Abstract
Nitrous oxide is a recreational drug gaining in popularity for its deemed innocence. However, side effects have recently been reported. In this case, a patient suffered major aortic arch thrombus resulting in arterial occlusion of his arm and temporary cerebral infarction and later deep venous thrombosis and pulmonary embolism. No common causes for thrombus in this high-flow vessel were identified. The authors state that the patient's chronic nitrous oxide abuse might have led to this thrombus, although it has never been described previously. This hypothesis is supported with laboratory tests at several presentations.
Case report
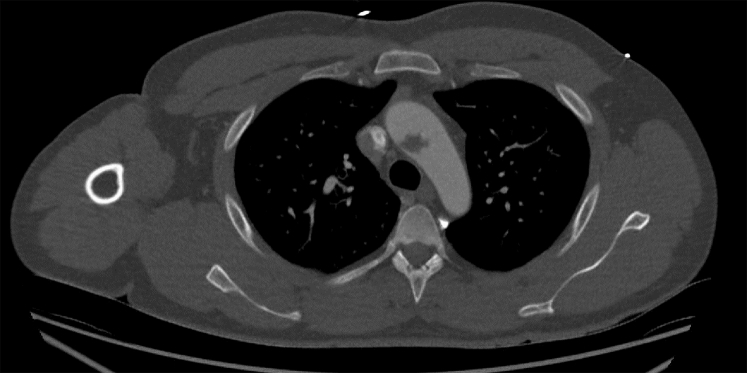
A 32-year-old man with no medical history presented to the emergency department with severe pain and numbness in his right arm. A pale limb without arterial pulses was seen, and emergency computed tomography (CT) angiography revealed an occlusion of the axillary artery, derived from two large free-floating mural thrombi in the ascending aorta (Figs 1 and 2). Embolectomy of the right brachial artery was performed successfully, and pathologic and microbiologic analysis of the blood clots revealed no abnormalities. Continuous heparin perfusion was started, to which we pragmatically added clopidogrel (Plavix). One day after surgery, he suffered an infarction in his right hemisphere, with paralysis of the facial nerve and left arm and leg, inadequate eye tracking movements, and dysarthria. On cerebral CT scan, we identified an infarction of the territory of the right medial cerebral artery. Because of clinical recovery within hours, lack of clarity with respect to the exact duration of this infarction, and adequate activated partial thromboplastin time because of heparin treatment, it was decided not to administer additional thrombolytic therapy. Finally, heparin was replaced by warfarin treatment and later rivaroxaban.
Fig 1.
Large free-floating thrombus in the aortic arch on computed tomography (CT) scan, transverse view.
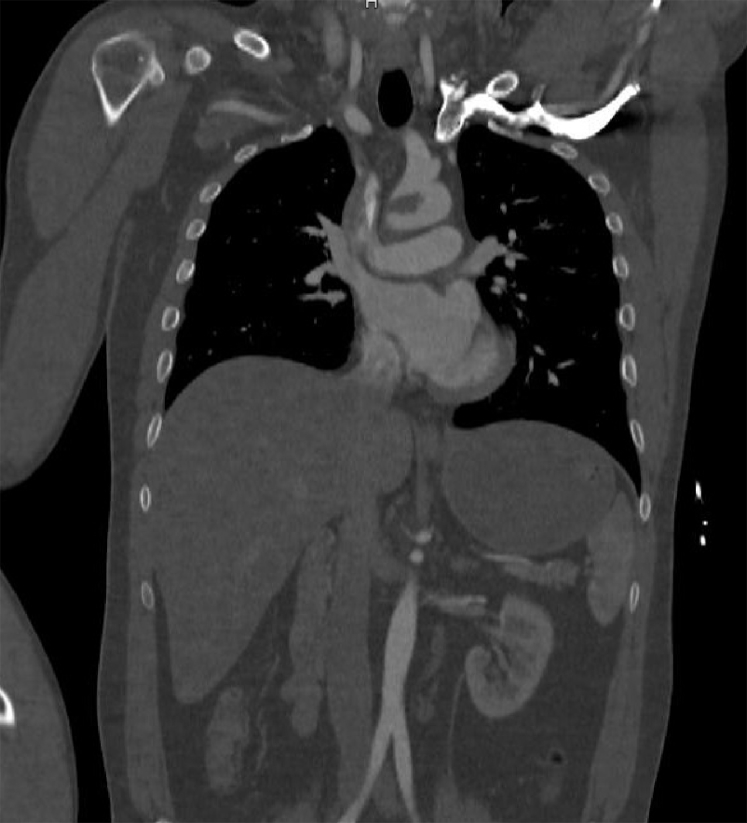
Fig 2.
Large free-floating thrombus in the aortic arch on computed tomography (CT) scan, coronal view.
The patient finally recovered from both events without any residual symptoms. In follow-up, CT and magnetic resonance angiography studies revealed complete resolution of the floating thrombus in the aortic arch in approximately 2 weeks, without signs of aortic wall irregularities, dissection, intimal flaps, or new occlusions. Transthoracic ultrasound examination of the heart also showed these thrombi initially without other structural abnormalities, such as a patent foramen ovale or valvulopathy. The analysis of potential risk factors revealed severe abuse of nitrous oxide, which he used for a more intense perception of sound while building speakers. Furthermore, a heterozygous factor II mutation was found. Consultation with a vascular medicine expert revealed no other signs of common hypercoagulable defects, such as factor V Leiden and antiphospholipid syndrome. Protein C and protein S were not analyzed because the patient had already started warfarin treatment. However, after the patient stopped using warfarin, he had normal activated partial thromboplastin time and partial thromboplastin time. Furthermore, there was no prolonged immobility and no family history of hypercoagulability, and there were no relatives with vascular events.
In this report, we discuss our hypothesis about the effect of chronic nitrous oxide abuse on blood coagulation. The patient's consent was obtained for publication of his data.
Discussion
The incidence of acute peripheral arterial occlusion is around 1.5 cases per 10,000 persons a year,1 but thrombus formation in high-flow vascular regions like the aortic arch are rare.2 Some cases of spontaneous aortic thrombus have been described previously, and those cases are characterized by a prolonged quest for a single identifiable cause.3, 4 As in those cases, we were not able to identify a single well-known cause for arterial thrombus. The heterozygous factor II mutation is related to increased risk for venous blood clot formation but has not been linked to thrombus formation in arterial high-flow regions. This leaves only the patient's chronic nitrous oxide abuse, with recurrent laboratory abnormalities concurring with nitrous oxide abuse and vascular events. Although this has never been linked to human vascular events before, this led to our hypothesis, with a plausible biologic background.
Nitrous oxide, known since the 19th century for its role in anesthetics,5 was already used centuries ago in “laughing gas parties” and is currently regaining popularity in the recreational scene because of its availability, presumed innocence, rapid action, and short duration. Furthermore, it can be simply obtained, for instance, from nitrous oxide whippets for whipped cream.
The relation between nitrous oxide abuse and thrombus formation might be explained by a combined two-step pathway, of which both steps are well known individually. First, nitrous oxide is known to decrease levels of active vitamin B12 when it is used chronically.6 This phenomenon is widely used to create animal models with vitamin B12 deficiency. Second, this inactivation of vitamin B12 is known to inhibit methionine synthase, which plays a key role in the metabolism of methionine and folate. This inhibited process results in hyperhomocysteinemia. This hyperhomocysteinemia might be the missing link to arterial thrombus formation in this case because it is a known long-term cardiovascular risk factor. It is related to venous thromboembolism and peripheral arterial disease by degradation of collagen, elastin, and proteoglycans.7
To test our hypothesis, we performed blood tests within 1 week after the patient's event, which indeed showed homocysteine levels to be above detection levels of our laboratory (>55 μmol/L) and an extremely low level of vitamin B12 (116 nmol/L) for a young and otherwise healthy man. During the first 6 months of follow-up, while the patient abandoned his nitrous oxide abuse completely, the homocysteine level dropped (19 μmol/L) and the vitamin B12 level increased (276 nmol/L) without any vitamin supplementation. MTHFR gene mutation was not assessed but seems unlikely because our patient had normal homocysteine levels between two periods of severe abuse. Other chronic vitamin B12 deficiency is unlikely because he had normal levels at some point too.
Unfortunately, our patient relapsed into his nitrous oxide abuse. He visited our emergency department several times with deep venous thrombosis, pulmonary embolism, and post-stroke epilepsy. At diagnosis of the deep venous thrombosis, vitamin B12 level had dropped again to 204 nmol/L, with a homocysteine level of >50 μmol/L. At diagnosis of pulmonary embolism, vitamin B12 levels were even lower, 162 nmol/L. Both times, there were no other laboratory abnormalities.
Conclusions
This report seems to indicate that chronic nitrous oxide use could be related to vitamin B12 deficiency in humans too. In addition to myelopathy described recently,8 this may lead to hyperhomocysteinemia, increasing the risk for cardiovascular events. This indicates a new potential risk of this popular and previously assumed innocent drug.
Our hypothesis is supported by combining two independent but well-known biologic mechanisms—nitrous oxide and vitamin B12 deficiency, and vitamin B12 deficiency and hyperhomocysteinemia. Laboratory tests support this hypothesis, with improving results in a period without abuse and worsening results in a renewed period with severe abuse. There might be new or other unrecognized factors that contributed to his hypercoagulable state. All common causes were excluded, however, except for a heterozygous factor II mutation. Because our patient suffered thrombi in a high-flow arterial region, there must be a strong additional factor, which we believe could be nitrous oxide in this case.
In this case, conservative treatment with regard to the aortic thrombi was successful. Only surgery for his ischemic arm was performed; the thrombi resolved with anticoagulants alone.
Although it is reasonably supported by the results in this case, appropriately designed future studies are needed to confirm the relation of chronic nitrous oxide use with thrombus formation. The abuse of nitrous oxide is gaining in popularity, and both national and international media repeatedly report about this phenomenon. This should result in the attention of health care professionals as well. Therefore, this report contributes to the awareness of the risks of this upcoming and until recently deemed innocent party drug.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Creager M.A., Kaufman J.A., Conte M.S. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366:2198–2206. doi: 10.1056/NEJMcp1006054. [DOI] [PubMed] [Google Scholar]
- 2.Jaworski L., Fijalkowski M., Rogowski J. Giant thrombus in ascending aorta and aortic arch. J Thorac Cardiovasc Surg. 2013;145:1668–1669. doi: 10.1016/j.jtcvs.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Rossi P.J., Desai T.R., Skelly C.L., Curi M.A., Glagov S., Schwartz L.B. Paravisceral aortic thrombus as a source of peripheral embolization—report of three cases and review of the literature. J Vasc Surg. 2002;36:839–843. [PubMed] [Google Scholar]
- 4.Sawada T., Shimokawa T. Giant thrombus in the ascending aorta that caused systemic embolism. Interact Cardiovasc Thorac Surg. 2011;12:1048–1050. doi: 10.1510/icvts.2011.266445. [DOI] [PubMed] [Google Scholar]
- 5.Emmanouil D.E., Quock R.M. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54:9–18. doi: 10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanarin I. The effects of nitrous-oxide on cobalamins, folates, and on related events. Crit Rev Toxicol. 1982;10:179–213. doi: 10.3109/10408448209037455. [DOI] [PubMed] [Google Scholar]
- 7.Selhub J., Jacques P.F., Bostom A.G., DAgostino R.B., Wilson P.W., Belanger A.J. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 8.Shoults K. Case report: neurological complications of nitrous oxide abuse. BCMJ. 2016;58:192–194. [Google Scholar]