Abstract
The basis for how we represent temporal intervals in memory remains unclear. One proposal, the mental time line theory (MTL), posits that our representation of temporal duration depends on a horizontal mental time line, thus suggesting that the representation of time has an underlying spatial component. Recent work suggests that the MTL is a learned strategy, prompting new questions of when and why MTL is used to represent temporal duration, and whether time is always represented spatially. The current study examines the hypothesis that the MTL may be a time processing strategy specific to centrally-located stimuli. In two experiments (visual eccentricity and prismatic adaptation procedures), we investigated the magnitude of the rightward bias, an index of the MTL, in central and peripheral space. When participants performed a supra-second temporal interval reproduction task, we observed a rightward bias only in central vision (within 3° visual angle), but not in the peripheral space (approximately 6–8° visual angle). Instead, in the periphery, we observed a leftward bias. The results suggest that the MTL may be a learned strategy specific to central space and that strategies for temporal interval estimation that do not depend on MTL may exist for stimuli perceived peripherally.
1. Introduction
An important question in time perception regards how we represent the duration of temporal intervals. One possibility is that magnitudes, including numbers and times, may be processed in the same way as spatial information (Walsh, 2003). Consistent with this idea, the relationship between time and space has been posited to be in the form of the mental time line (MTL) in which time information is represented along a mental horizontal line from left to right (e.g., Bonato, Zorzi, & Umilta, 2012; Di Bono et al. 2012; Ishihara, Keller, Rossetti, & Prinz, 2008; Oliveri et al., 2009; Vallesi, Binns, & Shallice, 2008). A common characteristic observed amongst experiments supporting the MTL is the tendency for those who read from left to right to report a shorter or earlier event when it is represented on the left hemispace, and longer or later when the event is represented on the right. This specific directional relationship is known as the rightward bias (the leftward bias is seen in those who read from right to left; Fuhrman & Boroditsky, 2010), and has been used as an index of the MTL (Note: Explanations for the correlation between reading direction and the MTL is offered by the hypothesis that reading experience influences time points to be implicitly associated with one side of space; Casasanto & Bottini, 2014). At the brain level, the rightward bias may be governed by the right parietal cortex and a possible rightward egocentric bias (Di Bono et al., 2012; Frassinetti, Magnani, & Oliveri, 2009; Vicario, Caltagirone, & Oliveri, 2007; Vicario et al., 2008; but see Ekstrom & Isham, 2017). Evidence of the rightward bias as supportive evidence for the MTL has been observed in different contexts beyond vision, including in participants with limited sight (Bottini, Crepaldi, Casasanto, Crollen, & Collignon, 2015) and when performing non-visual gesturing (Casasanto & Jasmin, 2012). Such evidence is often taken to support a domain-independent interpretation of the MTL as underlying many different forms of time perception (Anelli, Ciaramelli, Arzy, & Frassinetti, 2016; Frassinetti et al., 2009; Magnani, Pavani, & Frassinetti, 2012).
Yet, while there is cumulative evidence for the rightward bias to support the MTL as evidence for the close relationship between time and space, there is also evidence suggesting that a spatial component is not always associated with time in some contexts. For example, there are instances in which temporal and spatial representations dissociate during episodic memory tasks (e.g., memory for temporal order and spatial distances dissociate during a navigation task; Ekstrom, Copara, Isham, Wang, & Yonelinas, 2011), as do their underlying neural correlates (Ekstrom et al., 2011; Gauthier & van Wassenhove, 2016; Peer, Salomon, Goldberg, Blanke, & Arzy, 2015; Vass et al., 2016). This is also true, neurophysiologically in rodents, as neural representations for spatial location versus temporal interval dissociate in some instances (Kraus, Robinson, White, Eichenbaum, & Hasselmo, 2013). In addition, other neural models suggest that temporal interval estimation, particularly in the supra-second range, may not depend on an explicit spatial metric nor on parietal cortex (Akam & Kullmann, 2014; Buhusi & Meck, 2005; Gu, van Rijn, & Meck, 2015; Kononowicz & van Rijn, 2015). These observations thus suggest that a spatial rubric, including the MTL, need not always underlie temporal representations.
The different instances in which time and space associate and dissociate from one another prompts a general question of when might the MTL be used to represent time. From our literature review, we have observed that the rightward bias, a characteristic of the MTL, is commonly reported in studies where the experimental stimulus is centrally located (e.g., a 3° deviation experienced in the aftereffect of a prismatic adaptation, Frassinetti et al., 2009; a time bisection task with a stimulus deviating 5° from the midline, Vicario et al., 2008). In contrast, there is minimal evidence for the rightward bias in peripheral vision. This observation motivated the current hypothesis that the MTL may be unique to central space and not in the periphery.
Such perspective that the MTL may be unique to central space is also drawn from a different line of research which examines the relationship between time perception and retinal eccentricity. In a typical retinal eccentricity task, the position of the stimulus is physically varied such that the light information is projected on the different locations of the retina. Some of these studies have extended the object position as far as 20° (e.g., temporal order judgment task; Westheimer, 1983) yet these studies have not documented any directional biases in time judgments. Instead, they have generally reported the results of combined data drawn from both the left and right hemispaces, (Aedo-Jury & Pins, 2010; Kliegl & Huckauf, 2014; Long & Beaton, 1981; Westheimer, 1983), suggesting that the rightward bias may not be present. These findings add to the current hypothesis that there is a difference in the relationship between time perception in central and peripheral spaces.
In addition to the visual eccentricity research, the literature on the effect of near and far spaces (i.e., closer and further extended in the forward direction, respectively) motivates the question regarding whether time is perceived differently when the object is centrally and peripherally located. For example, participants performed differently on cognitive tasks in the near and far spaces (e.g., Vuilleumier, Valenza, Mayer, Reverdin, & Landis, 1998; Zach & Brugger, 2008). Although the near-far phenomenon is observed along a different spatial plane (vertical to the head) from the mental time line plane (horizontal to the head), it nevertheless hints to the possibility that central (i.e., near) and peripheral (i.e., far) spaces on the horizontal plane may affect cognitive processes that include the way we represent temporal magnitudes.
An extension to the current hypothesis that the MTL is specific to centrally-located stimuli is tied to the question of its purpose. Droit-Volet and Coull (2015) has demonstrated that the MTL is a learned process, developed around eight years of age. While the purpose of such development is unclear, one possible avenue to explore is whether the MTL is specific to centrally-located stimuli because these stimuli are often rich in details, and the MTL is a mental strategy developed to aid the processing of such details. In the same manner as Gestalt principles, the MTL may be learned so to achieve simplicity in the mental representation of time.
1.1. Current study
In the current study, we examine the magnitude of the rightward bias during a time reproduction task performed on stimuli located in central and peripheral spaces on the horizontal plane. Our study is an initial attempt to address whether the MTL is a strategy mostly applied to centrally-located stimuli, and whether this is because these stimuli possess greater resolution of information. If the rightward bias does not generalize across spatial location, and is only observed in central space, the result would support the perspective that the MTL is specific to centrally-located stimuli (visual eccentricity procedure; Experiment 1). Subsequently, we tested whether the MTL is specific to centrally-located stimuli because of their high-resolution, detailed information. To do so, we dissociated the spatial location and level of details (prismatic adaptation, Experiment 2). The results revealed that the MTL is specific to central location but not necessary to details.
2. Experiment 1
We examined the magnitude of the rightward bias, a signature of the mental time line theory, across different positions on the horizontal meridian. The experiment employed the visual eccentricity procedure and systematically varied the position of the stimulus across a range of 0–11° while holding visual fixation. Eye movements were monitored by an eyetracker to ensure fixation.
2.1. Methods
2.1.1. Participants
14 participants (age 18–25; 6 females; all right handed) volunteered for the study to satisfy partial course requirement. Informed consent was obtained. The protocol complied to and was approved by the Institutional Review Board at the University of California, Davis.
2.1.2. Apparatus and procedure
Participants were seated 60 cm in front of a monitor. During a trial, the observers fixated on a cross on the center of the screen while being monitored by an eyetracker (Eyelink II, SR Research, Ontario, Canada). During the Encoding phase, the participants continued to hold fixation at the center while covertly attending to a visual stimulus (yellow circle, subtended 4°) that appeared at one of the nine possible spatial locations (0°, 3°, 5.8°, 8.4°, 11.2°) along the horizontal meridian. The stimulus duration varied between 1600, 1800, 2000, 2200, and 2400 ms. The Time Reproduction phase followed immediately after encoding. In this second phase, the participants reported the perceived duration of the stimulus from the Encoding phase by pressing the spacebar on the computer keyboard twice using the index finger of the right hand; the first press indicated the beginning of the perceived duration and the second press indicated the end of the perceived duration. Note: During the time reproduction task, no visual stimulus is present on the screen, except for the fixation cross. We omitted a visual stimulus due to the possibility that time reproduction might be influenced by the location of the placeholder (a concern raised by Frassinetti et al., 2009). There were 45 unique trials (9 spatial positions × 5 durations) presented in random order across each of the seven blocks, totaling 315 trials. Fig. 1 depicts the Experiment 1 procedure.
Fig. 1.

Experiment 1 procedure. During ENCODING, participants viewed the standard stimulus appear on the screen. The position of the stimulus varied 0 and 11.2 degrees left and right of central fixation. No stimulus was present during reproduction.
2.2. Results
2.2.1. Perceived duration as a function of retinal eccentricity & manipulation check
We first examined all trials and ensured that fixation was held within 2° visual angel of the fixation cross. Of the 315 trials, the average number of trials excluded across participants ranged from 3 to 35 trials. Of the remaining trials, the data were subjected to a 5 Duration × 9 Spatial Location (–11.2°, –8.4°, –5.8°, –3.0°, 0°, 3.0°, 5.8°, 8.4°, 11.2°) within-subject ANOVA to explore the distribution over different visual eccentricity. This analysis also allowed us to examine the data and to validate the methodology by making a direct comparison to previous findings from the visual eccentricity literature.
As expected, the results showed a main effect for Duration, F(4,52) = 59.88, p < .001, η2 = 0.82, indicating that the participants were able to differentiate between the different durations tested. The reported duration means were overestimated at 2160.59, 2354.41, 2476.56, 2589.43, and 2718.26 ms (the actual intervals were 1600, 1800, 2000, 2200, and 2400 ms). Posthoc pairwise comparisons between each pair of durations were significant, p < .005. Moreover, there was a main effect of Spatial Location, F(8,104) = 3.33, p = .002, η2 = 0.20, indicating that the perceived duration varied with the stimulus’ spatial position. Further examination of this variation showed the function best fit a polynomial of order 5, F(1,13) = 13.39, p = .003, η2 = 0.51 (equation: y = 0.0028x5–0.0129x4–.4513x3 + 2.008x2 + 12.446x + 2416.1; R2 = 0.803). The results are consistent with the visual eccentricity literature that perceived duration increases with visual eccentricity (e.g., Long & Beaton, 1981); Fig. 2. We next examined whether there was a rightward bias.
Fig. 2.

Reported duration across retinal eccentricity (solid line) closely resembles a polynomial function of order 5 (dotted line).
2.2.2. Rightward bias analysis
A primary interest of this experiment was to observe whether the rightward bias would be observed for all spatial positions. We performed a pair sampled t-test comparing the reproduced durations of the stimulus seen on the left and the right hemispaces at each of the eccentricity tested: 3.0, 5.8, 8.4, 11.2°. As shown on Table 1, the reproduced interval was longer when presented 3.0° rightward compared to 3.0° leftward, suggesting a rightward bias, t = 3.12, df = 13, p < .008. At 8.4°, however, we observed the reverse of the bias such that the duration was judged as longer when the stimulus appeared on the left than the right side, t = –2.81, df = 13, p = .015.
Table 1.
The difference in the perceived duration in the left and right representational spaces across visual eccentricity.
| Eccentricity (degrees) | Left duration, SD | Right duration, SD | Difference in perceived duration (Right-Left; ms) |
|---|---|---|---|
| 3.0 | 2419.00 (134.82) | 2488.77 (143.91) | 69.77 (24.79)* |
| 5.8 | 2480.92 (139.41) | 2465.88 (140.28) | –15.03 (25.78) |
| 8.4 | 2526.65 (149.10) | 2442.55 (140.66) | –84.09 (26.93)* |
| 11.2 | 2473.18 (125.03) | 2462.64 (130.70) | –10.54 (34.01) |
Denotes a statistically significant difference.
2.3. Discussion
The rightward bias observed in the 3.0° condition was anticipated as this is consistent with the MTL literature. However, the fact that the rightward bias was only observed at this eccentricity, i.e., near fovea vision, appears to support our hypothesis that the MTL is unique to centrally-located stimuli. Beyond central space, our data showed that rightward bias is minimized, and in fact, reversed.
At this juncture, it is unclear whether the MTL is specific to the centrally-located stimuli because the MTL has been acquired to aid in the processing of detailed information, which is often associated with central vision. We explored this component further in Experiment 2 in which the stimuli presented were foveated and only their perceived location was manipulated.
In addition to the minimization of the rightward bias, the results from Experiment 1 also suggested a leftward bias at 8.4° eccentricity. This result was not predicted by our hypothesis, and although other studies have investigated peripheral space (e.g., Vicario et al., 2008), there is only one past report, to our knowledge, showing a leftward bias in temporal interval perception (e.g., Vicario, Martino, Pavone, & Fuggetta, 2011). In their study, participants tended to overestimate temporal intervals to a greater extent when their head was turned to the right, and therefore using left peripheral vision to view the stimuli, compared to when the head is straight or turned in the leftward direction, prompting the use of right peripheral vision. Given the limited number of reports of a leftward bias, and the different experimental design of Vicario et al., 2011, an important next step is to address whether the leftward results are replicable or whether it might have resulted from a sort of anomaly related to our paradigm. A replication and discussion followed in Experiment 2.
3. Experiment 2
In Experiment 1, we observed the rightward bias for stimuli located near central vision (3.0°). In Experiment 2, we examined whether this reflected the MTL as a strategy for processing centrally-located information in general, or whether the MTL is applied only during the processing of detailed information. To decouple central vision and detailed information, we employed a prismatic adaptation procedure similar to Frassinetti et al. (2009). The prismatic adaptation procedure allows the stimuli to be presented centrally according to the retinotopic map, and the amount of details were equated, but the perceived location varied according to the spatiotopic topography. Accordingly, we tested whether the MTL varied with the perceived spatial position while now holding the amount of details constant. If the rightward bias continued to be observed for stimuli perceived to be centrally-located and not for those perceived to be peripherally positioned, then it would imply that the MTL is specific to perceived spatial location but not to details (since in this experiment, the level of details is equated in all experimental conditions).
In addition, Experiment 2 was also an opportunity to test for reproducibility of the leftward bias observed in Experiment 1.
3.1. Methods
3.1.1. Participants
14 participants (age 18–38; 10 females; all right handed) volunteered for the study and received partial course credit. Informed consent was obtained. The protocol complied with and was approved by the Institutional Review Board at the University of California, Davis.
3.1.2. Sample size
We chose the number of participants based on Frassinetti et al. (2009). The anticipated sample size at the alpha level of 0.05 and power of 0.90 was 6 participants and at power value of 0.95 was 8 participants. We chose the average of 7 participants assigned to each level of the between-subjects variable (i.e., degree shift). Given that we had two between-subjects conditions, the sample size was 14.
3.1.3. Apparatus and procedure
Experiment 2 consists of two time-reproduction tasks that flanker a prismatic adaptation procedure. Fig. 3 summarizes the experimental structure.
Fig. 3.

Experiment 2 procedure consisting of two blocks of time reproduction task (Pre-adaptation and post-adaptation). The prismatic adaptation occurs in between the two blocks. Each participant was enrolled in two sessions (left and right adaptation); the sessions were two days apart.
A. Time Reproduction Task
The experiment followed the apparatus and procedure used by Frassinetti et al. (2009). Briefly, the participants performed a temporal duration report of a visual stimulus pre and post prismatic adaptation. During both pre and post adaptation phases, the participants encoded the temporal information and reproduced the duration in the following manner: A visual stimulus (i.e., a yellow circle) appeared at the center of the computer screen and subtended 3° from a 60-cm viewing distance. The circle was presented for a variable time interval: 1600, 1800, 2000, 2200, or 2400 ms. After the stimulus offset, the participants immediately performed a time reproduction task by pressing the spacebar on the computer keyboard twice using their right index finger. However, unlike Experiment 1, the first press initiated the onset of a placeholder stimulus (a yellow circle with a smiley face) and the second press offset the placeholder. The placeholder was incorporated because participants had a difficult time reproducing an empty interval during the pilot. There were 50 encoding-reproduction trials in each of the pre and post adaptation phases (10 trials per duration). The stimuli of different durations were randomly presented.
B. Prismatic Adaptation
As in the Frassinetti et al. (2009) procedure, the participants were seated at a table in front of a hollow box (height = 30 cm, depth = 34 cm at the center and 18 cm at the periphery, width = 72 cm). In each trial, the experimenter placed an object (a figurine) at the distal edge of the top surface of the box in one of the three positions in random order: central position (0°) and 21° to the left or right of the central position. Participants performed a pointing task directly to the center of the figurine object using only the index finger of the right hand. They were asked to maintain their right hand at the level of the sternum when not pointing. The end position of the participant’s pointing direction was recorded. The pointing task was performed in three phases (pre-adaptation, adaptation and post-adaptation) totaling 180 pointing trials. During the pre-adaptation trials, the participants pointed toward the direction of the figurine either above the top of the box (30 trials; pointing visible to the participants) or through the hollow space of the box (30 trials; pointing was not visible to the participants). The adaptation phase followed. The participants performed the pointing task (90 trials) while wearing prismatic lenses that induced a spatial shift to the left or the right (within-subjects, left and right order was counterbalanced and were tested at least one day apart). One group of the participants experienced a 10° shift and the other group experienced a 30° shift. Subsequently, in the post-adaptation phase, the participants removed the goggles and performed a pointing task inside the box.
C. Hemispace adaptation
Each participant was adapted to either the left or right adaptation in her first experimental session. The participant returned two days later to perform the same tasks but under a different directional adaptation. The order of left and right adaptation was counterbalanced.
3.2. Results
3.2.1. Prismatic adaptation at 10° and 30° resulted in 3° and 6° aftereffects
We followed the same analysis procedure as in Frassinetti et al. (2009). As illustrated in Fig. 4, after the adaptation procedure, the observers experienced an aftereffect in spatial orientation. A 10° adaptation shifted the orientation by approximately 3° whereas a 30° adaptation shifted visual orientation by approximately 6°. The degree of disorientation was subsequently used as an independent variable in the following analysis.
Fig. 4.


a. Mean displacement measured during the post-adaptation pointing task. An adaptation of 10° resulted in a displacement of 3.5–3.8°.
b. An adaptation of 30° resulted in a displacement of approximately 6°.
3.2.2. Manipulation check
We first examined whether the participants were able to differentiate between the different durations tested. The perceived duration data drawn from the post condition of both left and right adaptation condition indicated that the perceived duration systematically increased with the actual duration tested, F(4,48) = 74.87, p < .001, η2 = 0.86. Post-hoc pairwise comparisons for all duration pairs were statistically reliable, p < .04. These findings indicated that participants could in fact perceive and accurately represent the different temporal intervals.
3.2.3. Perceived duration (Rightward and leftward biases)
With the verification that the participants were able to differentiate between the different durations, we next analyzed the data in the same manner as Frassinetti et al. (2009). The normalized perceived duration was calculated as the difference between post-adaptation and baseline (i.e. perceived duration reported prior to the adaptation procedure). The more positive the net difference, the longer the perceived duration observed after adaptation. Subsequently, the perceived duration was subjected to a 5 Duration (1600, 1800, 2000, 2200, 2400 ms) × 2 Hemispace (aftereffect left or aftereffect right) × 2 Spatial Location (3° or 6° from central location) mixed ANOVA.
Critically, we found an interaction effect between Hemispace and Spatial Location, F(1,12) = 9.30, p = .010, η2 = 0.44, suggesting that interval estimations varied differently when under different combinations of hemispace (left or right) and spatial location (central location or extended into the periphery). Specifically, when the stimulus is perceived to be near the center of the visual scene (3°), a rightward bias was observed; that is, the reproduced duration of the object perceived to be on the right (M = 187.04 ms, SE = 46.27) was longer than the reproduced duration of the stimulus that was perceived to be on the left (M = 74.47 ms, SE = 58.57), t = 2.72, df = 6, p = .017. This particular result is consistent with the data reported by Frassinetti et al. (2009) and Experiment 1. In addition, when the stimulus’ spatial position was perceived to be in the periphery, (6°), participants did not experience a rightward bias, and instead experienced a leftward bias, consistent with Experiment 1. Specifically, the reproduced duration in the right hemispace (M = 25.23 ms, SE = 95.86) was shorter than the stimulus in the left hemispace (M = 195.75 ms, SE = 38.13), t = 2.05, df = 6, p = .043; Fig. 5. Consistent with the results from Experiment 1, the leftward bias prompts further questions about the generalizability of the MTL as well as suggests alternative approaches in time perception.
Fig. 5.
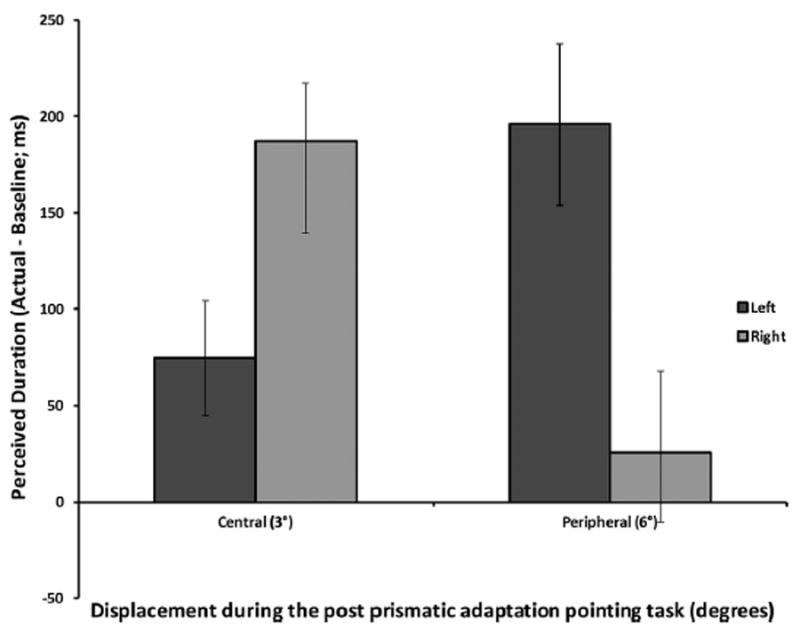
Perceived duration as a function of left and right aftereffect for the 3° and 6° shifts in Experiment 2. When the object’s position was perceived to be within central space, at 3° from central fixation, a rightward bias was observed. When the object’s position was perceived to be further into the periphery, at approximately 6°, a leftward bias was observed.
No other main effects or interactions were observed, p > .05.
3.3. Discussion
The results from Experiment 2 have two important implications. First, we replicated the rightward bias effect for temporal interval reproduction when the stimulus was perceived to be near central space. According to our results, the rightward bias was observed prominently in the centrally-located spatial position (at 3° aftereffect); this is consistent with data reported by Frassinetti et al. (2009) and in our Experiment 1. The results therefore support the working hypothesis that the MTL is specific to centrally-located stimuli. However, the data do not support the secondary hypothesis that the MTL is selective for detailed information. Stimuli in both the 3° and 6° aftereffect conditions were equated in details since they were both held constant at the fovea at the retinal level. Yet, the rightward bias was only observed for the 3° stimuli, suggesting that the MTL is not sensitive to details. Alternative hypotheses are discussed in the General Discussion below.
In addition to the rightward bias, the goal of Experiment 2 was also to further explore the leftward bias from Experiment 1. In Experiment 2, when the spatial position was further extended into the periphery, we observed a similar leftward bias such that longer time was perceived when the stimulus was in the left periphery compared to the right periphery. Such replication adds to the accumulating evidence for leftward bias in the periphery and suggests that the rightward bias is not generalizable beyond central vision; and that other strategies are employed when judging temporal duration of objects peripherally located.
4. General discussion
The current study examined whether the mental timeline (MTL) is a strategy applied under specific spatial parameters, namely when the event or object of interest is perceived to be centrally-located. Additionally, the current study also examined whether this may be attributed to centrally-located stimuli being enriched in details compared to those perceived in the periphery. To address the primary hypothesis regarding the MTL being specific to centrally-located stimuli, we employed two techniques (visual eccentricity and prismatic adaptation) to manipulate the perceived stimulus location. In both experiments, we assessed for the presence of the rightward bias, which is a common behavioral output showing that the magnitude of time is represented along a left-to-right horizontal time line. In both experiments, the participants performed a time reproduction task, and the results commonly suggest there was a rightward bias when the stimuli were perceived to be centrally-located (i.e., within 3 degrees of fixation). However, the rightward bias was not observed when the stimuli were perceived to be peripherally-located (approximately 6–8°). Instead, we observed an opposite effect, which we have called the leftward bias. In this case, our participants reported the stimuli perceived to be on the left to be longer than those perceived to be on the right.
One possible interpretation from these results suggests that the rightward bias is not a stable phenomenon. While many studies have used it to support the MTL, our data suggest that the rightward bias is specific to when the visual event is perceived to be centrally-located. Therefore, future studies should consider this when designing and interpreting their results. In addition, the observation of the leftward bias in the periphery also prompts further discussion on the MTL. If the mental timeline is defined such that the magnitude of time increases from left to right, the presence of leftward bias would challenge this definition of the MTL. This furthermore questions an important aspect of the MTL regarding whether such a relationship between time and space is constant across all situations and testing scenarios. The mechanisms of the leftward bias observed in the periphery may help answer some of these questions. It is beyond the scope of the current study, but we speculate that the leftward bias is the result of a different heuristic to process time information. For instance, studies on visual scanning report a left visual field bias such that the left side of the visual field is often the first to be inspected and for a longer viewing period (Butler et al., 2005; Guo, Meintz, Hall, Hall, & Mills, 2009; Mertens, Siegmund, & Grusser, 1993; Philips & David, 1997). Based on these findings, it might be possible that a mental left gaze was deployed to our stimuli presented at approximately 6–8°. The deployment subsequently could have resulted in a time dilation in two ways: (a) the deployment recruits greater attentional resources and that leads to the interpretation of time dilation (Zakay & Block, 1995); or, (b) the mental left gaze captures one’s attention to the left visual field and therefore delays a shift toward the right visual field. This can result in the observer missing the onset of a stimulus presented in the right hemispace, resulting in an underestimation of time compared to a stimulus presented in the left hemispace.
If it is true that leftward bias in the periphery relies on a different strategy, albeit with spatial basis, it would suggest then that time is not always represented on the mental timeline as posited by the MTL. Alternatively, we propose that we develop a set of heuristics, not just a single strategy, we ultimately defer to the method that is best suited under the specific circumstances, and this method doesn’t necessarily have to be the MTL. Moreover, it is possible that some of these procedures may even be spatial in nature, but it may not be specific to a mental number line. If it so happens that we do represent time according to the mental timeline, it would not necessarily suggest that time and space are innately bound. In this manner, it seems that it is not that time is naturally represented spatially, but it so happens that spatial component is one of the learned strategy associated with the well-practiced reading directionality. This interpretation is supported by the correlation between reading directionality and the MTL (Casasanto & Bottini, 2014) and by the results from Droit-Volet and Coull (2015) which offer that MTL is a developed process, rather than innate, as well as by dissociations observed in studies on episodic memory (e.g. Ekstrom et al., 2011).
Another working hypothesis in the current study is whether the MTL is applied toward centrally-located stimuli because these stimuli often are enriched with details compared to those in the periphery, and that the MTL could potentially serve as a strategy to simplify this. In Experiment 2, we employed a procedure where visual stimuli were physically located in the center, but were perceived to be diverged away from the central location. In this fashion, the level of details was held constant, but in one case the stimuli were perceived to be centered and in the other case they were perceived to be in the periphery. The rationale here is that if the MTL was specific to details, rather than location, then the rightward bias should have been observed in both spatial conditions. This is not what happened. It seemed that the MTL was specific to central location (by way of the presence of the rightward bias) despite the stimuli being equated in details, suggesting that the MTL may be elected as a strategy for this specific location. Future investigation could further explore the question regarding cognitive demand by manipulating the levels of details of the stimuli directly (e.g., enriched vs impoverished stimuli).
4.1. MTL and egocentric representation
The observation that the rightward bias is strongest in central space and declines as we move into the peripheral space may also serve as support that the MTL depends primarily on an egocentric mechanism for spatial representation. An egocentric representation relies on referencing the spatial information with respect to one’s own head, body, and eye positions such that the starting point is the self. An egocentric representation is observed in various navigational contexts (Ekstrom & Isham, 2017) and involves a representation biased toward a specific experienced or remembered directionality (Waller & Hodgson, 2006). Similarly, when reading, our eyes move from left to right, creating an egocentric bias in the left-to-right direction. Such bias in reading, along with the correlation between reading directionality and the MTL, collectively suggests the possibility that the MTL is also tied to an egocentric representation and is most prominent in the central space.
As we move further from the central space and into the periphery though, the egocentric bias may decline. It is possible that in this peripheral space, an alternative form of spatial representation is in use. It is unclear what this alternative method is, but one possibility is the allocentric representation. Differing from egocentric representation, an allocentric approach has two distinct attributes: the allocentric representation does not depend on updating one’s current position relative to a start point, and it is associated with coarser configural knowledge rather than direction-dependent higher resolution visual snapshots (Ekstrom & Isham, 2017; Waller & Hodgson, 2006). For stimuli perceived in the periphery, the brain may interpret the information as coarse, and it may be harder therefore to employ an egocentric based mechanism. While we cannot be sure of the exact alternative mechanism they do employ, an allocentric strategy would be inherently more flexible in terms of lacking a left to right bias. Future work will be needed to determine alternative strategies subjects might use to encode temporal intervals in the periphery.
4.2. Cortical and neural involvement
A series of studies have suggested that central and peripheral representations may be mediated differently beyond the visual cortex (Culham & Valyear, 2006; Malach, Levy, & Hasson, 2002; Rosenholtz, 2016). The difference in central versus peripheral processes may speak to the neural mechanisms of time perception, and the right and leftward biases observed in our study. As noted in the introduction, there are alternative theories pertaining to the neural mechanisms for temporal interval estimation and some do not depend on the MTL and a parietal representation for temporal intervals (Akam & Kullmann, 2014; Buhusi & Meck, 2005; Gu et al., 2015; Kononowicz & van Rijn, 2015). As such, another intriguing possibility is that peripherally represented temporal intervals depend on mechanisms outside of the parietal cortex and the well-studied dorsal “where” pathway in vision (Mishkin, Ungerleider, & Macko, 1983). Further investigations on what neural mechanisms might underlie temporal interval representation in central versus peripheral space would better our understanding of the current results.
4.3. Contributions to the retinal eccentricity literature
While our primary goal of the current study was to examine the MTL, the current results also add to the retinal eccentricity literature and how retinal location is related to time perception. Within the visual eccentricity literature, there is a discrepancy on whether the duration is perceived to increase or decrease as the stimulus location is shifted further in the periphery (e.g., see Kliegl & Huckauf, 2014; Long & Beaton, 1981). Consistent with previous observations by Long and Beaton (1981), the results from Experiment 1 show an overall increase in the perceived duration as retinal eccentricity increased. As mentioned earlier, the observed parabolic function could be due to the difference in the detection rate and accuracy in central and peripheral vision. The shorter perceived duration in central vision could reflect accuracy whereas the longer perceived duration in the periphery reflected the lower acuity, which in turn could be attributed to the lower resolution. In support of this speculation, Pease and Sticht (1965) observed different response times to visual stimuli presented in fovea and peripheral vision. In central vision, the response time to the onset and offset of the stimulus did not differ. In contrast, the response time was longer for the offset than the onset for peripheral stimuli. Thus, it is possible that the delayed response to the peripheral offset adds to the increasing perceived duration. Future investigation may wish to consider these possibilities, along with a more inclusive experimental design that include other sensory modalities beyond vision. Moreover, different timescales beyond the supraseconds should also be investigated.
4.4. Final remarks
The MTL is a prominent theory relating time with spatial information (e.g., Frassinetti et al., 2009). However, our results suggest that this spatio-temporal representation is specific to central visual space. The observation of the leftward bias in the periphery further suggests the possibility that a different set of mechanisms is responsible for the processing of peripheral stimuli, making the MTL not generalizable across spatial locations. Our findings advocate for the perspective that the MTL is strategy, amongst other spatially-based heuristics, developed to process temporal information, but the MTL is quite limited and applied only to stimuli perceived to be centrally-located.
Supplementary Material
Acknowledgments
The authors would like to thank Joy Geng, Bill Prinzmetal and Bruce Bridgeman for helpful discussions. We also would like to thank the following research assistants for experimental preparations and data collection: Hunter Trice, Ben Emerzian, Aimee Lynch, Krystal Wulf, Bevy John, and Tsz Ho Hui. The study was funded in part by UC Davis Center for Educational Effectiveness.
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nlm.2017.12.006.
References
- Aedo-Jury F, Pins D. Time compression increases with eccentricity: A magnocellular property. NeuroReport. 2010;21:84–89. doi: 10.1097/WNR.0b013e3283308d57. [DOI] [PubMed] [Google Scholar]
- Akam T, Kullmann DM. Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nature Reviews Neuroscience. 2014;15(2):111–122. doi: 10.1038/nrn3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli F, Ciaramelli E, Arzy S, Frassinetti F. Prisms to travel in time: Investigation of time-space association through prismatic adaptation effect on mental time travel. Cognition. 2016;156:1–5. doi: 10.1016/j.cognition.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Bonato M, Zorzi M, Umilta C. When time is space: Evidence for a mental time line. Neuroscience & Biobehavioral Reviews. 2012;36:2257–2273. doi: 10.1016/j.neubiorev.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Bottini R, Crepaldi D, Casasanto D, Crollen V, Collignon O. Space and time in the sighted and blind. Cognition. 2015;141:67–72. doi: 10.1016/j.cognition.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Butler S, Gilchrist ID, Burt DM, Perrett DI, Jones E, Harvey M. Are the perceptual biases found in chimeric face processing reflected in eye-movement patterns? Neuropsychologia. 2005;43:52–59. doi: 10.1016/j.neuropsychologia.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Casasanto D, Bottini R. Mirror-reading can reverse the flow of time. Journal of Experimental Psychology: General. 2014;143:473–479. doi: 10.1037/a0033297. [DOI] [PubMed] [Google Scholar]
- Casasanto D, Jasmin K. The hands of time: Temporal gestures in English speakers. Cognitive Linguistics. 2012;23:643–674. [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16(2):205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Di Bono MG, Casarotti M, Priftis K, Gava L, Umilta C, Zorzi M. Priming the mental time line. Journal of Experimental Psychology: Human Perception and Performance. 2012;38:838–842. doi: 10.1037/a0028346. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Coull JT. The developmental emergence of the mental time-line: Spatial and numerical distortion of time judgement. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage, 2011. 2011:18. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Isham EA. Human spatial navigation: Representations across dimensions and scales. Current Opinion in Behavioral Sciences. 2017;17:84–89. doi: 10.1016/j.cobeha.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassinetti F, Magnani B, Oliveri M. Prismatic lenses shift time perception. Psychological Science. 2009;20(8):949–954. doi: 10.1111/j.1467-9280.2009.02390.x. [DOI] [PubMed] [Google Scholar]
- Fuhrman O, Boroditsky L. Cross-cultural differences in mental representations of time: Evidence from an implicit nonlinguistic task. Cognitive Science. 2010;34:1430–1451. doi: 10.1111/j.1551-6709.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- Gauthier B, van Wassenhove V. Cognitive mapping in mental time travel and mental space navigation. Cognition. 2016;154:55–68. doi: 10.1016/j.cognition.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Gu BM, van Rijn H, Meck WH. Oscillatory multiplexing of neural population codes for interval timing and working memory. Neuroscience & Biobehavioral Reviews. 2015;48:160–185. doi: 10.1016/j.neubiorev.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Guo K, Meintz K, Hall C, Hall S, Mills D. Left gaze bias in humans, rhesus monkeys and domestic dogs. Animal Cognition. 2009;12:409–418. doi: 10.1007/s10071-008-0199-3. [DOI] [PubMed] [Google Scholar]
- Ishihara M, Keller P, Rossetti Y, Prinz W. Horizontal spatial representations of time: Evidence for the STEARC effect. Cortex. 2008;44:454–461. doi: 10.1016/j.cortex.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kliegl K, Huckauf A. Perceived duration decreases with increasing eccentricity. Acta Psychologica. 2014;150:136–145. doi: 10.1016/j.actpsy.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Kononowicz TW, van Rijn H. Single trial beta oscillations index time estimation. Neuropsychologia. 2015;75:381–389. doi: 10.1016/j.neuropsychologia.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: Time versus path integration. Neuron. 2013;78(6):1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G, Beaton R. The effects of stimulus numerosity, retinal location, and rod contrast on perceived duration of brief visual stimuli. Perception and Psychophysics. 1981;29(4):389–394. doi: 10.3758/bf03207349. [DOI] [PubMed] [Google Scholar]
- Magnani B, Pavani F, Frassinetti F. Changing auditory time with prismatic goggles. Cognition. 2012;125(2):233–243. doi: 10.1016/j.cognition.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends in Cognitive Sciences. 2002;6(4):176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Mertens I, Siegmund H, Grusser OJ. Gaze motor asymmetries in the perception of faces during a memory task. Neuropsychologia. 1993;31:989–998. doi: 10.1016/0028-3932(93)90154-r. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. Trends in Neurosciences. 1983;6:414–417. doi: 10.1016/0166-2236(83)90190-x. [DOI] [Google Scholar]
- Oliveri M, Koch G, Salerno S, Torriero S, Gerfo EL, Caltagirone C. Representation of time intervals in the right posterior parietal cortex: Implications for a mental time line. NeuroImage. 2009;15:1173–1179. doi: 10.1016/j.neuroimage.2009.03.042. [DOI] [PubMed] [Google Scholar]
- Pease V, Sticht T. Reaction time as a function of onset and offset stimulation of the fovea and periphery. Perceptual and Motor Skills. 1965;20:549–554. doi: 10.2466/pms.1965.20.2.549. [DOI] [PubMed] [Google Scholar]
- Peer M, Salomon R, Goldberg I, Blanke O, Arzy S. Brain system for mental orientation in space, time, and person. PNAS. 2015;112(35):11072–11077. doi: 10.1073/pnas.1504242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips ML, David AS. Viewing strategies for simple and chimeric faces: An investigation of perceptual bias in normal and schizophrenic patients using visual scan paths. Brain and Cognition. 1997;32:225–238. doi: 10.1006/brcg.1997.0939. [DOI] [PubMed] [Google Scholar]
- Rosenholtz R. Capabilities and limitations of peripheral vision. Annual Review of Vision Science. 2016;2(2):437–457. doi: 10.1146/annurev-vision-082114-035733. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Binns MA, Shallice T. Spatial temporal association of response codes effect: Understanding cognitive representation of time. Cognition. 2008 doi: 10.1016/j.cognition.2007.10.011. [DOI] [PubMed]
- Vass LK, Copara MS, Seyal M, Shahlaie K, Farias ST, Shen PY, Ekstrom AD. Oscillations go the distance: Low-frequency human hippocampal oscillations code spatial distance in the absence of sensory cues during teleportation. Neuron. 2016;89(6):1180–1186. doi: 10.1016/j.neuron.2016.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario CM, Caltagirone C, Oliveri M. Optokinetic stimulation affects temporal estimation in healthy humans. Brain and Cognition. 2007;39:1317–1328. doi: 10.1016/j.bandc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Vicario CM, Martino D, Pavone EF, Fuggetta G. Lateral head turning affects temporal memory. Perceptual and Motor Skills. 2011;113:3–10. doi: 10.2466/04.22.PMS.113.4.3-10. [DOI] [PubMed] [Google Scholar]
- Vicario CM, Pecoraro P, Turriziani P, Koch G, Caltagirone C, Oliveri M. Relativistic compression and expansion of experiential time in the left and right space. PlosONE. 2008 doi: 10.1371/journal.pone.0001716. [DOI] [PMC free article] [PubMed]
- Vuilleumier P, Valenza N, Mayer E, Reverdin A, Landis T. Near and far visual space in unilateral neglect. Annals of Neurology. 1998;4:406–410. doi: 10.1002/ana.410430324. [DOI] [PubMed] [Google Scholar]
- Waller D, Hodgson E. Transient and enduring spatial representations under disorientation and self-rotation. Journal of Experimental Psychology Learning, Memory, and Cognition. 2006;32(4):867–882. doi: 10.1037/0278-7393.32.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V. A theory of magnitude: Common cortical metrics of time, space and quantity. Trends in Cognitive Science. 2003;7(11):483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Temporal order detection for foveal and peripheral visual stimuli. Vision Research. 1983;23(8):759–763. doi: 10.1016/0042-6989(83)90197-9. [DOI] [PubMed] [Google Scholar]
- Zach P, Brugger P. Subjective time in near and far representational space. Cognitive Behavioral Neurology. 2008;21:8–13. doi: 10.1097/WNN.0b013e31815f237c. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block RA. An attentional-gate model of prospective time estimation. In: Richelle V, De Keyser G, d’Ydewalle S, Vandierendonck A, editors. Time and the dynamic control of behaviour. Liege, Belgium: Universite de Liege; 1995. pp. 167–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


