Abstract
Objectives
Despite the importance of verbal learning and memory in speech and language processing, this domain of cognitive functioning has been virtually ignored in clinical studies of hearing loss and cochlear implants in both adults and children. In this paper, we report the results of two studies that used a newly developed visually based version of the California Verbal Learning Test (CVLT-II), a well-known normed neuropsychological measure of verbal learning and memory.
Design
The first study established the validity and feasibility of a computer-controlled visual version ofthe CVLT-II, which eliminates the effects of audibility of spoken stimuli, in groups of young normal-hearing and older normal-hearing adults. A second study was then carried out using the visual CVLT-II format with a group of older postlingually deaf experienced cochlear implant (ECI) users (N=25) and a group of older normal hearing (ONH) controls (N=25) who were matched to ECI users for age, socioeconomic status, and non-verbal IQ. In addition to the visual CVLT-II, subjects provided data on demographics, hearing history, non-verbal IQ, reading fluency, vocabulary, and short-term memory span for visually presented digits. ECI participants were also tested for speech recognition in quiet.
Results
The ECI and ONH groups did not differ on most measures of verbal learning and memory obtained with the visual CVLT-II, but deficits were identified for ECI participants that were related to recency recall, the build-up of proactive interference (PI) and retrieval induced forgetting. Within the ECI group, nonverbal fluid IQ, reading fluency, and resistance to the build-up of PI from the CVLT-II consistently predicted better speech recognition outcomes.
Conclusions
Results from this study suggest that these underlying foundational neurocognitive abilities are related to core speech perception outcomes following implantation in older adults. Implications of these findings for explaining individual differences and variability and predicting speech recognition outcomes following implantation are discussed.
Introduction
Cochlear implants (CIs) work and often work very well for many children and adults with profound sensorineural hearing loss. There is no question in anyone’s mind at this time that CIs are “one of the great success stories of modern medicine,” representing a significant engineering and medical milestone in the treatment of sensorineural hearing loss (Wilson et al. 2011). Unfortunately, while many CI patients display substantial benefits in recognizing speech and biologically significant environmental sounds and understanding spoken language following implantation, a significant number of patients have poor outcomes and display less than optimal benefits following implantation, even after several years of experience with their CIs. The estimates of poor outcomes following cochlear implantation fall in the 25 to 30 percent range, depending on the behavioral criteria that are used to assess benefit and outcomes (Rumeau et al. 2015; Lenarz et al. 2012). Most patients who have received CIs get some benefit from their implants under idealized, highly controlled listening conditions in the audiology clinic or research laboratory, but in everyday real-world conditions they commonly report significant difficulties in listening to speech in noise, especially multi-talker babble, communicating over the telephone, or listening under conditions of high cognitive workload. Recognizing and understanding speech produced by non-native speakers or speakers who have marked unfamiliar regional dialects are also very serious challenges for most patients who have received CIs (see Tamati & Pisoni 2014). CI users’ abilities to rapidly adapt and adjust to highly variable adverse listening conditions are significantly compromised, compared to young normal-hearing listeners.
Understanding and explaining the reasons for poor outcomes following cochlear implantation is a challenging research problem, with significant gaps in our knowledge remaining, despite the pressing clinical significance of this issue (See Moberly et al. 2016a, b). Only a small number of conventional clinical factors have been identified as predictors of speech recognition outcomes, such as amount of residual hearing preoperatively, previous use of a hearing aid, patient age, and duration of hearing loss prior to implantation (Green, Bhatt, Mawman et al., 2007; Kelly, Purdy, & Thorne, 2005; Lazard et al., 2012; Leung et al., 2005). However, it still remains unclear why some patients do extremely well with their CIs while other patients struggle and fail to reach optimal levels of speech recognition performance even under quiet conditions in the clinic or laboratory. This issue represents a significant gap in our current knowledge of speech recognition outcomes following cochlear implantation and is an important barrier to progress in developing novel personalized interventions to help patients with suboptimal outcomes improve their speech recognition performance.
Patients with CIs generally have difficulty understanding speech in noise, recognizing music and non-speech biologically significant environmental sounds, and processing acoustic signals under challenging listening conditions (Limb & Roy; 2014; Shafiro, Gygi, Cheng, Vachhani, & Mulvey, 2011; Srinivasan, Padilla, Shannon, & Landsberger, 2013). Part of the difficulty they have stems from the nature and integrity of the electrical signal they receive through their implant, which is spectrally degraded, frequency-shifted, significantly underspecified, and sparsely-coded relative to the original unprocessed signal presented at the ear (Friesen, Shannon, Baskent, & Wang, 2001). The critical acoustic-phonetic cues encoded in the signal that support robust speech intelligibility are coarsely-coded, and the fine-grained phonetic and indexical details of the original speech signal are significantly compromised during neural encoding and transmission to higher neurocognitive information processing centers of the brain. While some of the elementary acoustic-phonetic cues needed for speech recognition in the quiet are encoded and preserved, these minimal phonetic attributes are fragile and poorly specified by the current generation of signal processing algorithms used in CIs clinically today. As a result, subsequent neural coding and memory representations in sensory and working memory are also fragile, lacking important episodic context cues and critical dynamic information about coarticulation that reflects the encoding of the motions of the talker’s vocal tract. These contextual variations in the speech waveform are essential for robust recognition of speech in noise and under challenging listening conditions or under high cognitive load (Mattys et al. 2012).
In addition to these considerations about the degraded nature of the signal a listener receives through a CI, several researchers have argued recently that a much broader based systems-approach should be adopted to begin investigating the underlying basis of the enormous individual differences and variability routinely observed after implantation (Pisoni & Kronenberger 2010). The variability universally observed in conventional speech recognition outcome measures not only reflects the efficiency and quality of early sensory registration and the fidelity of encoding of acoustic signals by the auditory nerve, but it also reflects the foundational contributions of the information processing system as a whole. Robust speech recognition and spoken language comprehension rely heavily on basic foundational cognitive information processing operations such as attention, learning, memory, and inhibitory control processes, which are actively used by listeners to support the initial encoding, processing, storage and retrieval of linguistic information carried by the speech waveform (Altman, 1990; Borden, Harris & Raphael, 1994; Pisoni & Remez, 2005). Speech scientists and acoustical engineers have known for more than 60 years that speech recognition and spoken language processing do not take place in isolation in the ear or at the auditory periphery (Flanagan, 1972; Liberman, 1996; Stevens, 1998). Robust spoken word recognition and speech understanding reflect the final product of a long series of information processing stages that routinely draw on multiple cognitive resources and many different sources of knowledge in long-term memory, based on the listener’s prior experiences and unique developmental histories (Pisoni & McLennan 2015).
In a similar vein, there is a growing consensus among many clinicians and researchers in the field of CIs that although there is enormous variability routinely observed in our current endpoint outcome measures of speech recognition following implantation, these endpoint measures do not help us to understand and explain the underlying foundational basis for these individual differences (Wilson & Dorman, 2012). The current battery of behaviorally based outcome measures which are used universally at all CI centers around the world was never designed to assess or investigate individual differences in speech recognition outcomes. Rather, this test battery was developed to establish efficacy of CIs for FDA approval; none of the individual tests on these assessment batteries were developed to study the effectiveness of CIs in complex real-world environments or to investigate the information processing mechanisms underlying individual differences and variability in speech recognition outcomes (Pisoni et al. 2008).
In this paper, we report the results of a new study undertaken to investigate verbal learning and memory following implantation in a group of post-lingually deaf adults who received a CI for acquired severe-to-profound hearing loss. Verbal learning and memory are defined as encoding, storage, and retrieval processes using words presented over multiple trials. The most distinctive hallmark of spoken language comprehension is rapid adaptation and adjustment to novel input signals in many different listening environments. In the case of a successful CI user, rapid perceptual learning and efficient adaptation processes must compensate and normalize for acoustic-phonetic variability, as well as for the compromised underspecified signals delivered to the brain by the CI. Despite the central importance that learning and memory processes play in the perceptual processing and recognition of novel input signals, basic and clinical research on verbal learning and memory processes has been neglected in the research efforts carried out on patients with CIs, even though these cognitive processes are central to understanding successful adaptive functioning in adverse listening environments. Most clinicians and researchers in the CI field believe that the variability observed in speech recognition outcomes following implantation is simply due to differences in early registration and encoding of sensory information in short-term and working memory. This is true in spite of the critical importance of long-term memory (LTM) in supporting robust adaptive functioning and speech recognition in challenging or adverse listening environments. In these situations, prior linguistic knowledge and experience play compensatory roles when the bottom-up sensory information is significantly degraded (see Rönnberg et al. 2013). Thus, the premise of this study was that the use of a non-auditory measure of rapid verbal learning and memory could provide valuable insight into the information-processing mechanisms of CI users. Moreover, we predicted that findings from this measure of learning and memory would be related to speech recognition outcomes.
The present study used a well-known clinical neuropsychological instrument, the California Verbal Learning Test – Second Edition (CVLT-II; Delis et al. 2000), to measure several underlying information processing components and cognitive processes used to encode, store, process, and retrieve lists of familiar words from both short- and long-term memory. The CVLT-II and a variety of other verbal learning and memory tests such as the Rey Auditory Verbal Learning Test (RAVLT; Schmidt 1996), the Hopkins Verbal Learning Test (HVLT; Brandt 1991), and the Wide Range Assessment of Memory and Learning Verbal Learning subtest (WRAML; Sheslow & Adams 1990) are routinely used by neuropsychologists to identify memory weaknesses and impairments in a broad range of clinical populations. In addition to recall and recognition measures, the CVLT-II also provides very detailed information about the control processes and self-generated organizational strategies that an individual patient uses in a well-defined experimental task — free recall of categorized word lists — which has been studied and modeled extensively by cognitive and mathematical psychologists over the last 60 years (see Delis et al. 2000; Atkinson & Shiffrin 1968; Wixted & Rohrer 1993). Moreover, the CVLT-II has been used with several different clinical populations, providing critical behaviorally based benchmarks for comparison to establish strengths, weaknesses, and developmental milestones across a number of core foundational domains underlying verbal learning and memory processes.
The CVLT-II is a multi-trial free recall (MTFR) neuropsychological test that is used to study repetition learning effects and organizational strategies for verbal learning and memory. The test protocol produces a large amount of clinically relevant data in a short period of time. The scores obtained from the CVLT-II provide important diagnostic information about basic core verbal learning and memory processing mechanisms that are related to domains of executive functioning and cognitive control (cognitive processes involved in active recruitment and direction of mental activity in the service of attaining goals), including controlled attention, fluency-speed, abstraction, and self-generated retrieval organization strategies, along with measures of word recognition, encoding, storage, and retrieval strategies. Specifically, the MTFR methodology used in the CVLT-II provides measures of foundational cognitive processes underlying verbal learning and memory, including repetition-based multi-trial free recall, primacy and recency effects, proactive and retroactive interference, memory storage and decay in both immediate and delayed free- and cued-recall, as well as self-generated organizational strategies in memory retrieval such as serial, semantic, and subjective clustering. These organization strategies are routinely used by subjects to increase the strength of memory representations and make the encoding of verbal items in memory more accessible for retrieval during free recall tasks (Delis et al. 2000). Thus, it is reasonable to predict that measures obtained using the CVLT-II would be related to speech recognition performance in CI users.
In the traditional clinical use of the CVLT-II, participants are read a list of 16 familiar words (List A) five times by a trained examiner to assess repetition learning processes and free recall after each study trial. The 16 words on List A come from four semantic categories (animals, vegetables, ways of travelling, furniture). After the list is presented, the participant is asked to recall as many of the words from List A as possible in any order. This free recall procedure is then repeated four additional times using the same List A, with items always presented in the same order, for a total of five repetition learning study trials. After the fifth presentation and recall trial of items from List A, participants are presented with a new list of 16 words from List B, the “interference list,” to measure proactive interference (PI). List B also contains words from four semantic categories. Half of the new words on List B share semantic categories with words on List A (animals, vegetables); the other half of the items are new words from two different semantic categories (musical instruments, rooms of a house). After recall of List B, participants are then asked to recall List A again (short-delay free recall) to measure retroactive interference (RI) effects produced by List B. Following a 20-minute delay period, during which the participant is engaged in a non-verbal distractor task, the participant is asked to recall the words from List A again (long-delay free recall) to measure memory decay after a retention interval. After each of the two List A delayed free recall tasks, the subjects are also given a cued recall task using the four original semantic categories from List A as retrieval cues. Finally, after the long delay recall tests are completed, a Yes/No recognition memory test is administered to assess storage of items from List A without any demands on retrieval.
The present study builds on the results reported in two earlier studies using the CVLT-II carried out with postlingually deaf adults who had severe-to-profound hearing losses (Heydebrand et al. 2007; Holden et al. 2013). Both studies used a non-standard version of the CVLT-II with simultaneous visual (printed words) and live-voice auditory presentation of the test lists. Heydebrand and colleagues (2007) reported that auditory-visual CVLT-II scores based on a composite score of several component measures of free recall performance accounted for 42% of the variance in CNC monosyllabic word recognition scores six months post-implantation. Based on their findings from the CVLT, the authors suggested the potential use of verbal learning and memory tasks in predicting speech recognition outcomes for post-lingually deaf CI users, but they provided few details about precisely what kinds of verbal learning measures would be clinically useful and which specific domains of verbal memory and learning should be investigated.
In a more recent follow-up study with a larger sample size encompassing a broader age range, Holden and her colleagues (2013) also found a significant relation between a similar CVLT-II composite free recall score and speech recognition outcomes, but this correlation was eliminated when they controlled for chronological age. However, despite use of combined visual-auditory presentation of stimuli, both of these studies were also limited in their conclusions because their CVLT-II assessment results may have been influenced by variability in auditory capabilities of the participants. For example, there may have been interference and competition from the auditory modality, or alternatively participants with stronger auditory processing may have received more benefit from the auditory signal resulting in uncontrolled variance in the free recall scores from the CVLT. Moreover, detailed analysis and assessments of the critical process and contrast measures of performance that can be derived from the CVLT-II, which was designed to measure specific capacities of verbal encoding, storage, retrieval, and self-generated organizational strategies used in retrieval, were unfortunately not reported in either of these earlier studies. The process measures obtained from the CVLT-II are highly informative about underlying cognitive information processing strategies, because they provide very detailed information and quantitative measures about what participants are doing with the verbal information they encode, store, and retrieve from memory in this task. Without examining the process measures provided by the CVLT-II, such as learning rates, proactive interference (PI) and retroactive interference (RI), retrieval inhibition, release from PI, and organizational strategies such as semantic, serial and subjective clustering of output responses, as well as response repetitions and intrusions in free recall, detailed insights cannot be gained into the possible differences in underlying information processing operations and neurocognitive mechanisms used by participants in carrying out the CVLT-II task protocol. Focusing on only the “first-order” primary free recall measures obtained from the immediate and delayed free recall trials provides only a global overall impression of the foundational information processing operations underlying verbal learning and memory for lists of categorized words in this clinical population. Without detailed information about the process measures on the CVLT-II, we obtain an incomplete picture of the strengths, weaknesses, and milestones in these patients.
Despite these concerns and reservations about the two earlier studies, the findings reported by Heydebrand et al. (2007) and Holden et al. (2013) suggest that measures of verbal memory and learning processes could serve as useful predictors of speech recognition outcomes in adult CI users and therefore might provide new process-based behavioral measures that could help explain the underlying basis of the enormous individual differences and variability observed in outcomes following implantation. The current study used a novel visual presentation version of the CVLT-II (the “V-CVLT”) to assess verbal memory and learning abilities in adults with postlingual severe-to-profound hearing loss who were experienced CI users. The use of the V-CVLT eliminated audibility issues and live-voice presentation by using visual presentation of test stimuli on a computer display screen. This visual-presentation format of the CVLT-II eliminates any concerns about the role of audibility and early auditory sensory processing as possible confounding factors influencing the verbal learning and memory measures obtained. Thus, any differences observed among CI users (or between CI users and normal-hearing control participants) cannot be due to modality-specific sensory effects related to audibility or early sensory processing and encoding. Rather, differences found must reflect levels of information processing that involve modality-general differences associated with verbal coding, phonological and lexical processing, storage, retrieval, and information processing operations that are not compromised by prior hearing loss or differences in audibility.
This report presents the results of two studies. Study 1 consisted of two phases (1a and 1b) that were performed to assess the validity and feasibility of the V-CVLT to ensure that the primary and process measures obtained from visual presentation of the CVLT-II would be equivalent to scores obtained from the conventional auditory, live-voice clinical version of the same task. Study 1a was carried out with two groups of young normal-hearing (YNH) adults at Indiana University in Bloomington; Study 1b was carried out with two groups of older normal-hearing (ONH) adults at The Ohio State University in Columbus. In each of these studies, we hypothesized that scores of primary and process measures of the V-CVLT would be equivalent to those obtained using the conventional live-voice auditory version of the CVLT-II, in both young YNH (Study 1a) and ONH (Study 1b) adults. Study 2, which was also carried out at Ohio State, was designed to compare performance on the new V-CVLT between adult experienced CI users (ECI) and age-matched older normal-hearing (ONH) control participants, and to investigate the relations between verbal learning and memory and speech recognition outcomes in the ECI users. In Study 2, we hypothesized that ECI participants would demonstrate deficits in some primary and process measures from the V-CVLT, compared with ONH peers, as a result of their experience of prolonged auditory deprivation. In particular, based on recent findings from Pisoni et al. (2016) with prelingually deaf adult CI users, we predicted that ECI participants would recall fewer list items over multiple presentations, as a result of less efficient encoding of verbal information, even using visual presentation. We also predicted that ECI participants would show less semantic clustering and more serial clustering than ONH peers because semantic clustering requires allocation of additional cognitive resources during verbal learning and memory, which would be too taxing for CI users who already allocated maximum resources for basic recall of list items. Additionally, we hypothesized that several measures from the V-CVLT would correlate with speech recognition outcomes in ECI users. Understanding speech through a CI requires rapid perceptual learning and adaptation to the underspecified degraded auditory signals; thus, ECI participants with good speech recognition outcomes (as compared with ECI users with poor speech recognition) might be expected to demonstrate highly effective learning of word lists over serial presentation on the V-CVLT, and might demonstrate more effective retrieval strategies, such as use of semantic clustering. Finally, in addition to the V-CVLT, several non-auditory visually based neurocognitive measures were also obtained from listeners in both studies to investigate the relations between measures of verbal learning and memory obtained from the V-CVLT and neurocognitive scores from tests of non-verbal fluid reasoning (IQ), reading fluency, immediate memory span, and vocabulary knowledge. These additional measures served to ensure equivalence between groups enrolled in the validation studies (1a and 1b), as well as to relate findings on these more traditional neurocognitive measures with scores on the V-CVLT.
Study 1a – Validation and Feasibility of V-CVLT in Young Normal-Hearing Adults
Methods
Participants and Procedures
Forty participants between the ages of 18 and 54 (mean 23.3 years) were recruited via flyers posted around the Indiana University Bloomington campus, listings on the IU Classifieds online advertisement system, and ads on IU’s paid subject pool website. Participants reported minimal to no history of any speech and hearing problems and received compensation at the rate of $5 per half hour for the time they were in the lab, for a total of $10. The present study consisted of measurement of verbal learning and memory and assessment of several domains of neurocognitive functioning. Participants were randomly assigned (1:1) to either the visual (V-CVLT) or the conventional auditory (A-CVLT) presentation condition. All participants completed the same visually-presented versions of all of the other measures in the study.
Materials
Visual and Auditory CVLT-II. Two new computer-controlled versions of the California Verbal Learning Test – Second Edition (CVLT-II) were developed specifically for this validity and feasibility study. The CVLT-II provides verbal learning and memory scores based on participants’ recall of lists of words using the procedure described in the Introduction. The various measures obtained from the CVLT-II are described in Table 1. The conventional clinical administration of the CVLT-II is carried out using live-voice presentation (see Delis et al. 2000). The current validity and feasibility study used two on-line computer-controlled versions of the CVLT-II that followed the same instructions and procedures of the live-voice CVLT-II as closely as possible. The first version, called the “Auditory” condition (A-CVLT), included the same words and followed the same order of trials as the original live-voice CVLT-II, but instead we used digital audio recordings of the test items that were created off-line by an experienced clinician. After the audio files were made, the test words were played out of a high-quality loudspeaker (Advent, Model AV-570) at a comfortable listening level next to a computer display screen, separated by 650 milliseconds. The second version of the CVLT was a “Visual” presentation condition (V-CVLT), in which the test words from both List A and List B were presented on the computer display screen visually in printed English for 1 second, with the same 650 msec interval between each word. For both presentation conditions, a research assistant sitting next to the participant also read aloud the printed instructions for each trial, which were modified slightly from the original CVLT-II instructions to match the computer-controlled versions of the two tasks. All participants also completed an on-line non-verbal distractor task using the Raven’s Progressive Matrices for a fixed 15-minute time period in between the short and long delay recall trials to block active rehearsal (Ravens; Raven et al. 1998). Scores on all CVLT-II trials were calculated as raw scores as well as percent correct scores depending on the comparisons of interest. Several normed scores were also obtained from the CVLT-II scoring software (see Table 1).
Table 1.
CVLT-II Scores and Descriptions
| Score | Description |
|---|---|
| List A Trial N | Number of words recalled following the Nth exposure to List A, where N=1 to 5 |
| List A Trials 1–5 | Total number of words recalled across all 5 List A exposure trials |
| List B | Number of words recalled following exposure to List B, the “interference list” |
| Short-Delay Free Recall (SDFR) | Immediately after List B recall, number of List A words recalled (List A is not read again for this trial or for the remainder of the test) |
| Short-Delay Cued Recall (SDCR) | Immediately after SDFR, number of List A words recalled when the subject is provided with each of the four semantic categories (furniture, vegetables, ways of traveling, animals) for List A as cues |
| Long-Delay Free Recall (LDFR) | Following a 20-minute delay after SDCR, number of List A words recalled |
| Long-Delay Cued Recall (LDCR) | Immediately after LDFR, number of List A words recalled when the subject is provided with each of the four semantic categories (furniture, vegetables, ways of traveling, animals) for List A as cues |
| Long-Delay Recognition | Immediately after LDCR, percentage of words identified accurately as List A words from a list of 48 words (16 List A words, 16 List B words, and 16 distractor words) |
| Primacy Recall | Percentage of the first four words on the list that are recalled on a trial or set of trials (note that this measure differs from the method of calculating Primacy Recall in the CVLT-II manual) |
| Pre-recency Recall | Percentage of the middle 8 words on the list that are recalled on a trial or set of trials |
| Recency Recall | Percentage of the last four words on the list that are recalled on a trial or set of trials (note that this differs from the method of calculating Recency Recall in the CVLT-II manual) |
| Serial Clustering | Chance adjusted score for recall clustering based on serial order, obtained by taking the difference between observed number of words recalled in the same serial order as presented and the the number of words that would be recalled in the same serial order as presented by chance alone |
| Semantic Clustering | Chance-adjusted score for recall clustering based on semantic category, obtained by taking the difference between observed number of words from the same semantic category recalled by the subject in serial contiguity and the expected number of words from the same semantic category that would occur in serial contiguity by chance alone |
| Subjective Clustering | When subject uses the same unique idiosyncratic clustering strategy in free recall across trials following each list presentation. |
| Intrusions | Words recalled by the subject that were not part of the target list |
| Perseverations (Repetitions) | Words repeated by the subject in response to the same trial |
Neurocognitive Measures. In addition to the Ravens Progressive Matrices that was completed during the 15-minute delay period in the CVLT protocol, all participants also completed three additional neurocognitive tasks to ensure that both groups of subjects were comparable, not only on non-verbal fluid IQ from the Raven's, but also reading fluency, vocabulary knowledge, and short-term memory capacity. First, the Test of Word Reading Efficiency, Second Edition (TOWRE-2; Torgesen et al. 2012), a reading assessment, was administered to obtain a measure of reading fluency and speed using visually presented words and non-words. Participants were given 45 seconds to read aloud as many words as accurately as possible from a list of 108 words that increased in difficulty as the list progressed. Next, a list of 66 non-words of increasing difficulty was presented. Participants were also given 45 seconds to read aloud as many non-words as possible. In both conditions of the TOWRE-2 test, all of the stimulus items were presented visually on the computer screen in columns. After the 45-second time period, the screen changed to blank. Raw scores were the number of words, non-words, and total number of items read aloud correctly.
Second, a visual word familiarity task, the WordFAM-150 test, was administered to measure vocabulary knowledge and lexicon size (Lewellen et al. 1993). One hundred and fifty-two words varying in frequency and subjective familiarity were presented visually on the computer display screen, one at a time, along with a rating scale ranging from one to five where participants recorded their familiarity with each word. In this task, a rating of 1 indicated low familiarity, whereas a rating of 5 indicated high familiarity. Each participant’s subjective word familiarity score was calculated by averaging his or her familiarity ratings over all words.
Finally, participants completed a forward Visual Digit Span test using visually presented sequences of digits. Random sequences of numbers from 2 to 13 digits in length were presented one digit at a time on the computer display screen. After each test sequence was presented, participants saw a 3 X 3 grid of numbers on the computer display and were instructed to use their mouse to click the numbers that they saw in the same order in which they were presented on the computer screen. An up-down adaptive testing algorithm was used to increase the sequence length if the subject correctly reproduced the test sequence items. Two sequences were presented at each length. If the subject correctly reproduced both sequences at a given list length, the algorithm increased the sequence length on the next trial. If the subject made two errors in a row, the algorithm decreased the length of the next test sequence. The longest span that each participant correctly recalled was recorded, along with the total number of individual digits recalled in the correct order, out of 180.
Data Analyses
Five of the original 40 subjects were removed from the final data analysis because they were considered outliers based on their List A Trials 1–5 T-Score. Only subjects who fell within two SDs from the mean normative score (i.e., T-Scores between 30 and 70) were included, while subjects with higher or lower T-Scores were excluded. This procedure eliminated three subjects from the V-CVLT group who were at ceiling above the normative mean and two subjects from the A-CVLT group who were at the floor below the norm mean, leaving Ns of 17 and 18, respectively, in each group. Independent-samples t-tests were then performed separately to establish equivalency between the two groups on age, Ravens scores, TOWRE-2 scores, Visual Digit Span, and word familiarity ratings. Following these initial analyses, additional t-tests were performed to assess group differences in performance on the primary, process, and contrast measures obtained from the CVLT-II protocol. Relations between the neurocognitive tests and the CVLT-II scores were assessed using correlational analyses. ANOVA and post-hoc paired-comparisons were carried out to test for differences between groups and experimental variables.
Results
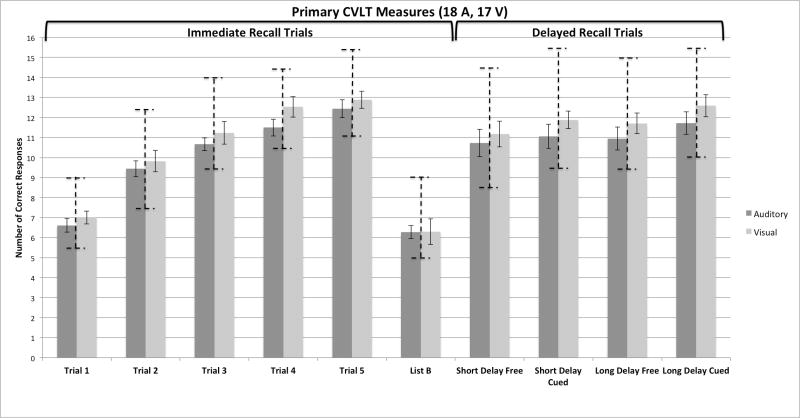
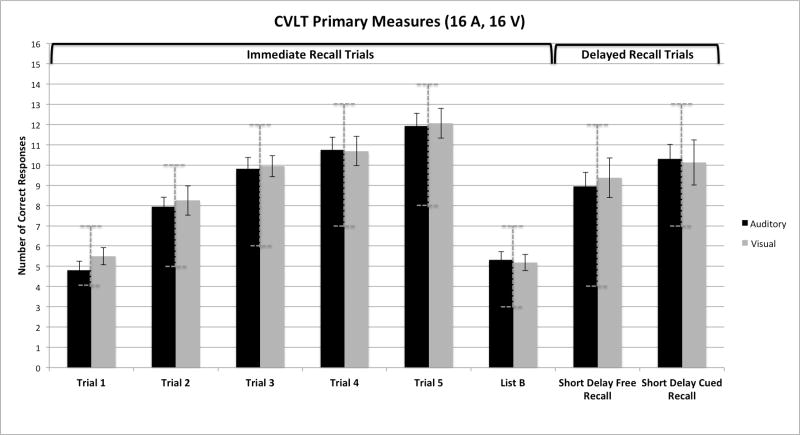
Figure 1 shows a global overall summary of the immediate and delayed free- and cued-recall scores obtained from the CVLT-II protocol. The figure is divided into two panels. On the left are the immediate free recall scores for the five repetitions of List A and the one presentation of List B (the interference list) obtained from the two groups of participants who were tested. The left set of bars in each panel of this figure shows the average free recall raw scores from the group of participants who were assigned to the A-CVLT; the right set of bars shows the scores from the group of participants who were assigned to the V-CVLT. Each bar represents the average number of correct responses out of the 16 items in each presentation of List A and List B. On the right of Figure 1 are the short-delay and long-delay free- and cued-recall scores obtained from both groups of subjects.
Figure 1.
Overall summary of the immediate and delayed free and cued recall scores obtained from the CVLT-II protocol in young normal-hearing adults. On the left are the immediate free recall scores for the five repetitions of List A and the single presentation of List B. On the right are the short-delay and long-delay free- and cued-recall scores obtained from both groups of subjects, who were tested using the auditory CVLT-II (A-CVLT) versus the visual CVLT-II (V-CVLT). The left set of bars in each panel shows the average free recall raw scores from the participants who were assigned to the A-CVLT; the right set of bars shows the scores from the participants who were assigned to the V-CVLT. The vertical dashed bars show the range of normative scores for each of the recall conditions based on age and gender.
In addition to the raw scores for each group, both panels in Figure 1 also display the range of normative scores for each of the recall conditions. These normative bars are shown as vertical dashed error bars superimposed on the raw scores and correspond to the normative range based on the gender and age of the participants included in each group. The normative range plotted here was found by first calculating the average age of males and females in each sample. The averaged ages were then used to look up the normed score for each trial at +/−1 SD from the mean in the CVLT-II manual. As shown in Figure 1, the mean of the two groups fell within +/− 1 SD from the normative means for all of the CVLT-II measures in both panels of the figure.
Inspection of the immediate free recall scores on the left in Figure 1 shows two main findings. First, both groups of subjects displayed robust repetition learning effects over the five presentations of List A. Performance improved continuously from Trial 1 to Trial 5 after each List A repetition (F= 99.29, p<.001). Second, inspection of this figure shows that both groups of participants showed comparable levels of immediate free recall performance after each repetition of List A over the five presentations. The main effect of group over the five learning trials was not statistically significant by a 2 X 5 ANOVA followed by a series of post-hoc paired-comparison tests (all p> .05). While the recall performance of both groups declined significantly overall for immediate free recall of the new words from List B compared to recall of words on List A Trial 1 (p< .001), both groups showed comparable free recall scores on this list, too. Although no group differences were found in immediate free recall for items on either List A or List B based on presentation modality, a significant linear trend was observed across the five study trials for List A as a function of repetition learning (F=258.29; p<.001).
The short- and long-delay scores for the free- and cued-recall conditions of List A items are shown on the right of Figure 1 for both groups of subjects. The first two sets of bars on the right show the results for the short-delay free-recall (SDFR) and short-delay cued-recall (SDCR) conditions; the remaining two sets of bars show the results for the long-delay free-recall (LDFR) and long-delay cued-recall (LDCR) conditions. Examination of the raw scores in the four conditions of this panel, followed by independent-samples t-test comparisons between groups, also revealed no differences in either free or cued recall between the groups based on presentation modality (p>.05).
CVLT-II Process Measures
Looking at the overall average first-order measures of immediate and delayed free and cued recall performance shown in Figure 1 provides only a global superficial picture of the underlying organizational and processing strategies that subjects use in carrying out a multi-trial free recall task with categorized word lists. In addition to providing total recall scores summed across all serial positions following the five repetitions of List A, and the one repetition of List B (the interference list), we also looked at several other more detailed measures of the cognitive processes underlying verbal learning and memory using this task. These measures included examination of serial position curves, primacy and recency effects, proactive and retroactive interference (PI and RI), retrieval inhibition, release from PI, self-generated organizational strategies (semantic, serial, or subjective clustering), recall intrusion errors, and Yes/No recognition memory. There were no significant differences in any of these process measures between the two groups. The results that are most relevant for interpretation of Study 2 (primacy and recency effects, PI, RI, retrieval inhibition, and release from PI) are reported below.
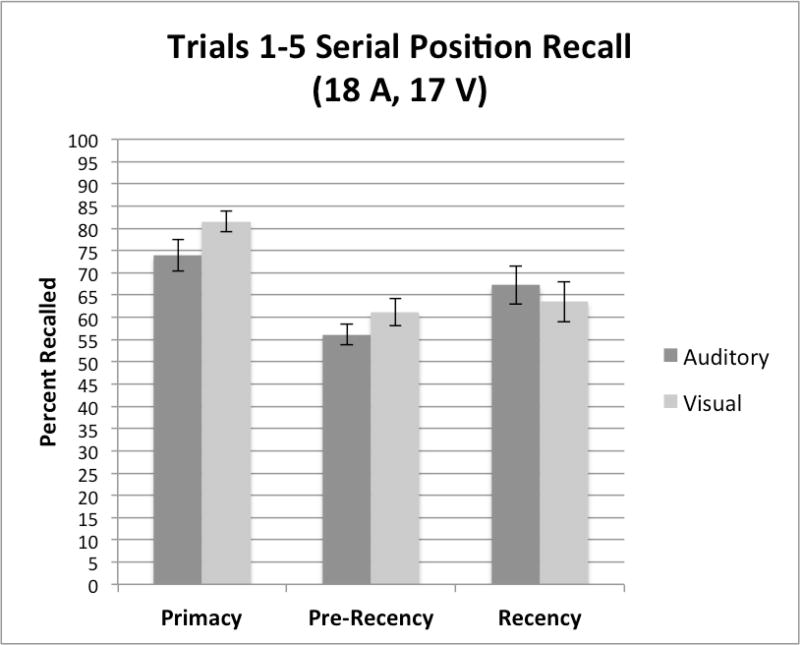
Primacy and Recency Effects
Analysis of recall of words from primacy (first four items of the list), pre-recency (middle 8 items), and recency (final four items) portions of List A were carried out. Recall of words from these three subcomponents of the serial position curve are assumed to reflect fundamentally different storage and retrieval processes used in carrying out free recall tasks (see Atkinson & Shiffrin 1971; Raaijmakers 1990). Figure 2 shows a summary of the free recall scores obtained from the primacy (left panel), pre-recency (center panel), and recency (right panel) components of the serial position curves of List A averaged over the five repetition learning trials for each group of subjects. Examination of this figure shows two patterns in the free recall scores. First, recall is better overall from primacy than from recency or pre-recency portions of the serial position curve (p<.005). Second, although there are reliable differences across the three serial positions, no differences in free recall are present in any of the three subcomponents between the two groups based on presentation modality. The absence of any differences due to presentation modality between the two groups in both the primacy and recency portions of the serial position curve suggests that early list items were successfully encoded and stored in memory and retrieved equivalently by both groups of subjects.
Figure 2.
Summary of free recall scores for the primacy (left panel), pre-recency (center panel), and recency (right panel) subcomponents of the serial position curves for List A averaged over the five repetition learning trials for the subjects, tested using the auditory CVLT-II (A-CVLT) versus the visual CVLT-II (V-CVLT).
Proactive and Retroactive Interference
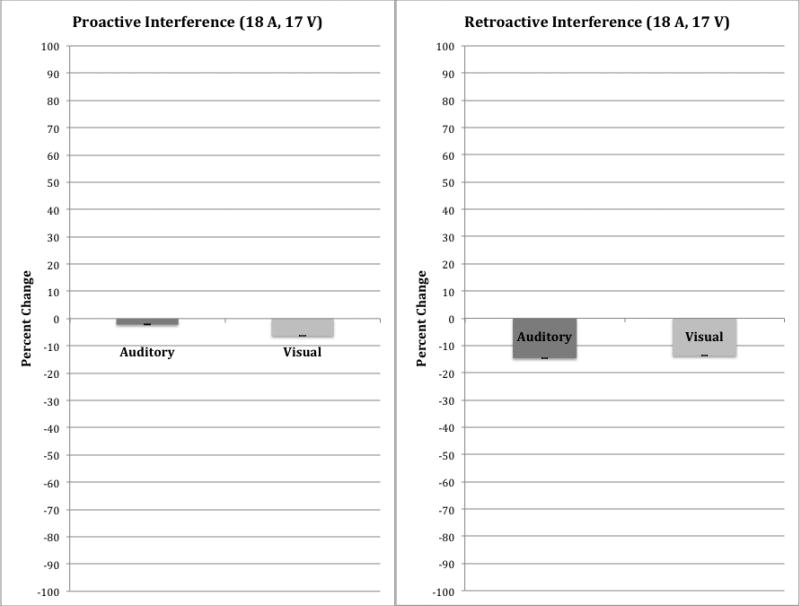
The free recall scores from List B, the interference list, are typically used to compute two measures of forgetting: a proactive interference (PI) index and a retroactive interference (RI) index. PI refers to forgetting of newly learned words on List B as a result of interference from previous learning of similar or related words from List A. In contrast, RI refers to the presence of forgetting that occurs when learning of new words on List B reduces the ability to remember words that were previously learned on List A. A measure of PI is obtained by subtracting the recall of words on List B from the immediate free- recall of words from List A Trial 1; a measure of RI is obtained by subtracting recall of short-delay free-recall of List A items from immediate free-recall of items on List A Trial 5. Figure 3 shows a summary of these two interference scores for both presentation conditions. The PI scores for both groups are shown in the left-hand panel while the RI scores are shown in the right-hand panel. Measures of PI (List B free-recall minus List A Trial 1 free-recall) and RI (List A short-delay free-recall minus List A Trial 5 free-recall) were obtained from the response protocols. Although we found slightly greater RI overall than PI, no differences were observed in either the PI or RI scores between the two groups of subjects based on presentation modality.
Figure 3.
Summary of the proactive interference (PI) and retroactive interference (RI) scores for auditory CVLT-II (A-CVLT) versus visual CVLT-II (V-CVLT). PI scores are shown in the left-hand panel; RI scores are shown in the right-hand panel.
Retrieval Induced Forgetting and Release from PI
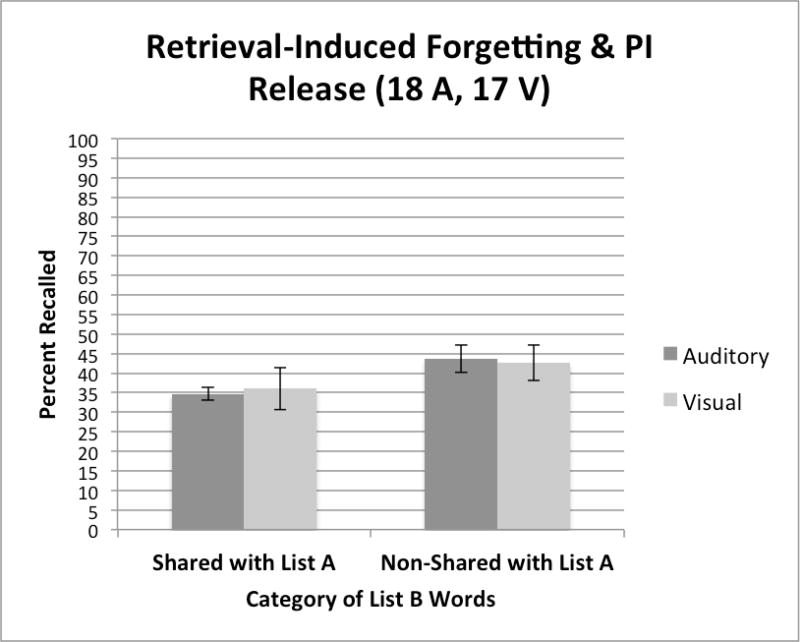
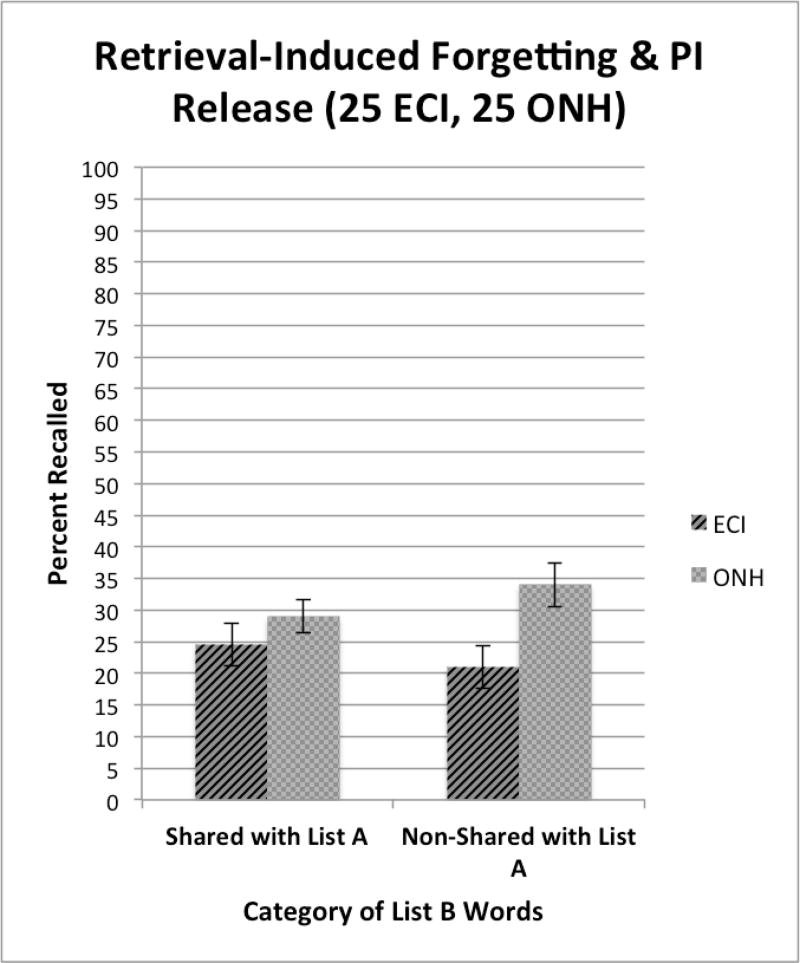
The composition of the specific test items used on List B of the CVLT-II, the interference list, was not only designed to measure the presence of PI and RI effects but was also created to assess several more subtle aspects of forgetting due to interference and inhibitory control processes in list learning experiments. Half of the test items on List B were new words that shared semantic categories with words on List A while the other half of the items on List B were new words that did not share any categories with the items on List A. Recall of List B words that share semantic features with words on List A provides a way to measure forgetting of new items due to “retrieval induced forgetting,” a form of forgetting that reflects interference or inhibitory control processes related to the activation of semantically similar items in memory (see Anderson et al. 1994). Retrieval induced forgetting has received a great deal of interest and attention by memory scientists in recent years because it demonstrates that sometimes the very act of remembering information and retrieving a memory trace from long-term memory can cause forgetting and memory loss (see Anderson et al. 1994; Storm et al. 2015). In contrast, recall of non-shared List B items on the CVLT provides a way to measure of the improvement in free recall due to a “release from PI,” which reflects the restoration of the capacity to encode and recall studied words from one semantic category after switching the semantic categories of new study words to be recalled (Wickens et al. 1963; Wickens 1970). Thus, an examination of the free recall scores for the shared and non-shared items on List B provides a way to assess both retrieval induced forgetting as well as release from PI at the same time.
Figure 4 shows the free recall scores for shared and non-shared items on List B for the two groups of subjects. Auditory presentation (A-CVLT) is shown by the solid dark bars on the left of each pair of bars; visual presentation (V-CVLT) is shown by the gray bars on the right of each pair of bars. Although performance was better overall for the non-shared items than the shared items on List B (p<.001), there were no differences in recall between the two groups based on presentation modality. The lower recall scores observed for the shared items in this figure is evidence for retrieval induced forgetting for new items on List B that share the same semantic categories as the items previously studied and retrieved from List A. In contrast, the advantage observed for non-shared items on List B is evidence of a release from PI, the improvement in recall of words from one semantic category after the category of the new materials is shifted to a different semantic category (Wickens et al. 1963).
Figure 4.
Free recall scores for Shared and Non-shared words on List B for the two groups of subjects, tested using the auditory CVLT-II (A-CVLT) versus the visual CVLT-II (V-CVLT). A-CVLT is shown by the solid dark bars on the left of each pair of bars; V-CVLT is shown by the gray bars on the right of each pair of bars.
Neurocognitive Measures
In addition to the measures of verbal learning and memory using the newly developed computer-controlled CVLT protocols, we also administered several neurocognitive measures to assess the strengths, weaknesses, and milestones in these two groups of subjects (see Kronenberger et al. 2016; Kronenberger & Pisoni 2016). The cognitive measures obtained from both groups of subjects included the following: Ravens Progressive Matrices, a non-verbal test of fluid reasoning (Raven et al. 1998), TOWRE-2, a visual word recognition test of reading fluency and speed (Torgesen et al. 2012), a forward Visual Digit Span test to measure verbal short-term memory capacity, and the WordFam-150 test, a visual word familiarity rating scale that has been used to measure vocabulary knowledge and lexicon size (Lewellen et al. 1993; Stallings et al. 2000). Table 2 presents a summary of the scores on these four tests for the two groups of subjects. No differences were observed in any of the neurocognitive scores between the two groups of subjects.
Table 2.
Demographic Variables for Study 1a
| Table 2 (Study 1A) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Auditory (N = 18) | Visual (N = 17) | |||||
|
| ||||||
| Demographics | Mean | SD | Range | Mean | SD | Range |
|
| ||||||
| Age | 22.61 | 1.95 | 18–54 | 24.00 | 1.73 | 18–42 |
|
| ||||||
| Gender Breakdown | 4 M | -- | -- | 6 M | -- | -- |
| 14 F | 11 F | |||||
|
| ||||||
| Measures | Mean | SD | Range | Mean | SD | Range |
|
| ||||||
| Raven’s Matrices (# Correct) | 44.94 | 1.30 | 32–55 | 41.41 | 1.70 | 24–52 |
|
| ||||||
| TOWRE-II Words (# Correct) | 90.28 | 2.38 | 73–108 | 93.00 | 1.71 | 83–107 |
|
| ||||||
| TOWRE-II Non-Words (# Correct) | 53.56 | 1.74 | 34–64 | 51.47 | 1.84 | 35–60 |
|
| ||||||
| TOWRE-II Total (# Correct) | 143.83 | 3.51 | 117–169 | 144.47 | 2.64 | 125–157 |
|
| ||||||
| Word Familiarity Score | 3.12 | 0.12 | 2.22–4.28 | 3.22 | 0.15 | 2.30–4.44 |
|
| ||||||
| Longest Digit Span | 7.56 | 0.33 | 5–10 | 8.41 | 0.39 | 5–11 |
|
| ||||||
| Digit Span Points | 104.00 | 4.05 | 55–132 | 104.00 | 4.53 | 70–134 |
We also carried out a series of bivariate correlations to investigate the relations between the neurocognitive scores and a subset of the primary first-order measures obtained from the A-CVLT or V-CVLT (List A Trial 1, List A Trial 5, List A Trial 1–5 total words correctly recalled, List B, and learning slope over the five List A repetition trials). No differences were found between the groups in the pattern of correlations among the measures. These findings provide converging evidence of common associations between measures of verbal learning and memory obtained from the CVLT-II and several verbal and non-verbal neurocognitive measures, suggesting that the same elementary information processing operations are shared by all these sets of measures independently of presentation modality. Again, no differences were observed between the two groups of subjects based on presentation modality of the CVLT-II.
Discussion
The present set of findings comparing auditory and visual presentation modalities using stimulus materials adapted from the conventional live-voice CVLT-II clinical protocol revealed no differences in primary or process measures as a function of presentation modality. Therefore, based on these findings, it may be appropriate to use the alternative V-CVLT when the conventional live-voice auditory presentation format would be inappropriate to study verbal learning and memory processes in some clinical populations, such as adults who have significant hearing loss. In addition to the absence of any significant differences on scores of the CVLT-II between the A-CVLT and V-CVLT groups, the correlations of the CVLT-II scores with the neurocognitive measures were comparable across the two groups of subjects.
Because the new V-CVLT test was developed specifically for postlingually deaf older adults with significant hearing loss who are CI users or potential candidates for CIs, we carried out an additional validity and feasibility study with two groups of older normal-hearing adults. This additional validation study was done because it was possible that the validity and feasibility results obtained with the young normal hearing adults in this study might not generalize robustly to an older population of healthy adults who may have other additional co-morbidities related to cognitive aging. To accomplish this objective, we recruited two new groups of older normal hearing (ONH) participants and compared their performance on the same computer-controlled auditory and visual presentation conditions of the CVLT-II used in Study 1a. Our objective was to establish validity and feasibility of the V-CVLT with a sample of ONH subjects who are representative of the age range of the target clinical population of CI patients we are currently studying.
Study 1b – Validation & Feasibility of V-CVLT in Older NH Adults
Methods
Participants
Thirty-two participants between the ages of 55 and 77 years (mean age of 67.6) were recruited via flyers posted at The Ohio State University Department of Otolaryngology and through a national research recruitment database, ResearchMatch. Participants reported no history of any speech and hearing problems. For compensation, they received $15 for participation. All participants underwent conventional audiological assessment immediately prior to testing. Normal hearing was defined as four-tone (.5, 1, 2, and 4 kHz) pure-tone average (PTA) of better than 25 dB HL in the better ear. Because many of these participants were elderly adults, this criterion was relaxed to 35 dB HL PTA, although only three participants had a PTA poorer than 25 dB HL. All participants also demonstrated scores within normal limits on the Mini-Mental State Examination (Folstein et al. 1983), a cognitive screening task, with raw scores all greater than 26 out of a possible 30. A summary of the demographics is provided in Table 3.
Table 3.
Demographic Variables for Study 1b
| Table 3 (Study 1B) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Auditory (N = 16) | Visual (N = 16) | |||||
|
| ||||||
| Demographics | Mean | SD | Range | Mean | SD | Range |
|
| ||||||
| Age | 66.00 | 0.70 | 60–71 | 69.00 | 1.40 | 55–77 |
|
| ||||||
| Gender Breakdown | 8 M | -- | -- | 5 M | -- | -- |
| 8 F | 11 F | |||||
|
| ||||||
| MMSE (raw score) | 29.25 | 0.27 | 26–30 | 29.31 | 0.22 | 27–30 |
|
| ||||||
| Measures | Mean | SD | Range | Mean | SD | Range |
|
| ||||||
| Raven’s Matrices (# Correct) | 14.50 | 1.55 | 6–26 | 11.44 | 1.22 | 6–22 |
|
| ||||||
| Longest Digit Span | 6.69 | 0.35 | 4–9 | 5.94 | 0.23 | 5–8 |
|
| ||||||
| Digit Span Points | 56.19 | 5.08 | 25–100 | 44.75 | 3.09 | 27–69 |
|
| ||||||
| TOWRE-II Words (# Correct) | 82.94 | 3.30 | 55–108 | 85.25 | 2.06 | 75–107 |
|
| ||||||
| TOWRE-II Non-Words (# Correct) | 45.88 | 2.78 | 20–59 | 43.44 | 2.70 | 19–57 |
|
| ||||||
| TOWRE-II Total (# Correct) | 128.81 | 4.82 | 95–164 | 128.69 | 3.89 | 95–150 |
|
| ||||||
| Word Familiarity Score | 5.36 | 0.24 | 4.12–6.46 | 5.43 | 0.17 | 3.85–6.36 |
Procedures and Materials
Participants were randomized to undergo testing using either the V-CVLT or the A-CVLT. Testing was performed as described above in Study 1a, except that an abridged version of each CVLT task was used, concluding with “Short Delay Cued Recall” followed by the Yes/No recognition task. The 20 minute distractor period and the long-delay free-recall and long-delay cued-recall tests were not used with these subjects to reduce testing time. As in Study 1a, four additional neurocognitive assessments were also performed: the Raven’s Progressive Matrices for a 10 minutes time period, the TOWRE-2, visual forward digit span, and the WordFam-150 test. This final test was similar to the earlier WordFam-150 test, except that it was completed on paper by participants at a later time and mailed back in, and participants rated their familiarity with words from 1 (not familiar at all) to 7 (very familiar).
Data Analyses
To evaluate the feasibility and equivalency of the A-CVLT and V-CVLT tests in older NH participants, independent-samples t-tests were performed separately to assess any differences between groups based on age, Ravens, TOWRE-2, Visual Digit Span, and word familiarity ratings. Following these analyses, a series of ANOVAs and post-hoc paired-comparison tests were performed to assess group differences in performance between auditory and visual presentation conditions on the primary and process measures of the CVLT-II.
Results
Figure 5 shows a summary of the immediate and short-delay free- and cued- recall CVLT scores obtained from the two groups of ONH subjects tested. As in Study 1a, the figure is divided into two panels. On the left are the immediate free-recall scores for the five repetitions of List A and the one repetition of List B. The panel on the right presents a summary of the short-delay free- and short-delay cued-recall scores from both groups of subjects. The left set of bars in each panel shows the average recall scores from the group of ONH participants who were assigned to the auditory presentation condition (A-CVLT); the right set of bars shows the scores from the group of ONH participants who were assigned to the visual presentation condition (V-CVLT).
Figure 5.
Overall summary of the immediate and short-delay free- and cued- recall CVLT-II scores obtained from two groups of older normal hearing (ONH) subjects using the auditory CVLT-II (A-CVLT) versus the visual CVLT-II (V-CVLT). On the left are the immediate free-recall scores for the five repetitions of List A and the one repetition of List B. On the right are the short-delay free- and cued-recall scores from both groups of subjects. The left set of bars in each panel shows the average recall scores from ONH participants assigned to the auditory presentation condition (A-CVLT); the right set of bars shows the scores from the group of ONH participants who were assigned to the visual presentation condition (V-CVLT). The vertical dashed bars show the range of normative scores for each of the recall conditions based on age and gender.
As in Study 1a with young adults, the immediate free recall scores displayed on the left in Figure 5 show two main findings. First, both groups of ONH subjects displayed robust repetition learning effects over the five presentations of List A. Using a 2 (modality) × 5 (trials) ANOVA, followed by paired-comparison tests, performance improved continuously for both groups from Trial 1 to Trial 5 after each repetition of List A (F=103.44, p<.001). Second, as in the initial study, both groups of participants showed comparable levels of immediate free recall performance after each repetition of List A over the five presentations. The main effect of presentation modality across the five learning trials was not statistically significant in a 2 × 5 ANOVA (p> .05). As in the first study, performance of both groups was also substantially lower for recall of the new items from List B compared to the items on List A Trial 1 (p<.001). When the free recall scores for both groups were plotted as a function of serial position for List A Trial 1 and List B, no significant differences were observed based on presentation modality. Both groups also showed comparable levels of recall on List B, List A short-delay free recall, and List A short-delay cued recall, replicating the results obtained with the younger subjects in Study 1a.
No differences between the two groups were found in primacy or recency recall, PI or RI, retrieval induced forgetting, or release from PI. Self-generated organizational strategies in free recall (i.e. semantic, serial, or subjective clustering) were also comparable across both groups of ONH subjects, as were recall intrusion errors and scores on Yes/No recognition memory. We also did not find any differences in performance between the two groups on any of the neurocognitive measures. The correlations between CVLT-II primary and process scores and the neurocognitive measures were again similar between A-CVLT and V-CVLT groups.
Discussion
The results of this validity and feasibility study using two groups of ONH controls in Study 1b replicated the initial findings obtained with young college-aged students in Study 1a. No differences were observed in any of the immediate or delayed free- and cued-recall measures or any of the primary or process measures obtained from the CVLT-II based on presentation modality. Taken together with the results obtained from Study 1a, we believe it is appropriate to use the new visual presentation format of the CVLT-II with hearing impaired older adults who have received CIs. Having a visual presentation format of the CVLT-II available to study foundational underlying processes of verbal learning and memory in this clinical population eliminates any concerns that could be raised about the role of audibility and any possible differences that may be due to early auditory sensory processing and hearing loss as possible confounding factors that might influence the verbal learning and memory measures obtained. The use of a visual CVLT protocol removes hearing and audibility from the equation and permits us to obtain pure measures of any disturbances in basic verbal learning and memory processes without the confounding influence of compromised hearing or speech perception.
Study 2 – Visual CVLT-II in older experienced CI users (ECIs) and older NH controls (ONHs)
After having confirmed the equivalency of the visual CVLT-II with the conventional auditory CVLT-II, a subsequent study was carried out to investigate verbal learning and memory processes in a group of post-lingual experienced CI users (ECI) and to compare their performance to a group of age- and nonverbal IQ-matched older normal-hearing controls (ONH). Our objective was to uncover and identify differences in core verbal learning and memory processes that could serve as reliable predictors of speech recognition outcomes following implantation. We also wanted to investigate the combined contributions to speech recognition outcomes of demographics and hearing history, neurocognitive factors, and several core measures of verbal learning and memory in this clinical population.
It is important to mention here that except for the two earlier studies by Heydebrand et al. (2007) and Holden et al. (2013), all of the previous research carried out on verbal learning and memory with post-lingual adult CI users has been concerned almost exclusively with short-term and working memory processes, not multi-trial verbal learning, or long-term memory (LTM) processes. Research on verbal learning and memory using supra-span lists that exceed the immediate processing capacity of STM has received very little attention by clinicians and researchers in the past despite the critical importance of LTM to speech recognition and spoken language processing. This is not surprising because most clinicians and researchers who are working in the field of CIs believe that the individual differences and variability routinely observed in speech recognition outcomes following implantation simply reflect differences in early registration and encoding sensory information in short-term and working memory. Research on storage and retrieval processes in LTM and interactions between encoding and retrieval processes has been minimal despite the critical importance of LTM in supporting robust adaptive functioning and speech recognition in challenging or adverse listening environments, situations in which the use of prior linguistic knowledge and experience play substantial compensatory roles when the bottom-up sensory information is significantly degraded (see Rönnberg et al. 2013).
Participants
Fifty adult participants between the ages of 53 and 81 years (mean age of 68.0) were recruited using flyers posted at The Ohio State University Department of Otolaryngology and through the use of ResearchMatch, a national research recruitment database. Half of the subjects were experienced CI users (ECIs) and half were older normal hearing control participants (ONH). All subjects received $15 as compensation for participation in this study. Socioeconomic status (SES) of participants was quantified using a metric developed by Nittrouer and Burton, consisting of occupational and educational levels each rated from 1 (lowest level) to 8 (highest level) and then multiplied, resulting in scores between 1 and 64 (Nittrouer & Burton, 2006).
Inclusion criteria for all participants were as follows: (1) native English speaker; (2) high school diploma or equivalency; (3) vision of 20/40 or better on a basic near-vision test; (4) Mini Mental State Examination (MMSE; Folstein & Folstein, 1975) score greater than or equal to 26, suggesting no evidence of cognitive impairment; (5) Wide Range Achievement Test (WRAT; Wilkinson & Robertson, 2006) word reading standard score ≥ 80; (6) age 50 or older. Inclusion criteria for the ECI sample were (1) onset of severe-to-profound hearing loss no earlier than age 12 years; (2) severe-to-profound hearing loss in both ears prior to implantation; (3) use of at least one cochlear implant; and (4) CI-aided thresholds better than 35 dB HL at .25, .5, 1, and 2 kHz, as measured by clinical audiologists within one year before enrollment in the present study. The inclusion criterion required specifically for the ONH sample was four-tone (.5, 1, 2, and 4 kHz) pure-tone average (PTA) of less than 35 dB HL in the better ear (the standard 25dB HL criterion for NH was relaxed to 35 dB HL PTA because the sample consisted of older adults, although only three participants had a PTA poorer than 25 dB HL).
The twenty-five ECI participants were recruited from the patient population of the Otolaryngology department at OSU and had diverse underlying etiologies of hearing loss and different ages of implantation (see Table 4). All but four of the CI users reported onset of hearing loss after age 12 years, meaning they were post-lingually deaf and had normal language development prior to the onset of their hearing loss (suggested by their normal hearing until the time of puberty). The other four CI users reported some degree of congenital hearing loss or onset of hearing loss during childhood but did not meet criteria for severe-to-profound hearing loss until age 12. All ECI participants had experienced early hearing aid intervention and typical auditory-only spoken language development during childhood, were mainstreamed in education, and experienced progressive hearing losses into adulthood. All of the CI users received their CIs after the age of 35 years, with mean age at implantation of 61.5 years (SD 10.4). All ECI participants had used their CIs for at least 2 years prior to testing, with mean duration of CI use 7.5 years (SD 6.7). All of the ECI participants except one used Cochlear Corporation (New South Wales, Australia) devices with an Advanced Combined Encoder processing strategy; one CI user had an Advanced Bionics (Valencia, California) device and used a Hi Res Optima-S processing strategy. Eleven participants had a right CI, four used a left implant, and nine had bilateral implants. Eight participants wore a contralateral hearing aid. During testing with auditory materials, participants wore their devices in their usual everyday modes, including any use of hearing aids, and settings were kept the same throughout the entire testing session. Twenty-five ONH participants were recruited from the same settings and did not differ from the ECI users in chronological age, Ravens scores (non-verbal fluid IQ), or socioeconomic status (SES) (Table 4).
Table 4.
Demographic Variables for Study 2
| Table 4 (Study 2) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Experienced CI (N = 25) | Older Normal Hearing (N = 25) | |||||||
|
| ||||||||
| Demographics | Mean | SD | Range | Mean | SD | Range | ||
|
| ||||||||
| Age | 67.0 | 1.58 | 55–81 | 69.0 | 1.44 | 53–81 | ||
|
| ||||||||
| Gender Breakdown | 14 M | -- | -- | 6 M | -- | -- | ||
| 11 F | 19 F | |||||||
|
| ||||||||
| Education | 6 High school or lower, | -- | -- | 4 High school or lower, | -- | -- | ||
| 2 Tech degree, | 0 Technical degree, | |||||||
| 14 College degree or some college, | 10 College degree or some college, | |||||||
| 3 Masters/PhD/post-graduate | 11 Masters/PhD/post-graduate | |||||||
|
| ||||||||
| SES | 25.00 | 3.18 | 6–64 | 34.88 | 3.03 | 9–64 | ||
|
| ||||||||
| Pure-Tone Average | 101.00 dB | 3.14 | 70–120 | 21 dB | 1.61 | 8–34 | ||
|
| ||||||||
| MMSE | 28.00 | 0.29 | 26–30 | 29.28 | 0.17 | 27–30 | ||
|
| ||||||||
| Age Hearing Loss Began | 27.00 | 3.96 | 0–61 | -- | -- | -- | ||
|
| ||||||||
| Etiology of Hearing Loss | 13 Genetic, | -- | -- | -- | -- | -- | ||
| 1 Menieres, | ||||||||
| 4 Progressive, | ||||||||
| 1 Otosclerosis, | ||||||||
| 1 Sepsis, | ||||||||
| 1 Chronic ear infections, | ||||||||
| 4 Unknown | ||||||||
|
| ||||||||
| Wear Hearing Aids Currently | 6 Y, 19 N | -- | -- | -- | -- | -- | ||
Procedures
All participants underwent testing using the computer-controlled visual presentation CVLT-II developed for Study 1. Testing was performed as described above in Study 1b, using the abridged shortened version of the original V-CVLT concluding with “Short Delay Free Recall” followed by the Yes/No Recognition test. Participants also completed the Raven’s Progressive Matrices for a fixed 10-minute time period, the TOWRE-2 (words and nonwords), and visual forward Visual Digit Span task. To decrease testing time, the WordFam-150 test was completed on paper at home and mailed back to the laboratory.
In addition to the V-CVLT and neurocognitive measures, the ECI participants also completed three different speech recognition tests to assess their ability to recognize spoken words in isolation and in sentence contexts. All test signals were presented in quiet at 68 dB SPL over a high-quality loudspeaker one meter in front of the participant at zero degrees azimuth in a sound attenuated booth. Percent correct keyword recognition scores were computed for both the word and sentence tests. In addition, percent correct whole sentence scores were also calculated for the two sentence tests.
To assess open-set recognition of isolated spoken words, one list of 50 CID W-22 words (Hirsh et al. 1952) was used. Test words were presented in the carrier phrase, “Say the word _______.” All test words were recorded by a single male talker who spoke with a mid-western regional dialect. To assess word recognition in sentences, two measures of sentence recognition in meaningful contexts were obtained using the following materials: (1) Harvard “Standard” test sentences which are relatively long, complex, and semantically meaningful sentences taken from the IEEE corpus (IEEE 1969; Egan 1948), such as “The wharf could be seen from the opposite shore”; (2) PRESTO (Perceptually Robust English Sentence Test Open-set) sentences, representing perceptually challenging, high-variability listening conditions (Gilbert et al. 2013; Tamati et al. 2013). To increase acoustic-phonetic and indexical variability in sentence recognition, each sentence on a given PRESTO test list was produced by a different talker, and all of the talkers used for a given test list were selected to span a wide range of regional dialects in the US. To reduce perceptual learning and adaptation, no talker was ever repeated in the same test list and each test sentence in a list was always a novel sentence. None of the sentences on any of the lists were ever repeated during the test.
Data Analyses
ANOVAs and t-tests were performed to assess group differences between ECI and ONH groups on the primary and process measures obtained from the CVLT-II protocol. In addition, correlational and regression techniques were performed for the ECI group to assess the relations among V-CVLT primary, process, and contrast measures with the speech recognition scores.
Results
Descriptive statistics comparing the ECI participants and ONH controls on Ravens, TOWRE-2, visual digit span, and WordFam-150 scores are shown in Table 5. No differences in the group means were found for any of the baseline cognitive test scores except word familiarity ratings, where ECI participants demonstrated slightly smaller vocabulary size (p<.05).
Table 5.
Summary of Performance Measure 5 for Study 2
| Table 5 (Study 2) | ||||||
|---|---|---|---|---|---|---|
| Experienced CI (N = 25) |
Older Normal Hearing (N = 25) |
|||||
| Cognitive Measures | Mean | SD | Range | Mean | SD | Range |
| Raven’s Correct Matrices | 10.08 | 0.89 | 3–20 | 11.84 | 1.08 | 5–26 |
| TOWRE-II Words (# Correct) | 77.72 | 2.68 | 45–95 | 82.96 | 1.62 | 66–107 |
| TOWRE-II Non-Words (# Correct) | 41.68 | 2.44 | 6–59 | 41.72 | 1.96 | 19–57 |
| TOWRE-II Total (# Correct) | 119.40 | 4.74 | 51–148 | 124.68 | 2.97 | 95–150 |
| Longest Digit Span | 5.68 | 0.26 | 3–8 | 5.8 | 0.20 | 4–8 |
| Digit Span Points | 42.68 | 3.53 | 14–79 | 43.40 | 2.60 | 20–69 |
| Word Familiarity Score | 4.69 | 0.19 | 3–7 | 5.30 | 0.15 | 3.57–6.36 |
| Speech Recognition | Mean | SD | Range | Mean | SD | Range |
| CID Phonemes Percent Correct | 78.56 | 3.90 | 28–100 | -- | -- | -- |
| CID Words Percent Correct | 69.04 | 4.63 | 18–96 | -- | -- | -- |
| Harvard Standard Keywords Percent Correct (N=24) | 79.46 | 3.70 | 11–95 | -- | -- | -- |
| Harvard Standard Sentences Percent Correct (N=24) | 48.47 | 4.49 | 0–80 | -- | -- | -- |
| PRESTO Keywords Percent Correct (N=24) | 61.00 | 4.24 | 8–89 | -- | -- | -- |
| PRESTO Sentences Percent Correct (N=24) | 27.78 | 3.75 | 3–63 | -- | -- | -- |
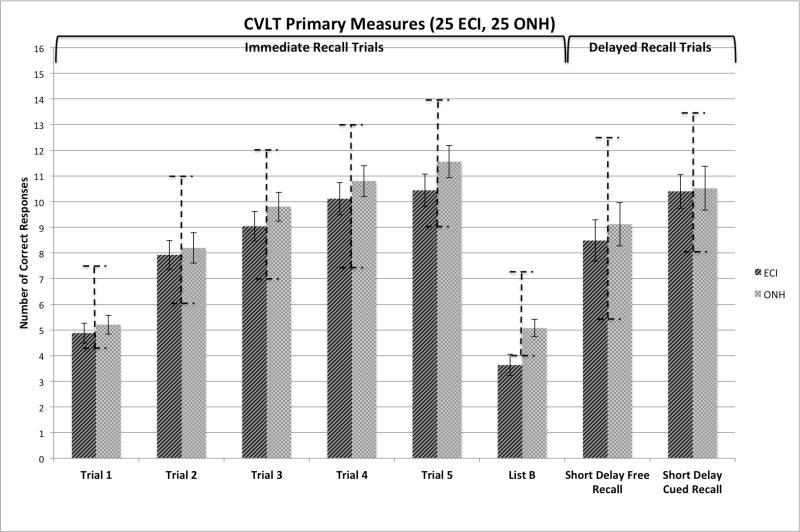
Figure 6 shows a summary of the immediate and short-delay free- and cued-recall scores obtained on the V-CVLT from the ECI and ONH controls. Except for recall of List B items by the ECI group, all of the scores fell within +/−1 SD of the normative range. A 2 × 5 ANOVA followed by a series of post-hoc pair-wise comparisons showed no group differences in recall on any of the List A immediate or short-delay recall measures. However, as shown in Figure 6, the groups did differ significantly in recall of items on List B; the ONH controls recalled significantly more List B words than the ECI group (p=.009). No other group differences were observed on any of the other primary measures from the CVLT.
Figure 6.
Summary of the immediate and short-delay free- and cued-recall scores obtained from the experienced CI users (ECI) and older normal hearing (ONH) controls, tested using the visual CVLT-II (V-CVLT). The panel on the left presents a summary of the immediate free recall scores for the five repetitions of List A and the one presentation of List B for both groups of subjects. The panel on the right presents a summary of the short-delay free- and short-delay cued-recall scores from both groups. The left set of bars in each panel shows the average recall scores from the ECI participants; the right set of bars shows the scores from the ONH controls. Bars representing the norm range scores are plotted on top of each recall condition based on age and gender.
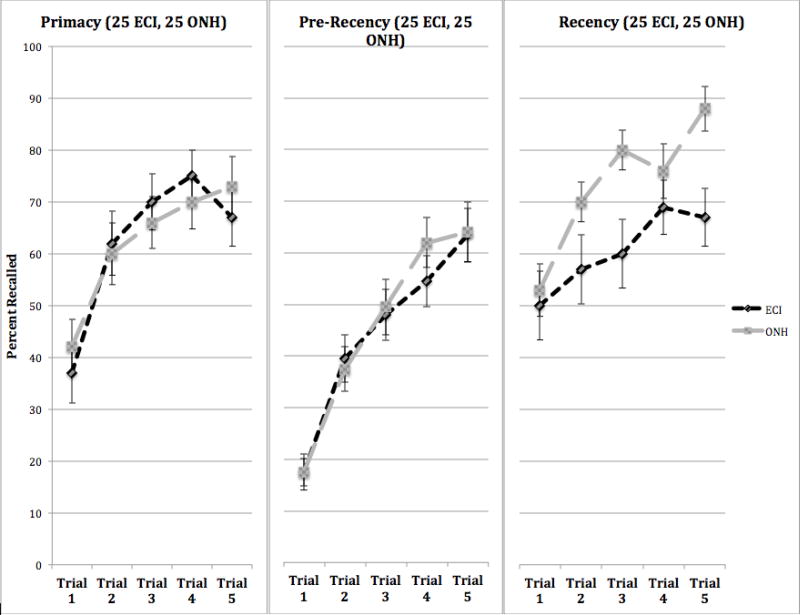
An examination of the process measures from the CVLT revealed that the ONH group recalled more List A words from the recency portion of the serial position curve than the ECI group, and this difference occurred consistently across all five study trials of List A, as shown in the right-hand panel of Figure 7. A 2 × 5 ANOVA on recall of words from the recency portions of List A revealed main effects for group and repetition learning trials (F= 5.83, p=.02; F=10.57, p<.001). Both groups showed comparable levels of free recall for words from the primacy (left-hand panel) and pre-recency (middle panel) subcomponents of the serial position curve (main effects for group and repetition learning trials on both of these subcomponents were not significantly different in a series of 2 × 5 ANOVAs).
Figure 7.
Percent correct free recall of words from primacy, pre-recency, and recency sub-components of the serial position curve for the experienced CI users (ECI) and older normal hearing (ONH) groups as a function of the five repetition study trials of List A, using the visual CVLT-II (V-CVLT). The panel of the left shows free recall of words from primacy, the middle panel shows free recall of words from pre-recency, and the right panel shows free recall of words from recency.
Forgetting Due to Proactive and Retroactive Interference
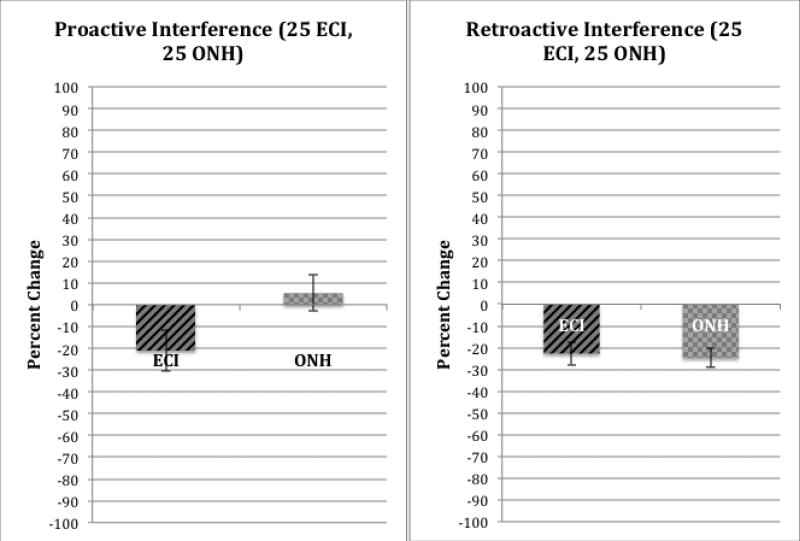
Figure 8 shows a summary of the secondary process measures of PI in the left-hand panel and RI in the right-hand panel obtained from the CVLT. The measure of PI was obtained by subtracting the recall of words on List B from the recall of words from List A Trial 1; the measure of RI is obtained by subtracting recall of short-delay free-recall of List A items from immediate free-recall of items on List A Trial 5. Both groups showed comparable RI effects. Although recall performance on short-delay free recall of List A items in both groups was reduced significantly by prior exposure to List B items (p<.001), there was no difference in RI between the two groups. In contrast, a different pattern of results was obtained for PI, as shown in the left-hand panel of Figure 8. While the ECI group showed significantly lower performance on List B items following the five repetitions of List A (p=.025), the ONH group showed a small increase in recall of List B items. A 2 (group) × 2 (List A Trial 1 vs. List B) ANOVA revealed a significant effect of trial (List A Trial 1 vs List B; p=.02) and marginal effect for group (ECI vs ONH; p=.058). There was also a marginally significant interaction between these two main effects (p=.053).
Figure 8.
Summary of measures of proactive interference (PI) and retroactive interference (RI) for the experienced CI users (ECI) and older normal hearing (ONH) groups, using the visual CVLT-II (V-CVLT). PI scores are shown in the left-hand panel; RI scores are shown in the right-hand panel.
Retrieval Induced Forgetting and Release from Proactive Interference
Figure 9 shows a summary of free recall scores for List B shared items (8 new words from List B that shared semantic categories with words on List A) on the left, and List B non-shared items (8 new words from List B that were selected from new semantic categories that were not shared with words on List A) on the right. As shown in the left-hand panel of Figure 9, there is a trend showing more retrieval induced forgetting (i.e., lower recall scores on List B) for the shared categories from List A for the ECI group than the ONH controls. In contrast, inspection of the right-hand panel of Figure 9 shows that while the ONH group displayed evidence of a release from PI for non-shared semantic categories from List A (i.e., higher recall scores), the ECI group declined slightly but not significantly relative to their recall of words from the shared category on the left. Although the difference between the two groups of subjects was not significant for the shared categories on List B (p>.05), the difference was statistically significant for the non-shared categories shown on the right (p=.01). The present results suggest that differences in basic underlying neurocognitive information processing operations related to retrieval induced forgetting and release from PI which rely on inhibitory control processes can be used to distinguish the verbal learning capacities and attributes of these two groups of subjects even with visually presented materials using the CVLT protocol and methodology.
Figure 9.
Summary of free recall scores for new words on List B that shared semantic features with words on List A (Shared) and new words on List B that did not share any semantic features with words on List A (Non-shared), for the experienced CI users (ECI) and older normal hearing (ONH) groups, using the visual CVLT-II (V-CVLT).
Self-Generated Organizational Strategies: Semantic, Serial, and Subjective Clustering
Figure 10 shows a summary of analyses of semantic clustering (top panel), serial clustering (middle panel), and subjective clustering (bottom panel) strategies used by both groups of subjects. A semantic organizational strategy is assumed to take place when there is a higher probability of recalling a sequence of items in succession that come from the same semantic category. A serial organization strategy is inferred when subjects recall words in the same sequential order in which they were presented on the list. A subjective organizational retrieval strategy is assumed when subjects adopt unique idiosyncratic methods of clustering that do not conform to either the standard semantic or serial clustering strategies. That is, recall is organized and systematic but is specific to an individual subject’s mnemonic strategies and response biases. As described in Study 1, the CVLT-II scoring program quantifies these different self-generated organizational strategies by using three different list-based clustering indices (see Delis et al. 2000; Stricker et al. 2002).
Figure 10.
Summary of semantic, serial, and subjective clustering scores for the experienced CI users (ECI) and older normal hearing (ONH) groups, using the visual CVLT-II (V-CVLT). The top panel shows the semantic clustering scores, the middle panel shows the serial clustering scores, and the bottom panel shows the subjective clustering scores for each group.
Inspection of Figure 10 reveals three patterns in the clustering results. First, as shown in the top panel, there is evidence of greater semantic clustering overall than serial clustering (middle panel) or subjective clustering (bottom panel). Second, both semantic (top panel) and subjective (bottom panel) clustering scores show consistent increases as a function of List A study trials and repetition, suggesting greater organizational clustering with more List A repetitions. And, third, comparing observed clustering between groups across all three panels in Figure 10, the scores were comparable for the two groups of subjects across all three types of clustering strategies No significant differences were observed between the two groups across the three types of clustering strategies.
Y/N Recognition Memory
No differences were found between the two groups of subjects in either hits or false alarm rates. Both groups displayed excellent recognition memory for the studied items from List A compared to the recognition foils. Additional more detailed analyses of the recognition memory data were carried out using measures of discriminability based on d-primes that considered both the hit and false alarm rates. Examination of these discriminability scores also revealed no differences between the two groups of subjects in total recognition, List A source recognition, semantic recognition, or novel item recognition.
Neurocognitive Measures and Speech Recognition Scores
Table 5 provides a summary of the scores obtained for the two groups on the neurocognitive tests: visual digit span, TOWRE-2, WordFam, and Ravens. Speech recognition scores are also summarized in the bottom half of Table 5 for the ECI group, including CID W-22 words, Harvard Standard sentences, and PRESTO sentences. A series of t-tests for independent samples established that almost all of the differences in the neurocognitive measures shown in Table 5 between the two groups were not significantly different from each other. However, there was one exception. WordFam-150 scores were significantly higher for the ONH group than the ECI group (p= 0.02).
Associations of Demographics and Hearing History with Neurocognitive Measures, CVLT-II, and Speech Recognition Outcomes
Next a series of bivariate correlation analyses was performed for the ECI group to assess the association of demographics/hearing history with neurocognitive measures, CVLT scores, and speech recognition outcomes. As shown in Table 6, moderate correlations were obtained between several of the core demographic/hearing history variables such as duration of hearing loss before CI and age at first CI with Ravens and TOWRE-2 scores. Table 7 shows the correlations between core demographics/hearing history and the CVLT primary and process scores. Two of the core demographic/hearing history variables, duration of hearing loss before CI and age at first CI, were correlated with the CVLT scores for List A Trial 5 and List B. Several of the other correlations of demographics with the CVLT scores shown in Table 7 approached significance. Finally, Table 8 shows the correlations between demographic/hearing history variables and the speech recognition outcome measures for the ECI group. Duration of hearing loss before CI, age at first CI, and years of CI use were all significantly correlated with the speech recognition outcome measures. Strong to moderate correlations were found for the CID W-22 words, Harvard Standard sentences, and PRESTO sentences. Correlations with the speech recognition outcomes were generally stronger overall for whole sentence scores compared to keywords correct.
Table 6.
Study 2 Experienced CI (ECI) Demographics/Neurocognitive Correlations (N = 25).
| Age | Best Pure Tone Average |
SES | Age at Hearing Loss Onset |
Duration of Hearing Loss Befor CI |
Age at First CI |
Years of CI Use |
|
|---|---|---|---|---|---|---|---|
| Raven's Number Correct | −0.536 (0.006)* | 0.213 (0.306) | −0.039 (0.857) | 0.229 (0.272) | −0.436 (0.029)* | −0.580 (0.002)* | 0.218 (0.295) |
| TOWRE-II Words | −0.296 (0.151) | 0.250 (0.228) | 0.145 (0.500) | 0.276 (0.182) | −0.423 (0.035)* | −0.430 (0.032)* | 0.408 (0.043)* |
| TOWRE-II Non-Words | −0.471 (0.018)* | 0.074 (0.725) | 0.196 (0.359) | 0.061 (0.773) | −0.252 (0.225) | −0.495 (0.012)* | 0.154 (0.463) |
| Longest Digit Span | 0.072 (0.733) | −0.367 (0.071) | 0.026 (0.904) | −0.332 (0.105) | 0.332 (0.105) | 0.082 (0.697) | −0.040 (0.848) |
| Digit Span Points | 0.010 (0.964) | −0.395 (0.051) | 0.068 (0.751) | −0.317 (0.123) | 0.290 (0.160) | 0.007 (0.975) | 0.006 (0.979) |
| WordFam Score | 0.277 (0.180) | −0.347 (0.089) | 0.678 (<0.001)* | 0.094 (0.654) | 0.034 (0.871) | 0.312 (0.129) | −0.145 (0.489) |
Note: upper value represents bivariate correlation coefficient (r); lower value represents p value. p values with * are significant at < 0.05 and bolded for clarity.
Table 7.
Study 2 Experienced CI (ECI) Demographics/CVLT Correlations (N = 25).
| Age | Best Pure Tone Average |
SES | Age at Hearing Loss Onset |
Duration of Hearing Loss Before CI |
Age at First CI |
Years of CI Use |
|
|---|---|---|---|---|---|---|---|
| List A Trial 1 | −0.228 (0.273) | 0.148 (0.480) | 0.005 (0.983) | −0.270 (0.191) | 0.106 (0.614) | −0.369 (0.070) | 0.412 (0.041)* |
| List A Trial 2 | −0.207 (0.321) | −0.062 (0.767) | 0.031 (0.886) | 0.070 (0.740) | −0.167 (0.424) | −0.282 (0.172) | 0.236 (0.257) |
| List A Trial 3 | −0.383 (0.059) | 0.079 (0.707) | −0.037 (0.862) | 0.032 (0.880) | −0.192 (0.358) | −0.428 (0.033)* | 0.191 (0.361) |
| List A Trial 4 | −0.375 (0.065) | 0.291 (0.158) | −0.012 (0.955) | 0.313 (0.127) | −0.461 (0.020)* | −0.454 (0.022)* | 0.280 (0.175) |
| List A Trial 5 | −0.185 (0.376) | 0.120 (0.567) | −0.056 (0.795) | 0.426 (0.034)* | −0.484 (0.014)* | −0.260 (0.209) | 0.233 (0.263) |
| List A Trials 1–5 | −0.316 (0.123) | 0.133 (0.526) | −0.019 (0.931) | 0.169 (0.418) | −0.308 (0.134) | −0.406 (0.044)* | 0.295 (0.152) |
| List B | −0.372 (0.067) | 0.108 (0.606) | −0.146 (0.497) | 0.275 (0.183) | −0.429 (0.032)* | −0.444 (0.026)* | 0.260 (0.210) |
| Short-Delay Free Recall | −0.159 (0.449) | 0.119 (0.572) | 0.173 (0.419) | 0.258 (0.213) | −0.321 (0.118) | −0.236 (0.257) | 0.232 (0.265) |
| Short-Delay Cued Recall | −0.285 (0.167) | 0.181 (0.387) | 0.020 (0.926) | 0.225 (0.280) | −0.331 (0.106) | −0.347 (0.089) | 0.217 (0.298) |
| PI | −0.153 (0.464) | −0.072 (0.732) | −0.256 (0.227) | 0.441 (0.027)* | −0.451 (0.024)* | −0.097 (0.644) | −0.118 (0.575) |
| RI | 0.063 (0.766) | 0.094 (0.654) | 0.464 (0.022)* | −0.040 (0.848) | 0.030 (0.886) | −0.032 (0.878) | 0.236 (0.256) |
| Semantic Clustering | −0.186 (0.374) | 0.383 (0.058) | −0.001 (0.996) | 0.145 (0.488) | −0.213 (0.306) | −0.211 (0.312) | 0.101 (0.631) |
| Serial Clustering | 0.265 (0.201) | −0.285 (0.167) | 0.132 (0.538) | −0.122 (0.561) | 0.215 (0.303) | 0.275 (0.183) | −0.078 (0.711) |
| Total Learning Slope | −0.138 (0.511) | 0.176 (0.399) | −0.083 (0.699) | 0.679 (<0.001)* | −0.666 (<0.001)* | −0.127 (0.546) | −0.002 (0.991) |
| Y/N Recognition Hits | −0.030 (0.888) | −0.258 (0.213) | −0.108 (0.615) | 0.546 (0.005)* | −0.505 (0.010)* | −0.024 (0.910) | −0.009 (0.966) |
Note: upper value represents bivariate correlation coefficient (r); lower value represents p value. p values with * are significant at < 0.05 and bolded for clarity.
Table 8.
Study 2 ECI Demographics/Speech Recognition Correlations (N = 25).
| Age | Best Pure Tone Average |
SES | Vision | Age at Hearing Loss Onset |
Duration of Hearing Loss Before CI |
Age at First CI |
Years of CI Use |
|
|---|---|---|---|---|---|---|---|---|
| CID Words | −0.492 (0.013)* | 0.202 (0.332) | −0.110 (0.609) | −0.384 (0.058) | 0.309 (0.133) | −0.538 (0.006)* | −0.645 (<0.001)* | 0.496 (0.012)* |
| CID Phonemes | −0.450 (0.024)* | 0.180 (0.390) | −0.150 (0.484) | −0.337 (0.099) | 0.354 (0.083) | −0.561 (0.004)* | −0.600 (0.002)* | 0.479 (0.015)* |
| Harvard Standard Keywords | −0.416 (0.043)* | −0.052 (0.808) | 0.068 (0.756) | −0.411 (0.046)* | 0.292 (0.166) | −0.472 (0.020)* | −0.498 (0.013)* | 0.243 (0.253) |
| Harvard Standard Sentences | −0.369 (0.076) | −0.113 (0.598) | 0.033 (0.879) | −0.285 (0.177) | 0.413 (0.045)* | −0.564 (0.004)* | −0.440 (0.031)* | 0.213 (0.318) |
| PRESTO Keywords | −0.257 (0.225) | 0.040 (0.852) | 0.063 (0.774) | −0.385 (0.063) | 0.192 (0.369) | −0.351 (0.093) | −0.429 (0.037)* | 0.449 (0.028)* |
| PRESTO Sentences | −0.258 (0.223) | 0.016 (0.940) | 0.166 (0.448) | −0.359 (0.085) | 0.188 (0.379) | −0.324 (0.122) | −0.377 (0.069) | 0.320 (0.127) |
Note: upper value represents bivariate correlation coefficient (r); lower value represents p value. p values with * are significant at < 0.05 and bolded for clarity.
Speech Recognition Outcomes: Correlations and Multiple Regression Analyses
One of the primary long-term objectives of the present study was to determine if we can successfully identify new visually based non-auditory cognitive and verbal learning and memory measures that could be used in addition to conventional demographic and hearing history variables to predict and explain speech recognition outcomes in a group of ECI users. Considering the relatively small size of our sample of ECI users, these analyses were considered exploratory in nature. However, identifying factors that correlate with our speech recognition outcome measures, even in this small sample, would provide evidence supporting their significant effects in this clinical population, and would provide a basis for continued study enrollment to enlarge our sample size for multivariate analyses. To accomplish this exploratory analysis goal, we adopted a two-stage analysis approach. In the first stage, we looked at the pattern of bivariate correlations among two distinct sets of predictor variables (the first set was the demographic/hearing history measures and the second set was the neurocognitive measures including Ravens non-verbal IQ scores and verbal learning and memory measures) and speech recognition outcome measures (CID W-22 words, Harvard-Standard sentences, and PRESTO Sentences) obtained from the sample of ECI users. These correlations are reported in Table 9 and will be discussed in the first section below.
Table 9.
Correlations of Speech Recognition with Cognitive and Verbal Learning and Memory Measures from Study 2
| N = 25 | W-22 Phonemes |
W-22 Words |
Harvard-S Keywords |
Harvard-S Sentences |
PRESTO Keywords |
PRESTO Sentences |
|---|---|---|---|---|---|---|
| Cognitive Measures: | ||||||
| Ravens | 0.64 | 0.64 | 0.71 | 0.60 | 0.62 | 0.68 |
| TOWRE-Words | 0.47 | 0.55 | - | 0.47 | 0.41 | 0.41 |
| TOWRE-NonWords | - | 0.41 | 0.49 | 0.48 | - | 0.48 |
| WordFam | - | - | - | - | - | 0.45 |
| Verbal Learning and Memory: | ||||||
| CVLT: List B | 0.51 | 0.47 | 0.48 | 0.56 | 0.52 | 0.52 |
| CVLT: List A Trial 1 | 0.40 | - | - | - | - | - |
| CVLT: List A Trial 5 | - | - | 0.46 | - | - | - |
In the second stage, following the bivariate correlational analyses, we carried out several analyses using hierarchical multiple regression techniques. The incremental contributions of the individual predictor variables from each of the two domains (i.e., demographics/hearing history and neurocognition) were examined together using multiple regression analysis. Variables were entered into two regression models in the following sequence: Model 1: demographic/hearing history factors; and Model 2: demographic/hearing history factors (retaining all significant variables from Model 1) + neurocognitive measures. In order to reduce the number of variables in the regression analysis, only predictor variables that were significantly correlated with the speech recognition outcome measures in the first stage bivariate analysis were eligible for inclusion in Model 1. For regression Model 1, predictor variables with a p-value of 0.10 or lower were entered into the model, and a p-value of 0.10 or lower was required for variables to remain in the model (Stepwise with p<0.10). This p-value of 0.10 was selected, rather than a more conservative value of p< 0.05, because this was an initial exploratory analysis based on a small sample size. For Model 2, Ravens scores and V-CVLT List B scores were entered using a forced entry method because the Ravens score was the neurocognitive variable displaying the highest and most consistent bivariate correlations with the speech recognition scores; likewise, CVLT-II List B score was the CVLT-II variable with the consistently highest bivariate correlations with the speech recognition scores. The additional variance observed in the speech recognition outcome measures that was accounted for by the predictor variables within each of the two domains (demographics/hearing history and neurocognitive) was summarized and evaluated by R-squared statistics for each of the models. It is important to note here that the sample size of the ECI participants in the current dataset is relatively small. Thus, the results of these initial multiple regression analyses should be considered as exploratory in nature at this time pending the addition of more subjects to the sample.
Correlational Analyses
Table 9 shows a summary of the significant bivariate correlations that were obtained between the conventional demographic/hearing history variables, and the neurocognitive and verbal learning and memory measures, with the speech recognition outcomes (only statistically significant correlations are reported). For the W-22 word recognition test, we used scores based on both words and phonemes correct; for the Harvard-Standard and PRESTO sentence tests, we used scores based on both keywords correct and whole sentences correctly recognized. Examination of Table 9 shows that several of the predictor variables in each of these domains were strongly correlated with each of the three speech recognition outcomes. Significant correlations within the demographic/hearing history variables included the following: Age at first CI, Duration of hearing loss before CI, Duration of CI use, and currently wearing a hearing aid (HA).
Table 9 also shows the correlations obtained between the Ravens and the verbal learning and memory measures obtained from the CVLT-II and the three sets of speech recognition outcome measures. Two consistent findings emerged from the correlational analyses of the neurocognitive and verbal learning and memory scores with speech recognition scores. First, the Ravens, a global measure of non-verbal fluid intelligence was found to be strongly and consistently correlated with all three of the speech recognition outcome measures. Second, the List B measure of free recall, a process measure obtained from the CVLT-II that reflects resistance to the build-up of PI from the prior presentation of five repetitions of the words on List A, was also found to be strongly correlated with all three speech recognition outcome domains. Because both the Ravens and the CVLT-II used visually presented stimulus materials, these effects are due to information processing operations beyond the initial sensory encoding and processing of the stimulus materials by the auditory system..
Multiple Regression Analyses
A summary of the results of the regression equations is provided in the three panels shown in Table 10. For all three speech recognition outcome measures, there was a consistent increase in the amount of variance accounted for by Model 1 (Demographics/Hearing History) by the addition of the neurocognitive predictor variables in Model 2. For the CID W-22 word recognition test shown in top panel, the R-square increased from Model 1 (R2= 0.48) to Model 2 (R2=0.58) for phonemes correct and from (R2=0.51) to (R2=0.59) for words correct. For the Harvard-Standard sentence test shown in the middle panel, the R-squared increased from Model 1 (R2=0.43) to Model 2 (R2=0.62) for keywords correct and from (R2=0.32) to (R2=0.55) for whole sentences correctly recognized. Finally, for the PRESTO sentence recognition test shown in the bottom panel, the R-squared increased from Model 1 (R2= 0.31) to Model 2 (R2=0.57) for keywords correct and from (R2=0.28) to (R2=0.65) for whole sentences correctly recognized. Thus, although these are still preliminary findings pending larger sample sizes, the addition of neurocognitive measures using the Ravens to assess non-verbal fluid intelligence and the CVLT to assess verbal learning and memory processes, specifically, free recall performance of List B items, provides substantial additional predictive power above and beyond the variance captured by conventional clinical measures related to demographics and hearing history in Model 1 alone.
Table 10.
| A. Regression coefficient estimates and 95% confidence intervals for factors associated with CID using multiple regression model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percent of Phonemes Correct | Percent of Words Correct | |||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||
| Estimate (95% CI) |
P Value |
Estimate (95% CI) |
P Value |
Partial Correlation |
Estimate (95% CI) |
P Value |
Estimate (95% CI) |
P Value |
Partial Correlation |
|
| Demographics | ||||||||||
| Age at first CI | −1.02 (−1.82, −0.23) | 0.014 | −0.5 (−1.41, 0.4) | 0.26 | −1.39 (−2.31, −0.47) | 0.005 | −0.85 (−1.91, 0.21) | 0.11 | ||
| Duration of hearing loss before CI | −0.34 (−0.65, −0.03) | 0.034 | −0.22 (−0.54, 0.11) | 0.18 | −0.35 (−0.71, 0.01) | 0.054 | −0.23 (−0.61, 0.15) | 0.22 | ||
| Measures | ||||||||||
| RAVENS: Number Correct | 1.5 (−0.19, 3.19) | 0.079 | 0.38 | 1.66 (−0.33, 3.66) | 0.097 | 0.36 | ||||
| CVLT: List B | 0.28 (−0.25, 0.82) | 0.28 | 0.23 | 0.24 (−0.39, 0.87) | 0.43 | 0.17 | ||||
| R square | 0.48 | 0.58 | 0.51 | 0.59 | ||||||
| B. Regression coefficient estimates and 95% confidence intervals for factors associated with Harvard Standard using multiple regression model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percent of Keywords Correct | Percent of Sentences Correct | |||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||
| Estimate (95% CI) |
P Value |
Estimate (95% CI) |
P Value |
Partial Correlation |
Estimate (95% CI) |
P Value |
Estimate (95% CI) |
P Value |
Partial Correlation |
|
| Demographics | ||||||||||
| Age at first CI | −0.41 (−0.73, −0.09) | 0.016 | −0.14 (−0.49, 0.21) | 0.41 | ||||||
| Duration of hearing loss before CI | −0.11 (−0.23, 0.003) | 0.056 | −0.05 (−0.16, 0.06) | 0.33 | −0.59 (−0.97, −0.21) | 0.004 | −0.28 (−0.67, 0.1) | 0.14 | ||
| Measures | ||||||||||
| RAVENS:Number Correct | 0.63 (0.1, 1.16) | 0.023 | 0.53 | 1.95 (0.23, 3.67) | 0.029 | 0.5 | ||||
| CVLT: List B | 0.1 (−0.08, 0.28) | 0.25 | 0.24 | 0.48 (−0.12, 1.08) | 0.11 | 0.4 | ||||
| R square | 0.43 | 0.62 | 0.32 | 0.55 | ||||||
| C. Regression coefficient estimates and 95% confidence intervals for factors associated with Presto Standard Using multiple regression model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percent of Keywords Correct | Percent of Sentences Correct | |||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||
| Estimate (95% CI) |
P Value |
Estimate (95% CI) |
P Value |
Partial Correlation |
Estimate (95% CI) |
P Value |
Estimate (95% CI) |
P Value |
Partial Correlation |
|
| Demographics | ||||||||||
| Age at first CI | −0.89 (−1.93, 0.15) | 0.089 | 0.21 (−0.89, 1.32) | 0.69 | −0.92 (−1.81, −0.03) | 0.044 | 0.3 (−0.58, 1.17) | 0.49 | ||
| Duration of hearing loss before CI | 2.29 (−0.27, 4.86) | 0.077 | 1.99 (−0.19, 4.16) | 0.071 | ||||||
| Wear HA currently | 15.63 (−0.32, 31.59) | 0.054 | 11.75 (−0.58, 23.69) | 0.053 | ||||||
| Measures | ||||||||||
| RAVENS: Number Correct | 2.36 (0.58, 4.15) | 0.012 | 0.53 | 2.51 (1.08, 3.94) | 0.002 | 0.62 | ||||
| CVLT: List B | 0.46 (−0.12, 1.05) | 0.11 | 0.35 | 0.49 (0.03, 0.95) | 0.039 | 0.41 | ||||
| R square | 0.31 | 0.57 | 0.28 | 0.65 | ||||||
Discussion
In this study, we used a newly developed visual presentation format of the CVLT-II with a group of ECI patients and a group of age-, SES-, and nonverbal IQ-matched ONH controls. Overall, the present results demonstrate that most characteristics of verbal learning and memory in ECI adults resemble those of ONH controls. For example, free recall of words following one to five exposures to a 16-word study list was equivalent across the two samples. Additionally, measures of short-delay free recall (after presentation of a distractor word list), short-delay cued recall, semantic and serial clustering, and Yes/No recognition did not differ significantly between the ECI and ONH samples.
On the other hand, several differences between the ECI and ONH groups emerged which provide additional insights into the underlying information processing operations and strategies used to carry out this multi-trial free recall task. First, looking at the primary immediate free recall scores obtained during the repetition learning trials of the protocol, we found that the ECI group displayed a selective weakness in recalling words from the recency portion of the serial position curve. The selective forgetting of items from recency was observed consistently from the first presentation of List A all the way to the final presentation, suggesting disturbances and weaknesses in verbal short-term memory capacity. This selective deficit in recall of words from the recency portion of the serial position curve suggests that Type I rote verbal rehearsal processes, control processes used to maintain verbal and lexical memory codes in active short-term and working memory dynamics, may be compromised in this clinical sample by a period of severe-to-profound hearing loss prior to cochlear implantation, even with stimulus materials presented visually. Further research should be carried out to pursue these initial findings on the nature of the experience- and activity-dependent changes in verbal learning and memory that take place during the period of auditory deprivation before a patient receives a CI.
Second, examination of the secondary process measures derived from the CVLT-II revealed that the ECI group showed more forgetting and greater build-up of PI following five repetitions of List A. While both groups of subjects showed equivalent amounts of RI following presentation of a new list of words (List B), the ECI group not only showed more PI but they also displayed greater retrieval induced forgetting for new words on List B that came from the same semantic categories as the words used on List A (i.e., the List B “Shared Words”). Moreover, the ECI group showed no evidence of a “release from PI” for new words that did not share any semantic features with the original studied items (i.e., List B “Non-shared Words”). In contrast, the ONH group showed less retrieval induced forgetting for the Shared items compared to the ECI group, and they also demonstrated the expected release from PI on the Non-shared items on List B. The greater retrieval induced forgetting for the shared items and the absence of release from PI for the non-shared items by the ECI participants are novel and important findings that should be examined in further detail in future studies with larger sample sizes because they suggest selective weaknesses and possible deficits in elementary information processing operations related to verbal rehearsal and cognitive control processes that are not directly dependent on the registration and encoding of auditory sensory information. These results were obtained with visual presentation of all stimulus materials.
It is important to emphasize here that the present results were obtained using visually presented verbal materials, suggesting that the ECI group as a whole may also have selective weaknesses and/or disturbances in cognitive control processes related to the control of inhibition of competing verbal and lexical representations that are activated from semantically similar words in long-term memory. The selective weaknesses observed here are not modality-specific; that is, they cannot be attributed to audibility or to early auditory sensory encoding of information in short-term memory. Instead, the group differences found in this study reflect the active use of self-generated control processes – verbal coding, rehearsal, and organizational strategies operating on modality-general abstract phonological and lexical memory representations – independent of auditory sensory input.
In addition to these novel findings that uncovered differences between the two groups in basic underlying neurocognitive processes, we also found significant correlations between several information-processing measures from the CVLT-II protocol and three different speech recognition measures in ECI participants: isolated words (CID W-22), meaningful Harvard Standard sentences, and high-variability PRESTO sentences. The correlations found in the ECI group replicated and extended earlier results reported by Heydebrand et al. (2007), and Holden et al. (2013), who used an earlier version of the CVLT-II with simultaneous auditory and visual presentation of test materials in hearing-impaired adults. Their presentation methods potentially confounded deficits in early auditory sensory processing and encoding with basic verbal memory and learning processes. In the present study, by using only visual presentation of the CVLT-II, we were able to dissociate early auditory sensory processing from verbal and lexical encoding, storage, and retrieval processes used in verbal learning and memory tasks.
In addition to the CVLT-II, we found that Ravens scores correlated strongly with all measures of speech recognition outcome. This strong relationship could result from several potential factors. First, both Ravens and some speech recognition outcome scores (CID W-22 and Harvard Standard Sentences) were significantly related to age, and therefore their intercorrelation may reflect age-based changes that occur on both Ravens and these speech recognition scores. Second, Ravens scores reflect global intellectual ability, and therefore a domain general effect of stronger intellectual functioning on speech recognition scores could be indicated by their intercorrelation. Finally, because our version of the Ravens test was timed, it incorporated an element of processing speed and efficiency, which was also likely influential in speech recognition scores. Future research should be carried out to further investigate and explain these associations between Ravens and speech recognition scores.
The preliminary results obtained from our exploratory regression analyses suggest that speech recognition outcomes in this clinical population of ECI participants rely on several domains of neurocognitive functioning above and beyond the variance captured by conventional clinical demographic and hearing history measures alone. Demographics and hearing history are, of course, really only “proxy variables” for more basic auditory declines and neurocognitive adaptations based on experience- and activity-dependent learning and exposure to speech and spoken language processing operations before and after implantation. There is now a growing body of converging evidence suggesting that speech recognition outcomes after implantation in both prelingually deaf early-implanted children and post-lingual adults with acquired hearing loss reflect the combined effects of multiple information processing systems and subsystems working together in an integrated fashion to support robust spoken word and sentence recognition following implantation (see Pisoni 2000, 2016; Kral et al. 2016; Moberly et al. 2016). Although correlations between CVLT-II measures (particularly List B) and speech recognition outcomes were statistically significant, CVLT-II List B scores significantly predicted only PRESTO sentences scores (the most challenging, high-variability, perceptually-robust measure of speech recognition outcomes in this study) in the regressions. The lack of significant results for CVLT-II List B predicting other speech recognition outcome scores in the regression equations may have been a result of low power or shared variance with Ravens scores. The new findings reported here also suggest that visually based process measures of neurocognitive functioning may have substantial clinical utility in identifying core weaknesses and underlying disturbances in verbal information processing in hearing impaired patients without the need for auditory presentation of stimulus materials.
General Discussion
In the present set of studies on verbal learning and memory using the CVLT-II, we demonstrated that a new visually based computer-controlled version of the CVLT-II provided results that were comparable with those obtained using a conventional auditory-based version of the CVLT-II. The CVLT-II was selected for this study because it is considered to be an efficient “high-yield” clinical neuropsychological instrument that provides detailed data about the information processing operations used in verbal learning and memory in a short period of time (Lezak 1983). The CVLT-II provides several quantitative “primary measures” that can be used to characterize the information processing strategies used in encoding, storage, and retrieval of verbal information from both STM and LTM. It also provides additional detailed “process measures” about repetition learning, primacy and recency effects, forgetting of verbal materials, such as RI and PI, retrieval induced forgetting, and release from the build-up of PI, semantic and serial clustering, intrusions, Yes/No recognition memory, and response bias. Moreover, and perhaps even more importantly, the CVLT-II provides extensive normative data over the lifespan so that the scores from an individual patient can be compared to benchmarks based on age, gender, and education to identify strengths, weaknesses, and milestones in verbal learning and memory performance. The present report does not include any detailed analyses of individual differences or outliers in our samples and was primarily focused on group differences; however, the topic of individual differences in outcomes following implantation is also critically important because of its clinical utility for decision making and intervention and will be covered in a future paper from our research group using an individual differences approach.
Our first study was carried out to establish validity and feasibility of the visual CVLT-II in two separate samples, one with young NH adults (Study 1a) and a second with older NH (ONH) adults (Study 1b). Having a valid visually based version of the CVLT-II then allowed us to investigate verbal learning and memory processes in a sample of post-lingually deaf experienced CI users (ECIs) in Study 2, without the potential confounds of auditory presentation of stimulus materials.
Two important findings were obtained from Study 2. First, we replicated and extended the previous CVLT-II findings reported by Heydebrand et al., (2007) and Holden et al. (2013), while eliminating any confounding effects from audibility and early auditory sensory registration and encoding. We found that several of the primary recall measures obtained from the CVLT-II correlated with three different speech recognition outcome measures including open-set word recognition and two different sentence recognition tests. All three speech recognition outcomes were significantly correlated with both the immediate free-recall (List A Trial 5 and total of List A trial 1 through 5) as well as short-delay free-recall and short-delay cued-recall scores obtained from the V-CVLT test. In the previous studies reported by Heydebrand et al. and Holden et al., the authors created a global composite measure based on the free recall scores obtained from their CVLT-II protocols and used these composite scores as their only assessment of CVLT-II performance. More importantly, neither of these two earlier studies examined any of the theoretically important process-based measures obtained from the CVLT-II protocol, which provide detailed information about the underlying elementary neurocognitive processing operations used to carry out the multi-trial free recall task. The present results suggest that in addition to encoding and retrieval interactions, the strength of phonological and lexical representations of words in LTM may also contribute to the enormous unexplained variance underlying individual differences in speech recognition outcomes following implantation.
The second major finding of Study 2 was that most of the primary and process measures of verbal learning and memory in ECI adults closely resembled measures obtained from a group of matched ONH controls. However, several theoretically important differences in recall between ECI and ONH samples were obtained. A large and consistent difference was identified between the groups on recall of items from the recency subcomponent of the serial position curve. This difference was present from List A Trial 1 through List A Trial 5. We also found a significance difference in free recall of List B items between ECI and ONH controls. List B is the “interference list” in the CVLT-II protocol, and it is used to uncover forgetting problems and weaknesses related to interference, either PI or RI. Both groups showed equivalent amounts of forgetting due to RI, but they differed on PI. They also differed on retrieval induced forgetting for Shared categories on List B, as well as release from PI on the Non-shared categories on List B. The observed weaknesses and information processing deficits in CI users in verbal learning and memory cannot be explained by poor sensory encoding and processing of spoken words. Instead, the differences reflect disturbances in elementary verbal coding, verbal rehearsal strategies, and information processing operations that are not modality-specific in nature. This is an important new finding both clinically and theoretically because it suggests that some modality-independent aspect of verbal coding and information processing used in speech recognition and spoken language comprehension (i.e., type I maintenance rehearsal, retrieval, and search strategies) may contribute an additional unique source of variance to the conventional speech recognition outcome measures routinely used to establish candidacy for implantation and to assess benefits and track progress after implantation.
Precisely why the ECI subjects have weaknesses in recall of List B items remains unclear, but the nature of the forgetting of List B items appears to be fundamentally different for the two groups, despite comparable performance on List A Trial 1. It is possible to explain these differences by appeal to the build-up of proactive interference and retrieval induced forgettting following the five presentations of List A. However, the precise mechanism of action responsible for the build-up of PI and the absence of release from PI in the ECI group remains an open question for future research. Despite the fact that the stimulus materials were presented visually, it is possible and likely that the differences we observed reflect more basic differences in rapid phonological recoding of visual words into phonological representations and verbal memory codes in active STM (Conrad 1979). Subtle changes and neural reorganization may have taken place in the ECI group during the period of auditory deprivation prior to implantation. This proposal is supported by the negative correlation of duration of hearing loss prior to implantation with all three of the speech recognition outcome measures, suggesting detrimental neuroplastic changes in verbal learning and memory prior to implantation. The detailed nature of the neural reorganization and changes in cognitive processing resulting from hearing loss and sensory deprivation prior to implantation are important theoretical and clinical issues to investigate in future studies.
Although both groups of subjects showed comparable levels of performance on reading words and non-words aloud on the TOWRE-2 test that assessed reading speed and fluency, the test items on the TOWRE-2 were presented one at a time without any additional cognitive load or processing demands on the capacity of immediate memory and active attentional control. The information processing demands placed on encoding, storage, and retrieval of items in the free recall protocol used in the CVLT-II are quite different from the processing demands of the TOWRE-2 test and may uncover differences between the two groups only under increased cognitive load when both STM and LTM are required to carry out the information-processing task. Greater forgetting of items from the recency portion of the serial position curve was observed consistently across the five study trials of List A, suggesting selective weaknesses in recall of items at the end of the list that are assumed to be “dumped” or “unloaded” from active primary memory before items at the beginning of the list are retrieved from secondary memory (Rundus & Atkinson 1970; Unsworth & Engle 2007).
The study of elementary foundational verbal learning and memory processes in this unique clinical population of adult CI users has received very little attention by clinicians and researchers in the past, despite the central importance of these core foundational processes to speech recognition, spoken language comprehension, and adaptive language and neurocognitive functioning in real-world environments. Being able to rapidly encode, retain, process, and learn new verbal information via the auditory sensory modality is critical for many aspects of real-world adaptive functioning that substantially impact on quality of life and psycho-social interactions. Moreover, several recent studies have suggested close links between hearing loss and cognitive aging effects associated with dementia and other neurodegenerative diseases (Lin 2011, 2012; Lin et al. 2011; Lin et al. 20011; Li et al. 2017). Findings from this study raise the possibility that changes in several specific subcomponents of verbal learning and memory processes may underlie these hearing loss-related declines in cognition that have been documented previously.
Understanding and Explaining Variability and Individual Differences after Implantation
The present findings on verbal learning and memory are directly relevant to several pressing clinical problems in the fields of hearing impairment and CIs, specifically, new research efforts focused on understanding and explaining the underlying basis for the enormous variability and individual differences in speech recognition and spoken language outcomes following implantation. The emphasis in past research studies on outcomes following implantation has been focused primarily on audibility and sensory factors at the level of registration and encoding of auditory input, not on cognition, information processing strategies, self-generated organizational strategies, learning and memory, or inhibitory control processes, which play significant roles in robust real-world adaptive functioning in adverse listening conditions. Our current lack of understanding of the causal mechanisms of action that underlie individual differences in outcomes represents a significant barrier to further progress in the fields of Otology and Cognitive Hearing Science in developing novel interventions to help poorly performing patients with CIs (see Moberly et al. 2016; Li et al. 2017). Without knowing precisely why or how an individual patient is performing poorly after implantation, it is impossible to recommend a specific medical intervention or develop an effective clinical treatment protocol that could help CI patients improve their speech recognition skills and reach optimal levels of performance. We believe that the study of verbal learning and memory, along with other foundational elementary neurocognitive information processing mechanisms underlying speech recognition, may be one of the “missing pieces of the puzzle” in understanding and explaining the enigma of variability and individual differences in speech recognition outcomes following cochlear implantation (Moberly et al. 2016; Pisoni et al. 2016). Cognition is the “interface” that links the ear and brain and supports a highly robust information processing system for speech communication under a wide range of adverse and challenging conditions (Pisoni 2000).
Limitations and Weaknesses of the Present Study
Although this study provides converging evidence for the roles of verbal memory and learning and neurocognitive functioning in speech recognition outcomes for adults with CIs, along with new findings documenting differences between CI users and NH controls on several foundational information processing skills, the sample size was relatively small. Clearly, testing multivariate regression models to explain speech recognition outcomes beyond the exploratory analyses performed here will require a much larger group of ECI participants. Furthermore, we were not able to use corrections for multiple statistical tests as a result of the small sample size and resultant adverse effects on statistical power; as a result, our correlational analyses should be regarded with some caution until they can be replicated in additional, larger samples. Nonetheless, the fact that significant relations were identified in our multivariate models suggests the robust nature of these relations even in a relatively small sample. Additionally, the exploratory nature of this study resulted in findings that were not necessarily predicted beforehand (such as some of the differences in the CVLT-II scores between ECI and ONH groups, like the recency effects, PI, retrieval induced forgetting, and release from PI), so appropriate additional converging measures were not collected. Future studies will be needed to further elucidate these processing differences, with inclusion of related converging measures like inhibitory control, selective attention, rapid verbal coding and rehearsal dynamics.
Summary and Future Research Directions
Results of this study provide further evidence that modality-general neurocognitive functions and core verbal memory and learning processes contribute to speech recognition outcomes in post-lingual adults with CIs, and that differences in these information processing functions and operations likely help explain some of the enormous variability and individual differences in outcomes experienced by CI patients. Based on the findings reported here, the new visual CVLT-II could serve a valuable role in identifying some important core information processing skills that contribute to speech recognition outcomes, with exploratory analyses suggesting that List B, the “interference list,” may provide diagnostic potential, relating to PI and inhibitory control in CI users, particularly those who are relatively poor performers on conventional speech recognition outcome measures. Additionally, studies are currently underway to investigate verbal learning and memory in elderly hearing impaired patients prior to cochlear implantation to determine if these visual measures of neurocognitive functioning can be used to predict speech recognition outcomes after implantation and identify those patients who may be at high risk for poor outcomes following implantation. Finally, ongoing participant enrollment continues to increase the sample size of ECI patients to extend our multivariate regression analyses beyond their current exploratory limitations.
Acknowledgments
Data collection and analysis were supported by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders (NIDCD) Career Development Award 5K23DC015539-02 and the American Otological Society Clinician-Scientist Award to ACM. This research was also supported by NIDCD research grant DC-000111 and the IU Grant Linking University-wide Expertise award to David Pisoni, and NIDCD research grant DC-015257 to David Pisoni and William Kronenberger. ResearchMatch, used to recruit some NH participants, is supported by National Center for Advancing Translational Sciences Grant UL1TR001070. The authors would also like to acknowledge Susan Nittrouer for providing some testing materials used in this study, Beth Miles-Markley for her administrative support, and Leticia DeLeon, Gabrielle Grose, and Eleanor Gulick for their assistance in data scoring for this study. We also thank Terren Green for her help in preparing the final manuscript for publication and overseeing the research project.
Financial Disclosures: This research was funded by the Federal NIH/NIDCD Grant 5R01 DC-000111 “Speech Perception and Spoken Word Recognition”
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Author Contribution Statement
All authors contributed to the completion of this research project. ACM, DBP, WGH, LRH and MSH conceptualized and designed the original study and research protocol. ACM and DBP supervised the data collection at both sites. DBP, ACM and WGK drafted, edited and revised the final manuscript. DBP, ACM, WGK, KDM and HX carried out the statistical analyses. DBP, ACM, LRH and KDM prepared the graphics and figures. All authors of this paper were involved in discussing the major results of the study and commented on the final manuscript in various stages of preparation.
References
- Anderson M, Bjork R, Bjork E. Remembering can cause forgetting: retrieval dynamics in long-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Altman GTM. Cognitive Models of Speech Processing: Psycholinguistics and Computational Perspectives. Cambridge, MA: The MIT Press; 1990. [Google Scholar]
- Atkinson RC, Shiffrin RM. Human Memory: A proposed system and its control processes. The Psychology of Learning and Motivation. 1968;2:89–195. [Google Scholar]
- Atkinson RC, Shiffrin RM. The control of short-term memory. Sci. Am. 1971;224:82–90. doi: 10.1038/scientificamerican0871-82. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clinical Neuropsychogist. 1991;5:125–142. [Google Scholar]
- Borden GJ, Harris KS, Raphael LJ. Speech Science Primer: Physiology, Acoustics, and Perception of Speech. 3. Baltimore: Williams & Wilkins; 1994. [Google Scholar]
- Conrad R. The Deaf Schoolchild. London: Harper & Row; 1979. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, et al. CVLT-II: California Verbal Learning Test- Second Edition, Adult Version. Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Egan JP. Articulation testing methods. Laryngoscope. 1948;58:955–991. doi: 10.1288/00005537-194809000-00002. [DOI] [PubMed] [Google Scholar]
- Flanagan JL. Speech Analysis Synthesis and Perception. 2. New York: Springer- Verlag; 1972. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. The Journal of the Acoustical Society of America. 2001;110(2):1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Gilbert JL, Tamati TN, Pisoni DB. Development, reliability and validity of PRESTO: A new high-variability sentence recognition test. Journal of the American Academy of Audiology. 2013;24(1):26–36. doi: 10.3766/jaaa.24.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Bhatt YM, Mawman DJ, O’Driscoll MP, Saeed SR, Ramsden RT, Green MW. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants International. 2007;8:1–11. doi: 10.1179/cim.2007.8.1.1. [DOI] [PubMed] [Google Scholar]
- Heydebrand G, Hale S, Potts L, et al. Cognitive predictors of improvements in adults’ spoken word recognition six months after cochlear implant activation. Audiology Neurotology. 2007;12:254–264. doi: 10.1159/000101473. [DOI] [PubMed] [Google Scholar]
- Hirsh IJ, Davis H, Silverman SR, et al. Development of materials for speech audiometry. Journal of Speech and Hearing Disorders. 1952;17:321–337. doi: 10.1044/jshd.1703.321. [DOI] [PubMed] [Google Scholar]
- Holden LK, Finley CC, Firszt JB, et al. Factors affecting Open-Set Word Recognition in adults with cochlear implants. Ear and Hearing. 2013;34(3):342–360. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IEEE. IEEE recommended practice for speech quality measurements. IEEE Report No 297 1969 [Google Scholar]
- Kelly AS, Purdy SC, Thorne PR. Electrophysiological and speech perception measures of auditory processing in experienced adult cochlear implant users. Clinical Neurophysiology. 2005;116:1235–46. doi: 10.1016/j.clinph.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Kral A, Kronenberger WG, Pisoni DB, et al. Neurocognitive factors in sensory restoration of early deafness: A connectome model. Lancet Neurology. 2016;15:610–621. doi: 10.1016/S1474-4422(16)00034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger WG, Castellanos I, Pisoni DB. Questionnaire-based assessment of executive functioning: Case studies. Applied Neuropsychology: Child. 2016;13:1–11. doi: 10.1080/21622965.2016.1200976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger WG, Pisoni DB. Neurocognitive assessment of children with cochlear implants. In: Eisenberg L, editor. Clinical management of children with cochlear implants. San Diego, CA: Plural Publishing; 2016. pp. 433–472. [Google Scholar]
- Lazard DS, Vincent C, Venail F, Van de Heyning P, Truy E, Sterkers O, Mawman D. Pre-, per-and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One. 2012;7:e48739. doi: 10.1371/journal.pone.0048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz M, Sonmez H, Joseph G, et al. Long-term performance of cochlear implants in postlingually deafened adults. Otolaryngology Head and Neck Surgery. 2012;147(1):112–118. doi: 10.1177/0194599812438041. [DOI] [PubMed] [Google Scholar]
- Leung J, Wang NY, Yeagle JD, Chinnici J, Bowditch S, Francis HW, Niparko JK. Predictive models for cochlear implantation in elderly candidates. Archives of Otolaryngology Head and Neck Surgery. 2005;131:1049–54. doi: 10.1001/archotol.131.12.1049. [DOI] [PubMed] [Google Scholar]
- Lewellen MJ, Goldinger SD, Pisoni DB, et al. Lexical familiarity and processing efficiency: individual differences in naming, lexical decision, and semantic categorization. Journal of Experimental Psychology: General. 1993;122(3):316–330. doi: 10.1037//0096-3445.122.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 2. New York: Oxford University Press; 1983. [Google Scholar]
- Li L, Blake C, Sung Y, et al. The Studying Multiple Outcomes After Aural Rehabilitative Treatment Study: Study Design and Baseline Results. Gerontology & Geriatric Medicine. 2017;3:1–10. doi: 10.1177/2333721417704947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman AM. Speech: A Special Code. Cambridge, MA: The MIT Press; 1996. [Google Scholar]
- Limb CJ, Roy AT. Technological, biological, and acoustical constraints to music perception in cochlear implant users. Hearing Research. 2014;308:13–26. doi: 10.1016/j.heares.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Lin FR. Hearing loss and cognition among older adults in the United States. Journals of Gerontology : Series A Biological Scienes and Medical Science. 2011;66(10):1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR. Hearing loss in older adults: who's listening? Journal of the American Medical Association. 2012;307(11):1147–1148. doi: 10.1001/jama.2012.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, Metter EJ, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Archives of Neurology. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattys SL, Davis MH, Bradlow AR, et al. Speech recognition in adverse conditions: A review. Language and Cognitive Processes. 2012;27(7–8):953–978. [Google Scholar]
- Moberly AC, Bates C, Harris MS, et al. The enigma of poor performance by adults with cochlear implants. Otology & Neurotology. 2016;37(10):1522–1528. doi: 10.1097/MAO.0000000000001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly AC, Lowenstein JH, Nittrouer S. Word recognition variability with cochlear implants: “Perceptual Attention” versus “Auditory Sensitivity”. Ear and Hearing. 2016a;37(1):14–26. doi: 10.1097/AUD.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly AC, Lowenstein JH, Nittrouer S. Early bimodal stimulation benefits language acquisition for children with cochlear implants. Otology & Neurotology. 2016b;37(1):24–30. doi: 10.1097/MAO.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S, Burton LT. The role of early language experience I the development of speech perception and phonological processing abilities: Evidence from 5-year-olds with histories of otitis media with effusion and low socioeconomic status. Journal of Communication Disorders. 2005;38:29–63. doi: 10.1016/j.jcomdis.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Pisoni DB. Cognitive factors and cochlear implants: Some thoughts on perception, learning, and memory in speech perception. Ear & Hearing. 2000;21:70–78. doi: 10.1097/00003446-200002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Conway CM, Kronenberger W, et al. Efficacy and effectiveness of cochlear implants in deaf children. In: Marschark M, Hauser P, editors. Deaf Cognition: Foundations and Outcomes. New York: Oxford University Press; 2008. pp. 52–101. [Google Scholar]
- Pisoni DB, Conway CM, Kronenberger WG, et al. Executive function, cognitive control and sequence learning in deaf children with cochlear implants. In: Marschark M, Spencer P, editors. Oxford Handbook of Deaf Studies, Language, and Education. New York: Oxford University Press; 2010. pp. 439–457. [Google Scholar]
- Pisoni DB, Kronenberger WG, Chandramouli SH, et al. Learning and memory processes following cochlear implantation: The missing piece of the puzzle. Frontiers in Psychology. 2016;7 doi: 10.3389/fpsyg.2016.00493. article 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, McLennan CT. Spoken word recognition: Historical roots, current theoretical issues, and some new directions. In: Hickok G, Small S, editors. Neurobiology of Language. New York: Elsevier; 2015. [Google Scholar]
- Pisoni DB. Speech perception in deaf children with cochlear implants. In: Pisoni DB, Remez RE, editors. Handbook of Speech Perception. Blackwell Publishers: 2005. pp. 494–523. [Google Scholar]
- Raaijmakers JGW. The two-store model of memory: Past criticisms, current status, and future directions. In: Meyer DE, Kronblum S, editors. Attention and Performance XIV: Synergies in Experimental Psychology, Artifical Intelligence, and Cognitive Neuroscience. MIT Press; 1993. pp. 467–488. [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford: Oxford Psychologists Press; 1998. [Google Scholar]
- Rönnberg J, Lunner T, Zekveld A, et al. The Ease of Language Understanding (ELU) model: theoretical, empirical, and clinical advances. Frontiers Systems Neuroscience. 2013;7:31. doi: 10.3389/fnsys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau C, Frere J, Montaut-Verient B, et al. Quality of life and sudiologic performance through the ability to phone of cochlear implant users. European Archives of Oto-Rhino-Laryngology. 2015;272(12):3685–3692. doi: 10.1007/s00405-014-3448-x. [DOI] [PubMed] [Google Scholar]
- Rundus D, Atkinson RC. Rehearsal procedures in free recall: A procedure for direct observation. Journal of Verbal Learning and Verbal Behavior. 1970;9:99–105. [Google Scholar]
- Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- Shafiro V, Gygi B, Cheng MY, Vachhani J, Mulvey M. Perception of environmental sounds by experienced cochlear implant patients. Ear and Hearing. 2011;32(4):511. doi: 10.1097/AUD.0b013e3182064a87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D, Adams W. Wide Range Assessment of Memory and Learning (WRAML) Wilmington, DE: Jastak; 1990. [Google Scholar]
- Srinivasan AG, Padilla M, Shannon RV, Landsberger DM. Improving speech perception in noise with current focusing in cochlear implant users. Hearing Research. 2013;299:29–36. doi: 10.1016/j.heares.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings LM, Kirk KI, Chin SB, et al. Parent word familiarity and the language development of pediatric cochlear implant users. Volta Review. 2000;102(4):237–258. [Google Scholar]
- Stevens KN. Acoustic Phonetics. Cambridge, MA: The MIT Press; 1998. [Google Scholar]
- Stricker JL, Brown GG, Wixted J, et al. New semantic and serial clustering indices for the California Verbal Learning Test-Second Edition: background, rationale, and formulae. Journal of the International Neurospychological Society. 2002;8(3):425–435. doi: 10.1017/s1355617702813224. [DOI] [PubMed] [Google Scholar]
- Storm BC, Angello G, Buchli DR, et al. A review of retrieval-induced forgetting in the contexts of learning, eye-witness memory, social cognition, autobiographical memory, and creative cognition. In: Ross B, editor. The Psychology of Learning and Motivation. Academic Press: Elsevier Inc; 2015. pp. 141–194. [Google Scholar]
- Tamati TN, Gilbert JL, Pisoni DB. Some factors underlying individual differences in speech recognition on PRESTO: A first report. Journal of the American Academy of Audiology. 2013;24:616–634. doi: 10.3766/jaaa.24.7.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamati TN, Pisoni Non-native listeners' recognition of high-variability speech using PRESTO. Journal of the American Academy of Audiology. 2014;25:869–892. doi: 10.3766/jaaa.25.9.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency. 2. Austin, TX: Pro-Ed; 2012. [Google Scholar]
- Unsworth N, Engle R. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114:104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Wickens DD. Encoding categories of words: an empirical approach to meaning. Psychological Review. 1970;77(1):1–15. [Google Scholar]
- Wickens DD, Born DG, Allen CK. Proactive inhibition and item similarity in short term memory. Journal of Verbal Learning and Verbal Behavior. 1963;2:440–445. [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test: Fourth Edition. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Wilson BS, Dorman MF. Better Hearing with Cochlear Implants: Studies at the Research Triangle Institute. San Diego: Plural Publishing; 2012. [Google Scholar]
- Wilson BS, Dorman MF, Woldorff MG, et al. Cochlear Implants matching the prosthesis to the brain and facilitating desired plastic changes in brain function. Progress in Brain Research. 2011;194:117–129. doi: 10.1016/B978-0-444-53815-4.00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Rohrer D. Proactive interference and the dynamics of free recall. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1993;19:1024–1039. [Google Scholar]