Abstract
Objective
The objective of this study was to explore the perspectives and experiences of patients with rheumatoid arthritis (RA) whose assessments of their disease differ from those of their rheumatology care provider.
Methods
A total of 20 adult RA patients with patient-provider discordance at their most recent rheumatology appointment (within 4 weeks) were recruited. Discordance was defined by an absolute difference of 25 or more between patient and provider global assessments on visual analog scales (VASs) of disease activity. For descriptive purposes, participants completed the Health Assessment Questionnaire II, pain VAS, and Patient Health Questionnaire-9 depression scale. Interviews were conducted in person and individually with each patient with a semistructured interview guide. Topics ranged widely, including participants’ perspectives and experiences with living with RA, clinical disease assessments, patient-provider communication, and psychosocial or other needs. Data from the interviews were analyzed using an interpretive phenomenological approach.
Results
Six major themes emerged from the patient interviews describing patient-provider discordance and disease assessment: being misunderstood by others, limitations of provider assessments, discrepancy with provider findings, inadequate active listening on the part of health care providers, unmet psychosocial needs, and lack of patient empowerment.
Conclusion
Patients described discordance in terms of symptom assessment and understanding how RA affects everyday life. Typical clinical assessments did not capture their experience. The resulting conceptual framework should inform future interventional studies seeking to enhance concordance of patient-physician communication and to optimize satisfaction with care and health-related quality-of-life outcomes for patients with RA.
Keywords: disease activity assessment, patient centeredness, patient-physician discordance, qualitative research
Introduction
Rheumatologic assessment of patients with rheumatoid arthritis (RA) is complex, incorporating multiple objective and subjective variables. Patients present with undifferentiated symptoms of pain, fatigue, weakness, sleep disturbance, and psychosocial distress. Rheumatology providers recognize that their assessments must extend beyond physical examination and biomarkers (eg, swollen joints, inflammatory biomarkers, and joint imaging), but they often encounter difficulty identifying the cause of their patients’ adverse health status given the complexity of multiple comorbidities.
Patient and provider global assessments are key domains of recommended composite disease activity measures (1). Patient global assessment measured with a visual analog scale (VAS) captures the patient’s experience of living with the disease and its health impact. Provider global assessment summarizes the provider’s estimation of disease activity according to measureable clinical variables. Patient-provider discordance occurs when patient and provider global assessments differ substantially (ie, by a difference of 25–30 points). Discordance occurs at about 40% of clinical visits (2). Previous studies have shown that patients rate their disease activity mostly on pain and fatigue (3–5), while providers emphasize objective assessments, such as swollen joint counts and inflammatory markers (3,6,7). However, much is unexplained about patient-provider discordance, representing a barrier to optimal health outcomes among patients with RA. Furthermore, discordance may undermine shared decision making with patients about treatment options (6,8), and discordance associates with patient dissatisfaction and decreased compliance with treatments in non-RA studies (9–11).
Successful outcomes from rheumatologic care depend not only on the diagnosis and treatment but also on meeting the unique care needs of patients with RA. Simply identifying discordance may not bring patients and providers closer without an in-depth understanding of the patient perspective. Qualitative studies have addressed this issue in the overall RA patient population. For example, Haugli and colleagues (12) emphasized the importance of treating the patient as a person rather than as a disease entity, of providing validation for the patient’s symptoms and concerns, and of establishing a trusting, reciprocal relationship. Lempp and others (13) identified the tremendous toll that RA takes on a person’s relationships, roles and responsibilities, and self-identity. Flurey and colleagues’ study (14) highlighted the fluctuating, unpredictable nature of RA and the challenging self-management that patients must undertake to balance their lives. Their findings positively contributed to a better understanding of the personal impact of RA on people. However, to our knowledge, no studies have explored the perspectives specifically of patients with RA in patient-provider discordance. The objective of the present study was to investigate the perspectives and experiences of RA patients affected by patient-provider discordance on various topics, including disease assessments, patient-provider communication, and psychosocial needs.
Patients and Methods
Study Design
The study design was grounded in interpretative phenomenological analysis (IPA), a qualitative methodology from social psychology that focuses on understanding individuals’ personal experiences and their social worlds (15). The approach is interpretive in that the research team used these personal accounts to understand not only each patient’s experience but also broader questions related to patient-provider communication and discordance. The aim was to use individual interviews with patients who were identifed as discordant with their providers in order to understand how patients make sense of their experiences, including how they interpret discordance in context. Individual interviews were conducted to maintain patient confidentiality and to foster a comfortable environment for in-depth responses. Data on patient characteristics were collected from the electronic health records. This study was approved by the Mayo Clinic Institutional Review Board.
Participants
IPA uses a purposeful sampling approach to identify individuals who are able to describe a particular experience, in this case the experience of being discordant with the RA care provider. Eligible participants were at least 18 years old, fulfilled the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA (16), and had patient-provider discordance at their most recent rheumatology appointment (within 4 weeks) as defined by at least 25 mm of absolute difference in global assessments of disease activity (3,6). The provider global assessment and physical examination were completed by the patient’s provider (physician, physician assistant, or nurse practitioner) at the clinic visit. All participating patients provided written informed consent before the interviews.
Data Collection
Data were gathered with a semistructured interview guide. The guide included open-ended questions and probes designed to explore factors that contribute to patient-physician discordance and patients’ overall perceptions of rheumatology assessments. Interview topics included patient perspectives and experiences on disease symptoms, the impact of RA on daily functioning, patient-physician communication about disease activity assessment, and how evaluator assessments match patient assessments.
Interviews were conducted in person during a study visit by trained qualitative researchers (G.B.A. and J.L.R.) not involved in patient care. Each interview lasted approximately 30 minutes, and interviews were audio recorded and transcribed verbatim. Data collection ended after 20 patient interviews when interviewers determined there was sufficient information for analysis and little new information was emerging from later interviews (ie, data saturation) (17,18).
Additional clinical data were collected, including rheumatoid factor level, anti–cyclic citrullinated peptide antibody level, and presence of erosions on radiographs of the hands or feet (18). From the most recent clinical visit, the Clinical Disease Activity Index (CDAI) and C-reactive protein level were collected. At the study visit, participants also completed the Health Assessment Questionnaire II (HAQ-II), pain VAS, and Patient Health Questionnaire-9 (PHQ-9) depression scale.
Qualitative Data Analysis
All transcripts were checked for accuracy. Data were analyzed thematically using principles of IPA (15,19,20). Study researchers first read the transcripts and noted discussion points. Each transcript was analyzed to maintain the individual patient perspective and develop themes that emerged within and across patients’ accounts of their experiences. The process included identification of important themes and patterns by an open coding process. A framework that represented inductively identified themes and a priori constructs that informed the interview guide was developed to assist with data reduction. The framework was independently applied to transcripts by G.B.A. and J.L.R., and discrepancies were discussed to further assess meaning in the data. Interview data management and analysis was facilitated by NVivo 10.1 software (QSR International Pty Ltd). Research and clinical team members were involved in data interpretation in consideration of the existing literature. Clinical characteristics were used to aid interpretation of individual patient accounts and data patterns.
Results
Patient Characteristics
Of the 20 patients, 18 (90%) rated their disease activity substantially higher than their care providers (ie, positive discordance), with a mean difference between the patient and provider global assessments of 43.6 (range, 27–70). Only 2 patients rated their disease activity substantially lower than their providers (ie, negative discordance), with mean differences between patient and provider global assessments of −28 and −35. The Table shows the patient characteristics, patient and provider global assessments, and scores for HAQ-II, CDAI, pain VAS, and PHQ-9. Overall, the mean age was 62 years (range, 38–84 years), and 14 patients (70%) were female. Median disease duration was 7.5 years (range, <1–26 years). Over half the patients had a moderate or high CDAI. Three patients had PHQ-9 scores of 10 or more, compatible with moderate to severe depressive symptoms. Four patients’ most recent rheumatology visits were with physicians, 8 were with rheumatology-trained physician assistants, and 8 were with rheumatology-trained nurse practitioners. In our previous quantitative study, there were no associations between provider type and discordance (21).
Table.
Characteristics of Study Patients With RA
| Patient | Age, y | Sex | RA Duration, y | Global Assessments | Discordancea | Scoreb | Education, y | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Patient | Physician | HAQ-II | Pain VAS | CDAI | PHQ-9 | ||||||
| 1 | 54 | F | 11 | 62 | 10 | 52 | 1.1 | 77 | 8.2 | 12 | 12 |
| 2 | 66 | F | 5 | 85 | 15 | 70 | 0.2 | 85 | 10.0 | 4 | 12 |
| 3 | 60 | F | <1 | 43 | 15 | 28 | 0.9 | 60 | 15.8 | 9 | 14 |
| 4 | 74 | F | 12 | 52 | 80 | −28 | 1.0 | 67 | 54.2 | 0 | 14 |
| 5 | 63 | F | 17 | 54 | 10 | 44 | 1.4 | 22 | 6.4 | 1 | 14 |
| 6 | 38 | F | <1 | 52 | 15 | 37 | 0.3 | 52 | 11.7 | 1 | 17 |
| 7 | 76 | F | 11 | 87 | 20 | 67 | 1.4 | 97 | 10.7 | 3 | 12 |
| 8 | 50 | M | 1 | 79 | 15 | 64 | 0.9 | 71 | 10.4 | 2 | 14 |
| 9 | 46 | F | 1 | 73 | 40 | 33 | 2.4 | 80 | 43.3 | 23 | … |
| 10 | 69 | M | 8 | 50 | 0 | 50 | 0.2 | 66 | 5.0 | 9 | 17 |
| 11 | 64 | F | 11 | 48 | 10 | 38 | 1.0 | 36 | 9.8 | 1 | 14 |
| 12 | 47 | M | 15 | 15 | 50 | −35 | 0 | 15 | 25.5 | 4 | 12 |
| 13 | 66 | F | 4 | 55 | 25 | 30 | 0.7 | 55 | 9.0 | 3 | 15 |
| 14 | 76 | F | 26 | 33 | 5 | 28 | 1.1 | 45 | 7.8 | 4 | 13 |
| 15 | 72 | F | 4 | 63 | 30 | 33 | 1.5 | 80 | 34.3 | 3 | 16 |
| 16 | 84 | F | 7 | 60 | 10 | 50 | 0.7 | 78 | 8.7 | 4 | 16 |
| 17 | 56 | M | 3 | 77 | 20 | 57 | 1.3 | 63 | 17.7 | 10 | 12 |
| 18 | 50 | M | 8 | 27 | 0 | 27 | 0.5 | 42 | 2.7 | 2 | 14 |
| 19 | 79 | M | 8 | 60 | 30 | 30 | 1.4 | 60 | 24.0 | 4 | 14 |
| 20 | 56 | F | 1 | 57 | 10 | 47 | 1.0 | 23 | 8.7 | 2 | 15 |
Abbreviations: CDAI, Clinical Disease Activity Index; HAQ-II, Health Assessment Questionnaire II; PHQ-9, Patient Health Questionnaire-9; RA, rheumatoid arthritis; VAS, visual analog scale.
Discordance is calculated as the patient global assessment minus the provider global assessment (possible range, −100 to 100).
CDAI was assessed at the last clinical visit (<4 weeks from the interview). HAQ-II, VAS pain, and PHQ-9 were assessed at the interview.
Qualitative Findings
Data analysis yielded 6 main themes describing patient perspectives on patient-provider discordance: 1) being misunderstood by others, 2) limitations of provider assessments, 3) discrepancy with provider findings, 4) inadequate active listening, 5) unmet psychosocial needs, and 6) lack of patient empowerment (Box).
Box. Patient-Provider Discordance Themes and Quotations From Qualitative Interviews of Patients With RA.
Being Misunderstood by Others
“Just because you’ve gone to school and you’ve learned all this stuff and you’ve worked with all this stuff doesn’t mean you know what people are going through.”
Woman, aged 60 years; CDAI, 15.8; PHQ-9, 9; pain VAS, 60
“You know, people with cancer are treated differently. People understand they have cancer; they’re going through chemo or radiation. But people with RA, I think they’re classified as being—I don’t know—like it’s all in their head. … I’ve lost a lot of friends. Lost a few relationships because of it. It’s a very life changing event—it’s just every aspect. It’s just so sad and nobody understands.”
Woman, aged 46 years; CDAI, 43.3; PHQ-9, 23; pain VAS, 80
Limitations of Provider Assessments
“It just seems very vague. I mean, you have a line, and you put on there what level you are at, and you know the worst possible—I don’t know what that looks like—I don’t know, it’s really hard to quantify, you know, like my feet might hurt in the morning, but then they don’t hurt all day.”
Woman, aged 38 years; CDAI, 11.7; PHQ-9, 1; pain VAS, 52
“The biggest problem I have with that form is that my symptoms are not constant. They come and go, and so always in the back of my mind is, do they want me to fill out on that form what my worst-case scenario symptoms are when I’m really hurting—that’s an 8—or what I’m feeling right now sitting in the waiting room—that’s a 5. Or what I felt yesterday, which was a 3. So it’s all over the place with me—I would say in my mind I probably try to take a mean, which is not something that’s easy to do, in your mind to kind of think back over your symptoms if it says the last 14 days or 7 days or last 2 months.”
Man, aged 69 years; CDAI, 5; PHQ, 9; pain VAS, 66
Discrepancy With Provider Findings
“I mean, a person is a person; we’re not a number. I mean, just because it says, X, Y, and Z on this blood test doesn’t mean something else isn’t causing the pain for me. He doesn’t see that there’s that much damage being done, so he doesn’t—I don’t think that he relates to how much it hurts—I just feel like he doesn’t.”
Woman, aged 38 years; CDAI, 11.7; PHQ-9, 1; pain VAS, 52
“I scored my pain at probably a 9—an 8 or a 9, and I think if he were to score it based on the inflamed joints and whatnot, he probably would have scored it at a 6. But the fatigue plays a big role in how painful it is—I just rate it how I feel. And I think a huge part of that is the stress. And, you know, fatigue, depression, so that to me rates how you are feeling, not only physically but mentally, emotionally.”
Woman, aged 46 years; CDAI, 43.3; PHQ-9, 23; pain VAS, 80
“They say, this is up and this level is up, but this isn’t up, and you think, I feel like they all should be.”
Woman, aged 56 years; CDAI, 8.7; PHQ-9, 2; pain VAS, 23
“I am just coming to my senses on that factor—sometimes when I feel like—I’ve got the pain, but see, I don’t feel the pain real well. I tolerate the pain very well, but in my reading I’ve noticed now that maybe my body has adjusted to it, so that’s why I don’t. So I think those days that I feel kinda well, like it was a rough day or whatever, I think that might be those days that I’ve got the pain and don’t realize it.”
Woman, aged 74 years; CDAI, 54.2; PHQ-9, 0; pain VAS, 67
Inadequate Active Listening
“But he is not really listening to the way I’m feeling, and it’s depressing.”
Woman, aged 60 years; CDAI, 15.8; PHQ-9, 9; pain VAS, 60
“It was like nobody is listening to me.”
Woman, aged 63 years; CDAI, 6.4; PHQ-9, 1; pain VAS, 22
“We don’t really go over how it has been since the last time I was here.”
Man, aged 50 years; CDAI, 10.4; PHQ-9, 2; pain VAS, 71
“I just think he hears it all the time, and so he doesn’t really take time to listen because he’s thinking about that next patient—I mean, I know he sees this every day, so I’m sure he’s kind of probably immune to what we are really feeling.”
Woman, aged 60 years; CDAI, 15.8; PHQ-9, 9; pain VAS, 60
Unmet Psychosocial Needs
“After 30 years I’m getting a divorce because she’s tired of paying for all of the bills.”
Man, aged 50 years; CDAI, 10.4; PHQ-9, 2; pain VAS, 71
“My knee—I was in so much pain—I only got a few hours of sleep, which then impacted Sunday when I was babysitting with my grandson, and it’s like, sorry buddy, we can’t do anything today—we are watching movies.”
Woman, aged 60 years; CDAI, 15.8; PHQ-9, 9; pain VAS, 60
“Work—a real major issue. I had to leave my last job after 29 years because I was unable to continue it.”
Man, aged 56 years; CDAI, 17.7; PHQ-9, 10; pain VAS, 63
Lack of Patient Empowerment
“I know his time is valuable. I don’t want to take up any more time than I need to—hard to fit everything in with a 30-minute appointment. But I’m sure if I tried to talk him, he would certainly listen—if I was asked, I would share it. But without being asked, I don’t share it.”
Woman, aged 46 years; CDAI, 43.3; PHQ-9, 23; pain VAS, 80
“I don’t know what else they can do because I know they don’t have time—well, I’m in pain all the time. Pain changes who you are. It changes how you respond to things, it changes how you get through your day, and it wears you down.”
Woman, aged 60 years; CDAI, 15.8; PHQ-9, 9; pain VAS, 60
“Because she is very understanding, and, you know, I just don’t want to argue about it.”
Woman, aged 76 years; CDAI, 10.7; PHQ-9, 3; pain VAS, 97
Abbreviations: CDAI, Clinical Disease Activity Index; PHQ-9, Patient Health Questionnaire-9; RA, rheumatoid arthritis; VAS, visual analog scale.
Being Misunderstood by Others
Patients in this study felt supported when family members provided affirmation for what it is like to have RA. In a few instances, patients assessed how they were coping by comparing their disease manifestations with those of friends or family members who have arthritis. This also helped patients know that they were not alone in their experiences.
Patients without these kinds of experiences felt unsupported. They described the fact that RA is not always as apparent as other more objectively defined diseases. When signs of the disease are not apparent, others may perceive that the patient is not really ill. Many patients emphasized the sense that medical education is focused on the biomedical aspects of RA and does not help providers understand the experiences of patients living with RA, a conception that has permeated into the nonmedical community. The experience of RA is difficult to objectively see or measure.
Patients also talked about feelings of being misunderstood by people in the nonmedical community, such as family members and friends. Loss of relationships and the psychologic burden of the disease were difficult for some patients, but those were not always the types of disease effects that patients discussed with their providers.
Limitations of Provider Assessments
Data suggested that disease activity assessments, such as the pain score, HAQ-II disability index, and global assessments have many limitations. Patients expressed frustration with quantifying their symptoms on the day of the appointment, considering that their symptoms inherently vary in intensity, location, and duration. Patients described difficulty with completing the pain scale, comparing their pain experiences with those of others when assessing their own pain. Some reported thinking of other health conditions when assessing their pain. The time frame for which patients completed their disease assessments was important and was considered to reflect a great deal of the differences in global assessment ratings. Patients said that they attend rheumatology appointments relatively infrequently, whereas RA symptoms may vary from day to day, so a static indicator is difficult to use. Patients viewed the ability to answer activities questions as sometimes helpful, especially for describing worsening difficulty and for prompting discussions about changes in function.
Patients were also unclear about the purpose of the assessments, including how providers use them—or whether they use them at all. Patients gave the sense that they complete a lot of paperwork at clinic appointments but do not necessarily know what happens to it or whether it is simply filed away. Patients expressed a desire for providers to discuss the results of these assessments with them and suggested that this would improve understanding between patients and providers and give patients a chance to explain the considerations that informed their assessment.
Discrepancy With Provider Findings
Patients who have experienced discordance commonly reported that the providers’ findings from physical examination of the joints and laboratory blood tests did not accurately represent the status of their disease and how they felt. Some patients identified pain and fatigue and other comorbidies as key reasons for the discrepancy between patient and provider global assessments. Patients expressed uncertainties about whether providers appreciated the pain that the patients were feeling when their physical assessment may not have reflected it. Patients questioned whether providers can appreciate the patient’s level of pain when the physical examination findings may not suggest any overt signs of inflammation or damage.
Furthermore, many patients said that they do not necessarily understand the purpose or meaning of blood tests and radiographs. Patients indicated that this lack of education leads to confusion, and they expressed a desire to better understand how comorbidities affect their disease as well as treatment options. They reported that making distinctions between pain caused by RA as opposed to other conditions, such as fibromyalgia or osteoarthritis, can be frustrating to patients as well as to providers. Some patients suggested a desire to communicate better with their providers about available options for improving their health status, including not only drug therapies but also potential lifestyle modifications.
Contrary to the majority of patients in the present study, 2 patients reported lower disease activity than their providers described. One of those 2 patients attributed the discrepancy to getting used to the pain.
Inadequate Active Listening
Patients attributed some of the discrepancy with rheumatology assessments to their provider’s failure to listen and express empathy. Furthermore, providers may see patients only once or twice a year, so they are not connecting with them often unless patients are seen for an acute matter. Patients thought that some providers seem uninterested in taking time to listen. Other patients viewed the discordance as lack of communication on their part and hence described the need to write down their list of concerns or keep a journal of their symptoms to bring to clinic visits.
Patients discussed being very much aware of the time constraints of clinic appointments. These patients described feeling that providers could not take the time to listen to their account of the disease since the prior visit. Some suggested that providers were already mentally “moving on” and thinking about subsequent patients. Some patients expressed an understanding of the time constraints providers face during visits. Patients conveyed a general sense that rheumatology providers often evaluate patients with acute, debilitating diseases, so they become impervious to chronic disease manifestations and judge other patients against the worst cases.
Although patients were sampled according to discordance criteria, some described how well their providers understood their experience, and those patients noted some common observations, including providers’ displays of empathetic concern and listening. Evidently, empathy requires communication that reflects how patients are coping in all aspects of their lives. If the provider seemed to care, it was easy to bring up examples of personal impact. However, even patients who acknowledged that their providers listen and show compassion toward them reported they do not always agree with provider assessments, and they acknowledged that providers cannot fully understand how patients feel, especially about their pain.
Unmet Psychosocial Needs
Patients expressed that discordance may be related to unmet psychosocial, emotional, or other needs that are not easily interpretable by providers. Some patients lost employment and relationships, and others had limited interactions with their families. According to patients, providers often do not ask about the impact of RA on patients’ mental and emotional health, yet this impact is crucial to how patients assess their disease activity on rating scales. When filling out questionnaires, patients think globally about their disease and related issues such as fatigue and depression. Patients expressed a desire to share with others the impact RA has on them. Many implied that these issues are not discussed enough during clinic visits.
Patients’ descriptions of their pain provided a greater understanding of the psychosocial toll that pain has on their lives. Patients identified pain as the main factor that limits their ability to engage in activities they previously enjoyed, and that changes them psychologically.
Lack of Patient Empowerment
We found that patients lack empowerment to engage in discussions with their providers. Many patients perceived provider time as valuable and limited, so they often hesitate to share more information or ask questions. Even though patients lacked empowerment to engage in discussions, they felt providers would be willing to take time to discuss their care in more detail if asked to do so. Interestingly, some patients engage in this discussion only if the providers initiate the discussion. However, some patients also felt uncomfortable informing the providers that they disagreed with their opinion.
Discussion
To our knowledge, this is the first qualitative interview study of RA patients whose global ratings of disease activity are discrepant with those of their rheumatology providers. Overall, our findings demonstrate that patient-provider discordance is a real phenomenon. Patients with discordance experience difficulties and frustrations with current paradigms of RA disease assessment as well as patient-provider communication, and their needs for psychosocial support and empathy are not always met by the health care system.
The findings suggest that patients experience great uncertainty when completing the global assessment of disease activity and other rating scales, owing to difficulty in comparing their own situation with the anchoring extreme values of a VAS. Nikiphorou and colleagues (22) recently evaluated the clinical value and limitations of the patient global assessment. Attempts to improve the validity of the patient global assessment with marker states have shown mixed results (23–27). Ward and colleagues (28) provided evidence that different standards for comparison of disease activity between patients and evaluators contribute to discordance, with patients favoring a social comparison as opposed to comparisons with their prior disease activity. Cognitive research should be performed to improve the usefulness and validity of the comparison standard for patient global ratings, which is expected to improve concordance.
The findings also underscore the problem of asking patients to complete their global assessment at a single time. Patients clearly describe difficulty in view of the temporal variability of their symptoms. Future research should determine whether the use of smartphones or web-based applications enabling daily monitoring could improve measurement of the daily experiences of patients in managing their disease and thereby lessen patient-provider discordance. Clinically, it is important to discuss the results of patient-reported outcome measures with patients; failing to do so is a missed opportunity to develop an empathetic relationship with the patient.
Our results highlight how unmet psychosocial and other needs often contribute to patient-provider discordance. Patients with RA encounter difficulties in everyday life related to social life and interpersonal relationships (29) and describe being a psychosocial burden to their partners (30). As previously suggested (31), these findings imply that a deeper understanding of the psychosocial burden of RA will enable health care providers to better manage or prevent patient-provider discordance. However, addressing this issue may present challenges because providers may not recognize psychosocial cues that patients present (32,33). When patients voice distress or other negative affective tones during rheumatology visits, their adherence to recommended treatments is predictably lower over time (34). Training in mindfulness and compassion-based strategies could improve the quality of patient-provider communication and lesson the impact of discordance on health outcomes (35).
Health outcomes that are important to individual patients should be considered because they may influence patient-specific treatment decisions. Sanderson and colleagues (36) identified unmet patient needs, including emotional health issues. In addition to their psychosocial needs, patients in our study placed considerable value on their emotional health. However, they indicated that emotional health is often not considered during routine RA assessment and suggested that this contributes to patient-provider discordance. More importantly, some of our patients expressed a need to share their emotions but lacked support. Future studies should determine the most efficient and effective approach for providing psychosocial support and counseling to patients with RA, especially in the context of patient-provider discordance.
A better understanding of patient-provider discordance could improve shared decision making in the management of RA (8). We identified patients’ unmet needs that contribute to discordance, including information sharing and patient education, as reported previously (37,38). For example, our results suggest that patients would benefit from a greater understanding of the value and limitations of various laboratory tests. Patients evidently want to engage in shared decision making, but providers may not always recognize that need. It is important to encourage and allow patients to engage in discussions about their health concerns because patient-provider discordance can undermine decision making about initiating or discontinuing therapies (6).
Developing clinical decision aids that provide information on patient preferences and goals and depicting treatment options for improvement in high-priority quality-of-life domains, for example, could help lessen discordance and improve patient-centered outcomes. The findings from our study underscore the need to better integrate comorbidity assessments into treatment discussions with patients and to develop aids that facilitate shared decision making about RA and comorbidities.
As we and others have shown (39), patients have many health care needs but often lack the courage to discuss them during clinic visits. However, some patients are willing to accept interpersonal difficulties if they believe their provider is an expert in RA care and research. Results from a recent study showed that communication during clinical visits mainly centers around symptoms and treatments and that patients avoid other important topics (40). This may be explained by our finding that patients perceive providers’ time as limited and valuable and therefore choose to prioritize their time and focus on their major symptoms and treatment concerns. Awareness of patient needs is important, but it may be more vital to develop methods of identifying those needs in the clinic or to encourage patients to share information.
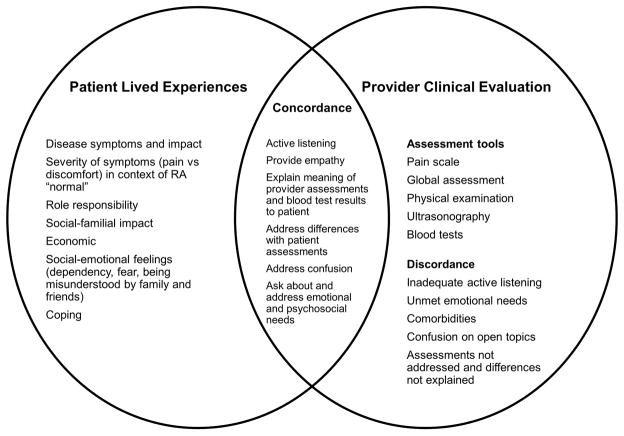
These findings inform the development of future interventions aimed at patient-provider concordance. The Figure provides a conceptual framework that lists all the components from patients’ lived experiences and providers’ clinical evaluations and identifies factors that contribute to patient-provider concordance. As shown in this framework, the patient experiences various effects of RA on role responsibilities, psychosocial functioning, and coping. The provider has several objective assessment tools for evaluating the biomedical aspects of the disease. Ultimately, the intersection of these 2 spheres contributes to concordance between the patient and care provider, characterized by active listening, providing empathy, asking about needs, and providing psychosocial support. If providers and their local health care systems have tools and support, patient experiences with care and health outcomes should be improved. This conceptual framework has implications for clinical practice and the provision of support to RA patients around discordant experiences.
Figure.
Conceptual framework of patient-centered factors that promote quality communication and concordance in disease assessment and decision making. RA indicates rheumatoid arthritis.
The strength of our study was the focus on patients determined to be discordant with their provider according to a standard definition comparing patient and provider global assessments. This interview-based qualitative study provided rich, important data on patients’ perspectives and lived experiences. A potential limitation of this study is participation bias, because the 20 patients who agreed to participate in the interviews were from a larger population of 103 consecutive patients with discordance. Nevertheless, the range of clinical characteristics of the study population, and the quantitative degree of patient-provider discordance, increases our confidence in the findings. Furthermore, the IPA methodological approach was aimed at in-depth understanding of patients’ experiences when they are discordant with provider assessments. Therefore, we interviewed only patients identified as discordant using the described methods. Another potential limitation is that patients completed their global ratings before their clinical appointment; no information was available about whether the clinicians provided any clarification to patients on how to complete their global assessment. Finally, it was beyond the scope of this qualitative study to interpret patient responses according to composite disease activity scores (eg, CDAI). Future studies could evaluate for differences in patient experiences according to disease activity states.
In conclusion, patient experiences of health care and patient-provider communication transcend clinical disease activity measures. Future research might explore constructs in the proposed conceptual model with patients identified according to different criteria, including patients who are concordant with their provider by traditional measures. The findings of this qualitative study highlight important psychosocial and behavioral cues for providers to consider during clinic visits. Awareness of these findings may help improve concordance of disease assessment and shared decision making. The insights from this study should inform the development of patient-centered models of shared decision making that could improve long-term outcomes among patients with RA.
Significance and Innovations.
In this qualitative interview study, patients with rheumatoid arthritis said that some providers do not adequately attend to psychosocial needs or concerns about health-related quality of life.
Efforts focusing on concordance of standard measures of disease pathophysiology may miss issues of discordance that matter most to patients with rheumatoid arthritis.
The developed conceptual framework for evaluating patient-provider discordance should help providers understand their patients’ assessments of disease and its impact on daily life, which would be expected to improve shared decision making and health outcomes.
Acknowledgments
Supported by the Mayo Foundation Eaton Family Career Development Award in Innovative Rheumatoid Arthritis Research as well as CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- CDAI
Clinical Disease Activity Index
- HAQ-II
Health Assessment Questionnaire II
- IPA
interpretative phenomenological analysis
- PHQ-9
Patient Health Questionnaire-9
- RA
rheumatoid arthritis
- VAS
visual analog scale
Footnotes
Conflict of interest: The authors have no financial conflicting interests to disclose.
Publisher: To expedite proof approval, send proof via email to scipubs@mayo.edu.
Author Contributions
Z.K. identified and recruited the patients and drafted the manuscript. G.B.A. and J.L.R. contributed to study design, conducted the patient interviews, and analyzed the qualitative data. C.S.C. contributed to the quantitative data collection and analysis. J.M.D. III designed the study and contributed to data analysis and interpretation. All authors contributed to manuscript preparation and gave approval for the final submitted version.
References
- 1.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016 Jan;68(1):1–26. doi: 10.1002/art.39480. Epub 2015 Nov 6. [DOI] [PubMed] [Google Scholar]
- 2.Desthieux C, Hermet A, Granger B, Fautrel B, Gossec L. Patient-physician discordance in global assessment in rheumatoid arthritis: a systematic literature review with meta-analysis. Arthritis Care Res (Hoboken) 2016 Dec;68(12):1767–73. doi: 10.1002/acr.22902. Epub 2016 Oct 28. [DOI] [PubMed] [Google Scholar]
- 3.Khan NA, Spencer HJ, Abda E, Aggarwal A, Alten R, Ancuta C, et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2012 Feb;64(2):206–14. doi: 10.1002/acr.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindstrom Egholm C, Krogh NS, Pincus T, Dreyer L, Ellingsen T, Glintborg B, et al. Discordance of global assessments by patient and physician is higher in female than in male patients regardless of the physician’s sex: data on patients with rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis from the DANBIO Registry. J Rheumatol. 2015 Oct;42(10):1781–5. doi: 10.3899/jrheum.150007. Epub 2015 Aug 1. [DOI] [PubMed] [Google Scholar]
- 5.Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res (Hoboken) 2015 Feb;67(2):264–72. doi: 10.1002/acr.22401. [DOI] [PubMed] [Google Scholar]
- 6.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010 Jun;62(6):857–64. doi: 10.1002/acr.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko Y, Kuwana M, Kondo H, Takeuchi T. Discordance in global assessments between patient and estimator in patients with newly diagnosed rheumatoid arthritis: associations with progressive joint destruction and functional impairment. J Rheumatol. 2014 Jun;41(6):1061–6. doi: 10.3899/jrheum.131468. Epub 2014 May 1. [DOI] [PubMed] [Google Scholar]
- 8.Studenic P, Radner H, Smolen JS, Aletaha D. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum. 2012 Sep;64(9):2814–23. doi: 10.1002/art.34543. [DOI] [PubMed] [Google Scholar]
- 9.Wartman SA, Morlock LL, Malitz FE, Palm E. Impact of divergent evaluations by physicians and patients of patients’ complaints. Public Health Rep. 1983 Mar-Apr;98(2):141–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg EE, Lussier MT, Beaudoin C. Lessons for clinicians from physician-patient communication literature. Arch Fam Med. 1997 May-Jun;6(3):279–83. doi: 10.1001/archfami.6.3.279. [DOI] [PubMed] [Google Scholar]
- 11.Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010 Mar;105(3):525–39. doi: 10.1038/ajg.2009.685. Epub 2009 Dec 8. [DOI] [PubMed] [Google Scholar]
- 12.Haugli L, Strand E, Finset A. How do patients with rheumatic disease experience their relationship with their doctors? A qualitative study of experiences of stress and support in the doctor-patient relationship. Patient Educ Couns. 2004 Feb;52(2):169–74. doi: 10.1016/s0738-3991(03)00023-5. [DOI] [PubMed] [Google Scholar]
- 13.Lempp H, Scott D, Kingsley G. The personal impact of rheumatoid arthritis on patients’ identity: a qualitative study. Chronic Illn. 2006 Jun;2(2):109–20. doi: 10.1177/17423953060020020601. [DOI] [PubMed] [Google Scholar]
- 14.Flurey CA, Morris M, Richards P, Hughes R, Hewlett S. It’s like a juggling act: rheumatoid arthritis patient perspectives on daily life and flare while on current treatment regimes. Rheumatology (Oxford) 2014 Apr;53(4):696–703. doi: 10.1093/rheumatology/ket416. Epub 2013 Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JA, Flowers P, Larkin M. Theory, Method and Research. London: Sage; 2009. p. 225. [Google Scholar]
- 16.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010 Sep;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 17.Patton MQ. Qualitative research and evaluation methods. 3. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- 18.Fusch PI, Ness LR. Are We There Yet? Data Saturation in Qualitative Research. Qualit Rep. 2015;20(9):1408–16. [Google Scholar]
- 19.Creswell JW. Research design: qualitative, quantitative, and mixed method approaches. 4. Thousand Oaks (CA): SAGE Publications; c2014. p. 273. [Google Scholar]
- 20.Braun V, Clarke V, Rance N. How to use thematic analysis with interview data. In: Vossler A, Moller N, editors. The counselling and psychotherapy research handbook. Los Angeles (CA): Sage; c2015. pp. 183–97. [Google Scholar]
- 21.Challa DN, Kvrgic Z, Cheville AL, Crowson CS, Bongartz T, Mason TG, 2nd, et al. Patient-provider discordance between global assessments of disease activity in rheumatoid arthritis: a comprehensive clinical evaluation. Arthritis Res Ther. 2017 Sep 26;19(1):212. doi: 10.1186/s13075-017-1419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikiphorou E, Radner H, Chatzidionysiou K, Desthieux C, Zabalan C, van Eijk-Hustings Y, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther. 2016 Oct 28;18(1):251. doi: 10.1186/s13075-016-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schunemann HJ, Griffith L, Stubbing D, Goldstein R, Guyatt GH. A clinical trial to evaluate the measurement properties of 2 direct preference instruments administered with and without hypothetical marker states. Med Decis Making. 2003 Mar-Apr;23(2):140–9. doi: 10.1177/0272989X03251243. [DOI] [PubMed] [Google Scholar]
- 24.Schunemann HJ, Armstrong D, Degl’innocenti A, Wiklund I, Fallone CA, Tanser L, et al. A randomized multicenter trial to evaluate simple utility elicitation techniques in patients with gastroesophageal reflux disease. Med Care. 2004 Nov;42(11):1132–42. doi: 10.1097/00005650-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Schunemann HJ, Goldstein R, Mador MJ, McKim D, Stahl E, Griffith LE, et al. Do clinical marker states improve responsiveness and construct validity of the standard gamble and feeling thermometer: a randomized multi-center trial in patients with chronic respiratory disease. Qual Life Res. 2006 Feb;15(1):1–14. doi: 10.1007/s11136-005-0126-x. [DOI] [PubMed] [Google Scholar]
- 26.Bremner KE, Tomlinson G, Krahn MD. Marker states and a health state prompt provide modest improvements in the reliability and validity of the standard gamble and rating scale in prostate cancer patients. Qual Life Res. 2007 Dec;16(10):1665–75. doi: 10.1007/s11136-007-9264-7. Epub 2007 Oct 3. [DOI] [PubMed] [Google Scholar]
- 27.Lati C, Guthrie LC, Ward MM. Comparison of the construct validity and sensitivity to change of the visual analog scale and a modified rating scale as measures of patient global assessment in rheumatoid arthritis. J Rheumatol. 2010 Apr;37(4):717–22. doi: 10.3899/jrheum.090764. Epub 2010 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward MM, Guthrie LC, Alba MI. Standards of compassion and discordance in rheumatoid arthritis global assessments between patients and clinicians. Arthritis Care Res (Hoboken) 2017 Aug;69(8):1260–5. doi: 10.1002/acr.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sverker A, Ostlund G, Thyberg M, Thyberg I, Valtersson E, Bjork M. Dilemmas of participation in everyday life in early rheumatoid arthritis: a qualitative interview study (The Swedish TIRA Project) Disabil Rehabil. 2015;37(14):1251–9. doi: 10.3109/09638288.2014.961658. Epub 2014 Sep 22. [DOI] [PubMed] [Google Scholar]
- 30.Matheson L, Harcourt D, Hewlett S. “Your whole life, your whole world, it changes”: partners’ experiences of living with rheumatoid arthritis. Musculoskeletal Care. 2010 Mar;8(1):46–54. doi: 10.1002/msc.165. [DOI] [PubMed] [Google Scholar]
- 31.Dobkin PL, De Civita M, Abrahamowicz M, Bernatsky S, Schulz J, Sewitch M, et al. Patient-physician discordance in fibromyalgia. J Rheumatol. 2003 Jun;30(6):1326–34. [PubMed] [Google Scholar]
- 32.Korsvold L, Mellblom AV, Lie HC, Ruud E, Loge JH, Finset A. Patient-provider communication about the emotional cues and concerns of adolescent and young adult patients and their family members when receiving a diagnosis of cancer. Patient Educ Couns. 2016 Oct;99(10):1576–83. doi: 10.1016/j.pec.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Stone AL, Tai-Seale M, Stults CD, Luiz JM, Frankel RM. Three types of ambiguity in coding empathic interactions in primary care visits: implications for research and practice. Patient Educ Couns. 2012 Oct;89(1):63–8. doi: 10.1016/j.pec.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Street RL, Jr, Marengo MF, Barbo A, Lin H, Gonzalez AG, Richardson MN, et al. Affective tone in medical encounters and its relationship with treatment adherence in a multiethnic cohort of patients with rheumatoid arthritis. J Clin Rheumatol. 2015 Jun;21(4):181–8. doi: 10.1097/RHU.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 35.Amutio-Kareaga A, Garcia-Campayo J, Delgado LC, Hermosilla D, Martinez–Taboada C. Improving communication between physicians and their patients through mindfulness and compassion-based strategies: a narrative review. J Clin Med. 2017 Mar 17;6(3) doi: 10.3390/jcm6030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanderson T, Morris M, Calnan M, Richards P, Hewlett S. What outcomes from pharmacologic treatments are important to people with rheumatoid arthritis? Creating the basis of a patient core set. Arthritis Care Res (Hoboken) 2010 May;62(5):640–6. doi: 10.1002/acr.20034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukoschek P, Fazzari M, Marantz P. Patient and physician factors predict patients’ comprehension of health information. Patient Educ Couns. 2003 Jun;50(2):201–10. doi: 10.1016/s0738-3991(02)00128-3. [DOI] [PubMed] [Google Scholar]
- 38.Bernatsky S, Feldman D, De Civita M, Haggerty J, Tousignant P, Legare J, et al. Optimal care for rheumatoid arthritis: a focus group study. Clin Rheumatol. 2010 Jun;29(6):645–57. doi: 10.1007/s10067-010-1383-9. Epub 2010 Feb 3. [DOI] [PubMed] [Google Scholar]
- 39.Barry CA, Bradley CP, Britten N, Stevenson FA, Barber N. Patients unvoiced agendas in general practice consultations: qualitative study. BMJ. 2000 May 6;320(7244):1246–50. doi: 10.1136/bmj.320.7244.1246. Erratum in: BMJ 2000 Jul 1;321(7252):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McInnes IB, Combe B, Burmester G. Understanding the patient perspective: results of the Rheumatoid Arthritis: Insights, Strategies & Expectations (RAISE) patient needs survey. Clin Exp Rheumatol. 2013 May-Jun;31(3):350–7. Epub 2013 Feb 13. [PubMed] [Google Scholar]