Abstract
Activation of central glucocorticoid receptors caused by the stress that is associated with a learning task facilitates storage of the acquired information. The molecular mechanism underlying this phenomenon is entirely unknown. Glucocorticoid receptors can influence transcription both through DNA binding-dependent and -independent mechanisms. To assess the importance of these two modes of action for spatial memory, we here used male mutant mice in which homodimerization and DNA binding of the glucocorticoid receptor is largely prevented (GRdim/dim) while protein–protein interactions still can take place. These mice showed a selective impairment of spatial memory in the water maze. Locomotion and anxiety-related parameters measured in an open field and a light/dark preference task were comparable for mutant and control mice. Mutant mice released more corticosterone than control mice under basal resting conditions and in response to swimming, which could have influenced memory processes of the mice. However, mimicking the task-related increase in corticosterone by supplementary injection of corticosterone (250 μg/kg, i.p.) in adrenalectomized mice, resulting in equal plasma corticosterone concentrations in both genotypes, improved spatial memory of control mice but had no effect on mutant mice. These findings suggest that task-related facilitating effects of corticosterone on spatial memory indeed depend on DNA binding of the glucocorticoid receptor rather than on protein–protein interactions of the receptor with other transcription factors. Although it cannot be excluded that both processes are involved in a coordinated way, interrupting the DNA-binding capacity of the receptor is sufficient to induce impairment.
Central actions of corticosteroids, which are secreted from the adrenal glands in high amounts after stress, are exerted via two receptors (1, 2): the high affinity mineralocorticoid (MR) and the lower affinity glucocorticoid receptors (GRs). The MR is already to a large extent activated under rest, whereas the GR becomes fully activated only at the circadian peak of corticosterone release and after stressful events (2). The two receptors are involved in specific aspects of information processing. MRs play a role in behavioral reactivity during novel situations (3). Importantly, activation of GRs caused by the stress associated with a learning task facilitates consolidation of information (4–6). Consequently, if one interferes with GR activation that occurs in association with a learning task by treatment with exogenous GR antagonists (7–9) or by knocking out the GR (10, 11), consolidation is impaired. Although the role of MR and GR activation in cognition thus is well established, the molecular mechanism underlying corticosteroid actions on learning and memory presently is entirely unknown.
Corticosteroid hormones act through nuclear receptors that can affect gene transcription in two ways: (i) through transactivation, a process that requires homodimerization of the receptors and binding of homodimers to the DNA, and (ii) through transrepression, which involves interaction of receptor monomers with other transcription factors and does not depend on binding of the steroid receptor to the DNA (12, 13). Recently, mice were created that are impaired in the DNA binding-dependent function of GR (GRdim/dim mice) (14). These mice carry the point mutation A458T, which largely prevents dimerization and DNA binding of the GR while functions requiring protein–protein interactions with other transcription factors still take place. Brain and liver GR protein levels were found unchanged (14). In the present study we used these GRdim/dim mice and wild-type control mice to investigate the mechanism responsible for the cognitive effects of corticosterone. In each mouse we examined spatial learning ability in a water maze. To determine the specificity of behavioral deficits we also observed general activity, exploratory behavior, and indices for anxiety. Corticosterone concentrations were determined under basal resting conditions and in response to swimming. To mimic the amount of circulating corticosterone in mutant and control animals, adrenalectomized mice of both genotypes were supplemented with the same amount of exogenous corticosterone and tested in the water maze.
Methods
Animals.
GR dimerization-deficient (GRdim) mice were generated by homologous recombination in the E14/1 ES cell line using the Cre/loxP method as described previously (13). Heterozygous GR+/dim were intercrossed to generate GRdim/dim mutants. Mice were housed singly 4 days before and during behavioral experiments. Food and water were available ad libitum. Animals were tested in the room in which they were housed (alternating 12-h light/dark cycle: lights on between 0700 and 1900 h). Behavioral tasks were run between 0800 and 1400 h. The experimenter was unaware of the mice's genotype. After the behavioral tests were completed, mice were decapitated and their genotype was verified by PCR. Animal care procedures were conducted in accordance with the EC Council Directive of November 1986 (86/609/EEC). All experiments were approved by the local Animal Experiment Committee (project DED14).
In the first series of experiments, 11 male mutant mice (4–5 months of age; mean bodyweight ± SEM = 30.9 ± 1.23 g), and their wild-type littermates (n = 9; 31.1 ± 0.7 g) were used. Three days before behavioral tests started, blood samples (100 μl) were collected via a small incision at the base of the tail (15) 1 h after lights on (circadian trough) and 1 h before lights off (circadian peak). In these samples, plasma corticosterone concentrations were measured by RIA (ICN). Circulating levels of plasma corticosterone were elevated significantly in GRdim/dim mice both at the trough and peak of the circadian hormone release pattern (mean corticosterone ± SEM: mutants, 38.6 ± 7.5 and 165.5 ± 17.6 ng/ml at the trough and peak, respectively; controls, 9.6 ± 1.6 and 99.8 ± 10.8 ng/ml; F(2,16) 8.800; P = 0.003). A second series of experiments was performed on 14 GRdim/dim mutant mice (4–6 months of age; bodyweight 30.9 ± 0.6 g) and 16 control mice (bodyweight 32.4 ± 0.9 g).
Behavioral Tasks.
Light/dark preference, open field behavior, and spatial learning and memory were tested sequentially within 14 days. Mice were taken from the home cage at the base of their tail and placed in the behavioral apparatus. To get the mice out of the apparatus, a grid was presented on which they could climb. Thereby, we prevented task-unrelated activation of the animal. The light/dark box and open field arena were swept clean between tests with 1% acetic acid solution to spread odors. Behavior was recorded on videotape and analyzed by EthoVision 1.95 (Noldus Information Technology, Wageningen, The Netherlands).
Light/Dark Preference.
Four animals were tested at the same time by using four separate alleys. These alleys were made of Plexiglas (40 cm long, 10 cm wide, and 35 cm high; half black/half white; black part covered with a lid). The mouse was placed in the dark part of an alley and allowed to explore the whole apparatus for 10 min. Rodents prefer dark surroundings. A long latency to leave the dark compartment, a short time spent, and short distance walked in the white compartment (600 lux) as well as a low number of crossings between the compartments can be used as indicators for anxiety.
Open Field.
In the center of a round arena (white Plexiglas: 80 cm in diameter; side wall 30 cm high; 300 lux), an object [piece of a hand brush 2.5 × 2.5 cm, which is an attractive stimulus for mice (N. Fentrop, personal communication)] was placed. The mouse was placed gently somewhere along the side wall and allowed to explore for 10 min. The open field was subdivided into three zones: the wall area (circle of 10 cm along the side wall; 37% of the arena), middle area (circle of 20 cm; 57% of the arena), and object area (circle of 10 cm around the center of the object; 6% of the arena). The walking distance as well as the time spent and entries into the object area and the distribution of activity over time are indicators of behavioral reactivity. General locomotor activity is given by the total walking distance and its change in time. Long latency to leave the area along the side wall might also indicate anxiety.
Water Maze.
A pool (white; 80 cm in diameter) was filled with warm water (26 ± 1°C) and made opaque by the addition of chalk. A platform (8 cm in diameter) was situated 8 mm below the surface of the water, invisible for the animal (spatial condition) or 8 mm above the water level (dark-colored rim; visible condition). During training, the pool was divided into four quadrants with the platform in the middle of one of the quadrants. For each trial, the mouse was placed in the water at a different location. A maximum of 60 sec was allowed, during which the mouse had to find the platform and climb onto it. It remained there for 20 sec (day 1) or 10 sec (other trials). If the animal did not find the platform, it was guided there with a grid and was allowed to stay for 20 sec on the platform. Four animals were run sequentially for the same trial during one session. After each trial mice were placed under a red-light warming lamp to dry. Before and after training, free swim trials were run in which the platform was removed. In these free swim trials, the mouse was placed into the water opposite to the former location of the platform and allowed to swim for 60 sec.
Schedule and Procedure.
On the day before spatial training in the water maze started, the pool was filled with 2 cm of warm water and a large flat object to climb on. This was the mice's first contact with water, and each mouse was allowed to move around for 120 sec (i.e., adaptation trial). The next day (day 1) started with a 120-sec free swim trial in the absence of the platform followed 60 min later by the first spatial training trial. By subjecting animals to a free swim trial before spatial training they were expected to be more motivated to search for escape from the novel “aversive” environment and accept the underwater platform as a “safe” place. Moreover, it allowed estimation of the swim ability of the mice as well as determination of the pretraining swim pattern of the animals (exploratory strategy) including putative basic preferences for a certain part of the pool. Training trials 2–4 were followed by another 60-min interval. On the following days the interval between trials was ≈5 min except when stated otherwise. On day 2, four training trials were run (trials 5–8), and days 3 and 4 contained two blocks of four training trials with an interval of 90 min (trials 9–16 and 17–24, respectively). During the second block of trials on day 4 (trials 21–24) the platform was visible. The visible platform was positioned in another quadrant than the submerged platform.
The second free swim trial was given after spatial training on day 4 (<5 min after trial 20) at a time when animals have made the transition from general exploratory to spatial search for the platform. The time or distance swum to reach the platform should be comparable or shorter than during the first free swim trial. Of particular interest is the swim pattern, which will depict the accuracy of search and a preference for a certain area of the pool.
For all platform training trials we assessed the swim velocity (cm/sec) as well as the distance swum (cm) and time needed (sec) to find and climb on the platform. Latency and distance to reach the position of the (now absent) platform were also analyzed for the free swim trials. The swim pattern was quantified by the cumulative distance to platform (16, 17), i.e., calculation of the position of the swimming mouse with respect to platform locations, estimated at five times per second. To assess the accuracy toward the trained submerged platform location, “arbitrary” platform locations in the other three quadrants were used. Crossings of the platform positions were counted as well. Additionally, we divided the pool into three circular areas (along the side wall, over the platform locations, and in the center) to assess the explorative and search patterns used. The total distance swum indicates the level of general activity.
Statistics.
Data were subjected to one-way ANOVA (F), when appropriate, with repeated measurements followed by a post hoc Tukey test. Significance was accepted at P < 0.05. Results are presented as mean ± SEM.
Results
Water Maze.
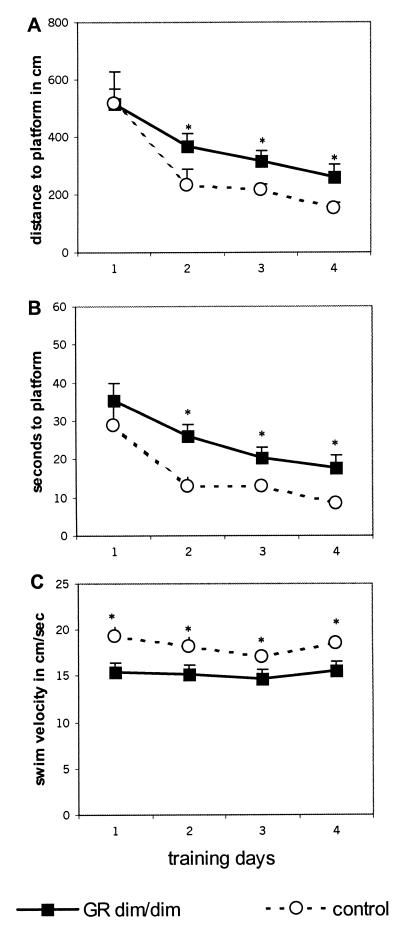
GRdim/dim mice showed impaired spatial memory, as is evident from the significantly longer swim distances and latencies required to locate the submerged platform compared with control mice (Fig. 1; distance F(1,17) 5.947 P = 0.026; latency F(1,17) 7.369 P = 0.015). Approach of the visible platform did not differ between the groups (distance in m: mean ± SEM GRdim/dim, 1.8 ± 0.4; control, 1.5 ± 0.2).
Figure 1.
Water maze performance of GRdim/dim and control mice. Distance in cm (A) and latency in sec (B) to reach the submerged platform were enhanced in GRdim/dim relative to control mice from day 2 onward. Swim velocity in cm/sec (C) was lower in GRdim/dim mice. Data are expressed as mean ± SEM. *, P < 0.05 between groups during spatial training trials.
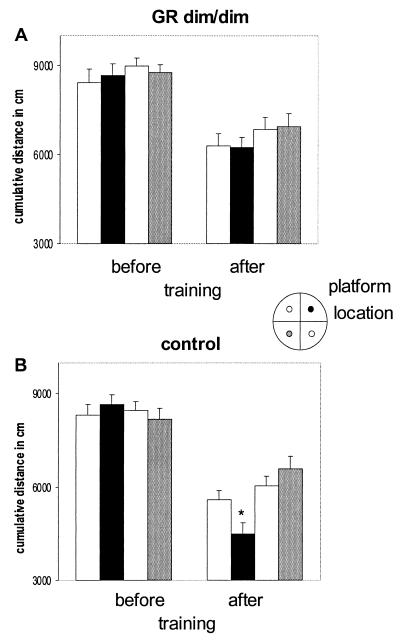
Free swimming (FS) after training revealed that the specificity of the swim pattern of GRdim/dim mice toward the learned platform position is impaired also compared with the control group. GRdim/dim as opposed to control mice did not show a clear preference to the (earlier) location of the underwater platform (Fig. 2; cumulative distance, F(1,17) 4.658 P = 0.045). Moreover, the swim distance to the (earlier) platform location was significantly longer in GRdim/dim mice than in controls (Table 1; F(1,16) 5.729 P = 0.013). Although the specificity of the swim pattern toward the learned platform position was apparently lost in GRdim/dim mice, they mastered some of the requirements of the task, i.e., to look for a platform in general at a certain distance from the side wall. This is supported by three observations. First, the cumulative distance toward the platform locations (16, 17) decreased significantly in both genotypes from 80–90 m during the FS before training to 50–60 m in the FS after training (see Fig. 2). In a second type of analysis, the pool was divided into three zones: along the side wall, a circle covering the “platform locations” (i.e., trained and three arbitrary locations), and the center. Before training, no difference was found in the distribution of time spent in these areas. FS after training revealed that both genotypes knew where to look for a platform, because they spent most time swimming in the platform area (FS before, 15 sec; FS after, >30 sec). Third, although crossings of the trained platform location were similar in GRdim/dim (mean number ± SEM, 5.7 ± 1.0) and control mice (6.9 ± 0.7), only control mice preferred to visit the trained compared with the arbitrary platform locations (data not shown).
Figure 2.
Cumulative distance in cm to trained platform and three arbitrary locations during free swim trials in the absence of the platform in GRdim/dim (A) and control mice (B). Cumulative distance is the measure of the position of the swimming mouse with respect to platform locations, calculated five times per second during the first 30 sec of the free swim trials. Spatial training markedly reduced the cumulative distance in both groups, with control mice expressing the shortest distance to the trained platform location. *, P < 0.05 trained platform location versus arbitrary platform locations in the other quadrants of the pool. (Inset) Training location of submerged platform (black) and other possible platform locations. The shading of the platform locations in the Inset corresponds with the bars in A and B.
Table 1.
Free swim trials
| Total swim distance, m
|
Distance to platform, m
|
Velocity, cm/sec
|
||||
|---|---|---|---|---|---|---|
| FS before | FS after | FS before | FS after | FS before | FS after | |
| GRdim/dim | 8.9 ± 0.7* | 10.0 ± 0.6* | 4.2 ± 1.1 | 2.5 ± 0.7* | 15.2 ± 1.2* | 16.8 ± 1.0* |
| Control | 11.4 ± 0.6 | 11.7 ± 0.5 | 4.1 ± 1.2 | 0.8 ± 0.2 | 19.2 ± 0.9 | 19.6 ± 0.9 |
GRdim/dim mice swim less and slower during the 60 sec of the free swim trials before and after water maze training. Data are expressed as mean ± SEM.
, P < 0.05 vs. control.
The relatively larger swim distance to the submerged (but not the visible) platform during platform training and the lack of preference for the learned location of the platform in the FS trial observed in GRdim/dim relative to control mice strongly support that mutant mice displayed impaired spatial memory. Both parameters are independent of possible disturbances in swim ability of GRdim/dim mice. This is important, because mutant mice indeed exhibited reduced swim velocity in the training sessions [F(1,17) 8.026 P = 0.01; Fig. 1], which in part explains the relatively long latency to locate the platform position. Moreover, separate analysis of the 120-sec swimming before training (divided into periods of 10 sec; data not shown) revealed that GRdim/dim mice swam less and slower than control mice [F(1,17) 4.853 P = 0.033]. This behavior result occurred mainly within the first 60 sec; only control mice decreased their swim distance over time [interaction genotype × period, F(11,187) 2.690 P = 0.003]. Also during the 60-sec analysis period of the FS trials before and after training (Table 1), GRdim/dim mice swam less and slower than control mice (distance, F(1,17) 6.380 P = 0.022; velocity, F(1,17) 6.312 P = 0.022).
Open Field and Light/Dark Preference.
The difference between GRdim/dim and control mice with respect to swim velocity was not a general disturbance in locomotion. Thus, in the open field mice of both groups walked comparable distances with similar velocity (Table 2). Distribution of activity along the side wall and the center of the open field as well as the latency to leave the side wall were similar. Both control and GRdim/dim mice spent ≈6% of their time exploring the object in the center. Dividing these open field parameters into periods of 2 min showed that the distribution of behavior within the total period of 10 min was comparable between the groups (data not shown).
Table 2.
Open field
| Genotype | Distance walked, m | Velocity, cm/sec | Time along sidewall, sec | Time in center, sec | Time to center, sec |
|---|---|---|---|---|---|
| GRdim/dim | 44.9 ± 8.3 | 14.8 ± 1.2 | 394.7 ± 26.5 | 37.5 ± 6.3 | 41.2 ± 14.2 |
| Control | 44.3 ± 8.4 | 14.8 ± 0.6 | 390.3 ± 31.6 | 39.4 ± 4.2 | 44.7 ± 14.9 |
GRdim/dim and control mice showed comparable locomotor activity (distance walked and velocity) and exploratory pattern (time along side wall and in center; latency to enter the center). Data are expressed as mean ± SEM.
When tested for light/dark preference, no significant difference was observed with respect to time spent in the light compartment in control (mean ± SEM percentage of time, 37.9 ± 3.9%) versus GRdim/dim mice (46.1 ± 3.5%). Also, the latency to leave the dark compartment for the first time (<50 sec), crossings between the compartments (≈20), and distance moved in the light compartment (≈5 m) were similar in the two groups (data not shown).
Water Maze in Corticosterone-Replaced Mice.
As found in the present and earlier studies (14), GRdim/dim mice showed increased plasma corticosterone concentrations both at the trough and peak of the circadian cycle compared with the wild-type controls. Circulating corticosterone levels, however, are known to influence learning and memory in the Morris water maze task (3–5). To address the question of whether the aberrant corticosterone concentrations of GRdim/dim mice rather than the missing transcriptional pathway contributed to the impaired water maze performance, a set of control experiments was performed. An independent group of mice was tested for (i) corticosterone responsiveness during swim trials in the water maze and (ii) water maze performance with fixed concentrations of corticosterone.
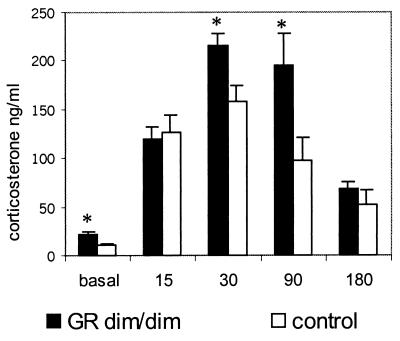
In this set of animals, basal resting concentrations of corticosterone were elevated significantly in GRdim/dim mice compared with the wild-type control group (mean corticosterone ng/ml ± SEM: mutant, 21.5 ± 3.2; control, 11.1 ± 1.4; P = 0.008; Fig. 3). To determine whether task-related corticosterone responses were increased as well, mice were subjected to a 1-min swim in a pool (140 cm in diameter; warm water of 26 ± 1°C; made opaque by chalk; the same pool was used later for water maze training), placed for 3 min under a heating lamp to dry, and then returned to their home cage. During blood sampling at 15, 30, 90, and 180 min after swimming (for methods of blood sampling and assay, see above), mice remained in their home cage. As shown in Fig. 3, mutant mice indeed released more corticosterone than control mice; the peak amplitude at 30 min (P = 0.02) was significantly higher as was the corticosterone concentration at 90 min (P = 0.02).
Figure 3.
Corticosterone response (ng/ml) to 1 min of swimming. Basal values were estimated on the day before swimming. The time points 15, 30, 90, and 180 min give corticosterone concentrations in plasma measured in the home cage after swimming. *, P < 0.05 GRdim/dim versus control group.
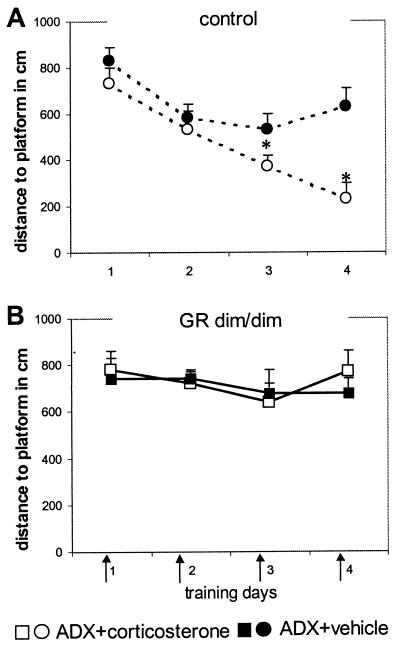
To exclude the influence of these increased corticosterone levels on water maze performance, mice were adrenalectomized bilaterally under isopentane anesthesia and supplemented with corticosterone in context with water maze training trials. In control mice, we determined the test conditions. Three days after adrenalectomy, water maze training started. Corticosterone (250 μg/kg i.p.; corticosterone-HBC complex, Sigma) or vehicle (0.2 ml of saline/25 g of bodyweight) was injected daily directly before the first trial of the day (days 1 and 2, four trials; days 3 and 4, 3 trials; 5-min intertrial interval; duration per trial, maximum of 60 sec; platform submerged in a fixed location). This training and injection paradigm was chosen to keep corticosterone elevated in close association with water maze performance. As expected, adrenalectomized control mice (Fig. 4A) treated with corticosterone improved their performance, as indicated by a decrease in distance swum to the platform and the significant difference from vehicle-treated controls (treatment distance, F(1,14) 10.159, P = 0.007; latency, F(1,14) 5.493 P = 0.034). The inferior performance of the adrenalectomized vehicle-treated control mice was evident also from the similar distances to the platform over days. In GRdim/dim mice (Fig. 4B), the same treatment and training schedule revealed no differences between the adrenalectomized corticosterone-treated or vehicle-treated mice. Two days after water maze training, mutant and control mice received an i.p. injection of 250 μg/kg corticosterone. Blood samples were taken at 15, 30, 60, and 180 min after injection. Plasma corticosterone concentrations in mutant and control mice were comparable (ng/ml at 15, 30, 60, and 180 min: mutant, 270.7 ± 34.0, 163.9 ± 8.6, 127.1 ± 17.0, and 52.7 ± 11.0; control, 279.2 ± 16.7, 176.5 ± 7.8, 142.7 ± 15.0, and 69.3 ± 10.1), indicating that no differences in clearance existed between the mutant and control mice.
Figure 4.
Water maze performance of adrenalectomized (ADX) control (A) and GRdim/dim (B) mice. Arrows indicate that the mice were injected with corticosterone (250 μg/kg i.p.; filled symbols) or vehicle (0.2 ml of saline/25 g of bodyweight; open symbols) before the daily training trials. Corticosterone improved the performance of adrenalectomized control but not of GRdim/dim mice. The data represent mean distance to platform in cm (± SEM)/day. *, P < 0.05 between groups.
Discussion
Our data in the water maze clearly show that spatial memory is impaired in GRdim/dim mutant mice. From day 2 onward GRdim/dim mice took longer distances to locate the platform than control animals. Although GRdim/dim mice slightly improved their performance over the days, they performed inferior to the control mice in this spatial task. Importantly, simplification of the water maze task by using a visible platform resulted in similar performance of GRdim/dim and control mice. That the swim distances to the platform on the first day of training in the spatial task are comparable furthermore shows that GRdim/dim and control mice displayed a similar short term acquisition of the task.
The GRdim/dim mice exhibited a reduced swim velocity during the training trials. Because these animals not only took more time but also swam longer distances, the differences in swim velocity did not confound our conclusion that spatial memory performance in the mutant mice was impaired. The impaired performance in the water maze spatial learning task did not signify a general behavioral deficit in the mutant mice. For instance, the locomotor activity of GRdim/dim mice in the “dry” tasks (i.e., open field and light/dark tasks) did not differ from control mice. Moreover, none of the parameters, which are related generally to anxiety, differed between the two groups. It is extremely important to monitor locomotion in connection with anxiety; increased locomotion as was observed in other mutant strains (10, 18, 19) may account for seemingly less anxious behavior when not controlled for.
Earlier studies in which pharmacological tools were applied to selectively activate corticosteroid receptors implicated GRs in memory consolidation and MRs in behavioral reactivity during novel situations (3, 8, 20). This points to coordinated MR- and GR-mediated actions on behavior. Therefore, it is of interest to know whether the mutation of GR affected the functionality of MR as was found previously in GR knockout and GR antisense mice (10, 18) but also with continuous infusion of GR antagonist in rats (21). However, the presently observed similar explorative patterns of GRdim/dim and control mice in the novel environments of the open field, the light/dark box, and the first exposure to the pool (in the absence of a platform) point to a functional MR. Thus, the unique experimental model of GRdim/dim mice allowed us to identify the molecular mechanism underlying GR-mediated effects on spatial memory in the face of unaltered functioning of the MR.
Previously (14) it was shown that in contrast to GR-mediated effects on corticotropin-releasing hormone expression, GR-dependent inhibitory feedback on proopiomelanocortin expression is impaired in GRdim/dim mice. In agreement, we and others (22) showed that circadian corticosterone concentrations were higher in GRdim/dim compared with control mice. Furthermore, GRdim/dim mice showed increased and prolonged corticosterone release in response to swimming, suggesting impaired GR-dependent inhibitory feedback. Finally, a similar observation was made in the restraint stress paradigm, in which mice also exhibited elevated corticosterone secretion after 20 min of immobilization (data not shown). Taken together, these findings imply that GRdim/dim mice are exposed to higher amounts of corticosterone in association with the learning task than control mice. On the other hand, elevated corticosterone concentrations associated with a learning situation in rats improved consolidation of learned information (5, 20), whereas rises in corticosterone level out of context impair cognitive function (for review see ref. 23). In agreement with the former, we presently found that mice receiving exogenous corticosterone in context with water maze performance respond with an improvement of spatial learning and memory. Importantly, the same amount of corticosterone was ineffective in GRdim/dim mice. Therefore, the deficit in spatial memory of GRdim/dim mice needs to be explained by the lack of DNA binding-dependent transcriptional regulation by GR rather than by the higher amount of corticosterone released in these animals.
The present data suggest that the memory-facilitating effect of GR activation in the context of a spatial task requires DNA binding of GR homodimers and thus the transactivation pathway of transcription. Transrepression via GR through protein–protein interactions seems insufficient to establish “normal” function of corticosteroid hormones on spatial memory, although it cannot be excluded that this pathway may contribute to hormonal modulation of behavior under different experimental conditions.
Acknowledgments
The help of Marc Fluttert and Leo Enthoven with the behavioral settings, Jeannette Grootendorst and Sergiu Dalm with the corticosterone RIA, and Peter Gass on discussing behavioral studies with mice is gratefully acknowledged. This study was supported by European Community EC Biotec Grant 96-0179 and Netherlands Drug Research Foundation Grant 014.80.005.
Abbreviations
- MR
mineralocorticoid
- GR
glucocorticoid receptor
- GRdim
GR dimerization-deficient
- FS
free swimming
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.McEwen B S, de Kloet E R, Rostene W. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet E R. Front Neuroendocrinol. 1991;12:95–164. [Google Scholar]
- 3.Oitzl M S, Fluttert M, de Kloet E R. Eur J Neurosci. 1994;6:1072–1079. doi: 10.1111/j.1460-9568.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandi C, Rose S P R. Brain Res. 1994;647:106–112. doi: 10.1016/0006-8993(94)91404-4. [DOI] [PubMed] [Google Scholar]
- 5.Sandi C, Loscertales M, Guaza C. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 6.Shors T. Neurobiol Learn Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- 7.Oitzl M S, de Kloet E R. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- 8.Sandi C, Rose S P R. Eur J Neurosci. 1994;6:1292–1297. doi: 10.1111/j.1460-9568.1994.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 9.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1997;2:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 10.Oitzl M S, de Kloet E R, Joëls M, Schmid W, Cole T J. Eur J Neurosci. 1997;9:2284–2296. doi: 10.1111/j.1460-9568.1997.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 11.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban P C, Bock R, Klein R, Schutz G. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 12.Beato M, Herrlich P, Schütz G. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 13.Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf H J, Herrlich P, Cato A C. EMBO J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichardt H M, Kaestner K H, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Amgel P, Schutz G. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 15.Durschlag M, Wurbel H, Stauffacher M, van Holst D. Physiol Behav. 1996;60:1565–1568. doi: 10.1016/s0031-9384(96)00307-1. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher M, Burwell R, Burchinal M. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 17.Dalm S, Grootendorst J, de Kloet E R, Oitzl M S. Behav Res Methods Instrum Comput. 2000;32:134–139. doi: 10.3758/bf03200795. [DOI] [PubMed] [Google Scholar]
- 18.Steckler T, Weis C, Sauvage M, Mederer A, Holsboer F. Behav Brain Res. 1999;100:77–89. doi: 10.1016/s0166-4328(98)00115-6. [DOI] [PubMed] [Google Scholar]
- 19.Steckler T, Holsboer F. Behav Brain Res. 1999;102:151–163. doi: 10.1016/s0166-4328(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 20.de Kloet E R, Oitzl M S, Joëls M. Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 21.Oitzl M S, Fluttert M, Sutanto W, de Kloet E R. Eur J Neurosci. 1998;10:3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 22.Karst H, Karten Y J G, Reichardt H M, de Kloet E R, Schütz G, Joëls M. Nat Neurosci. 2000;3:977–978. doi: 10.1038/79910. [DOI] [PubMed] [Google Scholar]
- 23.McEwen B S. Front Neuroendocrinol. 1999;20:49–70. doi: 10.1006/frne.1998.0173. [DOI] [PubMed] [Google Scholar]