Abstract
Stress can precipitate or worsen symptoms of many psychiatric disorders by dysregulating glutamatergic function within the prefrontal cortex (PFC). Previous studies suggest that antagonists of group II metabotropic glutamate (mGlu) receptors (mGlu2 and mGlu3) reduce stress-induced anhedonia through actions in the PFC, but the mechanisms by which these receptors act are not known. We now report that activation of mGlu3 induces long-term depression (LTD) of excitatory transmission in the PFC at inputs from the basolateral amygdala. Our data suggest mGlu3-LTD is mediated by postsynaptic AMPAR internalization in PFC pyramidal cells, and we observed a profound impairment in mGlu3-LTD following a single, 20-min restraint stress exposure. Finally, blocking mGlu3 activation in vivo prevented the stress-induced maladaptive changes to amygdalo-cortical physiology and motivated behavior. These data demonstrate that mGlu3 mediates stress-induced physiological and behavioral impairments and further support the potential for mGlu3 modulation as a treatment for stress-related psychiatric disorders.
Introduction
Stress, which is known to cause or exacerbate symptoms of mood disorders, alters synaptic function in the prefrontal cortex (PFC) and induces coincidental impairments to PFC-dependent motivational tasks1–4. Patients with major depressive disorder (MDD) exhibit reduced total PFC volume5, impaired PFC activation during cognitive performance6, and loss of dendritic branching of pyramidal cells7,8. Furthermore, findings from preclinical studies in rodent models align with clinical studies and suggest that dysfunction of PFC glutamatergic neurotransmission is a key substrate underlying the cognitive and motivational effects of stress exposure9–11.
Along with intra-cortical glutamate signaling, the PFC receives substantial excitatory input from the basolateral amygdala (BLA) and ventral hippocampus (VH)12. These afferents are thought to convey distinct components of motivation; specifically, BLA activity is associated with emotional state13,14 and VH activity regulates the expression and retrieval of previous memories15,16. This convergent excitatory signaling is processed, and Layer 5 pyramidal neurons provide the primary PFC output that encodes for the selection and execution of complex, goal-directed tasks17,18. Despite the accepted role of this motivational circuit, it is unknown to what extent physiological differences between the long-range glutamatergic afferents to the PFC may exist. A better understanding of these mechanisms is essential for the development of novel treatments for mental illnesses associated with motivational deficits19.
In recent years, metabotropic glutamate (mGlu) receptor subtype 3 (mGlu3) has emerged as a promising target for modulating glutamatergic transmission in the PFC9,20. Loss-of-function mutations in GRM3 are associated with PFC-related behavioral deficits in schizophrenia patients and healthy volunteers21,22. Conversely, recent studies suggest that activation of mGlu3 plays important roles in PFC-dependent working memory23,24 and extinction learning25. These data suggest that mGlu3 likely regulates key aspects of PFC function. Recent studies from our lab and others suggest that mGlu3 is postsynaptically localized in PFC pyramidal cells in both rodents and primates, where it modulates calcium and cAMP signaling23,25. Furthermore, activation of mGlu3 induces robust long-term depression (LTD) of excitatory transmission onto PFC pyramidal cells25–27. Each of these actions is likely to underlie the ability of mGlu3 to regulate PFC-mediated responses.
We now present a series of studies in which we found that mGlu3-LTD is restricted to excitatory transmission on pyramidal cells and the mechanism is consistent with postsynaptic AMPAR internalization. Furthermore, using projection-specific optogenetic techniques, we found that PFC mGlu3-LTD is selectively expressed at amygdalar but not hippocampal inputs. Remarkably, we found that a single, acute stressor impairs the induction of mGlu3-LTD, and that blocking mGlu3 activity in vivo prevents stress-induced perturbations in amygdalo-cortical function and motivated behavior. Together, these findings show that stress dysregulates postsynaptic synaptic plasticity in the amygdalar input to the PFC and that this can be prevented by administration of a selective mGlu3 NAM. These preclinical studies increase our understanding of the initial stress-induced physiological changes, and may provide mechanistic insights into changes in PFC function observed in patients. Furthermore, these studies raise the exciting possibility that mGlu3 modulators may provide therapeutic benefits for the treatment of stress-related psychiatric disorders.
Materials and Methods
Animals
Adult (>8week), male, C57Bl6/J mice, group-housed (2–5/cage) on a 12-hour cycle (lights on at 06:00), were used for all experiments. Food and water were available ad libitum. All protocols were approved by the Vanderbilt Institutional Animal Care and Use Committee. VU0650786 was administered via intraperitoneal (i.p.) injections in 10% Tween-80 vehicle (10μL/g).
Optogenetics
Channelrhodopsin-2 (ChR2) was virally expressed in glutamatergic neurons as described28. Mice were anesthetized with isoflurane and 250–400nL virus (AAV5-CaMKII-ChR2-EYFP, UNC) was delivered to: (mm) BLA (ML:−2.9, AP:−1.4, DV:−4.7) and VH (ML:−3.6, AP:−3.4, DV:−4.0).
Whole-cell electrophysiology
Mice were anesthetized with isoflurane and decapitated. Coronal slices (300μM) were prepared with NMDG-based cutting/recovery solution. Holding and recording chambers contained artificial cerebrospinal fluid (aCSF): (mM) 119NaCl, 2.5KCl, 2.5CaCl2, 1.3MgCl2, 1NaH2PO4, 11glucose, and 26NaHCO3. The recording chamber was perfused with warm (30±1°C), oxygenated (95/5%O2/CO2) aCSF at 2ml/min. Layer 5 prelimbic PFC neurons were filled with potassium-based internal solution: (mM) 125K-gluconate, 4NaCl, 10HEPES, 4MgATP, 0.3NaGTP, 10Tris-phosphocreatine. Local glutamate release was elicited at 0.1Hz with 0.1–0.15ms electrical stimulation from a concentric bipolar electrode in Layer 5. In ChR2-expressing slices, input-specific glutamate release was evoked with light stimulation (1–4ms, 470nm, LEDD1B, Thor labs). To preclude recording inhibitory currents, recordings were made at −70mV. Control recordings were interleaved with recordings under each experimental condition at an approximate ratio of one control cell per three experimental cells.
Progressive ratio task
Mice were trained on a progressive ratio schedule of reinforcement as described29. Assessments of drug and stress action occurred in a pseudo-random, counterbalanced, within-subjects design. Test day performance was normalized to the previous two sessions. Mice were not food-restricted and the experimenter was blind to all drug treatments.
Drugs
LY379268 and tetrodotoxin were purchased from Abcam. LY341495 and CNQX were purchased from Tocris. VU0650786 and MRK-8-29 were synthesized in-house. The D15 peptide (PPPQVPSRPNRAPPG) was prepared by Bio-Synthesis.
Statistics
The number of cells in each experiment is denoted by “n” and the mice by “N”. Data are presented as mean±SEM. Analyses were performed using GraphPad Prism. Two-tailed Student’s t-test and one-way ANOVA with Bonferonni post-tests were used as appropriate. Post-hoc power analyses ensured a sufficient number of cells and mice were used.
Results
Specific expression of mGlu3-LTD by excitatory transmission on PFC pyramidal cells
LTD induced by the activation of mGlu2/3 is often assumed to involve a reduction in presynaptic release probability. However, data from our lab and others suggest that mGlu3 has direct effects on excitability of PFC pyramidal cells25,30 and mGlu3-LTD in the PFC may involve postsynaptic signaling mechanisms23,25,30,31. To test this hypothesis, we utilized whole-cell patch-clamp electrophysiology. Single neurons in Layer 5 of the prelimbic PFC were classified by their firing properties. Regular-spiking neurons were identified by characteristic spike-firing adaptation (Figure 1a) in contrast to the rapid patterns of fast-spiking interneurons (Figure 1b). As reported previously, bath application of the mGlu2/3 receptor agonist LY379268 induced LTD of excitatory synaptic transmission on putative pyramidal cells (Figure 1a & 1c). Strikingly, LY379268 did not induce LTD at excitatory synapses onto fast-spiking interneurons (Figure 1b & 1d). In addition, LY379268 did not exert any effect on inhibitory postsynaptic currents (Figure S1). Together, these data are consistent with a postsynaptic locus of LTD specific to excitatory transmission onto pyramidal cells.
Figure 1. Specific expression of mGlu3-LTD by PFC pyramidal cells.
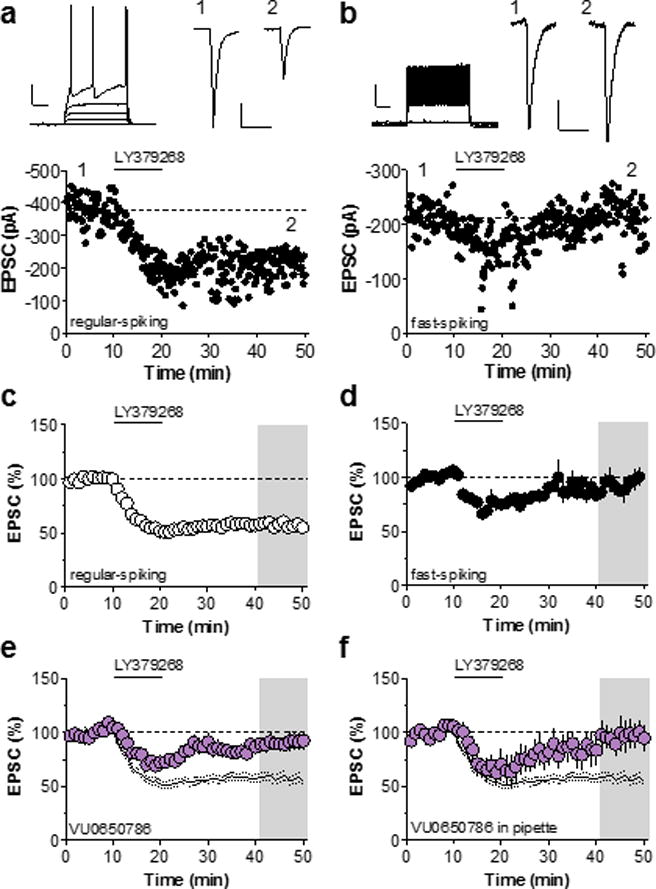
(a) Top left, representative input-output curve displaying characteristic spike-firing adaptation. Scale bars denote 250 ms and 20 mV. Top right, EPSCs recorded during baseline and after LTD induction. Scale bars denote 50 ms and 100 pA. Bottom, representative LTD time course in regular-spiking pyramidal cell. (b) Top right, representative input-output curve displaying fast-spiking properties characteristic of interneurons. Scale bars denote 250 ms and 20 mV. Top right, EPSCs recorded during baseline and after LTD induction. Scale bars denote 50 ms and 50 pA. Bottom, representative LTD time course in fast-spiking interneuron. (c) Summary of control time courses. Application of LY379268 induces LTD of EPSCs on PFC pyramidal cells (55 ± 3 % baseline, n/N = 17/14 cells/mice). (d) Summary of time course experiments in fast-spiking interneurons. LY379268 transiently depresses EPSCs on fast-spiking interneurons but does not induce LTD (93 ± 8 % baseline, n/N = 5/5). Black lines denote control LTD from panel C. (e) Bath application of the mGlu3 NAM VU0650786 blocks LTD induced by LY379268 (90 ± 7 % baseline, n/N = 9/8). (f) Restriction of VU0650786 to the patch pipette is sufficient to block mGlu3-LTD (96 ± 12 % baseline, n/N = 5/3). EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; NAM, negative allosteric modulator; PFC, prefrontal cortex.
We previously demonstrated that this LTD is lost following genetic deletion of mGlu3 and not mGlu225. Furthermore, we found that mGlu3 activation modulates calcium signaling in PFC pyramidal cells25, suggesting that mGlu3 may act postsynaptically on pyramidal cells to induce LTD. To confirm that mGlu3 mediates LTD in whole-cell configuration, we used the mGlu3 NAM VU0650786, which exhibits no off target activity at any other mGlu receptor32 (Figure 1e). Moreover, restriction of VU0650786 to the patch pipette was also sufficient to block LTD (Figure 1f), consistent with a postsynaptic site of action for mGlu3 signaling. Pretreatment with the mGlu2/3 antagonist LY341495 blocked both the initial depression and LTD (Figure S2), suggesting that mGlu2 mediates the transient decrease in EPSC amplitude. Taken together, these data suggest that mGlu3-LTD in the PFC is mediated by postsynaptic mGlu receptors located on pyramidal cells. We next performed studies to further understand the mechanism of action of mGlu3 in inducing PFC LTD.
PFC mGlu3-LTD is mediated by AMPAR internalization
In general, synaptic strength can be related to quantal size, neurotransmitter release probability, and/or the number of synapses33. We performed several analyses to identify which of these factors underlie mGlu3 LTD. The coefficient of variation of the EPSC is inversely proportional to both release probability and synapse number. For each control recording, we normalized the change in the coefficient of variation with the magnitude of LTD (Figure 2a). This analysis revealed a positive correlation, indicating that either a decrease in active synapse number and/or release probability is related to mGlu3-LTD expression. The paired-pulse ratio is thought to be related to release probability and not the number of active synapses. While we observed a trend towards a positive correlation between the change in paired-pulse ratio and the amount of LTD, the slope was significantly less than for the coefficient of variation analysis. We also sampled several additional interstimulus intervals and found no change in paired-pulse ratio following mGlu3-LTD (Figure 2b). The discrepancy between these two analyses is consistent with a rapid decrease in active synapse number underlying mGlu3-LTD.
Figure 2. mGlu3-LTD is mediated by AMPAR internalization.
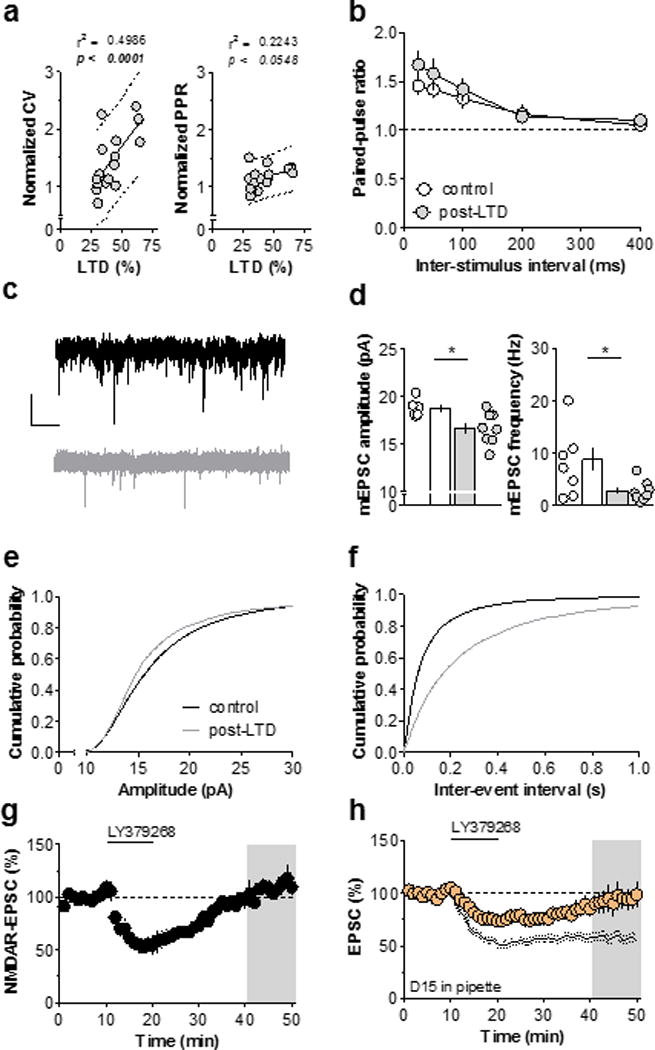
(a) Left, significant positive correlation between the change in CV and magnitude of mGlu3-LTD (r2 = 0.4986, p < 0.0015, n/N = 17/14 cells/mice). Dotted lines signify linear regression and 95% prediction limits. Right, trend towards a positive correlation between the change in PPR (50 ms ISI) and magnitude of mGlu3-LTD (r2 = 0.2243, p < 0.0548, n/N = 17/14). (b) No difference in PPR was observed across wide range of ISIs (n/N = 11/6, 9/5). (c) Representative traces of mEPSC currents. Scale bars denote 500 ms and 20 pA. (d) Expression of mGlu3-LTD is associated with a persistent decrease in mEPSC amplitude (left) and frequency (right) (n/N = 6/4, 8/4, *: p < 0.05, t-test). (e) Cumulative probability distribution of mEPSC amplitude for control cells and cells that underwent mGlu3-LTD. (f) Cumulative probability distribution of mEPSC interevent interval for control cells and cells that underwent mGlu3-LTD. (g) Activation of mGlu3 does not induce a long-term change in amplitude of NMDAR EPSCs (108 ± 5 % baseline, n/N = 4/3). (h) Inclusion of a dynamin dominant negative peptide, D15, in the patch pipette blocks mGlu3-LTD (99 ± 11 % baseline, n/N = 5/3). Black lines denote control LTD from panel 1C. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CV, coefficient of variation; EPSC, excitatory postsynaptic current; ISI, interstimulus interval; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; NMDAR, N-methyl-D-aspartate receptor; PPR, paired-pulse ratio; sEPSC, spontaneous EPSC.
Miniature EPSC (mEPSC) amplitude and frequency are commonly evaluated to measure changes in quantal size, release probability, and synapse number (Figure 2c). The mEPSC amplitude generally reflects quantal size, whereas mEPSC frequency is related to synapse number and release probability. Cells that underwent mGlu3-LTD exhibited a reduction in both mEPSC amplitude and frequency (Figure 2d/e/f). Furthermore, we observed a time-dependent decrease in spontaneous EPSC amplitude and frequency (Figure S3). These data suggest that mGlu3-LTD is mediated by rapid AMPAR internalization, culminating in a decrease in active synapse number. To further test this hypothesis, we isolated NMDA receptor (NMDAR) currents by removing Mg2+ from the aCSF to reduce the voltage-dependent block. The contribution of AMPAR currents was prevented with the antagonist CNQX. LY379268 induced a transient depression of the NMDAR current, however the response returned to baseline (Figure 2g), demonstrating that only AMPAR-mediated responses undergo LTD. In many brain regions, including the hippocampus34 and nucleus accumbens35, mGlu-LTD requires the internalization of AMPA receptors through dynamin-dependent endocytosis Therefore, we assessed the involvement of endocytotic machinery in mGlu3-LTD by using a well-characterized dominant negative peptide that blocks the interaction between dynamin and adapter proteins (D15)34. Inclusion of D15 in the patch pipette blocked mGlu3-LTD (Figure 2h), indicating that dynamin-dependent endocytosis is required for mGlu3-LTD. Together, these data strongly suggest that postsynaptic mGlu3 induces LTD in the PFC through AMPAR internalization.
Synapses from amygdalar, but not ventral hippocampal, afferents express mGlu3-LTD
The PFC receives excitatory input from several limbic structures involved in stress-related adaptations, notably including the BLA and VH (Figure 3a). To examine whether mGlu3-LTD is expressed at these long-range projections, we took a viral approach to exogenously express ChR2 in a regionally-specific manner. Monosynaptic optical EPSCs were evoked with light stimulation. We observed no difference in the coefficient of variation or paired-pulse ratio between BLA-PFC and VH-PFC synapses (Figure 3b & 3c) and examined mGlu3-LTD at each synapse. Consistent with electrical stimulation, the optically-activated BLA input underwent LTD following bath application of LY379268 (Figure 3d & 3g). In contrast, the VH-PFC synapse resisted both the initial and long-term depressions of synaptic transmission induced by mGlu2/3 activation (Figure 3e& 3g). To confirm that BLA LTD is also mediated by mGlu3, we returned to VU0650786. As expected the mGlu3 NAM blocked LTD at the amygdalo-cortical pathway, whereas the mGlu2 NAM MRK-8-2925 had no effect (Figure 3h), corroborating the mGlu3-dependent mechanism at the BLA-PFC synapse.
Figure 3. BLA, but not VH, afferents to PFC express mGlu3-LTD.
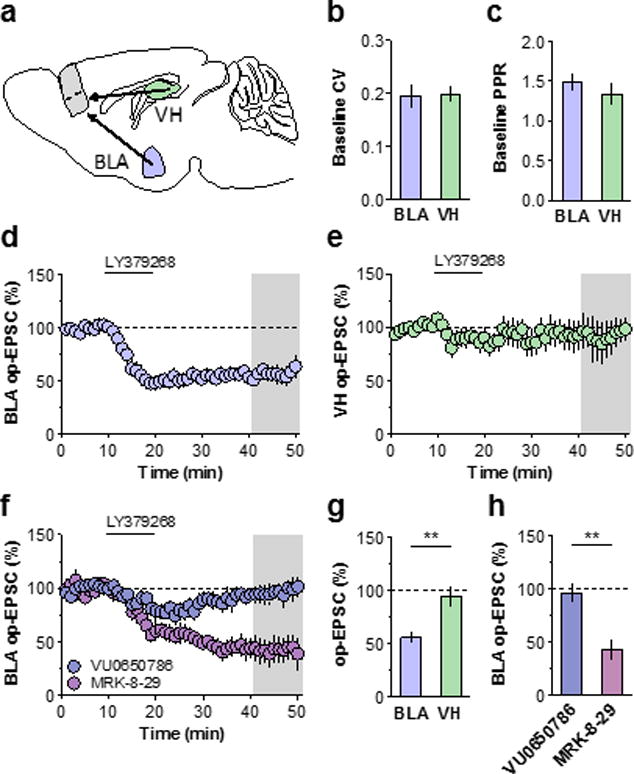
(a) Schematic displaying region-specific approach. AAV-CaMKII-ChR2 was injected into the BLA or VH of young mice and slice recordings were prepared 3–5 weeks later. (b) No difference in the baseline CV was observed across BLA or VH inputs (n/N = 13/7, 10/4 cells/mice). (c) No difference in PPR (50 ms ISI) was observed across inputs (n/N = 13/7, 7/4 cells/mice). (d) Summary time course of BLA-PFC recordings. LY379286 application induced LTD (56 ± 6 % baseline, n/N = 10/7). (e) LY3792678 did not depress excitatory transmission at VH-PFC synapses (94 ± 9 % baseline, n/N = 7/4). (f) Pharmacological confirmation of mGlu3-LTD at BLA-PFC input. The mGlu3 NAM VU0650786 blocked BLA-PFC LTD (96 ± 8 % baseline, n/N = 4/3) whereas LTD remained in the presence of the mGlu2 NAM MRK-8-29 (43 ± 9 % baseline, n/N = 5/4). (g) Summary of last 10 minutes of LTD across inputs (**: p < 0.01, t-test). (h) Summary of last 10 minutes of BLA-PFC pharmacological experiments (**: p < 0.01). BLA, basolateral amygdala; ISI, interstimulus interval; CV, coefficient of variation; mGlu, metabotropic glutamate receptor; op-EPSC optical excitatory postsynaptic current; PFC, prefrontal cortex; PPR, paired-pulse ratio; VH, ventral hippocampus.
Single exposure to restraint stress rapidly impairs PFC mGlu3-LTD
While many psychiatric disorders are associated with long-term and/or intense stress exposure, mild stressors occur on a day-to-day basis and affect motivated decision-making within the general population36. Moreover, acute stress can instigate relapse events in individuals with remitted psychiatric disorders. Human neuroimaging and mechanistic rodent studies have related these effects to impairments in PFC function3,4,36,37, therefore we aimed to assess whether acute stress modulates the induction of mGlu3 synaptic plasticity in the PFC. Mice were sacrificed 30 minutes after the termination of 20-minutes restraint stress (Figure 4a). Restraint stress did not affect excitability or basal membrane properties (Figure 4b), and the acute inhibition of EPSCs induced by LY379268 remained intact (Figure 4c &4d). However, mice exposed to acute stress displayed a significant impairment of mGlu3-mediated LTD (Figure 4d &4e). This impairment persisted for one day, but not three days, after stress exposure (Figure S4).
Figure 4. Acute restraint stress rapidly impairs PFC mGlu3-LTD.
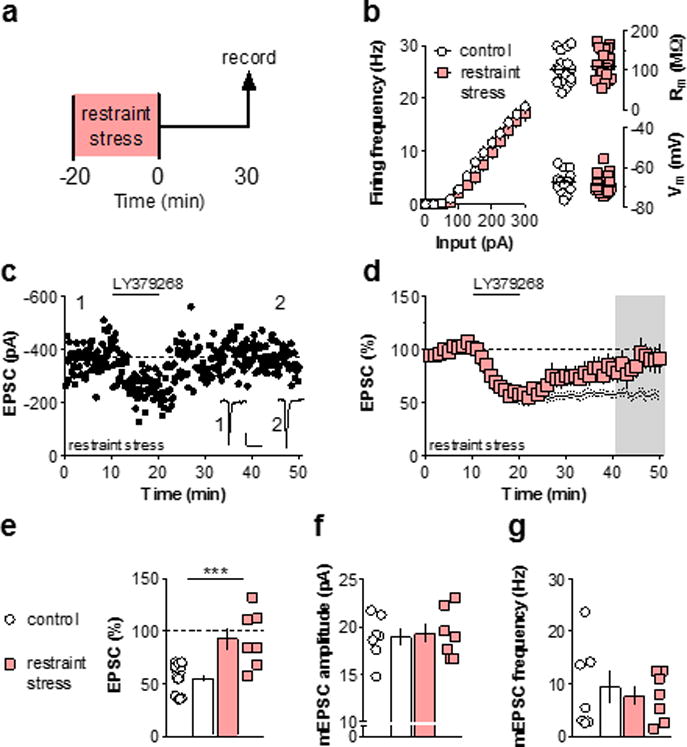
(a) Schematic displaying stress exposure paradigm. Mice were sacrificed for electrophysiology 30 minutes after the termination of 20-minutes immobilization stress. (b) Acute stress did not affect the basal membrane properties of PFC pyramidal cells (n/N = 23/12, 18/6 cells/mice). (c) Representative experiment displaying loss of LTD following restraint stress. Scale bars denote 100 pA, 50 ms. (d) Summary time course of long-term recordings following stress. While the acute depression during drug application remained intact, LTD did not occur following stress (93 ± 10 % baseline, n/N = 7/5). Black lines denote control data from figure 1C. (e) Summary of last 10 minutes of long-term recordings. Acute restraint stress impairs induction of LTD ex vivo (***: p < 0.001, t-test). (f) mEPSC amplitude does not differ between the restraint stress group and controls (n/N = 7/4, 7/3). (g) mEPSC frequency does not differ between the restraint stress group and controls (n/N = 7/4, 7/3). EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; mEPSC, miniature excitatory postsynaptic current; PFC, prefrontal cortex; Rm, membrane/input resistance; sac, sacrifice; Vm, resting membrane potential.
Loss of synaptic plasticity can generally be attributed to an impairment in the induction mechanism or to occlusion (i.e. a floor effect). To address this, we measured mEPSCs in pyramidal cells from control slices and slices from stressed mice, and found that stress exposure did not affect mEPSC amplitude or frequency (Figures 4f &4g). Moreover, the baseline coefficient of variation and paired-pulse ratio were not affected by stress (data not shown). These data suggest that the stress-induced LTD impairment was not caused by occlusion, and that a loss of function in mGlu3 or a downstream signaling partner is likely responsible.
mGlu3 NAM administration prevents stress-induced deficits in BLA-PFC mGlu3-LTD and motivation
Having demonstrated that acute stress dysregulated mGlu3 plasticity, we hypothesized that blocking mGlu3 activation during exposure to stress in vivo would prevent the maladaptive changes in PFC physiology and function. To test this hypothesis, we administered VU0650786 or vehicle 15 minutes prior to the acute stress (Figure 5a). In addition to high selectivity against other mGlu subtypes, VU0650786 exhibited no off-target activity in a screen against 68 clinically-relevant drug targets32. The basal synaptic properties of amygdalo-cortical transmission taken from vehicle- and VU0650786-pretreated, stressed mice were not different from each other, or from naïve control mice (Figures 5b &5c). As observed with electrical stimulation, acute stress disrupted mGlu3-LTD at BLA-PFC synapses (Figure 5d). Pretreatment with VU0650786 prevented the stress-induced impairment (Figures 5e & 5f), suggesting that mGlu3 activity in vivo is necessary for this stress-induced change to occur. Interestingly, treatment with VU0650786 immediately after stress exposure also prevented the deficits in ex vivo LTD (Figure S5).
Figure 5. Blocking mGlu3 activation in vivo prevents stress-induced deficits to BLA-PFC mGlu3-LTD and motivation.
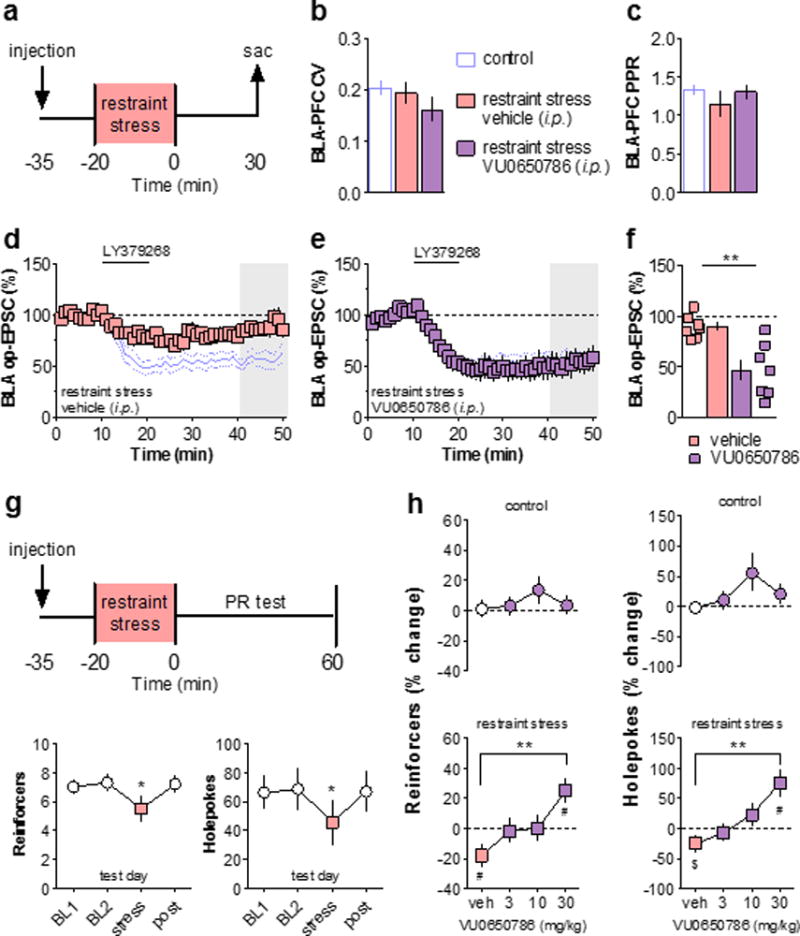
(a) Schematic displaying stress exposure paradigm. Mice were pretreated with i.p. injections of the mGlu3 NAM VU0650786 or vehicle 15 minutes prior to immobilization stress. Slices were prepared for electrophysiology 30 minutes after the stress ended. (b) Acute stress does not affect BLA-PFC CV. Control value taken from figure 3B. (c) Acute stress does not affect BLA-PFC PPR (50 ms ISI). Control value taken from figure 3C. (d) BLA-PFC LTD is impaired in cells from vehicle-treated mice exposed to restraint stress (89 ± 4 % baseline, n/N = 7/4 cells/mice). Blue lines denote control data from figure 3D. (e) Systemic pretreatment with the mGlu3 NAM rescues the stress-induced deficit in mGlu3-LTD (47 ± 10 % baseline, n/N = 7/3). (f) Summary of last 10 minutes of LTD recordings. (**: p < 0.01). (g) Top, schematic. Operant responding for liquid food was assessed on a PR schedule of reinforcement. Bottom, stress impaired performance on the PR schedule as assessed by the number of reinforcers earned and the number of holepokes elicited (N = 10, *: p < 0.05, Bonferonni post-tests vs. BL1, BL2, and post). (h) Effects of stress, VU0650786, and combination, on PR performance. The number of reinforcers earned on the test day is expressed as a percentage change relative to the two preceding baseline days. Top, injections of vehicle and VU0650786 did not alter the number of reinforcers earned on the test day in control mice. Bottom, vehicle-treated mice exposed to restraint stress exhibited a decrease in the number of reinforcers earned relative to baseline (N = 10, #: p < 0.05, one-sample t-test). Pretreatment with VU0650786 generated a dose-dependent reversal of the stress-induced impairment (N = 10, **: p < 0.01, Bonferonni post-test vs. veh). (i) PR performance in all conditions as measured by the number of holepokes elicited (N = 10, #: p < 0.05, $: p < 0.10, one-sample t-test; **: p < 0.01, Bonferonni post-test vs. veh). BL, baseline; BLA, basolateral amygdala; CV, coefficient of variation; EPSC, excitatory postsynaptic current; i.p., intraperitoneal; ISI, inter-stimulus interval; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; mEPSC, miniature excitatory postsynaptic current; NAM, negative allosteric modulator; PFC, prefrontal cortex; PPR, paired-pulse ratio; PR, progressive ratio schedule of reinforcement; sac, sacrifice; veh, vehicle.
PFC function regulates motivated behaviors and stress impairs motivation in clinical populations and animal models2,11,38,39. We sought to examine motivation by training mice to respond in an operant apparatus for delivery of a liquid reinforcer. Mice were then switched to a progressive ratio (PR) schedule, where the number of hole pokes required to earn a reinforcer increases exponentially during the task. The number of reinforcers earned on the PR schedule is decreased by lesions of the PFC but not of other cortical structures40. Once performance stabilized, acute restraint sessions were administered to mice immediately prior to the task (Figure 5g). Same-day acute restraint stress significantly decreased the number of reinforcers earned and the holepokes executed, consistent with a decreased motivational state. To measure the effects of VU0650786 on this stress-induced behavioral deficit, performance during restraint stress test sessions was normalized to the average of the two preceding baseline days. During control (no stress) test sessions, vehicle-treated mice displayed identical performance relative to baseline, and increasing doses of VU0650786 had no effect (Figure 5h & 5i, top). In contrast, during test sessions that immediately followed stress exposure, vehicle-treated mice recapitulated the deficit in PR performance (Figure 5h & 5i, bottom). This stress-induced deficit was prevented by pretreatment with the mGlu3 NAM VU0650786 in a dose-dependent manner, at doses consistent with the pharmacodynamic/pharmacokinetic relationship of this mGlu3 NAM32. These data indicate that blocking mGlu3 activation in vivo is sufficient to prevent decreased motivation following acute stress, and suggest that intact amygdalo-cortical neuroplasticity may underlie this phenomenon.
Discussion
Here we report a novel mechanism by which acute stress dysregulates PFC function in rodents. We found that mGlu3-LTD is specific to pyramidal cells and proceeds through a postsynaptic site of action. Moreover, the plasticity exists at long-range inputs from the BLA, but not VH, and is impaired by a single stress exposure. Finally, inhibiting mGlu3 function in vivo prevented the stress-induced deficit in BLA-PFC LTD as well as a motivational impairment in food-reinforced behavior. While the present studies are restricted to rodents, they are well-aligned with recent work describing a role for mGlu3 in regulating PFC function in non-human primates23,30, and with clinical studies on the impact of GRM3 mutations21,22 and stress on PFC function3,4. The present studies provide a strong mechanistic basis to guide future human studies aimed at evaluating how stress affects amygdalo-cortical function and the potential utility of mGlu3 NAMs as treatments for stress-related disorders.
Canonical plasticity induced by mGlu2/3 involves a decrease in presynaptic release probability41–45, and recent publications have shown that activation of mGlu2/3 can modify postsynaptic NMDAR receptor function under some circumstances46,47. However, the present data provide direct evidence that activation of mGlu3 induces LTD by postsynaptic AMPAR internalization in PFC pyramidal cells, similar to the mechanism by which mGlu5-LTD occurs in the hippocampus and nucleus accumbens48,49. These results raise the possibility that mGlu3 may regulate postsynaptic glutamatergic signaling in other brain regions and disease states50,51.
While changes in the hippocampus and other areas take several days or weeks to occur, stress-induced changes in dorsolateral/prelimbic PFC physiology can be observed following a single stress event3,4,37. Following chronic stress, profound reductions in AMPA receptor function are known to occur2,10,52. Having revealed the mechanism of mGlu3-LTD to involve AMPAR internalization and a reduction in active synapse number, we predicted that stress may impair mGlu3-LTD induction by occlusion (i.e. that stress usurps similar signaling mechanisms and initiates an LTD-like process in vivo). Based on that hypothesis, we expected to observe baseline differences in mEPSC frequency and amplitude, and were surprised to find no differences in pyramidal cell physiology between the control and stress groups. In contrast to our findings, a previous study found that acute stress enhanced AMPAR and NMDAR function in the PFC53. However, those experiments were performed in prepubertal rats and the physiological changes were measured 4-hours after the stress. The present data suggest that in adult animals, stress rapidly impairs glutamatergic synaptic plasticity in the PFC via desensitization of mGlu3 and/or its downstream signaling partners.
In general, stress-induced PFC impairments are thought to result from excessive glutamate signaling during stress exposure54,55. In this light, the loss of mGlu3-LTD following acute stress may provide a permissive initial step towards further impairments by exacerbating hyperactive glutamate signaling during future stress experiences. Testing whether long-term mGlu3 inhibition (i.e. pharmacological and/or genetic) prevents the development of chronic stress-related pathophysiology is an intriguing and important future experiment. Along those lines, mixed mGlu2/3 antagonists act as rapid-acting antidepressants in animal models of chronic stress exposure20,56–58. The efficacy of mGlu2/3 antagonists is thought to result from mechanisms like those of ketamine, involving a rapid re-potentiation of PFC glutamate transmission10,59,60. While neither ex vivo nor in vivo administration of VU0650786 had a profound effect on basal transmission in the present study, the situation may be quite different following chronic stress exposure. In that condition, where AMPAR function is impaired10,52, an mGlu3 NAM might exert fast-acting antidepressant actions by potentiating PFC glutamate signaling.
While evidence suggests that both amygdalar and hippocampal afferents to the PFC promote anxiety- and depressive-like behavior61,62, recent studies suggest that the two regions provide different contributions towards stress-related behavior63–65. We found that mGlu3 activation induced synaptic plasticity only at BLA-PFC synapses, and these exciting data suggest that mGlu3 may regulate emotional and motivational responses to stress exposure, while leaving memory-related components of stress experience intact. Consistent with that idea, inhibition of mGlu3 function modulates escape behavior in the forced swim test32, but does not disrupt the acquisition of conditioned freezing25. These findings raise many interesting questions related to the functional roles of the distinct limbic inputs into the PFC and whether specific neurotransmitter receptor signaling pathways may be exploited to have tailored therapeutic outcomes.
In addition to the varied sources of glutamate received by the PFC, the divergent flow of information out of the structure may provide a means of crafting nuanced treatment approaches. Pyramidal cells can be readily demarcated by their anatomical projection target and robust differences between these populations have been reported18,66,67. Because mGlu3-LTD occurred in every control cell examined, we find it unlikely that basal tract-specific differences in mGlu3 function exist. However, based on the variability observed following stress, mGlu3-LTD may be impaired in discrete pyramidal cell sub-populations, similar to reported tract-specific changes in spine morphology67. For example, it is conceivable that LTD could be differentially impacted in pyramidal cells projecting to stress or anxiety-related brain structures relative to neurons projecting to areas that promote motivated behavior. Additionally, PFC pyramidal neurons have been sub-classified based on the expression of cell surface receptors and mGlu3 function might be differentially dysregulated across those populations. For example, pyramidal cells expressing dopamine receptor subtypes D1 and D2 exert distinct effects on decision-making68–70. These and other genetically-classified pyramidal cell populations warrant further examination in the context of stress and mGlu function.
Alongside changes in motivated behavior, stress is known to disrupt several cognitive functions that require intact PFC function, such as working memory71, sustained attention72, and executive function11. Impairments in executive function can be caused by increases in rigid, perseverative behaviors. PFC mGlu3-LTD may comprise one mechanism that permits behavioral flexibility in the face of changing response contingencies or requirements40. Consistent with this notion, decreased expression of cortical mGlu2/3 has been observed in subjects with major depressive disorder73, loss of function mutations in GRM3 are associated with schizophrenia and low cognition in healthy controls21,22, and mGlu3 inhibition disrupts extinction learning in rodents25. Additionally, in a model of cocaine abuse, only rats that exhibited addiction-like drug-seeking displayed a loss of PFC mGlu3-LTD74. As such, mGlu3-LTD may function as a biological substrate that underlies comorbidities between stress, substance use disorders, and potentially schizophrenia75. Impairments in mGlu3-LTD might therefore comprise one mechanism by which dysregulated top-down control increases the likelihood of a stress-induced relapse event or psychotic episode. Further research into circuit-specific changes in PFC physiology will enhance our understanding of the behavioral ramifications of stress experience in the context of specific disease states. Clearly, continued holistic efforts to understand the molecular, circuit-level, and behavioral mechanisms underlying PFC dysfunction will be essential in efforts to translate novel preclinical mechanisms into efficacious therapies for stress-related psychiatric disorders. The data presented here reinforce that modulating mGlu3 function may be one such approach.
Supplementary Material
Figure S1. Group II mGlu activation does not alter inhibitory transmission in the PFC. (a) Representative traces and experiment. IPSCs were recorded with a high-chloride internal solution at −70 mV in the presence of CNQX. Scale bars denote 200 pA and 50 ms. (b) Summary timecourse of IPSC recordings during and after bath application of LY379268 (91 ± 6 % baseline, n/N = 5/2 cells/mice). IPSC, inhibitory postsynaptic current; mGlu, metabotropic glutamate receptor.
Figure S2. Transient depression is mediated by mGlu2. Both the transient and long-term depression of excitatory transmission induced by LY379268 are blocked by the mGlu2/3 antagonist, LY341495 (95 ± 10 % baseline, n/N = 4/3 cells/mice). EPSC, excitatory postsynaptic current; mGlu2/3, metabotropic glutamate receptor subtypes 2 and 3.
Figure S3. Immediate and long-term decreases in sEPSC amplitude and frequency during induction of mGlu3-LTD. (a) mGlu3-LTD coincides with sustained decrease in sEPSC amplitude (*: p < 0.05, Bonferonni post-test, n/N = 16/13 cells/mice). (b) mGlu3-LTD coincides with sustained decrease in sEPSC frequency (**: p < 0.01, Bonferonni post-test, n/N = 16/13). LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; sEPSC, spontaneous excitatory postsynaptic current.
Figure S4. Timecourse of stress-induced impairment in PFC mGlu3-LTD. (a) Summary time course of long-term recordings the day after acute stress exposure. LTD was impaired one day following acute stress (84 ± 9 % baseline, n/N = 5/3 cells/mice). (b) Summary time course of LTD recordings three days after acute stress exposure. LTD was not different from controls three days following acute stress (64 ± 13 % baseline, n/N = 5/2). (c) Summary of last 10 minutes of long-term recordings. Control and 30-min values taken from Figure 4e. (*: p < 0.05, ***: p < 0.001, Bonferonni post-tests vs. control). EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; PFC, prefrontal cortex; sac, sacrifice.
Figure S5. Posttreatment with VU0650786 rescues stress-induced deficits to BLA-PFC mGlu3-LTD. (a) Schematic displaying stress exposure paradigm. Mice were treated with i.p. injections of the mGlu3 NAM VU0650786 immediately following immobilization stress. Slices were prepared for electrophysiology 30 minutes later. (b) BLA-PFC LTD remained intact in stressed mice treated with VU0650786 (41 ± 3 % baseline, n/N = 5/3 cells/mice). BLA, basolateral amygdala; EPSC, excitatory postsynaptic current; i.p., intraperitoneal; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; NAM, negative allosteric modulator; PFC, prefrontal cortex; sac, sacrifice.
Figure S6. Diagram displaying hypothesized changes to BLA-PFC mGlu3-LTD following acute stress exposure. (Left) In PFC pyramidal cells, activation of mGlu3 generates LTD through a postsynaptic mechanism, consistent with the rapid AMPAR internalization and a decrease in the number of active synapses. This LTD occurs at inputs from the BLA and not from the ventral hippocampus. (Top) Others have shown that acute stress activates the BLA elevates glutamate levels in the PFC. The present data demonstrate that acute stress dysregulates mGlu3-LTD by impairing its mechanism (e.g. desensitization), as the baseline function of AMPARs was not changed. The LTD impairment occurred concomitant with a decrease in motivation as measured by performance in an operant behavioral task. (Bottom) Blocking mGlu3 activation with the NAM, VU0650786, prevented the maladaptive change in synaptic plasticity from occurring and rescued the stress-induced motivational deficit. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CV, coefficient of variation; BLA, basolateral amygdala; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; NAM, negative allosteric modulator; NMDAR, N-methyl-D-aspartate receptor; PFC, prefrontal cortex.
Acknowledgments
The authors thank Douglas Shaw, Jennifer Zachry, Weimin Peng, and Dr. Zixiu Xiang for their technical assistance and advice. This work was supported by National Institutes of Health (NIH) grants R01MH062646 and R37NS031373 (P.J.C.). M.E.J. was supported by NIH grant T32MH093366 and a postdoctoral fellowship through the Pharmaceutical Research and Manufacturers of America Foundation. Behavioral experiments were performed through the Murine Neurobehavior Core lab at the Vanderbilt University Medical Center.
Footnotes
Conflict of Interest
C.W.L. has been funded by the NIH, Johnson and Johnson, Bristol-Myers Squibb, AstraZeneca, Michael J. Fox Foundation, as well as Seaside Therapeutics. He has consulted for AbbVie and received compensation. P.J.C. has been funded by NIH, AstraZeneca, Bristol-Myers Squibb, Michael J. Fox Foundation, Dystonia Medical Research Foundation, CHDI Foundation, and Thome Memorial Foundation. Over the past three years he has served on the Scientific Advisory Boards for Michael J. Fox Foundation, Stanley Center for Psychiatric Research Broad Institute, Karuna Pharmaceuticals, Lieber Institute for Brain Development, Clinical Mechanism and Proof of Concept Consortium, and Neurobiology Foundation for Schizophrenia and Bipolar Disorder. C.W.L. and P.J.C. are inventors on patents that protect different classes of metabotropic glutamate allosteric modulators. M.E.J., C.I.S., and J.L.E. declare no potential conflicts of interest.
References
- 1.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;26:148. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnsten AFT, Raskind MA, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89–99. doi: 10.1016/j.ynstr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ossewaarde L, Qin S, Van Marle HJF, van Wingen GA, Fernández G, Hermans EJ. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage. 2011;55:345–352. doi: 10.1016/j.neuroimage.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 4.Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute Psychological Stress Reduces Working Memory-Related Activity in the Dorsolateral Prefrontal Cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, et al. Smaller Cingulate Volumes in Unipolar Depressed Patients. Biol Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased Amygdala and Decreased Dorsolateral Prefrontal BOLD Responses in Unipolar Depression: Related and Independent Features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 7.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression∗∗See accompanying Editorial, in this issue. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 13.Etkin A. Functional neuroanatomy of anxiety: A neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- 14.Anderson AK. Feeling emotional: The amygdala links emotional perception and experience. Soc Cogn Affect Neurosci. 2007;2:71–72. [Google Scholar]
- 15.Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JNP, Monyer H, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15:181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- 16.Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal–prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci. 2017 doi: 10.1038/nn.4553. advance on. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otis JM, Namboodiri VMK, Matan AM, Voets ES, Mohorn EP, Kosyk O, et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543:103–107. doi: 10.1038/nature21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaki S. MGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends Pharmacol Sci. 2017 doi: 10.1016/j.tips.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Bishop JR, Ellingrod VL, Moline J, Miller D. Association between the polymorphic GRM3 gene and negative symptom improvement during olanzapine treatment. Schizophr Res. 2005;77:253–260. doi: 10.1016/j.schres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Harrison PJ, Lyon L, Sartorius LJ, Burnet PWJ, Lane TT. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–22. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- 23.Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT, et al. mGluR2 versus mGluR3 Metabotropic Glutamate Receptors in Primate Dorsolateral Prefrontal Cortex: Postsynaptic mGluR3 Strengthen Working Memory Networks. Cereb Cortex. 2017 doi: 10.1093/cercor/bhx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lainiola M, Procaccini C, Linden AM. MGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and Y-maze cognitive tests. Behav Brain Res. 2014;266:94–103. doi: 10.1016/j.bbr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte Ka, Niswender CM, et al. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci U S A. 2015;112:1196–201. doi: 10.1073/pnas.1416196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otani S, Daniel H, Takita M, Crépel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–44. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CC, Yang PC, Lin HJ, Sen Hsu K. Repeated Cocaine Administration Impairs Group II Metabotropic Glutamate Receptor-Mediated Long-Term Depression in Rat Medial Prefrontal Cortex. J Neurosci. 2007;27:2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joffe ME, Grueter BA. Cocaine Experience Enhances Thalamo-Accumbens N-Methyl-D-Aspartate Receptor Function. Biol Psychiatry. 2016;80:671–681. doi: 10.1016/j.biopsych.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, et al. Role for the M1 Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice. ACS Chem Neurosci. 2015;6:1683–1695. doi: 10.1021/acschemneuro.5b00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin LE, Wang M, Yang S-T, Yang Y, Galvin VC, Lightbourne TC, et al. mGluR2/3 mechanisms in primate dorsolateral prefrontal cortex: evidence for both presynaptic and postsynaptic actions. Mol Psychiatry. 2016:1–11. doi: 10.1038/mp.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27:2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engers JL, Rodriguez AL, Konkol LC, Morrison RD, Thompson AD, Byers FW, et al. Discovery of a Selective and CNS Penetrant Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 3 with Antidepressant and Anxiolytic Activity in Rodents. J Med Chem. 2015;58:7485–7500. doi: 10.1021/acs.jmedchem.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–25. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, et al. Role of AMPA Receptor Cycling in Synaptic Transmission and Plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 35.Grueter Ba, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porcelli AJ, Delgado MR. Stress and decision making: effects on valuation, learning, and risk-taking. Curr Opin Behav Sci. 2017;14:33–39. doi: 10.1016/j.cobeha.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izquierdo A, Wellman C, Holmes A. Brief Uncontrollable Stress Causes Dendritic Retraction in Infralimbic Cortex and Resistance to Fear Extinction in Mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier SU, Makwana AB, Hare TA. Acute Stress Impairs Self-Control in Goal-Directed Choice by Altering Multiple Functional Connections within the Brain’s Decision Circuits. Neuron. 2015;87:621–631. doi: 10.1016/j.neuron.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Olausson P, Kiraly DD, Gourley SL, Taylor JR. Persistent effects of prior chronic exposure to corticosterone on reward-related learning and motivation in rodents. Psychopharmacology (Berl) 2013;225:569–577. doi: 10.1007/s00213-012-2844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niswender CM, Conn PJ. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, et al. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbe D, Bockaert J, Manzoni OJ. Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur J Neurosci. 2002;16:2231–2235. doi: 10.1046/j.1460-9568.2002.02273.x. [DOI] [PubMed] [Google Scholar]
- 44.Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73:1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- 45.Johnson KA, Lovinger DM. Presynaptic G Protein-Coupled Receptors: Gatekeepers of Addiction? Front Cell Neurosci. 2016;10:264. doi: 10.3389/fncel.2016.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg N, Gerber U, Ster J. Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors. 2016;36:11521–11531. doi: 10.1523/JNEUROSCI.1519-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trepanier C, Lei G, Xie Y-F, MacDonald JF. Group II metabotropic glutamate receptors modify N-methyl-D-aspartate receptors via Src kinase. Sci Rep. 2013;3:926. doi: 10.1038/srep00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- 49.Lüscher C, Huber KM. Group 1 mGluR-Dependent Synaptic Long-Term Depression: Mechanisms and Implications for Circuitry and Disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucas SJ, Bortolotto ZA, Collingridge GL, Lodge D. Selective activation of either mGlu2 or mGlu3 receptors can induce LTD in the amygdala. Neuropharmacology. 2013;66:196–201. doi: 10.1016/j.neuropharm.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Han JS, Bird GC, Neugebauer V. Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Neuropharmacology. 2004;46:918–926. doi: 10.1016/j.neuropharm.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated Stress Causes Cognitive Impairment by Suppressing Glutamate Receptor Expression and Function in Prefrontal Cortex. Neuron. 2011;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen B, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 55.Bagley J, Moghaddam B, Haven W. Temporal Dynamics of Glutamate Efflux in the Prefrontal Cortex and in the Hippocampus Following Repeated Stress : Effects of Pretreatment With Saline or Diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- 56.Fukumoto K, Iijima M, Chaki S. The Antidepressant Effects of an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacology. 2015;41:1046–1056. doi: 10.1038/npp.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koike H, Iijima M, Chaki S. Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol Biochem Behav. 2013;107:20–23. doi: 10.1016/j.pbb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR₂/₃ blockade. Neurospcyhopharmacology. 2012;15:429–34. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dwyer JM, Lepack AE, Duman RS. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol psychiatry. 2013;1:15. doi: 10.1186/2049-9256-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lodge D, Mercier MS. Ketamine and phencyclidine: The good, the bad and the unexpected. Br. J. Pharmacol. 2015;172:4254–4276. doi: 10.1111/bph.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron. 2016;89:857–866. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selden NRW, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 64.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 65.Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal Ensembles in Amygdala, Hippocampus, and Prefrontal Cortex Track Differential Components of Contextual Fear. J Neurosci. 2014;34:8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, et al. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci. 2014;34:3878–87. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jenni NL, Larkin JD, Floresco SB. Prefrontal dopamine D1 and D2 receptors regulate dissociable aspects of risk/reward decision-making via distinct ventral striatal and amygdalar circuits Output from. J Neurosci. 2012:10521. doi: 10.1523/JNEUROSCI.0030-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.St Onge JR, Abhari H, Floresco SB. Dissociable Contributions by Prefrontal D1 and D2 Receptors to Risk-Based Decision Making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Land BB, Narayanan NS, Liu R-J, Gianessi CA, Brayton CE, M Grimaldi D, et al. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014 doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The Prefrontal Cortex as a Key Target of the Maladaptive Response to Stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mcomish CE, Pavey G, Gibbons A, Hopper S, Udawela M, Scarr E, et al. Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J Affect Disord. 2016;190:241–248. doi: 10.1016/j.jad.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Kasanetz F, Manzoni OJ. Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. J Neurophysiol. 2009;101:2516–27. doi: 10.1152/jn.91039.2008. [DOI] [PubMed] [Google Scholar]
- 75.Joffe ME, Grueter CA, Grueter BA. Biological substrates of addiction. Wiley Interdiscip Rev Cogn Sci. 2014;5:151–171. doi: 10.1002/wcs.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Group II mGlu activation does not alter inhibitory transmission in the PFC. (a) Representative traces and experiment. IPSCs were recorded with a high-chloride internal solution at −70 mV in the presence of CNQX. Scale bars denote 200 pA and 50 ms. (b) Summary timecourse of IPSC recordings during and after bath application of LY379268 (91 ± 6 % baseline, n/N = 5/2 cells/mice). IPSC, inhibitory postsynaptic current; mGlu, metabotropic glutamate receptor.
Figure S2. Transient depression is mediated by mGlu2. Both the transient and long-term depression of excitatory transmission induced by LY379268 are blocked by the mGlu2/3 antagonist, LY341495 (95 ± 10 % baseline, n/N = 4/3 cells/mice). EPSC, excitatory postsynaptic current; mGlu2/3, metabotropic glutamate receptor subtypes 2 and 3.
Figure S3. Immediate and long-term decreases in sEPSC amplitude and frequency during induction of mGlu3-LTD. (a) mGlu3-LTD coincides with sustained decrease in sEPSC amplitude (*: p < 0.05, Bonferonni post-test, n/N = 16/13 cells/mice). (b) mGlu3-LTD coincides with sustained decrease in sEPSC frequency (**: p < 0.01, Bonferonni post-test, n/N = 16/13). LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; sEPSC, spontaneous excitatory postsynaptic current.
Figure S4. Timecourse of stress-induced impairment in PFC mGlu3-LTD. (a) Summary time course of long-term recordings the day after acute stress exposure. LTD was impaired one day following acute stress (84 ± 9 % baseline, n/N = 5/3 cells/mice). (b) Summary time course of LTD recordings three days after acute stress exposure. LTD was not different from controls three days following acute stress (64 ± 13 % baseline, n/N = 5/2). (c) Summary of last 10 minutes of long-term recordings. Control and 30-min values taken from Figure 4e. (*: p < 0.05, ***: p < 0.001, Bonferonni post-tests vs. control). EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; PFC, prefrontal cortex; sac, sacrifice.
Figure S5. Posttreatment with VU0650786 rescues stress-induced deficits to BLA-PFC mGlu3-LTD. (a) Schematic displaying stress exposure paradigm. Mice were treated with i.p. injections of the mGlu3 NAM VU0650786 immediately following immobilization stress. Slices were prepared for electrophysiology 30 minutes later. (b) BLA-PFC LTD remained intact in stressed mice treated with VU0650786 (41 ± 3 % baseline, n/N = 5/3 cells/mice). BLA, basolateral amygdala; EPSC, excitatory postsynaptic current; i.p., intraperitoneal; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; NAM, negative allosteric modulator; PFC, prefrontal cortex; sac, sacrifice.
Figure S6. Diagram displaying hypothesized changes to BLA-PFC mGlu3-LTD following acute stress exposure. (Left) In PFC pyramidal cells, activation of mGlu3 generates LTD through a postsynaptic mechanism, consistent with the rapid AMPAR internalization and a decrease in the number of active synapses. This LTD occurs at inputs from the BLA and not from the ventral hippocampus. (Top) Others have shown that acute stress activates the BLA elevates glutamate levels in the PFC. The present data demonstrate that acute stress dysregulates mGlu3-LTD by impairing its mechanism (e.g. desensitization), as the baseline function of AMPARs was not changed. The LTD impairment occurred concomitant with a decrease in motivation as measured by performance in an operant behavioral task. (Bottom) Blocking mGlu3 activation with the NAM, VU0650786, prevented the maladaptive change in synaptic plasticity from occurring and rescued the stress-induced motivational deficit. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CV, coefficient of variation; BLA, basolateral amygdala; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; NAM, negative allosteric modulator; NMDAR, N-methyl-D-aspartate receptor; PFC, prefrontal cortex.


