Abstract
Background
The diagnosis of prosthetic joint infection (PJI) is still a challenge in some patients after total joint replacement. Interleukin-6 (IL-6) strongly participates in the arrangement of the host-bacteria response. Therefore, increased levels of IL-6 should accompany every PJI.
Purpose
The aim of the study was to show diagnostic characteristics of serum IL-6 for the diagnosis of prosthetic joint infection (PJI). We also compared the diagnostic values of serum IL-6 with synovial IL-6 (sIL-6) and synovial C-reactive protein (sCRP).
Study design
We performed a prospective study of 240 patients in whom serum IL-6 was determined before total hip (n = 124) or knee (n = 116) reoperations. The PJI diagnosis was based on the MSIS (Musculoskeletal Infection Society) criteria (2011). Receiver operating characteristic plots were constructed for IL-6, sIL-6, and sCRP.
Results
PJI was diagnosed in 93 patients, and aseptic revision was diagnosed in 147 patients. The AUC (area under curve) for IL-6 was 0.938 (95% CI; 0.904–0.971). The optimal IL-6 cut-off value for PJI was 12.55 ng/L. Positive and negative likelihood ratios for IL-6 were 8.24 (95% CI; 4.79–14.17) and 0.15 (95% CI; 0.09–0.26), respectively. The optimal sIL-6 and sCRP cut-off values were 20,988 ng/L and 8.80 mg/L, respectively. Positive and negative likelihood ratios for sIL-6 were 40.000 (95% CI; 5.7–280.5) and 0.170 (95% CI; 0.07–0.417), respectively. Negative likelihood ratio for sCRP was 0.083 (95% CI; 0.022–0.314).
Conclusions
The present study identified the cut-off values for serum/synovial IL-6 and synovial CRP for diagnostics of PJI at the site of THA and TKA and separately for each site. The diagnostic odds ratio for serum/synovial IL-6 and synovial CRP is very good. Simultaneous positivity of serum IL-6 either with synovial IL-6 or synovial CRP almost excludes false negative detection of PJI at the site of interest.
Introduction
Prosthetic joint infection (PJI) is a feared complication of total joint arthroplasty (TJA). PJI accounts for almost 50% of failed total knee arthroplasties (TKA), [1], and around 17% of reoperated total hip arthroplasties (THA), [2]. The presence or absence of PJI has a crucial impact on the orthopaedic surgeon’s decision about the further treatment strategy. However, discrimination between infected and aseptic failed total joint arthroplasties can be difficult in some cases, as the physical examination does not reveal pathology except pain, and laboratory results may be equivocal. On the other side of the clinical presentation spectrum are patients with increased suspicion of PJI with painful joints and cloudy dense yellow/white viscous joint fluid who may be negative for PJI [3].
Interleukin-6 (IL-6) is a soluble mediator expressed as part of host defense against a wide range of environmental stresses including microorganism invasion [4]. This is why it is one of the key cytokines, which is strongly up-regulated during septic inflammation. IL-6, among others, contributes to the expression and release of CRP (C-reactive protein). It is also known that the serum/local expressions of IL-6 in patients with PJI differ detectably from those with aseptic failure [5]. Importantly, the postoperative decrease of serum IL-6 is rapid for applying the test early postoperatively [6, 7]. A number of studies have examined the diagnostic behavior of pre-operative detection of serum/synovial IL-6 in patients with PJI [8–13]. Diagnostic accuracy of the serum IL-6 test for PJI has been examined also in the meta-analysis/systematic reviews of these studies [14, 15]. The most recent one of them concludes that serum IL-6 is less sensitive than the synovial fluid IL-6 test but still could have a value for patients with prosthetic failure due to its high specificity.
The purpose of the current diagnostic study is to show the diagnostic characteristics of serum interleukin-6 for the pre-operative diagnosis of PJI either as a single test or in combination of synovial IL-6 (sIL-6) and CRP (sCRP), to assess the optimal threshold value, and to compare these results with the currently available diagnostic standards.
Materials and methods
The Ethical Committee of the Faculty of Medicine and Dentistry, Palacky University Olomouc and the Teaching Hospital Olomouc approved this study as part of the Internal Grant Agency, Ministry of Health Czech Republic project No. NT11049-5
Patients and controls
We prospectively collected blood/synovial fluid samples from 240 patients who underwent revisions of total hip or knee replacements at our Department (Table 1).
Table 1. Basic characteristics of the group of patients reoperated due to PJI and controls (median; range).
| THA+TKA | THA | TKA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PJI | Controls | p-value | PJI | Controls | p-value | PJI | Controls | p-value | |
| Gender, F/M | 49/44 (52.7%/47.3%) | 90/57 (61.2%/38.8%) | 0.192 | 22/12 (64.7%/35.3%) | 66/24 (73.3%/26.7%) | 0.379 | 27/32 (45.8%/54.2%) | 24/33 (42.1%/57.9%) | 0.712 |
| Age at the time of the revision, yrs. | 73.3 (40.6–90.9) | 70.7 (44.3–86.3) | 0.248 | 73.7 (40.6–90.9) | 70.4 (44.3–86.3) | 0.260 | 72.5 (47.8–87.0) | 70.9 (49.9–85.4) | 0.749 |
| BMI | 30.5 (20.5–48.9) | 29.7 (19.5–50.2) | 0.873 | 28.2 (21.5–45.0) | 28.6 (19.5–50.2) | 0.920 | 31.0 (20.5–48.9) | 31.0 (22.3–40.9) | 0.411 |
| Primary OA/Secondary OA | 55/38 (59.1%/40.9%) | 84/63 (57.1%–42.9%) | 0.760 | 16/18 (47.1%/52.9%) | 39/51 (43.3%/56.7%) | 0.710 | 39/20 (66.1%/33.9%) | 45/12 (78.9%/21.1%) | 0.122 |
| Stability of implant | |||||||||
| 0 –both components stable | 61 (65.6%) | 72 (49.0%) | <0.0001 | 19 (55.9%) | 35 (38.9%) | <0.0001 | 42 (72.4%) | 37 (64.9%) | 0.106 |
| 1 –FC stable, TC non-stable | 9 (9.7%) | 17 (11.6%) | 0 (0.0%) | 8 (8.9%) | 9 (15.5%) | 9 (15.8%) | |||
| 2 –FC loosened, TC stable | 2 (2.2%) | 54 (36.7%) | 0 (0.0%) | 45 (50.0%) | 2 (3.4%) | 9 (15.8%) | |||
| 3 –both components loosened | 7 (7.5%) | 4 (2.7%) | 2 (5.9%) | 2 (2.2%) | 5 (8.6%) | 2 (3.5%) | |||
| 5 –cup loosened, stem stable | 9 (9.7%) | 0 (0.0%) | 9 (26.5%) | 0 (0.0%) | |||||
| 6 –cup stable, stem loosened | 4 (4.3%) | 0 (0.0%) | 4 (11.8%) | 0 (0.0%) | |||||
| Type of pt. | |||||||||
| A | 35 (37.6%) | 32 (56.1%) | 0.024 | 12 (35.3%) | 46 (51.1%) | 0.265 | 23 (39.0%) | 32 (56.1%) | 0.006 |
| B | 52 (55.9%) | 19 (33.3%) | 17 (50.0%) | 35 (38.9%) | 35 (59.3%) | 19 (33.3%) | |||
| C | 6 (6.5% | 6 (10.5%) | 5 (14.7%) | 9 (10.0%) | 1 (1.7%) | 6 (10.5%) | |||
| Local status | |||||||||
| 1 –uncompromised | 50 (53.8%) | 37 (25.2%) | 0.290 | 18 (52.9%) | - | - | 32 (54.2%) | 37 (64.9%) | 0.218 |
| 2 –compromised | 42 (45.2%) | 19 (12.9%) | 15 (44.1%) | 27 (45.8%) | 19 (33.3%) | ||||
| 3 –significantly compromised | 1 (0.7%) | 1 (2.9%) | 0 (0.0%) | 1 (1.8%) | |||||
| Time from index surg. (months) | 22.21 (0.26–236.12) | 116.2 (0.6–387.9) | - | 17.5 (0.3–236.1) | 151.0 (0.6–387.9) | - | 24.5 (0.3–200.6) | 47.2 (0.6–265.0) | - |
| Preop. clin. score# | - | - | - | 34.5 (5–90) | 43.0 (4–93) | 0.068 | Pain: 44.0 (0–77) | Pain: 53.0 (8–100) | Pain: 0.0004 |
| Function: 25.0 (-20–80) | Function: 50.0 (5–100) | Function: <0.0001 | |||||||
| CRP (mg/L) | 74 (1–435) | 3 (1–152) | <0.0001 | 50 (3–386) | 3 (1–47) | <0.0001 | 107 (1–435) | 3.5 (1–152) | <0.0001 |
| ESR (mm/h) | 60 (1–120) | 17 (2–94) | <0.0001 | 55 (10–120) | 17 (2–75) | <0.0001 | 61 (1–120) | 14.5 (2–94) | <0.0001 |
| Culture positive/negative | 76/17 (81.7%/18.3%) | 34/107 (24.1%/75.9%) | <0.0001 | 27/7 (79.4%/20.6%) | 14/76 (15.6%/84.4%) | <0.0001 | 49/10 (83.1%/16.9%) | 11/46 (19.3%/80.7%) | <0.0001 |
| Histology positive/negative | 40/53 (43.0%/57.0%) | 48/99 (32.7%/63.7%) | 0.105 | 14/20 (41.2%/58.8%) | 31/59 (34.4%/56.6%) | 0.487 | 26/33 (44.1%/55.9%) | 17/40 (29.8%/70.2%) | 0.112 |
THA = total hip arthroplasty, TKA = total knee arthroplasty, PJI = prosthetic joint infection, F = female, M = male, BMI = body mass index, OA = osteoarthritis, FC = femoral component, TC = tibial component, Charnley typology of the patient: A = non-compromised, B = compromised (1–2 compromising factors), C = severely compromised (more than 2 compromising factors), CRP = C-reactive protein, ESR = erythrocyte sedimentation rate
# = Harris hip score at the case of THA; pain/function part of Knee Society Score at the case of TKA.
Every patient who underwent a revision knee or hip arthroplasty at our institution between October 2010 and June 2016 was potentially eligible for the current study (Fig 1). We enrolled participants who underwent revision knee or hip arthroplasties for PJI or for aseptic reasons at the same locations (aseptic loosening, periprosthetic osteolysis, instability, pain for uncertain reason, recurrent effusions, polyethylene wear). Sixteen of the included patients had rheumatoid arthritis (9 of them belonged to the group of PJI). Fourteen of the included patients had gout (4 of them were infected). Five of the included patients had psoriasis (3 of them were infected). Four patients suffered from other systemic inflammatory diseases (idiopathic ulcerative colitis, systemic lupus erythematosus, autoimmune vasculitis with polyarthritis and autoimmune syndrome with antinuclear antibodies). There was no difference between the patients with systemic inflammatory disease and those without it in any parameter except the patients with rheumatoid arthritis. These had higher ESR (98 mm per hour versus 60 mm per hour) and serum IL-6 (568.3 versus 45.2 ng/L). We did not exclude patients revised within the first 6 weeks after the primary implantation of the prosthesis, either. Neither patients with antibiotic pretreatment administered prior to the revision surgery were excluded. Forty-six patients (48.9%) received antibiotic pretreatment for a median of 7 days (range, 0–197 days) prior to the revision surgery. The former had significantly higher (p = 0.008) synovial fluid white cell count compared to those not treated (median; 71.5 and 37.7x109/L, respectively).
Fig 1. Flowchart showing the number of patients included and excluded as well as their distribution in the infected and non-infected groups.
THA = total hip arthroplasty, TKA = total knee arthroplasty, TJA = total joint arthroplasty, PJI = prosthetic joint infection, IL-6 = interleukin 6, MSIS = Musculoskeletal Infection Society.
PJI was classified in terms of early (<3M), delayed (between 3 and 12M), hematogenous (>12 to 24M), positive intraoperative culture, and PJI from direct or contiguous spread (Table 2). Recurrent PJI was diagnosed in 5 patients: once with the same bacterium that was isolated during the previous revision, four times with different bacteria.
Table 2. Modes infection among the patients from the group of PJI.
| Mode of infection | Number of patients | Percent |
|---|---|---|
| Acute | 20 | 21.5% |
| Delayed | 25 | 26.9% |
| Haematogenous | 28 | 30.1% |
| Positive intraoperative culture | 3 | 3.2% |
| Recurrent infection (same bacterium) | 1 | 1.1% |
| Recurrent infection (different bacteria) | 4 | 4.3% |
| Direct or contiguous spread | 12 | 12.9% |
| Total | 93 | 100.0% |
PJI = prosthetic joint infection.
All the revisions were carried out under standard conditions with written informed patient consent, and the study was approved by the local Ethics Committee as part of the Internal Grant Agency, Ministry of Health Czech Republic project No. NT11049-5.
Protocol of the study
In the morning preceding the revision surgery, blood samples were taken and sent to the Department of Biochemistry where the serum IL-6 levels were determined.
Synovial joint fluids (SJFs) were collected from the participants preoperatively at an office visit and/or intraoperatively just before opening the joint cavity. Sterile tubes with synovial fluid were immediately transported to the collaborating facilities. If there were two sources of synovial results (i.e. from that taken at the office and just before capsule incision) for a single patient, we used the intraoperative one for further statistical analysis. The reason for this rule is simple: we always try to synchronize intraoperative sampling (i.e. joint fluid with sampling of tissues, implants).
The index tests were the levels of serum IL-6, sIL-6 and sCRP. The clinical information and the laboratory results needed for assessment of the diagnosis of PJI were not available to the readers of the serum/synovial IL-6/CRP tests (JP), SWCC (synovial fluid white cell count) and neutrophil/lymphocyte percentages (JJ), neither the microbiologist nor the pathologist at the time of their contribution. All the participants enrolled in the study were classified as PJI positive/negative according to the MSIS (Musculoskeletal Infection Society) definition of PJI by a trained orthopaedic surgeon (MS) who did not lead either the surgeries (sampling) or treatment.
Definition of PJI
The reference standard was a set of diagnostic criteria according to the MSIS recommendation [16]. Both the major and minor criteria were employed accordingly. Therefore, the diagnosis of PJI was not built using serum IL-6, sIL-6, and sCRP.
Determination of serum IL-6, sIL-6, and sCRP
All index tests, i.e. serum IL-6, sIL-6, and sCRP were determined on the modular analyzer system Cobas8000 (Roche/Hitachi). The determination of sCRP was based on the immunoturbidimetric assay. Electrochemiluminescence immunoassay (ECLIA) was used to determine IL-6. Data are shown in the Table 3.
Table 3. Data for IL-6 and synovial analysis (median; range) for the group of patients reoperated due to PJI and controls.
| THA+TKA | THA | TKA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PJI | Controls | Difference | PJI | Controls | Difference | PJI | Controls | Difference | |
| IL-6 (ng/L) | 48 (4–17,673) | 5 (2–130) | <0.0001 | 41 (5–808) | 5.1 (1.5–130) | <0.0001 | 664 (22–17,673) | 4.6 (1.5–76.9) | <0.0001 |
| sCRP (mg/L) | 33 (1–114) | 1.0 (0.0–8.0) | <0.0001 | 21 (1–114) | 1.0 (0.0–7.0) | 0.002 | 34 (9–86) | 1.0 (0.0–8.0) | <0.0001 |
| sIL-6 (ng/L) | 34,476 (295–50,000) | 565 (7–24,839) | <0.0001 | 44,950 (586–50,000) | 770 (7–24,839) | 0.004 | 30,732 (295–50,000) | 467 (7–4,738) | <0.0001 |
| SWCC (cells/μL) | 52,000(800–416,600) | 1,000 (0–39,900) | <0.0001 | 51,700 (1,000–416,600) | 1,100(0–39,900) | <0.0001 | 56,200 (800–328,700) | 910(30–5,720) | <0.0001 |
| Neutrophils (%) | 91 (42–98) | 51 (6–94) | <0.0001 | 90 (52–97) | 51 (12–92) | <0.0001 | 92 (42–98) | 54 (6–94) | <0.0001 |
| Lymphocytes (%) | 5 (0–59) | 47 (4–89) | <0.0001 | 5 (1–38) | 49 (8–89) | <0.0001 | 5 (0–59) | 45 (4–85) | <0.0001 |
IL-6 = interleukin 6, sCRP = synovial C-reactive protein, sIL-6 = synovial interleukin 6, SWCC = synovial white cell count, THA = total hip arthroplasty, TKA = total knee arthroplasty, PJI = prosthetic joint infection.
Culture
Synovial fluid and tissue cultures were analyzed in the patients under study after opening of the reoperated joint. Retrieved implants were sent for sonication in special transport boxes. After sampling for microbial examination, systemic or local antibiotics were administered to all the patients with the exception of 45 (47.9%) persons who received antibiotic treatment before the surgery. Overall, we collected at least 5 samples (range, 5–9) per patient. All the materials were transported to the laboratory immediately after sampling. The specimens were processed by the laboratory within 2 hours from the collection according to the standardized laboratory protocols for aerobic and anaerobic cultivation in PJI [17]. Sonication was performed according to the protocol published elsewhere [18].
Joint fluid aspirations were considered positive if there were any signs of growth, including in enrichment broth; however, concordance between these and the intraoperative findings was required. The intraoperative samples were considered positive for infection if the same bacterium was cultured from at least two different operative sites [19].
Histological examination
After opening the joint, the granulation tissue was sampled from the most susceptive sites. An experienced pathologist, who collaborated with our team on other studies, performed the histological analysis of the tissue samples. The conclusion was positive when a mean of >5 polymorphonucleocytes (PMNs) was seen on at least ten high power fields (HPFs) at 400-times magnification [20].
Synovial white cell count and differential count of leukocytes in synovial fluid
The SWCC was performed using the Sysmex XE-5000 (Kobe, Japan) automated hematology analyzer in the body fluid mode. The resulting values are given in number of cells/μL. The differential count of leukocytes (i.e. neutrophil/lymphocyte percentage) was determined manually by microscope: smears of the joint fluid samples were performed, stained by Pappenheim’s panoptic staining method, and then evaluated under microscope per 100 cells (leukocytes) at 1,000-times magnification. The resulting values are given in percentage [21].
Statistical analysis
The data were collected and continuously entered into electronic spreadsheets (Microsoft Excel, Microsoft Corp., WA, USA). The intended sample size was 150 participants. One of the main goals of the current study was to determine the optimal cut-off values for serum IL-6, sIL-6, and sCRP. To achieve this goal, we used the receiver operating characteristic curve (ROC). True positive rate (sensitivity) was plotted against the false positive rate (1 –specificity). The area under the curve (AUC) serves as a single measure characterizing the discriminative ability of the IL-6, sIL-6, and sCRP tests across the full range of cut-offs. An optimal cut-off value was determined using the Youden index (J), [22]. All the statistical calculations including sensitivity, specificity, positive and negative predictive values (PPV, NPV), positive and negative likelihood ratios (LR+, -), diagnostic odds ratio (DOR) and their confidence intervals were performed using IBM SPSS Statistics v.22. In addition, we determined the diagnostic accuracy of the combined tests of serum interleukin 6 with synovial CRP and IL-6. Other parameters were evaluated according to their type by a particular test using the same statistical package. Statistical significance was set at p = 0.05.
Results
Culture
The most frequent pathogen was Staphylococcus aureus followed by coagulase-negative staphylococci, beta-haemolytic streptococci and viridans streptococci. Table 4 provides a list of all the pathogens cultivated from the participants from the infected group.
Table 4. List of the pathogens cultivated from the sites of prosthetic joint infection.
| Pathogen | Frequency | Percentage |
|---|---|---|
| Staphylococcus aureus | 23 | 24.7% |
| Coagulase-negative Staphylococci | 23 | 24.7% |
| Haemolytic Streptococci | 13 | 14.0% |
| Anhaemolytic or viridans Streptococci | 3 | 3,2% |
| Enterobacteriacae | 6 | 6.5% |
| Pseudomonades | 1 | 1.1% |
| Enterococci | 2 | 2.2% |
| Polymicrobial finding | 3 | 3.2% |
| Negative culture | 17 | 18.3% |
| Others | 2 | 2.2% |
| Total | 93 | 100% |
Serum IL-6 in PJI
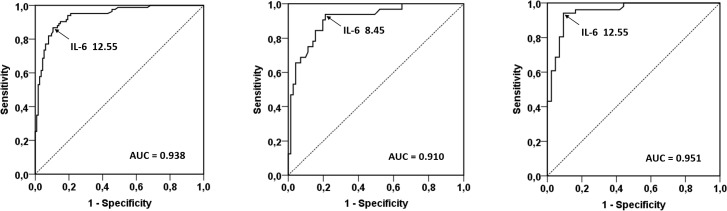
Serum IL-6 was significantly higher in patients with PJI (median, 48 ng/L; range, 4.0–17,673 ng/L; p<0.0001) than in those with aseptic failure (median, 5.0 ng/L; range, 2.0–130.0 ng/L). The ROC plots to determine an optimal cut-off value for IL-6 had the area under curve of 0.938 (95% CI; 0.904–0.971) for all the groups together (regardless of the site). According to the Youden index J method, the cut-off value for IL-6 with an optimal balance of the true positive rate (sensitivity) and false positive rate (1-specificity) was 12.55 ng/L. These cut-off values for serum IL-6 were 8.45 ng/L and 12.55 ng/L (positive = values greater than or equal to them; Table 5) when analyzed separately for THAs and TKAs, respectively (Fig 2).
Table 5. Cross tabulation of the IL-6 test results by the distribution of the patients to the aseptic and infected groups according to the MSIS criteria.
| IL-6 positive | IL-6 negative | Sum | |
|---|---|---|---|
| PJI | 72 | 11 | 83 |
| Aseptic reoperation | 12 | 102 | 114 |
| Sum | 84 | 113 | 197 |
IL-6 was not determined in all the patients included in this study (see Fig 1 and the Discussion). MSIS = Musculoskeletal Infection Society, PJI = prosthetic joint infection, IL-6 = interleukin 6.
Fig 2. The ROC plot of the serum IL-6 diagnostic test.
This ROC plot applies for both THA and TKA (a); THA (b); and TKA (c). ROC = receiver operating characteristic curve, THA = total hip arthroplasty, TKA = total knee arthroplasty, TJA = total joint arthroplasty.
The results were the same when the ROC analysis was done after exclusion of the patients with systemic inflammatory disease (cut-off value for the whole group was 12.55 ng/L; 8.45 and 12.55 ng/L for THA and TKA, respectively).
When the ROC analysis was done after exclusion of the patients treated preoperatively with antibiotics the cut-off value for IL-6 was 10.4 ng/L. These cut-off values for serum IL-6 were 8.45 ng/L and 12.85 ng/L (positive = values greater than or equal to them) when analyzed separately for THAs and TKAs, respectively.
Diagnostic utility of serum IL-6
Positive and negative likelihood ratios for THA+TKA were 8.24 (95% CI; 4.79–14.17) and 0.15 (95% CI; 0.09–0.26), respectively. Serum IL-6 works better at the site of the knee comparing to the THA. The complete list of the diagnostic characteristics for the IL-6 test is showed in the Table 6.
Table 6. Diagnostic characteristics of the serum IL-6 test in the patients with THA and/ or TKA.
| IL-6 ≥ | THA+TKA | THA | TKA | |||
|---|---|---|---|---|---|---|
| cut-off 12.55 | cut-off 8.45 | cut-off 12.55 | ||||
| 95% CI | 95% CI | 95% CI | ||||
| Sensitivity | 0.867 | 0.775–0.932 | 0.937 | 0.792–0.992 | 0.941 | 0.837–0.988 |
| Specificity | 0.895 | 0.823–0.944 | 0.789 | 0.676–0.877 | 0.907 | 0.779–0.974 |
| PPV | 0.857 | 0.777–0.912 | 0.667 | 0.558–0.759 | 0.923 | 0.825–0.968 |
| NPV | 0.903 | 0.842–0.942 | 0.966 | 0.879–0.991 | 0.929 | 0.812–0.975 |
| LR+ | 8.24 | 4.79–14.17 | 4.44 | 2.81–7.02 | 10.120 | 3.97–25.8 |
| LR- | 0.15 | 0.09–0.26 | 0.079 | 0.021–0.305 | 0.065 | 0.022–0.195 |
| DOR | 55.6 | 23.3–133.1 | 56.0 | 12–261 | 156.0 | 33–739 |
Diagnostic characteristics of the serum IL-6 test assessed in the patients with THA or TKA separately. The cut-off values were the following: 12.55 for THA+TKA; 8.45 for THA, and 12.55 for TKA. IL-6 = interleukin 6, THA = total hip arthroplasty, TKA = total knee arthroplasty, CI = confidence interval, PPV = positive predictive value, NPV = negative predictive value, LR = likelihood ratio, DOR = diagnostic odds ratio.
Synovial IL-6 and CRP in PJI
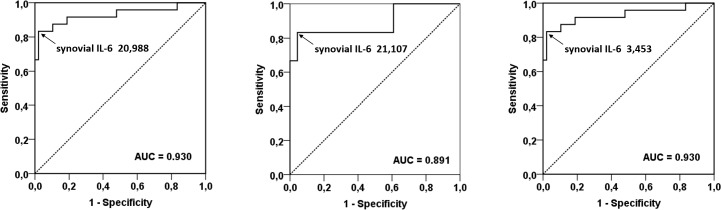
Synovial IL-6 was significantly higher in the patients with PJI (median, 34,477 ng/L; range, 295–50,000 ng/L; p<0.0001) than in those with aseptic failure (median, 565.0 ng/L; range, 7.0–24,839 ng/L). The ROC plots to determine an optimal cut-off value for synovial IL-6 had the area under curve of 0.930 (95% CI; 0.852–1.000) regardless of the site. The cut-off value for synovial IL-6 was 20,988 ng/L (Fig 3). Cut-off values were 21,107 ng/L and 3,453 ng/L (positive = values greater than or equal to them) when analyzed separately for THAs and TKAs, respectively. The corresponding cut-off values were 21,349 ng/L, 22,865 ng/L, and 3,453 ng/L when the patients with systemic inflammatory were excluded. A subanalysis for the patients not treated with antibiotics preoperatively was not possible as the number of suitable data was low.
Fig 3. The ROC plot of the synovial IL-6 diagnostic test.
This ROC plot applies for both THA and TKA (a); THA (b); and TKA (c). ROC = receiver operating characteristic curve, THA = total hip arthroplasty, TKA = total knee arthroplasty, IL-6 = interleukin 6.
Synovial CRP was significantly higher in patients with PJI (median, 33.2 ng/L; range, 1.1–113.7 ng/L; p<0.0001) than in those with aseptic failure (median, 0.9 mg/L; range, 0.1–8.3 ng/L). The ROC plots to determine an optimal cut-off value for synovial CRP had the area under curve of 0.974 (95% CI; 0.936–1.000) regardless of the site. The cut-off value for synovial CRP was 8.80 mg/L (Fig 3). When analyzed separately for THAs and TKAs, the cut-off values were 8.80 mg/L and 11.90 mg/L (positive = values greater than or equal to them), respectively. The corresponding cut-off values were 11.25 ng/L (for the whole group), 11.90 ng/L (for THA), and 11.25 ng/L (for TKA) when the patients with systemic inflammatory were excluded.
Diagnostic utility of sIL-6 and sCRP
The likelihood ratio for positive and negative result of sIL-6 for both THA and TKA were 40.00 (95% CI; 5.7–280.5) and 0.170 (95% CI; 0.07–0.417), respectively. Because of 100% specificity of synovial CRP, it was impossible to calculate likelihood ratios and DOR. The complete list of diagnostic characteristics is shown in the Table 7. In order to estimate the LR+, LR-, and DOR, we added 1.0 to all the cells with 0, then the following figures were calculated for all the patients (i.e. THA+TKA): 60.5, 0.085, and 715.0 for LR+, LR-, and DOR, respectively.
Table 7. Diagnostic characteristics of serum IL-6 when combined with other tests in patients with THA and/or TKA under assumption that both the tests are positive.
| Test | Location | Sensitivity | Specificity | PPV | NPV | LR+ | LR- | DOR |
|---|---|---|---|---|---|---|---|---|
| sIL-6 | THA+TKA | 0.680 | 0.954 | 0.850 | 0.886 | 40.000 | 0.170 | 235.0 |
| 95% CI | 0.465–0.851 | 0.871–0.990 | 0.645–0.946 | 0.814–0.932 | 5.7–280.5 | 0.07–0.417 | 24.7–2,236 | |
| THA | 0.833 | 0.956 | 0.833 | 0.956 | 19.17 | 0.174 | 110.0 | |
| 95% CI | 0.359–0.996 | 0.781–0.999 | 0.416–0.972 | 0.756–0.992 | 2.73–134.7 | 0.029–1.045 | 5.8–2,074 | |
| TKA | 0.889 | 0.960 | 0.941 | 0.923 | 22.2 | 0.116 | 192.0 | |
| 95% CI | 0.653–0.986 | 0.796–0.999 | 0.699–0.991 | 0.764–0.978 | 3.23–152.7 | 0.031–0.429 | 16–2,298 | |
| sCRP | THA+TKA | 0.917 | 1.000 | 1.000 | 0.970 | - | 0.083 | - |
| 95% CI | 0.730–0.989 | 0.945–1.000 | - | 0.896–0.992 | - | 0.022–0.314 | - | |
| THA | 0.667 | 1.000 | 1.000 | 0.939 | - | 0.333 | - | |
| 95% CI | 0.223–0.957 | 0.888–1 | - | 0.833–0.979 | - | 0.108–1.034 | - | |
| TKA | 0.944 | 1.000 | 1.000 | 0.971 | - | 0.056 | - | |
| 95% CI | 0.727–0.999 | 0.897–1 | - | 0.835–0.996 | - | 0.008–0.373 | - | |
| IL-6+sCRP | THA+TKA | 0.737 | 1.000 | 1.000 | 0.889 | - | 0.263 | - |
| 95% CI | 0.488–0.909 | 0.912–1 | - | 0.790–0.944 | - | 0.124–0.558 | - | |
| THA | 0.667 | 1.000 | 1.000 | 0.905 | - | 0.333 | - | |
| 95% CI | 0.223–0.957 | 0.824–1 | - | 0.754–0.967 | - | 0.108–1.034 | - | |
| TKA | 0.846 | 1.000 | 1.000 | 0.913 | - | 0.154 | - | |
| 95% CI | 0.545–0.981 | 0.839–1 | - | 0.746–0.974 | - | 0.043–0.55 | - | |
| IL-6+sIL-6 | THA+TKA | 0.444 | 1.000 | - | 0.889 | - | 0.556 | - |
| 95% CI | 0.276–0.627 | 0.969–1.000 | - | 0.851–0.918 | - | 0.396–0.778 | - | |
| THA | 0.625 | 1.000 | - | 0.956 | - | 0.375 | - | |
| 95% CI | 0.306–0.863 | 0.944–1.000 | - | 0.898–0.982 | - | 0.153–0.917 | - | |
| TKA | 0.667 | 1.000 | - | 0.906 | - | 0.333 | - | |
| 95% CI | 0.417–0.848 | 0.926–1.000 | - | 0.824–0.952 | - | 0.163–0.682 | - |
The cut-off values for serum IL-6 were the following: 12.55 for THA+TKA; 8.55 for THA, and 12.55 for TKA. The cut-off values for sIL-6 and sCRP were the following: 20,988 and 8.80 for THA+TKA; 21,107 and 8.80 for THA; 3,453 and 11.90 for TKA, respectively. IL-6 = interleukin 6, sCRP = synovial C-reactive protein, sIL-6 = synovial interleukin 6, CRP = C-reactive protein, THA = total hip arthroplasty, TKA = total knee arthroplasty, PPV = positive predictive value, NPV = negative predictive value, LR = likelihood ratio, DOR = diagnostic odds ratio.
When the patients with systemic inflammatory diseases were excluded sensitivity, specificity, LR+, LR-, and DOR (for sIL-6 and cut-off value of 21,349 ng/L) were as follows: 0.789, 0.977, 34.74, 0.22, and 161.3, respectively. The same parameters for sCRP and cut-off value of 11.25 were as follows: 0.947, 1.000, not applicable, 0.05, and not applicable.
Diagnostic utility of serum IL-6 combined with sCRP and sIL-6
Since we had all the data available from many patients from whom serum/joint fluid samples were taken preoperatively/intraoperatively, it was possible to interpret them collectively. The combinations of serum and synovial IL-6/CRP led to the improvement of specificity, however, at the expense of a decrease in sensitivity. The complete list of diagnostic characteristics for each particular combination of the serum IL-6 with sCRP/sIL-6 is shown in Table 7.
Discussion
In this study, we present diagnostic characteristics for serum and synovial IL-6, synovial CRP, and their combinations. They were tested against the composite reference standard (MSIS criteria). Serum IL-6 as well as sIL-6/sCRP showed separately good diagnostic characteristics for changing significantly the pre-test probability of PJI. Our results identified the serum IL-6 cut-off value of 12.55 ng/L for the entire group. When the anatomical location was included, then the same figures were 8.55 ng/L for THA and 12.55 ng/L for TKA. The combination of serum IL-6 with sIL-6/sCRP achieved specificity 1.000 while sensitivity decreased. As a result, the patients having both positive serum IL-6 and sIL-6 or sCRP are almost certain to have the correct diagnosis of PJI.
The Musculoskeletal Infection Society (MSIS) introduced a definition of PJI based on the presence of major and/or minor diagnostic criteria including threshold values for the laboratory methods [16]. Almost all the diagnostic methods have had determined relevant characteristics in the diagnostic studies. Based on this data, we might be able to differentiate non-infective cases from PJI even on the outcome of a single test. However, none of the diagnostic tests provides perfect diagnostic accuracy [23]. As a result, there still is a number of patients who have a substantial risk for false negative/positive results in relation to the chosen diagnostic method. The solution lies in an appropriate combination of the diagnostic tests together with applying of modern informative approaches enabling calculation of probability of PJI. The basic step for success in any such movement is the quality of reported diagnostic studies. STARD (Standards for Reporting Diagnostic Accuracy Studies) statements have been published recently in order to assist in the improvement of the quality of diagnostic studies [24]. Here we conducted a prospective study of a very simple and practical design [25]. A well-known index test was evaluated against international diagnostic reference standard (“gold standard”), and appropriate statistics were employed too.
In relation to the previous studies, sensitivity and specificity for serum IL-6 are slightly below the best achieved performance published for the method [26]. However, they are higher than pooled sensitivity (0.72) and specificity (0.89) for all the published studies [14]. The optimal IL-6 cut-off points in our study are in accordance, especially at the site of the knee [14]. The reason for the difference in relation to the hip is unclear. It may lie at least partly in the definition of PJI used in particular studies as well as in differences among the patients included. Some role might be played by the laboratory methodology used in the particular studies. A design-related bias is another potential source of inter-study discrepancies. Contrary to some authors who excluded all patients with chronic inflammatory joint diseases, our study did not exclude patients with chronic inflammatory diseases like crystal arthropathies (e.g. gout, pseudogout) either patients with chronic systemic inflammatory arthritis. The same approach chose also other authors examining diagnostic characteristics of serum IL-6 or synovial markers (sIL-6, sCRP) in relation to PJI [8, 27–29].
Several studies examined sCRP and sIL-6 and reported a relatively wide range of the published cut-off points (Table 8). The optimum cut-off for sIL-6 could be about 2.3 ng/mL [14], which is close to the cut-off for the whole group in our study. On the other hand, there is a relatively big discrepancy in the cut-off value between the hip and knee. The reasons for that are not clear to date but they may be close to those mentioned above in relation to serum IL-6. In addition, the causative agent should play a role as low-virulent pathogens could induce host response of lower intensity [30]. In fact, the timing of the synovial fluid test could play a role in the studies as it is well known that the half-life of many biologically potent signals is very short. A recent meta-analysis showed a similar pooled AUC for sIL-6 (0.95) and slightly lower for sCRP (0.90) compared to our study [31]. Even though the pooled sensitivity for sIL-6 and sCRP were slightly lower in the mentioned meta-analysis comparing to our data, the authors of the meta-analysis emphasized the role for synovial biomarkers of PJI. As a result, a lateral flow immunoassay (Quickline IL-6) for sIL-6 was recently developed–similarly to alpha defensin–for pre- and intraoperative decision-making [32]. However, an independent study evaluating the diagnostic performance of Quickline IL-6 is not available to date.
Table 8. Cut-off points of sCRP (mg/L) and sIL-6 (ng/L) and their LR+/LR- for PJI diagnosing in several diagnostic studies.
| Author | Location | Synovial IL-6 | Synovial CRP | ||||
|---|---|---|---|---|---|---|---|
| Cut-off | LR+ | LR- | Cut-off | LR+ | LR- | ||
| Frangiamore [33] | Shoulder | 359.3 | 8.45 | 0.15 | |||
| Lenski [34] | Not mentioned | 30,750 | 17.27 | 0.10 | |||
| Randau [13] | Hip or Knee | 2,100 | 4.37 | 0.44 | |||
| 9,000 | 19.70 | 0.54 | |||||
| Nilsdotter-Augustinsson [27] | Hip | 10,000 | 9.86 | 0.33 | |||
| Deirmengian [28] | Hip or Knee | 13,350 | — | 0.00 | |||
| Jacovides [35] | Hip or Knee | 4,270 | — | 0.129 | |||
| Gollwitzer [36] | Hip or Knee | 1,896.56 | — | 0.00 | |||
| Deirmengian [37] | Hip or Knee | 2,300 | 8.82 | 0.034 | 12.2 | 9.7 | 0.033 |
| Frangiamore [38] | Hip or Knee | 8,671 | 22.18 | 0.20 | |||
| Parvizi [39] | Knee | 3.65 | 6.51 | 0.164 | |||
| Ronde-Oustau [40] | Knee | 5.365 | 9.9 | 0.11 | |||
| Tetreault [41] | Hip or Knee | 6.6 | 5.87 | 0.141 | |||
| De Vecchi [42] | Hip or Knee | 10.0 | 13.81 | 0.197 | |||
| Our study | Hip and knee | 20,988 | 40.0 | 0.170 | 8.80 | — | 0.083 |
LR = likelihood ratio, PJI = prosthetic joint infection. If sensitivity of a test is 1.00, LR- is 0.00. If specificity of a test is 1.00, LR+ is impossible to calculate (division by zero). These cases are marked with—.
Diagnostic accuracy of a single test is not the only expected outcome of the diagnostic studies. On the one side, a test with excellent diagnostic accuracy may necessarily be the test of choice in clinical practice neither [43]. On the other side, the performance of even the most accurate test is not stable under all clinical situations. It can change for instance with changing pre-test probability, the definition of PJI, or reference standard tests. The solution could lie in an appropriate combination of high-performance tests. When serum IL-6 was combined with synovial CRP/synovial IL-6 in this study, specificity of the examination achieved 1.00 while sensitivity decreased. This trade-off is typical for many tests. Hence, a combination of the tests and optimization of the diagnostic strategy is one of the most challenging tasks. In addition, it is not clear whether tests detecting the same type of response (here, the inflammatory anti-infective one) can be combined either, and if yes, then rules for a combination of such tests are not known. In our case, the biological closeness of IL-6 and CRP on both the systemic or local levels is enormous. Thus, theoretically a combination of these tests may be inappropriate, and probability based on the combination of these tests can be useless. A solution could lie in the usage of continuous data coming from the diagnostic methods as they are via appropriate computational/graphical methods instead of dichotomizing them into positive/negative cases [24].
Limitations, sources of potential bias
Every patient was assigned either to the group of PJI or to the group of aseptic revisions according to the MSIS criteria. These were applied to all the patients who underwent revision hip or knee arthroplasty at our department during the period under study. A source of bias could be the missing results. These were histology if the patient was operated by an orthopaedic surgeon who was not collaborating on this study, SWCC and leukocyte differential in patients with dry aspiration similarly like for other synovial tests. In case of missing results that prevented us from assessing the MSIS criteria in a particular patient, we excluded such patients from the study. Thus, there were no inconclusive or missing MSIS results in the study. On the other hand, missing serum IL-6 could be if the patient was admitted to our hospital by a colleague who was not collaborating on this study.
Conclusion
The present study identified the cut-off values for serum/synovial IL-6 and synovial CRP for the diagnostics of PJI. The optimal serum IL-6 cut-off for PJI should be about 12.55 ng/L. The optimum cut-off value of synovial IL-6 for PJI was 20,988 ng/L. The optimum cut-off value of sCRP for PJI could be about 8.80 mg/L. Conjoined negativity of serum IL-6 either with synovial IL-6 or synovial CRP could further increase the diagnostic sureness for ruling out PJI. On the other hand, the differences in the optimum cut-off values between THA and TKA cases remain to be explained.
Supporting information
(XLSX)
Acknowledgments
The authors thank Yvona Loveckova, MD, PhD, Department of Microbiology, and Tomas Tichy, MD, Department of Pathology, Faculty of Medicine and Dentistry, Palacky University Olomouc, Teaching Hospital Olomouc, Czech Republic for histopathological and microbiological analyses performed as part of the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The corresponding author received grant support from the Agency of Health Research, Ministry of Health Czech Republic project No. VES 17-29680A. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Le DH, Goodman SB, Maloney WJ, Huddleston JI. Current modes of failure in TKA: infection, instability, and stiffness predominate. Clinical orthopaedics and related research. 2014;472(7):2197–200. Epub 2014/03/13. doi: 10.1007/s11999-014-3540-y ; PubMed Central PMCID: PMCPMC4048402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gundtoft PH. Prosthetic Joint Infection following Total Hip Arthroplasty—Incidence, Mortality and Validation of the Diagnosis in the Danish Hip Arthroplasty Register. Dan Med J. 2017;64(9). Epub 2017/09/07. . [PubMed] [Google Scholar]
- 3.Yi PH, Cross MB, Moric M, Levine BR, Sporer SM, Paprosky WG, et al. Do serologic and synovial tests help diagnose infection in revision hip arthroplasty with metal-on-metal bearings or corrosion? Clinical orthopaedics and related research. 2015;473(2):498–505. Epub 2014/08/31. doi: 10.1007/s11999-014-3902-5 ; PubMed Central PMCID: PMCPMC4294906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesquida M, Molins B, Llorenc V, de la Maza MS, Adan A. Targeting interleukin-6 in autoimmune uveitis. Autoimmun Rev. 2017;16(10):1079–89. Epub 2017/08/06. doi: 10.1016/j.autrev.2017.08.002 . [DOI] [PubMed] [Google Scholar]
- 5.Worthington T, Dunlop D, Casey A, Lambert R, Luscombe J, Elliott T. Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to short-chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci. 2010;67(2):71–6. Epub 2010/07/31. . [DOI] [PubMed] [Google Scholar]
- 6.Ugras AA, Kural C, Kural A, Demirez F, Koldas M, Cetinus E. Which is more important after total knee arthroplasty: Local inflammatory response or systemic inflammatory response? Knee. 2011;18(2):113–6. Epub 2010/05/15. doi: 10.1016/j.knee.2010.03.004 . [DOI] [PubMed] [Google Scholar]
- 7.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. International orthopaedics. 2000;24(4):194–6. Epub 2000/11/18. doi: 10.1007/s002640000136 ; PubMed Central PMCID: PMCPMC3619890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Gotze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. The Journal of bone and joint surgery British volume. 2007;89(1):94–9. doi: 10.1302/0301-620X.89B1.17485 . [DOI] [PubMed] [Google Scholar]
- 9.Buttaro MA, Tanoira I, Comba F, Piccaluga F. Combining C-reactive protein and interleukin-6 may be useful to detect periprosthetic hip infection. Clinical orthopaedics and related research. 2010;468(12):3263–7. doi: 10.1007/s11999-010-1451-0 ; PubMed Central PMCID: PMCPMC2974855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Cesare PE, Chang E, Preston CF, Liu CJ. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. The Journal of bone and joint surgery American volume. 2005;87(9):1921–7. doi: 10.2106/JBJS.D.01803 . [DOI] [PubMed] [Google Scholar]
- 11.Elgeidi A, Elganainy AE, Abou Elkhier N, Rakha S. Interleukin-6 and other inflammatory markers in diagnosis of periprosthetic joint infection. International orthopaedics. 2014;38(12):2591–5. doi: 10.1007/s00264-014-2475-y . [DOI] [PubMed] [Google Scholar]
- 12.Ettinger M, Calliess T, Kielstein JT, Sibai J, Bruckner T, Lichtinghagen R, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61(3):332–41. doi: 10.1093/cid/civ286 . [DOI] [PubMed] [Google Scholar]
- 13.Randau TM, Friedrich MJ, Wimmer MD, Reichert B, Kuberra D, Stoffel-Wagner B, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PloS one. 2014;9(2):e89045 Epub 2014/03/04. doi: 10.1371/journal.pone.0089045 ; PubMed Central PMCID: PMCPMC3931684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie K, Dai K, Qu X, Yan M. Serum and Synovial Fluid Interleukin-6 for the Diagnosis of Periprosthetic Joint Infection. Scientific reports. 2017;7(1):1496 Epub 2017/05/06. doi: 10.1038/s41598-017-01713-4 ; PubMed Central PMCID: PMCPMC5431429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad SS, Shaker A, Saffarini M, Chen AF, Hirschmann MT, Kohl S. Accuracy of diagnostic tests for prosthetic joint infection: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3064–74. doi: 10.1007/s00167-016-4230-y . [DOI] [PubMed] [Google Scholar]
- 16.Parvizi J, Gehrke T, International Consensus Group on Periprosthetic Joint I. Definition of periprosthetic joint infection. The Journal of arthroplasty. 2014;29(7):1331 doi: 10.1016/j.arth.2014.03.009 . [DOI] [PubMed] [Google Scholar]
- 17.Gallo J, Kolar M, Dendis M, Loveckova Y, Sauer P, Zapletalova J, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. The new microbiologica. 2008;31(1):97–104. Epub 2008/04/29. . [PubMed] [Google Scholar]
- 18.Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29(4):617–22. doi: 10.1002/jor.21286 . [DOI] [PubMed] [Google Scholar]
- 19.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36(10):2932–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clinical orthopaedics and related research. 1976;(117):221–40. . [PubMed] [Google Scholar]
- 21.Gallo J, Juranova J, Svoboda M, Zapletalova J. Excellent AUC for joint fluid cytology in the detection/exclusion of hip and knee prosthetic joint infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(3):310–9. Epub 2017/05/23. doi: 10.5507/bp.2017.021 . [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Xiong C. Youden index and Associated Cut-points for Three Ordinal Diagnostic Groups. Commun Stat Simul Comput. 2013;42(6):1213–34. doi: 10.1080/03610918.2012.661906 ; PubMed Central PMCID: PMCPMC3685301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahi A, Tan TL, Kheir MM, Tan DD, Parvizi J. Diagnosing Periprosthetic Joint Infection: And the Winner Is? The Journal of arthroplasty. 2017;32(9S):S232–S5. Epub 2017/07/18. doi: 10.1016/j.arth.2017.06.005 . [DOI] [PubMed] [Google Scholar]
- 24.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527 doi: 10.1136/bmj.h5527 ; PubMed Central PMCID: PMCPMC4623764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dendukuri N, Schiller I, de Groot J, Libman M, Moons K, Reitsma J, et al. Concerns about composite reference standards in diagnostic research. BMJ. 2018;360:j5779 Epub 2018/01/20. doi: 10.1136/bmj.j5779 . [DOI] [PubMed] [Google Scholar]
- 26.Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. The Journal of bone and joint surgery American volume. 2010;92(11):2102–9. doi: 10.2106/JBJS.I.01199 . [DOI] [PubMed] [Google Scholar]
- 27.Nilsdotter-Augustinsson A, Briheim G, Herder A, Ljunghusen O, Wahlstrom O, Ohman L. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta orthopaedica. 2007;78(5):629–39. Epub 2007/10/30. doi: 10.1080/17453670710014329 . [DOI] [PubMed] [Google Scholar]
- 28.Deirmengian C, Hallab N, Tarabishy A, Della Valle C, Jacobs JJ, Lonner J, et al. Synovial fluid biomarkers for periprosthetic infection. Clinical orthopaedics and related research. 2010;468(8):2017–23. doi: 10.1007/s11999-010-1298-4 ; PubMed Central PMCID: PMC2895851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clinical orthopaedics and related research. 2014;472(11):3254–62. doi: 10.1007/s11999-014-3543-8 ; PubMed Central PMCID: PMC4182392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deirmengian CA, Citrano PA, Gulati S, Kazarian ER, Stave JW, Kardos KW. The C-Reactive Protein May Not Detect Infections Caused by Less-Virulent Organisms. The Journal of arthroplasty. 2016;31(9 Suppl):152–5. Epub 2016/04/21. doi: 10.1016/j.arth.2016.01.060 . [DOI] [PubMed] [Google Scholar]
- 31.Lee YS, Koo KH, Kim HJ, Tian S, Kim TY, Maltenfort MG, et al. Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis. The Journal of bone and joint surgery American volume. 2017;99(24):2077–84. Epub 2017/12/20. doi: 10.2106/JBJS.17.00123 . [DOI] [PubMed] [Google Scholar]
- 32.Wimmer MD, Ploeger MM, Friedrich MJ, Bornemann R, Roessler PP, Gravius S, et al. The QuickLine IL-6 lateral flow immunoassay improves the rapid intraoperative diagnosis of suspected periprosthetic joint infections. Technol Health Care. 2016;24(6):927–32. Epub 2016/08/09. doi: 10.3233/THC-161247 . [DOI] [PubMed] [Google Scholar]
- 33.Frangiamore SJ, Saleh A, Kovac MF, Grosso MJ, Zhang X, Bauer TW, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. The Journal of bone and joint surgery American volume. 2015;97(1):63–70. Epub 2015/01/09. doi: 10.2106/JBJS.N.00104 . [DOI] [PubMed] [Google Scholar]
- 34.Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. The Journal of arthroplasty. 2014;29(6):1105–9. Epub 2014/02/25. doi: 10.1016/j.arth.2014.01.014 . [DOI] [PubMed] [Google Scholar]
- 35.Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. The Journal of arthroplasty. 2011;26(6 Suppl):99–103 e1. Epub 2011/05/17. doi: 10.1016/j.arth.2011.03.025 . [DOI] [PubMed] [Google Scholar]
- 36.Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. The Journal of bone and joint surgery American volume. 2013;95(7):644–51. Epub 2013/04/05. doi: 10.2106/JBJS.L.00205 . [DOI] [PubMed] [Google Scholar]
- 37.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid alpha-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. The Journal of bone and joint surgery American volume. 2014;96(17):1439–45. doi: 10.2106/JBJS.M.01316 . [DOI] [PubMed] [Google Scholar]
- 38.Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial Cytokines and the MSIS Criteria Are Not Useful for Determining Infection Resolution After Periprosthetic Joint Infection Explantation. Clinical orthopaedics and related research. 2016;474(7):1630–9. Epub 2016/01/29. doi: 10.1007/s11999-016-4710-x ; PubMed Central PMCID: PMCPMC4887354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry Award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clinical orthopaedics and related research. 2012;470(1):54–60. Epub 2011/07/26. doi: 10.1007/s11999-011-1991-y ; PubMed Central PMCID: PMCPMC3237977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ronde-Oustau C, Diesinger Y, Jenny JY, Antoni M, Gaudias J, Boeri C, et al. Diagnostic accuracy of intra-articular C-reactive protein assay in periprosthetic knee joint infection—a preliminary study. Orthop Traumatol Surg Res. 2014;100(2):217–20. Epub 2014/03/04. doi: 10.1016/j.otsr.2013.10.017 . [DOI] [PubMed] [Google Scholar]
- 41.Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clinical orthopaedics and related research. 2014;472(12):3997–4003. Epub 2014/07/30. doi: 10.1007/s11999-014-3828-y ; PubMed Central PMCID: PMCPMC4397770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Vecchi E, Villa F, Bortolin M, Toscano M, Tacchini L, Romano CL, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2016;22(6):555–60. Epub 2016/04/05. doi: 10.1016/j.cmi.2016.03.020 . [DOI] [PubMed] [Google Scholar]
- 43.Mustafa RA, Wiercioch W, Cheung A, Prediger B, Brozek J, Bossuyt P, et al. Decision making about healthcare-related tests and diagnostic test strategies. Paper 2: a review of methodological and practical challenges. J Clin Epidemiol. 2017;92:18–28. Epub 2017/09/17. doi: 10.1016/j.jclinepi.2017.09.003 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.