |
| Crossley 2014[50] | Adult | Hypothermia** | 2012 | 15 | High | 1 | 18 | 2 |
| Li 2014[51] | Adult | Moderate hypothermia | 2012 | 11 | Mod | 1 | 19 | 5 |
| Madden 2015[52] | Adult | Hypothermia** | 2009–2013 | 2 | Low | 1 | 33 | 0 |
| Zhang 2015[53] | Paed. | Hypothermia** | 2014 | 4 | Mod | 1 | 31 | 0 |
| 4. Intracranial, Cerebral Perfusion and Blood Pressure management | ||||||||
| Hypertonic saline and/or mannitol | ||||||||
| §Roberts 1998[32] | All | Mannitol vs. no mannitol | 1996 | 1 | Low | 0 | 17 | 0 |
| Banks 2008[54] | All | HTS | 2007 | 4 | Low | 0 | 14 | 0 |
| §Meyer 2010[31] | All | Mannitol, and/or HTS | 1980–2008 | 10 | Low | 6 | 0 | 2 |
| Wakai 2013[55] | All | Mannitol | 2009 | 4 | High | 2 | 12 | 0 |
| Kamel 2011[56] | All | Mannitol vs. HTS** | 2010 | 1 | Mod | 2 | 14 | 1 |
| §Lu 2012[19] | Adult | Mannitol, and/or HTS | 2011 | 5 | Low | 3 | 2 | 8 |
| Rickard 2014[57] | Adult | Mannitol vs. HTS** | ?2012 | 3 | Mod | 0 | 14 | 1 |
| Lourens 2014[58] | All | HTS vs. saline/Lactated Ringers** | 2011 | 3 | High | 1 | 13 | 1 |
| Li 2015[59] | Adult | Mannitol vs. HTS** | 2014 | 3 | Mod | 0 | 15 | 0 |
| Management guided by intracranial pressure | ||||||||
| Mendelson 2012[60] | Adult | ICP-directed therapy ** | 2011 | 0 | Mod | 1 | 1 | 0 |
| Sadaka 2013[61] | Adult | Placement of ICP monitors | 1993–2011 | 0 | Low | 0 | 2 | 0 |
| Su 2014[62] | All | ICP-directed therapy | 2013 | 1 | Mod | 0 | 1 | 0 |
| Yuan 2015[63] | Adult | ICP Monitoring** | 2013 | 1 | Mod | 0 | 1 | 0 |
| †Forsyth 2015[64] | All | ICP-directed therapy | 2015 | 1 | High | 0 | 1 | 0 |
| Cerebrospinal fluid drainage | ||||||||
| §Roberts 1998[32] | All | CSF drainage vs no drainage | 1996 | 0 | Low | 1 | 0 | 0 |
| §Meyer 2010[31] | All | CSF drainage | 1980–2008 | 1 | Mod | 0 | 0 | 0 |
| Posture | ||||||||
| Fan 2004[65] | All | Therapeutic body positioning** | 2003 | 1 | Low | 0 | 0 | 1 |
| §Meyer 2010[31] | All | Adjusting head posture | 1980–2008 | 2 | Mod | 0 | 0 | 0 |
| §Meyer 2010[31] | All | Body rotation | 1980–2008 | 0 | Mod | 0 | 2 | 0 |
| Pressure: other | ||||||||
| †Muzevic 2013[66] | All | The Lund concept | 2013 | 0 | High | 0 | 0 | 0 |
| 5. Nutrition and glucose management | ||||||||
| Nutrition: timing, delivery route and nutritional elements | ||||||||
| Krakau 2006[67] | Adult | Feeding timing, routes, content** | 1993–2003 | 8 | Mod | 8 | 5 | 1 |
| Perel 2006 [68] | All | Feeding timing & routes | 2006 | 7 | High | 2 | 13 | 0 |
| §Lu 2012[19] | Adult | Early nutritional support | 2011 | 3 | Low | 2 | 5 | 12 |
| Wang 2013[69] | All | Feeding timing, routes, elements** | 2012 | 10 | High | 0 | 8 | 4 |
| Wang 2015[70] | All | Sm. intestine vs gastric feeding** | 2013 | 3 | Mod | 0 | 18 | 1 |
| Nutrition: Insulin | ||||||||
| Lei 2012[71] | Adult | Tight vs. conv. glycaemic control | 2011 | 4 | Mod | 1 | 0 | 0 |
| §Lu 2012[19] | Adult | Insulin therapy | 2011 | 3 | Low | 1 | 1 | 0 |
| 6. Pharmacological therapies not elsewhere defined | ||||||||
| Progesterone | ||||||||
| §Meyer 2010[31] | All | Progesterone | 1980–2008 | 2 | Low | 5 | 0 | 0 |
| §Lu 2012[19] | Adult | Progesterone | 2011 | 2 | Low | 5 | 0 | 0 |
| Ma 2012[47] | All | Progesterone vs. placebo | 2012 | 2 | High | 5 | 0 | 0 |
| Wang 2015[72] | All | Progesterone** | 1980–2015 | 5 | High | 0 | 0 | 2 |
| †Zeng 2015[73] | All | Progesterone | 2015 | 6 | High | 0 | 1 | 0 |
| Bradykinin antagonists | ||||||||
| §Meyer 2010[31] | All | Bradykinin antagonists | 1980–2008 | 3 | Low | 1 | 0 | 0 |
| §Lu 2012[19] | Adult | Bradykinin antagonists | 2011 | 1 | Low | 0 | 1 | 2 |
| Calcium channel blockers | ||||||||
| Langham 2003[74] | All | Calcium channel blockers | 2005 | 4 | Mod | 0 | 0 | 0 |
| §Lu 2012[19] | Adult | Calcium channel blockers | 2011 | 3 | Low | 0 | 0 | 1 |
| Antifibrinolytic agents | ||||||||
| Perel 2010[75] | All | Haemostatic agents | 2009 | 2 | High | 2 | 0 | 0 |
| §Lu 2012[19] | Adult | Haemostatic agents | 2011 | 1 | Low | 1 | 0 | 2 |
| †Zehtabchi 2014[76] | All | Tranexamic acid | 2014 | 2 | High | 0 | 2 | 0 |
| †Ker 2015[77] | All | Antifibrinolytic agents | 2015 | 2 | High | 0 | 2 | 0 |
| Monoaminergic agonists | ||||||||
| Siddall 2005[78] | All | Methylphenidate | 2004 | 0 | Low | 0 | 0 | 0 |
| §Meyer 2010[31] | All | Dopamine targeting agents** | 1980–2008 | 0 | Mod | 0 | 0 | 0 |
| †Forsyth 2006[79] | All | Monoaminergic agonists | 2009 | 0 | High | 0 | 0 | 0 |
| Frenette 2012[80] | All | Dopamine agonists | 2010 | 0 | Mod | 0 | 0 | 0 |
| §Lu 2012[19] | Adult | Monoaminergic agonists | 2011 | 0 | Low | 0 | 0 | 0 |
| Aminosteroids | ||||||||
| †Roberts 1999[81] | All | Aminosteroid vs. Placebo | 2006 | 1 | High | 0 | 0 | 0 |
| §Lu 2012[19] | Adult | Tirilazad | 2011 | 1 | Low | 0 | 0 | 0 |
| Pharmacological therapies not elsewhere defined: various (single topic) | ||||||||
| §Meyer 2010[31] | All | Dimethyl sulphoxide | 1980–2008 | 0 | Low | 0 | 0 | 0 |
| §Lu 2012[19] | Adult | Pegogortein | 2011 | 1 | Low | 0 | 0 | 0 |
| †Alali 2014[82] | Adult | Beta-blockers | 2013 | 1 | High | 0 | 0 | 0 |
| Shen 2015[83] | All | Anticoagulants** | 2013 | 2 | Mod | 0 | 0 | 0 |
| †Zeiler 2014[84] | All | Tromethamine** | 2014 | 3 | High | 0 | 0 | 0 |
| Sanfilippo 2015[85] | Adult | Neuromuscular blocking agents | 2014 | 3 | Low | 0 | 0 | 0 |
| 7. Glutamate receptor antagonists | ||||||||
| Magnesium | ||||||||
| Arango 2008[86] | All | Magnesium vs. control | 2008 | 1 | High | 2 | 0 | 0 |
| Li 2015[87] | All | Magnesium** | 2013 | 3 | Mod | 0 | 0 | 0 |
| Glutamate receptor agonists: general | ||||||||
| Willis 2003[88] | All | EAAI vs. control** | 2002 | 2 | High | 5 | 0 | 0 |
| §Meyer 2010[31] | All | Cannabinoids | 1980–2008 | 2 | Low | 0 | 5 | 0 |
| §Lu 2012[19] | Adult | EAAI | 2011 | 4 | Low | 1 | 0 | 2 |
| 8. Prehospital and systems of care | ||||||||
| Prehospital intubation | ||||||||
| §Lu 2012[19] | Adult | Pre-hospital RSI | 2011 | 1 | Low | 0 | 0 | 0 |
| †Bossers 2015[89] | Adult | Prehospital intubation** | 2015 | 1 | High | 0 | 0 | 0 |
| Specialist versus general hospital transfer or care | ||||||||
| Pickering 2015[90] | All | Prehospital transfer strategies | 1998–2012 | 0 | Mod | 1 | 0 | 0 |
| †Fuller 2014[91] | Adult | Specialist neuroscience care | 2013 | 0 | High | 0 | 1 | 0 |
| 9. Sedation, Pain management, Anaesthesia and Arousal | ||||||||
| Sedative agents | ||||||||
| §Meyer 2010[31] | All | Opiods, propofol, midazolam | 1980–2008 | 3 | Mod | 2 | 1 | 6 |
| Roberts 2011[92] | All | Range of sedative agents | 2010 | 10 | High | 2 | 0 | 0 |
| Gu 2014[93] | All | Midazolam vs. propofol | 2013 | 2 | Mod | 0 | 10 | 0 |
| Ketamine | ||||||||
| Zeiler 2014[94] | All | Ketamine** | 2013 | 2 | High | 1 | 0 | 0 |
| Wang 2014[95] | All | Ketamine vs opiods** | 2014 | 2 | Mod | 0 | 0 | 1 |
| Cohen 2015[87] | Adult | Ketamine** | 2014 | 3 | Mod | 0 | 0 | 0 |
| Barbiturates | ||||||||
| §Roberts 1998[32] | All | Barbiturates vs. no barbiturates | 1996 | 2 | Low | 0 | 5 | 2 |
| §Meyer 2010[31] | All | Barbiturates | 1980–2008 | 3 | Low | 2 | 1 | 3 |
| §Lu 2012[19] | Adult | Barbiturates | 2011 | 2 | Low | 1 | 1 | 5 |
| Roberts 2012[96] | All | Barbiturates | 2012 | 6 | High | 0 | 1 | 2 |
| Stimulation | ||||||||
| §Meyer 2010[31] | All | Stimulation; sensory, electrical | 1980–2008 | 3 | Mod. | 1 | 0 | 0 |
| †Wong 2013[97] | All | Acupuncture | 2012 | 0 | High | 0 | 0 | 0 |
| Burst suppression | ||||||||
| Zeiler 2015[98] | All | Burst suppression** | 2015 | 1 | High | 0 | 18 | 2 |
| 10. Seizure prophylaxis | ||||||||
| Anti-epileptic agents | ||||||||
| Schierhout 1998[99] | All | Anti-epileptic agents | 1996 | 4 | Mod | 5 | 3 | 1 |
| Teasell 2007[100] | All | Any seizure interventions | 1980–2005 | 4 | Mod | 3 | 4 | 2 |
| Zafar 2012[101] | All | Phenytoin vs. levetiracetam | 2011 | 0 | High | 0 | 12 | 1 |
| Thompson 2015[102] | All | Anti-epileptic, neuroprot. agents** | 2015 | 8 | High | 1 | 4 | 0 |
| 11. Steroids | ||||||||
| Corticosteroids | ||||||||
| §Roberts 1998[32] | All | Corticosteroids vs. no corticost. | 1996 | 11 | Low | 2 | 3 | 3 |
| Alderson 2005[103] | All | Corticosteroids vs. control | 2008 | 16 | High | 1 | 2 | 0 |
| §Meyer 2010[31] | All | Corticosteroids | 1980–2008 | 7 | Low | 1 | 5 | 6 |
| §Lu 2012[19] | Adult | Corticosteroids | 2011 | 6 | Low | 1 | 0 | 12 |
| 12. Surgery | ||||||||
| Surgery: compared with no surgery and/or with different surgical techniques | ||||||||
| Sahuquillo 2006[104] | All | Decompressive craniectomy | 2008 | 1 | High | 4 | 5 | 0 |
| §Meyer 2010[31] | All | Decompressive craniectomy | 1980–2008 | 2 | Mod | 7 | 0 | 1 |
| Jacob 2011[105] | Paed. | Decompressive craniectomy** | 1997–2008 | 0 | Low | 0 | 9 | 1 |
| Guresir 2012[106] | Paed. | Decompressive craniectomy | 2010 | 0 | Low | 0 | 9 | 1 |
| Bor-Seng-Shu 2012[107] | All | Decompressive craniectomy** | 2010 | 1 | Low | 1 | 8 | 0 |
| §Lu 2012[19] | Adult | Decompressive craniectomy | 2011 | 3 | Low | 6 | 0 | 1 |
| Wang 2015[108] | All | Decompressive craniectomy** | 2015 | 3 | Mod | 2 | 4 | 1 |
| Surgery: timing of surgery (n = 10 RCTs in total) | ||||||||
| Kim 2014[109] | All | Time to surgery** | 1990–2013 | 2 | Low | 0 | 8 | 0 |
*Post-dates the systematic review: RCT published subsequent to the systematic review, but would otherwise have met the review inclusion criteria (score of 0 means the review is ‘current’)
^Out of scope: RCT did not meet the systematic review inclusion criteria irrespective of when it was published. RCTs that post-dated a review but would not have met the inclusion criteria were coded to this category.
#True Missing: RCT was published within review search dates and looks to have met the review inclusion criteria (score of 0 means the review is ‘complete’)
†Systematic review was judged to be current, complete and high quality
§Multi-intervention review (included more than one intervention type)
**Included one or more outcomes as inclusion criteria
Abbreviations: Conv. = conventional; Corticost. = corticosteroids; CPP = central perfusion pressure; CSF = cerebrospinal fluid; EAAI = excitatory amino acid inhibitors; Endotrach = endotracheal, HTS = hypertonic saline; ICP = intracranial pressure; Includ. = included, Mod. = moderate; Neuroprot = neuroprotective agents; Normo. = normothermia; Normovent. = normoventilation; Paed. = paediatric; PbtO2 = brain tissue oxygen; Pop. = population; PD = post-date the review; Qual. = quality; RBC = red blood cells, RCTs = randomised controlled trials; RSI = rapid sequence intubation; Rx = treatment; S = out of scope; Sm = small, T = true missing, Vs. = versus
Currency
Of the 80 single-intervention reviews, approximately half (n = 44, 55.0%) were judged as current, meaning they included the most recently published eligible RCTs (see Table 1; Fig 1). The remainder lacked currency, as there was one RCT (n = 13, 16.3%) two RCTs (n = 8, 10%) or 3 or more RCTs (n = 15, 18.8%) that were published subsequent to the review. When the most recently published systematic review in each intervention area (n = 25) was considered (owing to the inherent disadvantage in assessing currency for older systematic reviews) these numbers improved considerably, with nearly three-quarters of reviews (n = 18, 72.0%) found to be current. The majority (n = 5) of those found to be not current were missing one RCT only. For the five multi-intervention reviews currency was similar, with just under half found to be current (n = 17, 42.5%).
Fig 1. Currency, completeness, and quality of single-intervention systematic reviews.
Each bubble represents a single-intervention systematic review (n = 80). The ideal scenario is for bubbles to sit in the bottom right corner (denoting high quality and completeness), and be green in colour (denoting currency). Abbreviations: RCT = randomised controlled trial, SR = systematic review.
Completeness
Of the 80 single-intervention reviews, approximately two-thirds (n = 52, 65.0%) were judged as complete, meaning they included all published RCTs that met their inclusion criteria, relative to when their date of search (see Table 1; Fig 1). The remainder were missing one RCT (n = 16, 20.0%) or two or more RCTs (n = 11, 15.0%) that we judged should have been included. The five multi-intervention reviews fared more poorly on completeness, with half of the 40 individual interventions assessed found to be complete (n = 20, 50%).
Quality
Methodological quality of the systematic reviews was variable, with AMSTAR scores ranging from 0 to 10 out of 11 (see S2 Table). Of the 85 systematic reviews included, just under half (n = 38, 44.7%) were rated as high quality, with approximately one-third (n = 31, 36.5%) found to be moderate quality, with the remaining 16 (18.8%) judged as low quality (see Table 1; Fig 1). The five multi-intervention reviews were rated as low [19, 31, 32] or moderate quality [31].
The quality items in which the reviews scored best were the provision of comprehensive search details (n = 74, 87.1%), providing a detailed account of included studies (n = 81, 95.3%), assessing study quality (n = 69, 81.2%) and using appropriate methods for pooling studies (n = 69, 81.2%). Between half to one-third of systematic reviews reported using two independent reviewers (n = 53, 62.4%) or including unpublished studies (n = 45, 52.9%). Similar numbers of systematic reviews were found to have used their quality assessment ratings to interpret review findings (n = 58, 68.2%), or to have explicitly considered publication bias (n = 41, 48.2%). Only one-third of systematic review authors reported a study protocol (n = 29, 34.1%) and provided a full account of included and excluded studies (n = 30, 35.3%). No systematic review included both review-level and included study-level conflict of interest/funding information.
Combined currency, completeness and quality of systematic reviews
Across the 80 single-intervention reviews, 16 (20.0%) were judged as meeting all three criteria of being current, complete and high quality (see Table 1). Five of these reviews, on moderate hypothermia [44], the Lund concept [66], monoaminergic agonists [79], specialist neuroscience care [91], and acupuncture [97], did not contain any RCTs. They were either empty reviews, or none of their included studies met our definition of an RCT. No multi-intervention reviews were judged as being current, complete and high-quality.
As such, the following 11 interventions are underpinned by current, complete and high quality systematic review(s) that include one or more RCT: red blood cell transfusion [39], hypothermia [49], management guided by intracranial pressure [64], various pharmacological agents (progesterone [73], antifibrinolytic agents [76, 77], aminosteroids [81], beta-blockers [82], tromethamine [84]), and prehospital intubation [89].
Within the following intervention categories we found no systematic reviews including one or more RCT that are current, complete and high quality: airway, ventilation and oxygenation strategies; nutrition and glucose management; glutamate receptor antagonists; sedation, pain management, anaesthesia and arousal; seizure prophylaxis; corticosteroids; and surgery.
Randomised controlled trials not included in any systematic reviews
Of the 213 RCTs included, over three-quarters (n = 167, 78.4%) were included in one or more systematic review, leaving 46 RCTs (21.6%) that were not included in any systematic reviews (see Table 2). For approximately two thirds of these RCTs (n = 29, 63.0%), this was because there was no existing systematic review on that intervention topic. The remaining third of these RCTs, (n = 17, 37.0%) post-date the most recently published systematic review in that area, or they were found to be out of the scope of existing systematic reviews.
Table 2. Randomised controlled trials not included in any systematic review, with reasons.
| Reason | RCT intervention or topic* | RCTs (n =) |
|---|---|---|
| 1. Airway, ventilation and oxygenation strategies | ||
| No SR exists | Early trache.[110], temperature-corrected (pH-stat) blood gas-guided ventilatory Mx [111], normobaric hyperoxia[112] | 3 |
| SR exists^ | Hyperbaric oxygen therapy[113], Brain tissue oxygen guided Mx[114] | 2 |
| 2. Fluid management | ||
| No SR exists | Fresh frozen plasma[115] | 1 |
| SR exists^ | Nil | 0 |
| 3. Hypothermia | ||
| No SR exists | Normothermia (fever control)[116] | 1 |
| SR exists^ | Hypothermia x 6 [117–122] | 6 |
| 4. Intracranial, cerebral and blood pressure management | ||
| No SR exists | Vasopressin vs. catecholamines[123] | 1 |
| SR exists^ | CBF- vs. ICP-targeted Mx[124], hypertonic saline + dextran x 2[125, 126] | 3 |
| 5. Nutrition and glucose management | ||
| No SR exists | Nil | 0 |
| SR exists^ | Glycaemic control[127], vit. C[128], probiotics [129], jejunal vs. gastric feed[130], high protein feed[131] | 5 |
| 2. Pharmacological therapies not elsewhere defined | ||
| No SR exists | Erythropoetin x 3[132–134], Cyclosporine x 2[135, 136], Statins x 2 [137, 138], Prostacyclin[139, 140], Metoclompromide[141], Cerebrolysin[142] | 10 |
| SR exists^ | Anatibant (different doses) [143] | 1 |
| 3. Glutamate receptor antagonists | ||
| No SR exists | Nil | 0 |
| SR exists^ | Nil | 0 |
| 4. Prehospital and systems of care | ||
| No SR exists | Physician prehospital Mx[144] | 1 |
| SR exists^ | Bypass to neurosurg. centre [145] | 1 |
| 5. Sedation, pain management, anaesthesia and arousal | ||
| No SR exists | Nil | 0 |
| SR exists^ | Thiopental vs. propofol[146], phenobarbitol + phenytoin[147], auditory stim.[148] | 3 |
| 6. Seizure prophylaxis | ||
| No SR exists | Nil | 0 |
| SR exists^ | Lacosamide vs. fosphenytoin[149] | 1 |
| 7. Corticosteroids | ||
| No SR exists | Nil | 0 |
| SR exists^ | Hydrocortisone + fludrocortisone[150], dexamethasone[151] | 2 |
| 8. Surgery | ||
| No SR exists | Nil | 0 |
| SR exists^ | Early surgery[152], decomp. crani. x 2[153, 154], decomp. crani. plus cerebellar incision vs. decomp. crani.[155], min. invasive surgery [156] | 5 |
RCTs are grouped together under the 12 broad intervention categories and further classified by whether they meet the inclusion criteria of an existing systematic review, but were omitted for some reason (SR exists) or not (No SR exists).
*Interventions are compared to placebo, control or standard care, unless otherwise stated
^One or more systematic review exists on this topic, but the RCT was published after all systematic review on this topic or was deemed to be out of scope of the existing systematic reviews. Due to the differing inclusion criteria between systematic reviews within a single intervention area, some RCTs were judged as ‘out of scope’ for one systematic review on that topic, whereas they post-dated the publication of another systematic review in the same topic area. Given this, and the fact that the two reasons are both ‘legitimate’ explanations for an RCT to be omitted from a systematic review, we collapsed these two reasons together.
Abbreviations: CBF = Cerebral blood flow, crani. = craniectomy, decomp. = decompressive, FiO2 = fraction of inspired oxygen, ICP = Intracranial pressure, Min = minimally, Mx = management, Neurosurg. = neurosurgical, SR = systematic review, Trache. = tracheostomy, vs. = versus
Discussion
We identified 85 systematic reviews and 213 RCTs in acute management of moderate to severe TBI. The most frequently reviewed interventions were hypothermia, hypertonic saline and/or mannitol and surgery. Approximately half of the systematic reviews lacked currency, in that they did not include most recently published eligible RCT, and one-third of reviews were incomplete, meaning they appeared to miss one or more eligible RCT. When considering only the most recently published systematic review in each intervention, currency increased to approximately 75%. Approximately one-quarter of the RCTs in the acute management of moderate to severe TBI are not included in any systematic review, thus limiting their ability to impact upon practice.
In this study, that less than half of all systematic reviews in acute management of moderate to severe TBI were rated as high quality, with nearly 20% judged as low quality. This is consistent with recent examinations of systematic review quality in biomedical research more broadly [9, 10]. It is therefore not surprising that one-third of systematic reviews referred to a review protocol, and two-thirds lacked transparency around inclusion and exclusion decisions. Additionally, the number of reviews that missed RCTs suggests that searches are not sufficiently comprehensive. It is notable that no review reported both study-level and review-level funding or conflicts of interest. This is particularly problematic given the association between industry funding and favourable results, for both RCTs [157] and systematic reviews [11]. The implication of poor quality systematic reviews is that they may not provide trustworthy evidence to inform clinical practice. While there is meta-epidemiological research showing the correlation between risk of bias in RCTs and overestimation of treatment effects [158], there is limited methodological research into the relationship between systematic review quality and direction or strength of review results.
Despite 85 systematic reviews in this area, the only interventions underpinned by current, complete and high quality evidence are red blood cell transfusion, hypothermia, management guided by intracranial pressure, pharmacological agents (various) and prehospital intubation. Contrasting this is a picture of research waste, with examples of duplication (17 systematic reviews on hypothermia), redundancy (four systematic reviews on hyperventilation with only one RCT ever published) and potentially misleading reviews due to poor quality and/or missing RCTs. The implications for practice recommendations underpinned by such reviews are of concern.
This study is significant in that we have compiled what we believe to be the broadest and most comprehensive record of published, English-language systematic reviews and RCTs in acute management of moderate to severe TBI, highlighting strengths and weakness. Clinicians, decision makers and trialists may use our analysis of a cohort of systematic reviews to inform decision-making, clinical practice guidelines and future research.
We acknowledge a number of limitations. First, to align our work with that of Bragge [14], we excluded non-English and unpublished systematic reviews and RCTs, meaning our evidence map does not encompass these. While we undoubtedly excluded some non-English language RCTs, most of which were in Chinese [14], there are perhaps fewer non-English language systematic reviews, given the propensity for Chinese authors to publish systematic reviews in English [9]. Second, due to resource limitations, all the data extraction, and approximately 20% of the quality assessment, was undertaken by one reviewer, which may have introduced errors [159]. Third, we did not contact systematic review authors directly and therefore may have incorrectly categorised a number of RCTs as missing when in fact they were screened and excluded by the authors. This does, however, reinforce the importance of complete reporting of review methods as recommended by PRISMA [23]. Finally, we did not re-run the searches immediately prior to publication, but given the ‘state-of-the-science’ nature of this work it is unlikely to have influenced the conclusions of the review.
By its nature as an evidence map, this study provides the foundational work for a strategic research agenda [22]. A more nuanced assessment of systematic review and RCT quality [10, 160] and generalisability, explicit consideration of the potential impact of any new (or missing) RCTs on existing review conclusions and consideration of the clinical importance of the question [161] is warranted before new reviews are undertaken. Similarly, with regards to the RCTs on topics not covered by existing systematic reviews, consideration should be given to the clinical relevance and importance of these questions to stakeholders before undertaking new reviews [8].
This study highlights the ongoing challenge for the research community to produce rigorous and comprehensive systematic reviews that incorporate the latest evidence [162, 163]. A number of solutions have been proposed to improve systematic review quality, many of which focus on improved use, training and mandating of reporting checklists, such as PRISMA, by authors, journal editors and peer reviewers [10, 164]. While such approaches have shown promise in improving systematic review quality and completeness of reporting [164], others have called for a more radical change to the way in which secondary research is produced, with closer links between primary and secondary researchers resulting in prospective meta-analyses [9].
One such new approach is Living Systematic Reviews, defined as up to date online summaries of health care research that are updated as new research becomes available [165]. Living Systematic Reviews have been proposed as a way to maintain currency and quality of reviews, while reducing research waste [166, 167]. Living Systematic Reviews are currently being piloted by Cochrane [168] and explored by a number of research teams internationally [169–171]. In TBI, Living Systematic Reviews are being piloted within the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) project [172, 173]. In the CENTER-TBI model, teams of reviewers, with support from methodologists and content experts, are re-running searches every three months and publishing updates in the supplementary material of the published review [12, 174]. Others are making use of larger collaborations [171], citizen science [168], open data platforms [175], and machine learning and other technological enablers [165] to make a ‘living’ evidence model feasible, scalable and sustainable.
For systematic reviews to inform clinical practice, or to influence the primary research agenda, a careful assessment of the nature, strength and credibility of their findings is required [5]. The next logical steps are translation of review findings, if results are conclusive, or more primary research, if review findings are inconclusive [176]. This makes the case for a new piece of work examining the robustness of systematic review conclusions in TBI. We currently have in preparation a formal overview of systematic reviews in acute management of moderate to severe TBI, in which we build on the work presented here [177].
Conclusion
A substantial number of published systematic reviews of acute management of moderate to severe TBI lack currency, completeness and quality. These shortcomings could affect the robustness of review findings, yielding potentially unreliable evidence underpinning practice recommendations. We highlight both evidence gaps in this area, where consideration could be given to new systematic reviews, and considerable research waste, with much duplicative and redundant effort. Living systematic reviews are being piloted in TBI and offer an opportunity to improve the evidence base informing clinical care and future research in this area.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the European Union FP 7th Framework program (Grant 602150). The funder provided support in the form of salary for AS, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. DKM is supported by the National Institute for Health Research (UK). The specific roles of AS and DKM are articulated in the ‘author contributions’ section.
References
- 1.Institute of Medicine. Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research Eden J, Levit L, Berg A, Morton S, editors. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 2.Oxman AD, Cook DJ, Guyatt GH. Users' guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. Jama. 1994;272(17):1367–71. [DOI] [PubMed] [Google Scholar]
- 3.Kredo T, Bernhardsson S, Machingaidze S, Young T, Louw Q, Ochodo E, et al. Guide to clinical practice guidelines: the current state of play. International journal for quality in health care: journal of the International Society for Quality in Health Care. 2016;28(1):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine. Current Best Practices and Proposed Standards for Development of Trustworthy CPGs: Part 1, Getting Started In: Robin Graham MM, Wolman Dianne Miller, Greenfield Sheldon, and Steinberg Earl, editor. Clinical practice guidelines we can trust Washington DC: Institute of Medicine; 2011. p. 75–108. [Google Scholar]
- 5.Robinson KA, Saldanha IJ, McKoy NA. Development of a framework to identify research gaps from systematic reviews. Journal of clinical epidemiology. 2011;64(12):1325–30. doi: 10.1016/j.jclinepi.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 6.Macleod MR, Michie S, Roberts I, Dirnagl U, Chalmers I, Ioannidis JPA, et al. Biomedical research: increasing value, reducing waste. The Lancet. 2014;383(9912):101–4. [DOI] [PubMed] [Google Scholar]
- 7.Lancet T. REWARD (REduce research Waste And Reward Diligence) 2017 [Available from: http://www.thelancet.com/campaigns/efficiency.
- 8.Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. The Lancet. 2014;383(9912):156–65. [DOI] [PubMed] [Google Scholar]
- 9.Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. The Milbank quarterly. 2016;94(3):485–514. doi: 10.1111/1468-0009.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. PLOS Medicine. 2016;13(5):e1002028 doi: 10.1371/journal.pmed.1002028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebrahim S, Bance S, Athale A, Malachowski C, Ioannidis JP. Meta-analyses with industry involvement are massively published and report no caveats for antidepressants. Journal of clinical epidemiology. 2016;70:155–63. doi: 10.1016/j.jclinepi.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 12.Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M, et al. Epidemiology of Traumatic Brain Injury in Europe: A Living Systematic Review. Journal of neurotrauma. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan F, Baguley IJ, Cameron ID. 4: Rehabilitation after traumatic brain injury. Medical Journal of Australia. 2003;178:290–5. [DOI] [PubMed] [Google Scholar]
- 14.Bragge P, Synnot A, Maas AI, Menon DK, Cooper DJ, Rosenfeld JV, et al. A State-of-the-Science Overview of Randomized Controlled Trials Evaluating Acute Management of Moderate-to-Severe Traumatic Brain Injury. Journal of neurotrauma. 2016;33(16):1461–78. doi: 10.1089/neu.2015.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. The Lancet. 2012;380(9847):1088–98. [DOI] [PubMed] [Google Scholar]
- 16.Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2016. [DOI] [PubMed] [Google Scholar]
- 17.Lei J, Gao G-y, Jiang J-y. Is management of acute traumatic brain injury effective? A literature review of published Cochrane Systematic Reviews. Chinese Journal of Traumatology. 2012;15:17–22. [PubMed] [Google Scholar]
- 18.Gultekin R, Huang S, Clavisi O, Pattuwage L, Konig TC, Gruen R. Pharmacological interventions in traumatic brain injury: Can we rely on systematic reviews for evidence? Injury. 2016;47(3):516–24. doi: 10.1016/j.injury.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Gary KW, Neimeier JP, Ward J, Lapane KL. Randomized controlled trials in adult traumatic brain injury. Brain Injury. 2012;26(13–14):1523–48. doi: 10.3109/02699052.2012.722257 [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Gary KW, Copolillo A, Ward J, Niemeier JP, Lapane KL. Randomized Controlled Trials in Adult Traumatic Brain Injury: A Review of Compliance to CONSORT Statement. Archives of Physical Medicine and Rehabilitation. 2015;96(4):702–14. doi: 10.1016/j.apmr.2014.10.026 [DOI] [PubMed] [Google Scholar]
- 21.Bragge P, Clavisi O, Turner T, Tavender E, Collie A, Gruen RL. The Global Evidence Mapping Initiative: Scoping research in broad topic areas. BMC Medical Research Methodology 2011;11(92). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Systematic Reviews. 2016;5(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009;6(7):e1000097 doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefebvre C, Manheimer E, Glanville JobotCIRMG. Chapter 6: Searching for studies: Box 6.3.a: Cochrane definitions and criteria for randomized controlled trials (RCTs) and controlled clinical trials (CCTs) In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions: Version 510 [updated March 2011]: The Cochrane Collaboration; 2011. [Google Scholar]
- 25.National Trauma Research Institute. EvidenceMap.org n.d. [Available from: http://neurotrauma.evidencemap.org/.
- 26.Epistemonikos. Epistemonikos n.d. [11 January 2017]. Available from: http://www.epistemonikos.org/en/.
- 27.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieper D, Buechter RB, Li L, Prediger B, Eikermann M. Systematic review found AMSTAR, but not R(evised)-AMSTAR, to have good measurement properties. Journal of clinical epidemiology. 2015;68(5):574–83. doi: 10.1016/j.jclinepi.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 29.Ryan R, Santesso N, Hill S, Lowe D, Kaufman C, Grimshaw J. Consumer-oriented interventions for evidence-based prescribing and medicines use: an overview of systematic reviews. Cochrane Database of Systematic Reviews. 2011(5):CD007768 doi: 10.1002/14651858.CD007768.pub2 [DOI] [PubMed] [Google Scholar]
- 30.Higgins J, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews (Version 1.02) London: Cochrane; 2016. [Google Scholar]
- 31.Meyer M, Megyesi J, Meythaler J, Murie-Fernandez M, Aubut J, Foley N, et al. Acute management of acquired brain injury part II: an evidence-based review of pharmacological interventions. Brain Injury. 2010;24:706–21. [DOI] [PubMed] [Google Scholar]
- 32.Roberts I, Schierhout G, Alderson P. Absence of evidence for the effectiveness of five interventions routinely used in the intensive care management of severe head injury: a systematic review. Journal of Neurology, Neurosurgery & Psychiatry. 1998;65(5):729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonagh M, Helfand M, Carson S, Russman BS. Hyperbaric oxygen therapy for traumatic brain injury: a systematic review of the evidence. Archives of Physical Medicine and Rehabilitation. 2004;85(7):1198–204. [DOI] [PubMed] [Google Scholar]
- 34.Bennett M, Trytko B, Jonker B. Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database of Systematic Reviews. 2012;Issue 12. Art. No.: CD004609. doi: 10.1002/14651858.CD004609.pub3 [DOI] [PubMed] [Google Scholar]
- 35.Roberts I, Schierhout G. Hyperventilation therapy for acute traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 1997; (4). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000566/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nangunoori R, Maloney-Wilensky E, Stiefel M, Park S, Andrew Kofke W, Levine J, et al. Brain Tissue Oxygen-Based Therapy and Outcome After Severe Traumatic Brain Injury: A Systematic Literature Review. Neurocrit Care. 2012;17(1):131–8. doi: 10.1007/s12028-011-9621-9 [DOI] [PubMed] [Google Scholar]
- 37.Lazaridis C, Andrews C. Brain Tissue Oxygenation, Lactate-Pyruvate Ratio, and Cerebrovascular Pressure Reactivity Monitoring in Severe Traumatic Brain Injury: Systematic Review and Viewpoint. Neurocrit Care. 2014;21(2):345–55. doi: 10.1007/s12028-014-0007-7 [DOI] [PubMed] [Google Scholar]
- 38.Nishijima DK, Zehtabchi S, Berrong J, Legome E. Utility of platelet transfusion in adult patients with traumatic intracranial hemorrhage and preinjury antiplatelet use: A systematic review. Journal of Trauma and Acute Care Surgery. 2012;72:1658–63. doi: 10.1097/TA.0b013e318256dfc5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boutin A, Chassé M, Shemilt M, Lauzier F, Moore L, Zarychanski R, et al. Red Blood Cell Transfusion in Patients With Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Transfusion medicine reviews. 2016;30:15–24. doi: 10.1016/j.tmrv.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 40.Harris OA, Colford JM Jr, Good MC, Matz PG. The role of hypothermia in the management of severe brain injury: A meta-analysis. Archives of Neurology. 2002;59(7):1077–83. [DOI] [PubMed] [Google Scholar]
- 41.McIntyre LA, Fergusson DA, Hébert PC, Moher D, Hutchison JS. Prolonged therapeutic hypothermia after traumatic brain injury in adults: A systematic review. Jama. 2003;289(22):2992–9. doi: 10.1001/jama.289.22.2992 [DOI] [PubMed] [Google Scholar]
- 42.Henderson W, Dhingra V, Chittock D, Fenwick J, Ronco J. Hypothermia in the management of traumatic brain injury. Intensive Care Med. 2003;29(10):1637–44. doi: 10.1007/s00134-003-1848-2 [DOI] [PubMed] [Google Scholar]
- 43.Peterson K, Carson S, Carney N. Hypothermia Treatment for Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Journal of neurotrauma. 2008;25:62–71. doi: 10.1089/neu.2007.0424 [DOI] [PubMed] [Google Scholar]
- 44.Saxena M, Andrews Peter JD, Cheng A, Deol K, Hammond N. Modest cooling therapies (35°C to 37.5°C) for traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2014; (8). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006811.pub3/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database of Systematic Reviews [Internet]. 2009; (2). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001048.pub4/abstract. [DOI] [PubMed] [Google Scholar]
- 46.Fox JL, Vu EN, Doyle-Waters M, Brubacher JR, Abu-Laban R, Hu Z. Prophylactic hypothermia for traumatic brain injury: a quantitative systematic review. Canadian Journal of Emergency Medicine. 2010;12:355–64. [DOI] [PubMed] [Google Scholar]
- 47.Sadaka F, Veremakis C. Therapeutic hypothermia for the management of intracranial hypertension in severe traumatic brain injury: A systematic review. Brain Injury. 2012;26(7–8):899–908. doi: 10.3109/02699052.2012.661120 [DOI] [PubMed] [Google Scholar]
- 48.Georgiou AP, Manara AR. Role of therapeutic hypothermia in improving outcome after traumatic brain injury: a systematic review. British Journal of Anaesthesia. 2013;110(3):357–67. doi: 10.1093/bja/aes500 [DOI] [PubMed] [Google Scholar]
- 49.Harris B, Andrews P, Murray G, Forbes J, Moseley O. Systematic review of head cooling in adults after traumatic brain injury and stroke. Health Technol Assess. 2012;16(45). doi: 10.3310/hta16450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crossley S, Reid J, McLatchie R, Hayton J, Clark C, MacDougall M, et al. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Critical Care Medicine. 2014;18:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li P, Yang C. Moderate hypothermia treatment in adult patients with severe traumatic brain injury: a meta-analysis. Brain Inj. 2014;28(8):1036–41. doi: 10.3109/02699052.2014.910609 [DOI] [PubMed] [Google Scholar]
- 52.Madden LK, DeVon HA. A Systematic Review of the Effects of Body Temperature on Outcome After Adult Traumatic Brain Injury. The Journal of neuroscience nursing: journal of the American Association of Neuroscience Nurses. 2015;47(4):190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang BF, Wang J, Liu ZW, Zhao YL, Li DD, Huang TQ, et al. Meta-analysis of the efficacy and safety of therapeutic hypothermia in children with acute traumatic brain injury. World neurosurgery. 2015;83(4):567–73. doi: 10.1016/j.wneu.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 54.Banks CJ, Furyk JS. Review article: hypertonic saline use in the emergency department. Emergency medicine Australasia: EMA. 2008;20(4):294–305. doi: 10.1111/j.1742-6723.2008.01086.x [DOI] [PubMed] [Google Scholar]
- 55.Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database of Systematic Reviews. 2013;Issue 8. Art. No.: CD001049. [Google Scholar]
- 56.Kamel H, Navi B, Nakagawa K, Hemphill Jr, Ko N. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Critical Care Medicine. 2011;39:554–9. doi: 10.1097/CCM.0b013e318206b9be [DOI] [PubMed] [Google Scholar]
- 57.Rickard AC, Smith JE, Newell P, Bailey A, Kehoe A, Mann C. Salt or sugar for your injured brain? A meta-analysis of randomised controlled trials of mannitol versus hypertonic sodium solutions to manage raised intracranial pressure in traumatic brain injury. Emergency Medicine Journal. 2014;31(8):679–83. doi: 10.1136/emermed-2013-202679 [DOI] [PubMed] [Google Scholar]
- 58.Lourens A, Botha JC. Hypertonic saline (HTS) versus standard (isotonic) fluid therapy for traumatic brain injuries: A systematic review. African Journal of Emergency Medicine. 2015;4(4):188–94. [Google Scholar]
- 59.Li M, Chen T, Chen SD, Cai J, Hu YH. Comparison of equimolar doses of mannitol and hypertonic saline for the treatment of elevated intracranial pressure after traumatic brain injury: a systematic review and meta-analysis. Medicine. 2015;94(17):e736 doi: 10.1097/MD.0000000000000736 25929908 [Google Scholar]
- 60.Mendelson AA, Gillis C, Henderson WR, Ronco JJ, Dhingra V, Griesdale DEG. Intracranial Pressure Monitors in Traumatic Brain Injury: A Systematic Review. Canadian Journal of Neurological Sciences. 2012;39:571–6. [DOI] [PubMed] [Google Scholar]
- 61.Sadaka F, Kasal J, Lakshmanan R, Palagiri A. Placement of intracranial pressure monitors by neurointensivists: Case series and a systematic review. Brain Injury. 2013;27(5):600–4. doi: 10.3109/02699052.2013.772238 [DOI] [PubMed] [Google Scholar]
- 62.Su S-H, Wang F, Hai J, Liu N-T, Yu F, Wu Y-F, et al. The Effects of Intracranial Pressure Monitoring in Patients with Traumatic Brain Injury. PLoS ONE. 2014;9(2):e87432 doi: 10.1371/journal.pone.0087432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan Q, Wu X, Sun Y, Yu J, Li Z, Du Z, et al. Impact of intracranial pressure monitoring on mortality in patients with traumatic brain injury: a systematic review and meta-analysis. Journal of neurosurgery. 2015;122(3):574–87. doi: 10.3171/2014.10.JNS1460 [DOI] [PubMed] [Google Scholar]
- 64.Forsyth RJ, Raper J, Todhunter E. Routine intracranial pressure monitoring in acute coma. The Cochrane database of systematic reviews. 2015;11:CD002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan J-Y. Effect of Backrest Position on Intracranial Pressure and Cerebral Perfusion Pressure in Individuals with Brain Injury: A Systematic Review. Journal of Neuroscience Nursing. 2004;36:278–88. [DOI] [PubMed] [Google Scholar]
- 66.Muzevic D, Splavski B. The Lund concept for severe traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2013; (12). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010193.pub2/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krakau K, Omne-Pontén M, Karlsson T, Borg J. Metabolism and nutrition in patients with moderate and severe traumatic brain injury: A systematic review. Brain Injury. 2006;20(4):345–67. doi: 10.1080/02699050500487571 [DOI] [PubMed] [Google Scholar]
- 68.Perel P, Yanagawa T, Bunn F, Roberts Ian G, Wentz R. Nutritional support for head-injured patients. Cochrane Database of Systematic Reviews [Internet]. 2006; (4). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001530.pub2/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Dong Y, Han X, Qi X-Q, Huang C-G, Hou L-J. Nutritional Support for Patients Sustaining Traumatic Brain Injury: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE. 2013;8(3):e58838 doi: 10.1371/journal.pone.0058838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D, Zheng SQ, Chen XC, Jiang SW, Chen HB. Comparisons between small intestinal and gastric feeding in severe traumatic brain injury: a systematic review and meta-analysis of randomized controlled trials. Journal of neurosurgery. 2015:1–8. [DOI] [PubMed] [Google Scholar]
- 71.Lei Q, Maode W, Xin L, Tuo W, Gaofeng X, Qi L, et al. A meta-analysis of tight versus conventional glycemic control in critically ill brain injured adults. Journal of Medical Colleges of PLA. 2012;27(1):20–37. [Google Scholar]
- 72.Wang Z, Shi L, Ding W, Shao F, Yu J, Zhang J. Efficacy of Progesterone for Acute Traumatic Brain Injury: a Meta-analysis of Randomized Controlled Trials. Molecular neurobiology. 2015. [DOI] [PubMed] [Google Scholar]
- 73.Zeng Y, Zhang Y, Ma J, Xu J. Progesterone for Acute Traumatic Brain Injury: A Systematic Review of Randomized Controlled Trials. PloS one. 2015;10(10):e0140624 doi: 10.1371/journal.pone.0140624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langham J, Goldfrad C, Teasdale G, Shaw D, Rowan K. Calcium channel blockers for acute traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2003; (4). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000565/abstract. [DOI] [PubMed] [Google Scholar]
- 75.Perel P, Roberts I, Shakur H, Thinkhamrop B, Phuenpathom N, Yutthakasemsunt S. Haemostatic drugs for traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2010; (1). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007877.pub2/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zehtabchi S, Abdel Baki SG, Falzon L, Nishijima DK. Tranexamic acid for traumatic brain injury: a systematic review and meta-analysis. The American Journal of Emergency Medicine. 2014;32(12):1503–9. doi: 10.1016/j.ajem.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. The Cochrane database of systematic reviews. 2015;5:CD004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siddall O. Use of methylphenidate in traumatic brain injury. Ann Pharmacother. 2005;39:1309–13. doi: 10.1345/aph.1E637 [DOI] [PubMed] [Google Scholar]
- 79.Forsyth R, J., Jayamoni B, Paine Tom C, Mascarenhas S. Monoaminergic agonists for acute traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2006; (4). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003984.pub2/abstract. [DOI] [PubMed] [Google Scholar]
- 80.Frenette AJ, Kanji S, Rees L, Williamson DR, Perreault MM, Turgeon AF, et al. Efficacy and Safety of Dopamine Agonists in Traumatic Brain Injury: A Systematic Review of Randomized Controlled Trials. Journal of neurotrauma. 2012;29:1–18. doi: 10.1089/neu.2011.1812 [DOI] [PubMed] [Google Scholar]
- 81.Roberts I. Aminosteroids for acute traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 1999; (3). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001527/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alali A, McCredie V, Golan E, Shah P, Nathens A. Beta Blockers for Acute Traumatic Brain Injury: A Systematic Review and Meta-analysis. Neurocrit Care. 2014;20(3):514–23. doi: 10.1007/s12028-013-9903-5 [DOI] [PubMed] [Google Scholar]
- 83.Shen X, Dutcher SK, Palmer J, Liu X, Kiptanui Z, Khokhar B, et al. A Systematic Review of the Benefits and Risks of Anticoagulation Following Traumatic Brain Injury. J Head Trauma Rehabil. 2014;0:1–9. doi: 10.1097/HTR.0b013e318281966e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeiler FA, Teitelbaum J, Gillman LM, West M. THAM for Control of ICP. Neurocrit Care. 2014;21(2):332–44. doi: 10.1007/s12028-014-9978-7 [DOI] [PubMed] [Google Scholar]
- 85.Sanfilippo F, Santonocito C, Veenith T, Astuto M, Maybauer MO. The role of neuromuscular blockade in patients with traumatic brain injury: a systematic review. Neurocrit Care. 2015;22(2):325–34. doi: 10.1007/s12028-014-0061-1 [DOI] [PubMed] [Google Scholar]
- 86.Arango M, F., Bainbridge D. Magnesium for acute traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2008; (4). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005400.pub3/abstract. [DOI] [PubMed] [Google Scholar]
- 87.Cohen L, Athaide V, Wickham ME, Doyle-Waters MM, Rose NGW, Hohl CM. The Effect of Ketamine on Intracranial and Cerebral Perfusion Pressure and Health Outcomes: A Systematic Review. Annals of Emergency Medicine. 2015;65(1):43–51.e2. doi: 10.1016/j.annemergmed.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 88.Willis C, Lybrand S, Bellamy N. Excitatory amino acid inhibitors for traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2003; (1). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003986.pub2/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bossers SM, Schwarte LA, Loer SA, Twisk JW, Boer C, Schober P. Experience in Prehospital Endotracheal Intubation Significantly Influences Mortality of Patients with Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. PloS one. 2015;10(10):e0141034 doi: 10.1371/journal.pone.0141034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pickering A, Cooper K, Harnan S, Sutton A, Mason S, Nicholl J. Impact of prehospital transfer strategies in major trauma and head injury: Systematic review, meta-analysis, and recommendations for study design. The journal of trauma and acute care surgery. 2015;78(1):164–77. doi: 10.1097/TA.0000000000000483 [DOI] [PubMed] [Google Scholar]
- 91.Fuller G, Pallot D, Coats T, Lecky F. The effectiveness of specialist neuroscience care in severe traumatic brain injury: A systematic review. British Journal of Neurosurgery. 2014;28(4):452–60. doi: 10.3109/02688697.2013.865708 [DOI] [PubMed] [Google Scholar]
- 92.Roberts D, Hall R, Kramer A, Robertson H, Gallagher C, Zygun D. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit Care Med. 2011;39:2743–51. doi: 10.1097/CCM.0b013e318228236f [DOI] [PubMed] [Google Scholar]
- 93.Gu J-w, Yang T, Kuang Y-q, Huang H-d, Kong B, Shu H-f, et al. Comparison of the safety and efficacy of propofol with midazolam for sedation of patients with severe traumatic brain injury: A meta-analysis. Journal of Critical Care. 2014;29(2):287–90. doi: 10.1016/j.jcrc.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 94.Zeiler FA, Teitelbaum J, West M, Gillman LM. The Ketamine Effect on ICP in Traumatic Brain Injury. Neurocrit Care. 2014;21(1):163–73. doi: 10.1007/s12028-013-9950-y [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Ding X, Tong Y, Zong J, Zhao X, Ren H, et al. Ketamine does not increase intracranial pressure compared with opioids: meta-analysis of randomized controlled trials. Journal of Anesthesia. 2014;28(6):821–7. doi: 10.1007/s00540-014-1845-3 [DOI] [PubMed] [Google Scholar]
- 96.Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2012; (12). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000033.pub2/abstract. doi: 10.1002/14651858.ED000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong V, Cheuk Daniel KL, Lee S, Chu V. Acupuncture for acute management and rehabilitation of traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2013; (3). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007700.pub3/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeiler FA, Akoth E, Gillman LM, West M. Burst Suppression for ICP Control: A Systematic Review. Journal of intensive care medicine. 2015:1–10. [DOI] [PubMed] [Google Scholar]
- 99.Schierhout G, Roberts I. Prophylactic antiepileptic agents after head injury: a systematic review. J Neurol Neurosurg Psychiatry. 1998;64:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teasell R, Bayona N, Lippert C, Villamere J, Hellings C. Post-traumatic seizure disorder following acquired brain injury. Brain Injury. 2007;21(2):201–14. doi: 10.1080/02699050701201854 [DOI] [PubMed] [Google Scholar]
- 101.Zafar SN, Khan AA, Ghauri AA, Shamim MS. Phenytoin versus Leviteracetam for Seizure Prophylaxis after brain injury–a meta analysis. BMC Neurology. 2012;12:30 doi: 10.1186/1471-2377-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thompson K, Pohlmann-Eden B, Campbell LA, Abel H. Pharmacological treatments for preventing epilepsy following traumatic head injury. The Cochrane database of systematic reviews. 2015;8:CD009900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alderson P, Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2005; (1). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000196.pub2/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sahuquillo J. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database of Systematic Reviews [Internet]. 2006; (1). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003983.pub2/abstract. [DOI] [PubMed] [Google Scholar]
- 105.Jacob AT, Heuer GG, Grant R, Georgoff P, Danish SF, Storm PB, et al. Decompressive Hemicraniectomy for Pediatric Traumatic Brain Injury: Long-Term Outcome Based on Quality of Life. Pediatric Neurosurgery. 2011;47(2):81–6. doi: 10.1159/000329624 [DOI] [PubMed] [Google Scholar]
- 106.Güresir E, Schuss P, Seifert V, Vatter H. Decompressive craniectomy in children: single-center series and systematic review. Neurosurgery. 2012;70:881–8. doi: 10.1227/NEU.0b013e318237a6a6 [DOI] [PubMed] [Google Scholar]
- 107.Bor-Seng-Shu E, Figueiredo EG, Amorim RLO, Teixeira MJ, Valbuza JS, de Oliveira MM, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. Journal of neurosurgery. 2012;117(3):589–96. doi: 10.3171/2012.6.JNS101400 [DOI] [PubMed] [Google Scholar]
- 108.Wang R, Li M, Gao WW, Guo Y, Chen J, Tian HL. Outcomes of Early Decompressive Craniectomy Versus Conventional Medical Management After Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Medicine. 2015;94(43):e1733 doi: 10.1097/MD.0000000000001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim Y-J. The impact of time to surgery on outcomes in patients with traumatic brain injury: A literature review. International Emergency Nursing. 2014;22(4):214–9. doi: 10.1016/j.ienj.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 110.Bouderka MA, Fakhir B, Bouaggad A, Hmamouchi B, Hamoudi D, Harti A. Early tracheostomy versus prolonged endotracheal intubation in severe head injury. The Journal of trauma. 2004;57(2):251–4. [DOI] [PubMed] [Google Scholar]
- 111.Schibler A, Humphreys S. Increased brain tissue oxygen tension in children with traumatic brain injury using temperature-corrected guided ventilation during prophylactic hypothermia. Crit Care Resusc. 2012;14(1):20–4. [PubMed] [Google Scholar]
- 112.Taher A, Pilehvari Z, Poorolajal J, Aghajanloo M. Effects of Normobaric Hyperoxia in Traumatic Brain Injury: A Randomized Controlled Clinical Trial. Trauma Monthly. 2016;21(1):e26772 doi: 10.5812/traumamon.26772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qiansuokai W. Effect of early hyperbaric oxygenation therapy with a ventilator on the incidence of stress ulcer in severely brain-injured patients. Journal of neurotrauma. 2011;28(5):A45–A6. [Google Scholar]
- 114.Lin CM, Lin MC, Huang SJ, Chang CK, Chao DP, Lui TN, et al. A prospective randomized study of brain tissue oxygen pressure-guided management in moderate and severe traumatic brain injury patients. BioMed Research International. 2015:Article ID 529580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Etemadrezaie H, Baharvahdat H, Shariati Z, Lari SM, Shakeri MT, Ganjeifar B. The effect of fresh frozen plasma in severe closed head injury. Clinical neurology and neurosurgery. 2007;109(2):166–71. doi: 10.1016/j.clineuro.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 116.Sadaka F, Palagiri A, Lakshmanan R, Kasal J, Plisco M. Therapeutic hypothermia for refractory intracranial hypertension in traumatic brain injury patients: The mercy experience. Neurocrit Care. 2013. a;19(1):S283. [Google Scholar]
- 117.Beca J, McSharry B, Erickson S, Yung M, Schibler A, Slater A, et al. Hypothermia for Traumatic Brain Injury in Children-A Phase II Randomized Controlled Trial. Critical care medicine. 2015;43(7):1458–66. doi: 10.1097/CCM.0000000000000947 [DOI] [PubMed] [Google Scholar]
- 118.Maekawa T, Yamashita S, Nagao S, Hayashi N, Ohashi Y, Brain-Hypothermia Study G. Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: a randomized controlled trial. Journal of neurotrauma. 2015;32(7):422–9. doi: 10.1089/neu.2013.3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flynn LMC, Rhodes J, Andrews PJD. Therapeutic Hypothermia Reduces Intracranial Pressure and Partial Brain Oxygen Tension in Patients with Severe Traumatic Brain Injury: Preliminary Data from the Eurotherm3235 Trial. Therapeutic Hypothermia and Temperature Management. 2015;5(3):143–51. doi: 10.1089/ther.2015.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yan Y, Tang W, Deng Z, Zhong D, Yang G. Cerebral oxygen metabolism and neuroelectrophysiology in a clinical study of severe brain injury and mild hypothermia. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2010;17(2):196–200. [DOI] [PubMed] [Google Scholar]
- 121.Cheng SX, Shen YF, Zhang S. The effect of moderate hypothermi a on amyloid-(beta) in cerebrospi nal fluid after severe traumatic brain injury. Journal of neurotrauma. 2011;28(5):A86. [Google Scholar]
- 122.Gal R, Smrcka M, Slezak M, Colonova M, Mrlian A. Mild hypothermia therapy for patients with severe brain injury: Clinical study. Brain Injury. 2012;26(4–5):629–30. [Google Scholar]
- 123.Proctor K. Vasopressin Versus Catecholamines for Cerebral Perfusion Pressure Control in Brain Injured Trauma Patients (AVP) 2014. [Available from: http://clinicaltrials.gov/show/NCT00795366. [Google Scholar]
- 124.Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, Cormio M, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27(10):2086–95. [DOI] [PubMed] [Google Scholar]
- 125.Morrison LJ, Baker AJ, Rhind SG, Kiss A, MacDonald RD, Schwartz B, et al. The Toronto prehospital hypertonic resuscitation—head injury and multiorgan dysfunction trial: feasibility study of a randomized controlled trial. J Crit Care. 2011;26(4):363–72. doi: 10.1016/j.jcrc.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 126.Baker AJ, Rhind SG, Morrison LJ, Black S, Crnko NT, Shek PN, et al. Resuscitation with hypertonic saline-dextran reduces serum biomarker levels and correlates with outcome in severe traumatic brain injury patients. Journal of neurotrauma. 2009;26(8):1227–40. doi: 10.1089/neu.2008.0868 [DOI] [PubMed] [Google Scholar]
- 127.Blair D, Norton R, Dhingra V, Foster D, Hebert P, Henderson W, et al. Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: long-term follow-up of a subgroup of patients from the NICE-SUGAR study. Intensive Care Med. 2015;41(6):1037–47. doi: 10.1007/s00134-015-3757-6 [DOI] [PubMed] [Google Scholar]
- 128.Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, Mehrafshan A, Jamali M, Malekpour B, et al. Administration of vitamin C and vitamin E in severe head injury: A randomized double-blind controlled trial. Clinical Neurosurgery. 2011;58:133–7. [DOI] [PubMed] [Google Scholar]
- 129.Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Critical care. 2011;15(6):R290 doi: 10.1186/cc10579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grecu I, Nicolau M, Ologoiu D, Tiganiuc L, Constantinescu A. Jejunal versus gastric feeding in patients with severe head trauma. Nutritional Therapy & Metabolism. 2008;26(4):199–203. [Google Scholar]
- 131.Twyman D, Young AB, Ott L, Norton JA, Bivins BA. High protein enteral feedings: a means of achieving positive nitrogen balance in head injured patients. JPEN Journal of parenteral and enteral nutrition. 1985;9(6):679–84. doi: 10.1177/0148607185009006679 [DOI] [PubMed] [Google Scholar]
- 132.Robertson C, Hannay H, Yamal J, Gopinath S, Goodman J, Tilley B, et al. Effect of Erythropoietin and Transfusion Threshold on Neurological Recovery After Traumatic Brain Injury: A Randomized Clinical Trial. JAMA: the journal of the American Medical Association. 2014;312(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, et al. Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. The Lancet. 2015;386(10012):2499–506. [DOI] [PubMed] [Google Scholar]
- 134.Aloizos S, Evodia E, Gourgiotis S, Isaia EC, Seretis C, Baltopoulos GJ. Neuroprotective Effects of Erythropoietin in Patients with Severe Closed Brain Injury. Turkish neurosurgery. 2015;25(4):552–8. doi: 10.5137/1019-5149.JTN.9685-14.4 [DOI] [PubMed] [Google Scholar]
- 135.Hatton J, Rosbolt B, Empey P, Kryscio R, Young B. Dosing and safety of cyclosporine in patients with severe brain injury. Journal of neurosurgery. 2008;109(4):699–707. doi: 10.3171/JNS/2008/109/10/0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mazzeo AT, Brophy GM, Gilman CB, Alves OL, Robles JR, Hayes RL, et al. Safety and tolerability of cyclosporin a in severe traumatic brain injury patients: results from a prospective randomized trial. Journal of neurotrauma. 2009;26(12):2195–206. doi: 10.1089/neu.2009.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tapia-Perez JH, Sanchez-Aguilar M, Torres-Corzo JG, Gordillo-Moscoso A, Martinez-Perez P, Madeville P, et al. Effect of rosuvastatin on amnesia and disorientation after traumatic brain injury (NCT003229758). Journal of neurotrauma. 2008;25(8):1011–7. doi: 10.1089/neu.2008.0554 [DOI] [PubMed] [Google Scholar]
- 138.Naghibi T, Madani S, Mazloomzadeh S, Dobakhti F. Simvastatin's effects on survival and outcome in traumatic brain injury patients: A comparative study. Turkish Journal of Medical Sciences. 2016;46(1):1–5. doi: 10.3906/sag-1404-125 [DOI] [PubMed] [Google Scholar]
- 139.Koskinen LO, Eklund A, Sundstrom N, Olivecrona M. Prostacyclin influences the pressure reactivity in patients with severe traumatic brain injury treated with an ICP-targeted therapy. Neurocrit Care. 2015;22(1):26–33. doi: 10.1007/s12028-014-0030-8 [DOI] [PubMed] [Google Scholar]
- 140.Olivecrona M, Rodling-Wahlstrom M, Naredi S, Koskinen LO. Prostacyclin treatment in severe traumatic brain injury: a microdialysis and outcome study. Journal of neurotrauma. 2009;26(8):1251–62. doi: 10.1089/neu.2008-0605 [DOI] [PubMed] [Google Scholar]
- 141.Nursal TZ, Erdogan B, Noyan T, Cekinmez M, Atalay B, Bilgin N. The effect of metoclopramide on gastric emptying in traumatic brain injury. Journal of clinical neuroscience. 2007;14(4):344–8. doi: 10.1016/j.jocn.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 142.Khalaf MM, Enab E, Samir N, Heikal AA. Efficacy of cerebrolysin in the treatment of traumatic brain injuries. Egyptian Journal of Neurology, Psychiatry and Neurosurgery. 2011;48(1):43–8. [Google Scholar]
- 143.Shakur H, Andrews P, Asser T, Balica L, Boeriu C, Quintero JD, et al. The BRAIN TRIAL: a randomised, placebo controlled trial of a Bradykinin B2 receptor antagonist (Anatibant) in patients with traumatic brain injury. Trials. 2009;10:109 doi: 10.1186/1745-6215-10-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garner AA, Mann KP, Fearnside M, Poynter E, Gebski V. The Head Injury Retrieval Trial (HIRT): A singlecentre randomised controlled trial of physician prehospital management of severe blunt head injury compared with management by paramedics only. Emergency medicine journal. 2015;32(11):869–75. doi: 10.1136/emermed-2014-204390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lecky F, Russell W, Fuller G, McClelland G, Pennington E, Goodacre S, et al. The head injury transportation straight to neurosurgery (HITS-NS) randomised trial: A feasibility study. Health technology assessment. 2016;20(1):1–110, xiii. doi: 10.3310/hta20010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yaghoobi S, Khezri MB, Alamouti AM. A pilot study of cerebral and hemodynamic changes during sedation with low dose of thiopental sodium or propofol in patients with acute brain injury. Journal of Clinical and Diagnostic Research. 2015;9(8):UC05–UC7. doi: 10.7860/JCDR/2015/13955.6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Penry J, White B, Brackett C. A controlled prospective study of the pharmacologic prophylaxis of posttraumatic epilepsy. Neurology. 1979;29:600–1. [Google Scholar]
- 148.Parveen Y, Dhandapani M, Dhandapani S, Gupta SK. A randomized controlled trial to assess the efficacy of auditory stimulation on selected parameters of comatose patients with traumatic brain injury. Indian Journal of Neurotrauma. 2015;12(2):128–34. [Google Scholar]
- 149.Szaflarski J, Shutter LA, Mendoza L, Szaflarski M. Prospective randomized single-blinded trial of lacosamide versus fosphenytoin for seizure prophylaxis in traumatic brain injury. Epilepsy Currents. 2015;15:144 doi: 10.5698/1535-7597-15.3.14426316856 [Google Scholar]
- 150.Asehnoune K, Seguin P, Allary J, Feuillet F, Lasocki S, Cook F, et al. Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomised placebo-controlled trial. The Lancet Respiratory medicine. 2014;2(9):706–16. doi: 10.1016/S2213-2600(14)70144-4 [DOI] [PubMed] [Google Scholar]
- 151.Fanconi S, Kloti J, Meuli M, Zaugg H, Zachmann M. Dexamethasone therapy and endogenous cortisol production in severe pediatric head injury. Intensive Care Med. 1988;14(2):163–6. [DOI] [PubMed] [Google Scholar]
- 152.Mendelow AD, Gregson BA, Rowan EN, Francis R, McColl E, McNamee P, et al. Early Surgery versus Initial Conservative Treatment in Patients with Traumatic Intracerebral Hemorrhage (STITCH[Trauma]): The First Randomized Trial. Journal of neurotrauma. 2015;32(17):1312–23. doi: 10.1089/neu.2014.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. New England Journal of Medicine. 2016;375(12):1119–30. doi: 10.1056/NEJMoa1605215 [DOI] [PubMed] [Google Scholar]
- 154.Moein H, Sanati MA, Fard SA, Moein P, Hasheminasab SM. Outcome of decompressive craniectomy in patients with severe head injury: A pilot randomized clinical trial. Neurosurgery Quarterly. 2012;22(3):149–52. [Google Scholar]
- 155.Li ZM, Wang LX, Jiang LC, Zhu JX, Geng FY, Qiang F. Surgical treatment of transtentorial herniation after traumatic brain injury. Neurosurgery Quarterly. 2012;22(1):26–9. [Google Scholar]
- 156.Semenov A, Sorokovikov V, Zhivotenko A. The experience of minimal invasive evacuation of the intracranial hematomas in treatment the patients with multiple trauma. Journal of neurotrauma. 2012;29(10):A37–A8. [Google Scholar]
- 157.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ: British Medical Journal. 2003;326(7400):1167-. doi: 10.1136/bmj.326.7400.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of internal medicine. 2012;157(6):429–38. doi: 10.7326/0003-4819-157-6-201209180-00537 [DOI] [PubMed] [Google Scholar]
- 159.Tendal B, Higgins JPT, Jüni P, Hróbjartsson A, Trelle S, Nüesch E, et al. Disagreements in meta-analyses using outcomes measured on continuous or rating scales: observer agreement study. BMJ. 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Journal of clinical epidemiology. 2016;69:225–34. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Garner P, Hopewell S, Chandler J, MacLehose H, Schünemann HJ, Akl EA, et al. When and how to update systematic reviews: consensus and checklist. The BMJ. 2016;354:i3507 doi: 10.1136/bmj.i3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shojania K, Sampson M, Ansari M, Ji J, Doucette S, Moher D. How Quickly Do Systematic Reviews Go Out of Date? A Survival Analysis. Annals of Internal Medicine. 2007;147:224–33. [DOI] [PubMed] [Google Scholar]
- 163.Créquit P, Trinquart L, Yavchitz A, Ravaud P. Wasted research when systematic reviews fail to provide a complete and up-to-date evidence synthesis: the example of lung cancer. BMC Medicine. 2016;14(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8(12):e83138 doi: 10.1371/journal.pone.0083138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elliott JH, Turner T, Clavisi O, Thomas J, Higgins JPT, Mavergames C, et al. Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap. PLOS Medicine. 2014;11(2):e1001603 doi: 10.1371/journal.pmed.1001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vandvik PO, Brignardello-Petersen R, Guyatt GH. Living cumulative network meta-analysis to reduce waste in research: A paradigmatic shift for systematic reviews? BMC Med. 2016;14:59 doi: 10.1186/s12916-016-0596-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Page MJ, Moher D. Mass Production of Systematic Reviews and Meta-analyses: An Exercise in Mega-silliness? The Milbank quarterly. 2016;94(3):515–9. doi: 10.1111/1468-0009.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Living Systematic Review Methods Symposium. 24th Cochrane Colloquium; 2016 26 October; Seoul, South Korea.
- 169.Rahal AK, Badgett RG, Hoffman RM. Screening Coverage Needed to Reduce Mortality from Prostate Cancer: A Living Systematic Review. PLoS One. 2016;11(4):e0153417 doi: 10.1371/journal.pone.0153417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Créquit P, Trinquart L, Ravaud P. Live cumulative network meta-analysis: protocol for second-line treatments in advanced non-small-cell lung cancer with wild-type or unknown status for epidermal growth factor receptor. BMJ Open. 2016;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Charidimou A, Soo Y, Heo JH, Srikanth V. A call for researchers to join the META-MICROBLEEDS Consortium. The Lancet Neurology.15(9):900 doi: 10.1016/S1474-4422(16)30124-7 [DOI] [PubMed] [Google Scholar]
- 172.Synnot A, Gruen RL, Menon D, Steyerberg EW, Buki A, Peul W, et al. A new approach to evidence synthesis in traumatic brain injury: living systematic reviews. Journal of neurotrauma. 2015;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): A Prospective Longitudinal Observational Study. Neurosurgery. 2015;76(1):67–80. doi: 10.1227/NEU.0000000000000575 [DOI] [PubMed] [Google Scholar]
- 174.Cnossen MC, Scholten AC, Lingsma HF, Synnot A, Tavender E, Gantner D, et al. Adherence to Guidelines in Adult Patients with Traumatic Brain Injury: A Living Systematic Review. Journal of neurotrauma. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Badgett RG, Vindhyal M, Stirnaman JT, Gibson C, Halaby R. A living systematic review of nebulized hypertonic saline for acute bronchiolitis in infants. JAMA Pediatrics. 2015;169(8):788–9. doi: 10.1001/jamapediatrics.2015.0681 [DOI] [PubMed] [Google Scholar]
- 176.Bragge P, Chau M, Pitt V, Bayley M, Eng J, Teasell R, et al. An overview of published research about the acute care and rehabilitation of traumatic brain injured and spinal cord injured patients. Journal of neurotrauma. 2012;29(8):1539–47. doi: 10.1089/neu.2011.2193 [DOI] [PubMed] [Google Scholar]
- 177.Lunny C, Synnot A, Volovici V, Mondello S, Cnossen M, Bragge P, et al. An overview of reviews to explore evidence for effectiveness of acute management strategies for moderate to severe traumatic brain injury. CRD42016048571: PROSPERO; 2016. [Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016048571. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.