Abstract
The composition and mechanical properties of the extracellular matrix are dramatically altered during the development and progression of pulmonary fibrosis. Recent evidence indicates that these changes in matrix composition and mechanics are not only end-results of fibrotic remodeling, but active participants in driving disease progression. These insights have stimulated interest in identifying the components and physical aspects of the matrix that contribute to cell activation and disease initiation and progression. This review summarizes current knowledge regarding the biomechanics and dynamics of the ECM in mouse models and human IPF, and discusses how matrix mechanical and compositional changes might be non-invasively assessed, therapeutically targeted, and biologically restored to resolve fibrosis.
Homeostasis in Lung Extracellular Matrix Composition and Mechanics
The lung’s extracellular matrix (ECM) plays important roles in lung health from the earliest stages of development and throughout adulthood. For example, during lung development fibronectin deposition is essential to branching morphogenesis in utero (1), and elastin deposition pivotal in alveologenesis (2). Reciprocal interactions between epithelium and mesenchymal compartments are essential in forming the ECM scaffold that serves the local physical and biochemical needs of the developing tissue (3–5). Beyond these early dynamic phases of lung development, the lung continues to mature and eventually reaches a stable state of homeostasis. Importantly, homeostasis does not imply the absence of ongoing matrix synthesis; rather, it reflects the relative balance between synthesis, deposition, degradation and clearance of matrix components (Figure 1). Such dynamic balance is emphasized by the observation that both rapid turnover and stable collagen pools are present in the lung (6, 7), with rapid turnover pools likely representing collagen that is degraded without ever being deposited. While the rationale for such collagen turnover can only currently be speculated at, it may provide a primed system ready to respond rapidly to injury, reflecting the dependence of the lung on ECM for its integrity and function in conducting gases to and from the delicate alveolar-capillary interface. The balance between matrix synthesis and degradation in the healthy lung reflects a homeostatic system maintained by a vast array of pathways, proteases, anti-proteases (3, 4, 8, 9), and must include feedback mechanisms by which cells sense the need for shifts in the balance to accommodate system perturbations. The control system for matrix homeostasis remains far beyond our current understanding, but important elements include cell-matrix adhesion signaling to interpret cues regarding the content and mechanical properties of the matrix (10), as well as cell signaling mechanisms responsive to proteolytic matrix products that reflect matrix degradation (11, 12). A major question in the field is the nature of the defects in this homeostatic feedback system that allow the lung to escape from balance and undergo chronic and progressive fibrotic and degenerative remodeling processes.
Figure 1.
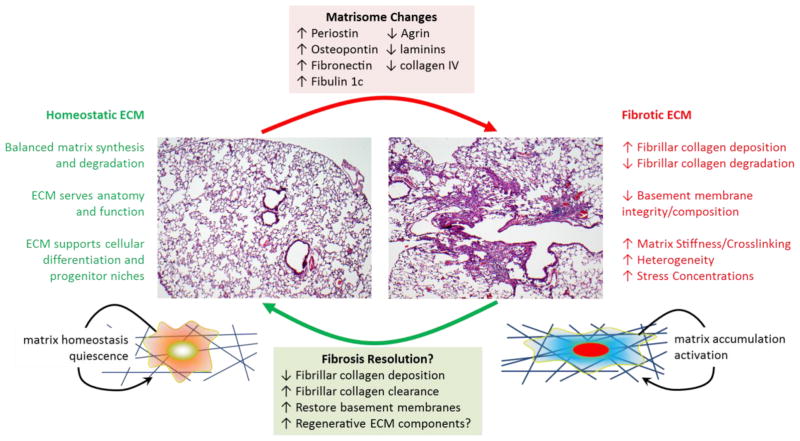
Summary of ECM biochemical and biomechanical changes that accompany the shift from a homeostatic ECM present in normal adult lung tissue to a fibrosis promoting ECM present in individuals with pulmonary fibrosis. The hematoxylin and eosin stained histological images shown are from control and bleomycin exposed mouse lungs, and emphasize the heterogeneous appearance of fibrotic remodeling often observed in both mouse models and human pulmonary fibrosis.
The composition, architecture and mechanical properties of the extracellular matrix are not uniform across the lung. Rather, they are adapted to the physiological and biomechanical requirements that the matrix must serve within anatomical compartments. For example, the alveolar compartment includes bands of collagen and elastin in alveolar ducts (13) that likely bear stresses and maintain structural stability (14), while the alveolar walls themselves exhibit shared basement membranes reflecting the need for close apposition of endothelial and epithelial barriers to support efficient gas exchange (15). The conducting airways (16) and vessels (17) of the lung exhibit more pronounced fibrous ECM structures of organized collagen and elastin, with the vascular structures in particular demonstrating variations in wall composition and thickness that reflect the position of the vessel within the vascular tree, and hence the decreasing pressures to which it is exposed during the cardiac cycle as the pulmonary vascular pressure decreases while transiting across the arteries, capillaries and veins (17). These differences in ECM architecture are paralleled by variations in mechanical properties across the anatomical compartments of the lung, including the airways (18), vessels (19), alveoli, and pleura (20–22), with the airways and vessels demonstrating the highest elastic moduli, followed by the pleura and finally the alveolar parenchyma. The cellular programs controlling local ECM composition remain to be defined, and may be key to understanding the local ECM signaling environments present in distinct anatomical compartments of the lung, and how these environments are perturbed during lung injury, repair, and fibrosis.
Pulmonary Fibrosis Effects on ECM Composition
Fibrillar collagen deposition is the dominant architectural feature of human lung fibrosis. However, the application of proteomic approaches has opened a new perspective by defining in broader terms the lung’s matrisome, those proteins composing or tightly associated with the extracellular matrix compartment (23–26). These proteomic approaches have begun to define how lung injury and fibrosis globally influences ECM composition. Early ECM proteomic efforts assessed the matrix composition of the lungs from humans who were asymptomatic or diagnosed with idiopathic pulmonary fibrosis (IPF) (27). More recently, this general approach has been applied in a comprehensive fashion to characterize the changing composition of the murine lung matrisome during the imitation, progression and resolution of bleomycin-induced lung injury, fibrosis and repair (28). Further stimulating efforts to decode the cues present in the ECM was evidence that the decellularized ECM of the fibrotic lung is itself a dominant signal that promotes a matrix synthetic phenotype in naïve cells, including enhanced translation of collagen encoding genes, whether cells originated from healthy or fibrotic lungs (29). While powerful in the scope of information provided, the proteomic characterization of the matrisome still must be regarded as largely a hypothesis generating tool. Rigorous follow up work is required to determine the localization of such matrisome components within the lung architecture and anatomical compartments, and to understand how the heterogeneous disease process of fibrosis alters ECM composition and distribution. Tissue clearance methodologies and multiplex analyses may open the door to adding spatial information crucial to understanding how local ECM cues are distributed in normal and pathological ECM (30). Beyond such descriptive work, functional loss and gain of function studies are necessary to determine if the matrisome components contribute to cellular or tissue level dysfunction. Alternatively, it must be considered that some changes in both murine models and human disease are compensatory in nature and essential to the survival of the host. Detailed mechanistic studies have begun to reveal matrisome components that may contribute to the pathologic progression of pulmonary fibrosis. These candidates include fibulin 1c (31–34), fibronectin (35, 36), osteopontin (37, 38), and periostin (39, 40), with evidence pointing toward enhanced expression of each in animal models or human IPF, and potential for each to augment cellular activation and fibrosis. Further work is needed to define whether tractable strategies can be developed to interrupt the signaling cues derived from such matrix components, with the obvious challenges that therapeutics may need to cross epithelial or endothelial barriers in order to reach their interstitial targets, and that such ECM targets may also be expressed constitutively in areas of normal tissue.
An interesting aspect of the matrisome that has not received as much attention is the components that may be lost as a function of pathologic tissue remodeling. Loss of basement membrane integrity and continuity has been suggested as a “point of no return” that separates reversible lung injury from pathological remodeling (41). Consistent with this notion, early proteomic analyses of human IPF matrices demonstrated loss of alveolar basement membrane components (27), including multiple laminin α, β and γ subunits, as has a more recent analysis of lung ECM from subjects with systemic sclerosis-related interstitial lung disease (42). Modeling pulmonary injury and fibrosis with bleomycin also appears to generate a loss of basement membrane components during the injury phase, while genetic deletion of basement membrane component α3 laminin in the lung worsens bleomycin-induced fibrosis (43). Interestingly, expression of basement membrane components appears to be regained at later time points as the fibrosis resolves, suggesting that restoration of basement membrane is key to resolution (28) (Figure 1). The contrasting fate of the mouse model, which exhibits spontaneous reversibility, and IPF, which while highly variable in its course but generally progresses without improvement, remains an unresolved mystery. Whether there are specific components or features of the ECM that promote resolution with their return in mouse models remains very much a possibility. An intriguing development from the field of cardiac regeneration lends some support to the possibility that ECM can promote regenerative healing. The ECM protein agrin was recently described as a component of the neonatal but not adult heart ECM; re-exposure of the adult heart to this matrix component engaged adult cardiomyocyte proliferation and promoted regenerative cardiac healing (44, 45), suggesting that developmental cues residing in the matrix may have important implications for tissue repair and regeneration. Intriguingly, agrin was found to be lost from the ECM in both IPF and systemic sclerosis affected lungs (27, 42). Along these same lines, a recent study of neonatal and adult lung matrices identified fibrillin-2 and tenascin-C as enriched in enriched in neonatal lung ECM, and as supportive of lung epithelial stem cell expansion (46). Together these studies implicate ECM composition in tissue regeneration, and suggest that identification of ECM components that are missing when injury transitions from normal tissue to pathological fibrosis may identify important ECM components essential to non-scarring resolution of lung injury.
Pulmonary Fibrosis Effects on Lung Mechanics
Pulmonary mechanics in IPF are typically assessed non-invasively using pulmonary function testing, such as by measurement of forced vital capacity (FVC), and changes in this metric have been used for evaluation of experimental therapies (47). Reductions in FVC reflect the reduced compliance (increased elastance) of the respiratory system. Such changes can occur through changes in lung tissue mechanics, surface tension, or chest wall mechanics, though in IPF such changes are typically ascribed to changes tissue mechanics. Pathological remodeling of the ECM in IPF is heterogeneously distributed, with varying distribution and proportion of normal and fibrotic appearing lung tissue (Figure 1). Hence global functional assessments can fail to capture the full extent of localized mechanical changes. Recently, invasive micro-scale mechanical characterization of lung tissue by atomic force microscopy has been used to assess the degree of tissue and ECM stiffening in human IPF. General agreement across multiple labs and publications demonstrates the highly compliant nature of normal lung tissue and the highly heterogeneous increases in Young’s modulus (more commonly referred to as stiffness) in IPF tissue, spanning a median of ~1.6 kiloPascals (kPa) in normal lungs to a median of ~16 kPa in IPF tissues, with localized increases to 50–100 kPa (20, 27). Comparison of intact and decellularized IPF matrices demonstrate minimal differences, suggesting that the bulk of the changes in tissue stiffness are accounted for by alterations in the ECM (27). Interestingly, the murine bleomycin model, which generates fibrosis within 14–21 days, is largely able to recapitulate the scope of changes in ECM stiffness seen in human IPF, though without the extremes of very high localized stiffness changes (48, 49). It should be noted that such measurements are not without limitations, as they require isolation of the lung tissue from its physiologic pre-stressed and perfused condition, and inherently disturb the surface tension forces present in the normal lung (50). Nevertheless, this approach provides the most detailed picture of local changes in the biomechanical properties of the lung ECM that occurs in IPF.
Measuring Lung Matrix Composition and Mechanics Non-Invasively
One of the major limitations in studying ECM composition and mechanics in pulmonary fibrosis is the inability to assess changes non-invasively. This challenge becomes particularly limiting when therapeutic interventions are being evaluated, especially those intended to directly target the ECM. Moreover, given the highly heterogeneous nature of IPF tissue destruction, non-invasive but organ wide assessments of lung ECM could be extremely valuable in assessing disease progression, and ultimately regression.
In the realm of non-invasive ECM imaging, recent efforts have described a number of approaches that may provide insight into matrix deposition, accumulation and degradation. To detect matrix deposition, the major focus has thus far been on detection of fibrillar type I collagen deposition. Molecular probes for detection of type I collagen by magnetic resonance (MR) imaging after intravenous injection have been described and validated to correlate with hydroxyproline levels in a mouse bleomycin model (51). Translating this approach to positron emission tomography (PET), a peptide-based probe (68Ga-CBP8) that targets collagen type I has also been described and validated for high target specificity and correlation with hydroxyproline levels, and also shows utility in tracking a response to therapy (52). To assess active collagen formation and cross-linking, novel MR probes that react with allysine, a necessary precursor of collagen crosslinks, have been described and demonstrated to sensitively detect collagen deposition and therapeutic response (53, 54). On the matrix degradation side, an activatable fluorescent probe that detects matrix metalloprotease activity has been developed and tested in a murine bleomycin model and can detect alterations in MMP activity that correlate with fibrosis status post-treatment (55). Similarly, a small molecule probe for the cysteine cathepsins, intracellular proteases implicated in fibrosis, has also been described and tested in a bleomycin model, and can identify areas of active macrophages within human pulmonary fibrotic tissue using clinical PET imaging (56). Together these studies open new prospects for non-invasively monitoring ECM deposition and degradation.
Non-invasive measurements of lung ECM mechanics are also undergoing intensive investigation, primarily using ultrasound and MR modalities. These approaches are challenged by the inherently weak imaging signal the lung provides due to its normally air-filled architecture. Ultrasound imaging has been applied in two approaches to characterize alterations in lung mechanics at the lung’s pleural surface, taking advantage of the predominant fibrosis formation at these sites and the limited depth of lung imaging available with ultrasound. Ultrasound measurements on the pleural surface appear capable of distinguishing mechanical strains occurring during respiration, and a proof of concept study in an experimental fibrosis model demonstrated the capacity of this method to detect reduced local compliance of the fibrotic lung (57). Lung ultrasound surface wave elastography generates waves on the lung surface from a local harmonic vibration excitation on the chest. The resulting surface wave propagation on the lung is detected using an ultrasound probe through the intercostal space (58). The wave speed in tissue is directly proportional to its elastic modulus, and this method also appear capable of discriminating different wave speeds in healthy subjects and those with interstitial lung disease (59).
A more global assessment of lung tissue mechanics may be made using magnetic resonance elastography. This approach uses MR imaging to detect the propagation of externally applied mechanical deformations through the tissue, and has already demonstrated substantial practical utility in the assessment of liver fibrosis (60). Overcoming the inherent MR imaging and motion issues with the lung has proven challenging, but a series of studies have now validated MR elastography approaches to assess lung mechanics non-invasively in animal models and human tissue (61–63). In a recent study the suitability of this approach for non-invasively assessing lung mechanics in IPF patients was validated (64). Interestingly, this study documented the non-linear mechanical properties of the lung, with substantial increases in tissue stiffness noted at total lung capacity (at end of maximal inspiration) versus residual volume (at end of maximal expiration). Also of note, the range of elastic modulus measured in normal and IPF subjects was similar to prior AFM studies (20, 27), though with lower mean and maximum levels, likely reflecting the heterogeneity in the disease spatial distribution and the larger spatial averaging performed in the MR (mm scale) vs AFM (μm scale) imaging modalities. Further development of this methodology may provide clinically relevant assessments of local changes in lung tissue mechanics relevant to the study of disease progression and experimental assessments of the efficacy of therapeutic strategies. Given the heterogeneous nature of IPF, the addition of such non-invasive and organ-wide mechanical measurements could be a powerful addition to existing pulmonary function testing and non-mechanical imaging modalities.
How Do ECM Alterations Contribute to IPF Progression?
While progressive changes in lung ECM and respiratory mechanics have long been associated with pulmonary fibrosis, only recently has it been discovered that the biochemical and mechanical changes in the ECM may contribute to disease progression. Studies with decelluarized matrices from IPF lungs (29, 65), and reductionists approaches using hydrogels (49, 66–68) have been instrumental in demonstrating key effects of ECM composition and mechanics on the activation state of lung fibroblasts, the drivers of fibrotic matrix deposition. We briefly review the effects of ECM composition and mechanics on lung fibroblasts and other lung resident cell types that may support fibrogenesis, and then consider a dynamic mechanism by which alterations in lung mechanical properties may propagate injury and fibrosis progression.
As discussed above, injury and fibrosis profoundly alter the composition of ECM and matrix-associated proteins in the lung (28). Decellularized IPF lung matrices promote fibroblast expression of alpha-smooth muscle actin (27), and engage a pro-fibrotic program of ECM gene expression via suppression of miR-29 (29) (Figure 1). Whether these responses reflect alterations in matrix-associated pro-fibrotic growth factors, such as TGF-beta or CTGF, signaling from matrix components themselves (69), or some combination of biochemical and biophysical stimuli remains to be determined, though in the case of Booth et al. the effect appeared to be independent of matrix-associated TGF-beta (27). ECM effects on lung resident cells are not restricted to fibroblasts, as alveolar epithelial cells respond to matrix cues with suppressed expression of epithelial markers and enhanced expression of mesenchymal markers (48, 70). This process can contribute to injury and fibrosis through the generation of paracrine fibroblast-stimulating signals (71), as well as through impaired differentiation and function of the epithelium itself. The activation of mesenchymal gene expression in alveolar epithelial cells requires interactions with ECM proteins such as fibrin and fibronectin (48, 72), again reinforcing a potential role of altered cell-ECM interactions in cellular signaling and the progression of fibrosis.
Beyond the compositional changes to the ECM, the mechanical properties of the ECM also clearly influence resident cell functions in support of fibrosis. The detailed mechanisms by which matrix stiffness signals to fibroblasts have been reviewed elsewhere (69, 73), and will thus only be briefly summarized here. Cell matrix interactions occur through integrin-based adhesions (74), which assemble as multiprotein focal adhesions structures that exhibit innate mechanosensitivity (75). Multiple integrins, including α6(76), αvβ6 (77, 78), αvβ1 and αv integrins more generally (79, 80) have been implicated in fibrosis, and substantial potential has been shown for strategies targeting these integrin to ameliorate fibrosis in experimental model systems. Integrin-mediated mechanosensitivity is thought to arise through the competing and reversible dynamics of integrin adhesion, talin recruitment, and talin-actin interactions conferring matrix stiffness sensitivity (81). As scaffolding for a host of adhesome proteins and signaling pathways, mechanosensitive assembly and recruitment of proteins into these complexes, with additional force dependent effects on protein structure and activity, generates intracellular biochemical signals (74, 82, 83). Downstream integration of these signals confers mechanosensitive cellular activation via a number of transcriptional effectors, including prominent contributions from myocardin-related transcription factors (66) and the transcriptional co-factors YAP and TAZ (20, 84, 85), both individually and in combination (86, 87). Matrix stiffness and mechanical forces also directly deform the nucleus (88), and may mediate changes in gene expression via altered nuclear deformation (89), altered transport of transcriptional effectors across the nuclear membrane (90), or as a result of physical effects on nuclear chromatin state (91–93). Together, the matrix stiffness-sensitive activation of these transcriptional pathways is necessary and sufficient to promote fibroblast activation to a contractile, proliferative and matrix synthetic state (20, 66, 84, 85), and also contributes to epithelial and endothelial phenotypes that may influence lung injury and fibrosis (94–96). Myofibroblast contractility can further amplify cellular activation through matrix stiffness and contractile force-dependent effects on TGF-beta activation from the ECM (97). Together these signals generate robust mechanical signaling to sustain fibrogenic activation. Direct targeting of these pathways can override mechanical activation and restore fibroblast quiescence (20, 66, 68), while restoring mechanical properties to normal compliance can similarly inactivate cells (67, 98), opening both ECM mechanics and mechanical signaling as targets for therapeutic intervention.
A final mechanism to consider in linking ECM to the pathogenesis of pulmonary fibrosis is the effect of non-uniform lung injury on the distribution of stresses within the lung tissue. The heterogeneous distribution of injury and fibrosis within the lungs of individuals with pulmonary fibrosis is well known, and evidence from AFM and MRE studies support the mechanical heterogeneity of the fibrotic lung as well (20, 27, 64). The lung is a mechanically active tissue, with distention of the tissue inherent in its gas exchange function. The generation of non-uniform mechanical properties (i.e. locally stiff regions) within a compliant surrounding lung has been analyzed in other contexts, with demonstration that concentrations of high stress and deformation levels in such scenarios are localized at the interface between stiff and compliant regions (99). Lung tissue is known to be sensitive to large deformations which can disrupt epithelial and endothelial barrier function (100), and the generation of lung injury and fibrosis after exposure to excessive tidal deformation of the lung is also well known (101, 102). Vascular leak contributes to both lung injury and pulmonary fibrosis (103), and high stretch levels can activate TGF-beta from the lung ECM (104). Together, these observations suggest the possibility that localized high levels of mechanical stress arising from the tidal stretching of heterogeneous and closely apposed fibrotic and relatively normal lung tissues may contribute to ongoing local lung injury and pro-fibrotic signaling that propagates pulmonary fibrosis (Figure 1).
How might ECM mechanics and composition be restored?
The accumulating evidence that the ECM in pulmonary fibrosis contributes to the progression of the disease has sparked interest in therapeutically targeting the matrix. Initial efforts have focused on ECM crosslinking, with the rationale that crosslinking increases the resistance of the ECM to proteolytic degradation and also contributes to increased matrix stiffness. Identification of the collagen crosslinking lysyl oxidase family member LOXL2 as a key enzyme in pathological matrix deposition led to development of an antibody-based therapeutic approach that, while promising in pre-clinical models (105), was ineffective in a clinical trial for IPF (106). Nevertheless, preclinical efficacy of LOXL2 and LOX inhibitory strategies across a number of tissue contexts continues to stimulate interest (107–110), including the development of small molecule LOXL2 inhibitors (111).
While inhibiting ECM deposition and crosslinking is a reasonable goal to slow or arrest progression of fibrosis, a more ambitious and potentially more clinically meaningful goal is to degrade and clear the fibrotic matrix (112), and ultimately restore the ECM to a state that better supports homeostasis and organ function (Figure 1). While the reversibility of fibrosis in IPF remains very much in question, particularly in advanced disease, abundant evidence from animal models, and from fibroproliferative human lung injury, now support the concept that fibrotic ECM deposition in the lung can be reversed (113, 114). Based on the heterogeneity of the fibrotic lung and the essential role of the ECM in supporting tissue integrity and mechanical function, it is difficult to envision a direct and organ-wide proteolytic strategy that could be therapeutically effective. Rather, the focus has been on identifying the cellular and molecular mechanisms that locally degrade and clear ECM proteins, with the goal of promoting these processes in support of resolving fibrosis.
Matrix metalloproteases are the major class of enzymes tasked with ECM degradation. However, these multi-functional proteins have multiple substrates that extend far beyond the ECM, and thus regulate cellular functions and pathobiology in diverse and sometimes unanticipated ways (3, 8, 115–117). Macrophages express a wide variety of MMPs, and have been shown to play a major role in collagen breakdown (118, 119), with a recently described pivotal role for MMP10 in directing macrophages toward a matrix degrading state (120). Further support for a functional role of macrophages in collagen turnover in the lung comes from a study of the protein Mfge8 (milk fat globule epidermal growth factor 8), a soluble glycoprotein shown to decrease the severity of pulmonary fibrosis by facilitating macrophage binding and targeting of collagen for cellular uptake (121).
While macrophage roles in fibrosis progression and resolution have been the focus of intense interest (122, 123), macrophages and fibroblasts can cooperate in managing matrix degradation and resorption (124), and fibroblast possess their own proteolytic machinery that can also contribute to matrix clearance. Cathepsin K is an intracellular protease unique among the cathepsins in its ability to degrade type I collagen (125). Cathepsin K knockout mice exhibit increased lung matrix deposition following bleomycin injury, and fibroblasts from cathepsin K deficient mice exhibit impaired collagenolytic activity, consistent with a role for fibroblast intracellular proteolysis in managing ECM homeostasis after lung injury (126) . Lung fibroblasts also possess potent extracellular collagenolytic activity through MMP14, also known as MT1-MMP (127). Fibroblast MMP14 is essential for fibrosis resolution in the dermis, and MMP14-deficient fibroblasts accumulate enhanced collagen type I deposition in vitro without increased de novo synthesis, pointing to a selective role in degradation (128). Expression of MMP14 and collagenolytic activity by lung fibroblasts decreases on matrices of increasing stiffness (49, 129), suggesting that matrix stiffness can impair fibroblast capacity to clear ECM, and that targeting the mechano-activation of fibroblasts may reverse this phenotype. MMP14 also plays an essential role in collagen phagocytosis into lysosomes for intracellular degradation (130). Linking extracellular proteolysis and intracellular degradation are surface scavenger receptors expressed by fibroblasts such as Endo180, also known as uPARAP (urokinase plasminogen activator receptor-associated protein)(131–133). Loss of uPARAP in the lung enhances collagen deposition and stiffens the lungs after bleomycin injury, suggesting roles for fibroblast mediated collagen internalization in normal collagen clearance (131). Ongoing work continues to identify novel mediators of collagen internalization and degradation (134), potentially generating new avenues for therapeutic intervention. Despite the progress in understanding ECM degradation and clearance mechanism, significant questions remain to be resolved relating to the issues of cell-type and spatially and temporally appropriate protease activation before therapeutic ECM clearance might be realized. As a cautionary example, collagen degradation products in patient sera have been explored as IPF biomarkers, with increasing degradation products indicative of increased disease severity and progression (135), emphasizing the need to understand where, when, how and by which proteases matrix degradation may be beneficial in the setting of pulmonary fibrosis.
Beyond clearance of the fibrotic ECM, true tissue repair will require restoration of normal tissue architecture, requiring the re-generation of an ECM that supports homeostatic cell-cell and cell-matrix interactions. As discussed above, pulmonary fibrosis is associated with aberrant deposition of fibrillar collagens, and loss of epithelial and endothelial basement membrane components (Figure 1). In parallel with clearance of fibrotic scar, restoration of appropriate epithelial and endothelial basement membrane will likely be essential to regenerative healing (41). Restoration of basement membranes will support normal healing and function of these critical barriers, but also re-establish appropriate niches (136, 137) for resident progenitor cells such as Krt5+/p63+ basal-like cells (138, 139) , type II alveolar epithelial cells (140) and Integrin alpha-6+ lung stem cells (141, 142) required to sustain tissue homeostasis.
Key to efforts aimed at functional restoration of the ECM may be our growing understanding of the mesenchymal resident populations of the lung and their roles as matrix producing and paracrine signaling support cells. Multiple studies of lung organoids have demonstrated that co-culture of lung epithelial progenitors with lung-derived mesenchymal cells promotes clonal proliferation and tissue-specific epithelial differentiation (143–146). A prominent example is that of Pdgfra+ lung fibroblasts, which in organoid culture promote type II alveolar cell self-renewal and differentiation into type I alveolar cells (140), and which in vivo engage in dynamic and reciprocal epithelial-mesenchymal interactions critical for alveolar septation and compensatory lung growth (147). Efforts at lineage tracing mesenchymal populations during experimental lung injury and fibrosis have identified fibrosis-generating contributions from pericytes as well as resident fibroblasts (148), with further study demonstrating reversible differentiation of ECM producing myofibroblasts from lipofibroblasts (149, 150), a sub-population of interstitial cells thought to play critical roles during lung development (151, 152). Further studies have continued to refine these concepts, with the recent description of distinct Lgr5+ and Lgr6+ mesenchymal populations that support alveolar and airway compartments respectively (153), and the description of mesenchymal alveolar niche cells and Axin2+ myofibrogenic progenitor cells with contrasting roles in alveolar homeostasis versus scar formation after injury (154). Continued exploration of these mesenchymal cell subpopulations, their plasticity and control mechanisms, and the molecular networks by which they engage cell-cell interactions and deposit ECM will be critical in efforts to promote functional ECM restoration and resolution of fibrosis.
Conclusions
While much remains to be learned, it is increasingly clear from the studies summarized above that the ECM in pulmonary injury and fibrosis is an active contributor to cellular activation and fibrosis progression, and a potential barrier to effective resolution and repair. The ongoing development and refinement of tools to define local molecular scale changes in ECM and to non-invasively track alterations in ECM biochemical and biomechanical states offers new opportunities to delineate the cues and signals that drive disease, and to assess the utility of therapeutic approaches targeting the ECM. Further investigation of the basic mechanisms by which cells degrade and clear ECM, receive cues from the ECM that promote fibrogenesis, and engage in reciprocal interactions to deposit and manage a homeostatic ECM will dramatically enhance our understanding of lung injury and repair. Ultimately these efforts will be essential to our efforts to not only arrest, but also reverse fibrosis and restore lung function to individuals with pulmonary fibrosis.
Highlights.
ECM composition and mechanics are dramatically and dynamically altered during lung fibrosis.
Changes in the fibrotic lung ECM promote cell activation and fibrogenesis, prompting efforts to therapeutically target ECM-derived cues.
Novel techniques are enabling the dynamic state of the lung ECM to be quantified in vivo.
Tantalizing evidence of fibrosis reversibility supports targeting fibrotic scar clearance and ECM restoration.
Acknowledgments
Funding: NIH HL092961 (DJT), HL133320 (DJT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423(6942):876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 2.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res. 2005;57(5 Pt 2):38R–46R. doi: 10.1203/01.PDR.0000159630.35883.BE. [DOI] [PubMed] [Google Scholar]
- 3.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12) doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341(1):126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decaris ML, Gatmaitan M, FlorCruz S, Luo F, Li K, Holmes WE, Hellerstein MK, Turner SM, Emson CL. Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Mol Cell Proteomics. 2014;13(7):1741–52. doi: 10.1074/mcp.M113.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAnulty RJ, Laurent GJ. Collagen synthesis and degradation in vivo. Evidence for rapid rates of collagen turnover with extensive degradation of newly synthesized collagen in tissues of the adult rat. Coll Relat Res. 1987;7(2):93–104. doi: 10.1016/s0174-173x(87)80001-8. [DOI] [PubMed] [Google Scholar]
- 8.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44–46:113–21. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, Pardo A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L562–74. doi: 10.1152/ajplung.2000.279.3.L562. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–12. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40(6–7):1101–10. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. J Clin Invest. 2016;126(9):3176–84. doi: 10.1172/JCI83147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda M, Fung YC, Sobin SS. Collagen and elastin fibers in human pulmonary alveolar mouths and ducts. J Appl Physiol (1985) 1987;63(3):1185–94. doi: 10.1152/jappl.1987.63.3.1185. [DOI] [PubMed] [Google Scholar]
- 14.Wagner W, Bennett RD, Ackermann M, Ysasi AB, Belle J, Valenzuela CD, Pabst A, Tsuda A, Konerding MA, Mentzer SJ. Elastin Cables Define the Axial Connective Tissue System in the Murine Lung. Anat Rec (Hoboken) 2015;298(11):1960–8. doi: 10.1002/ar.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccaro CA, Brody JS. Structural features of alveolar wall basement membrane in the adult rat lung. J Cell Biol. 1981;91(2 Pt 1):427–37. doi: 10.1083/jcb.91.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol. 2016;240(4):397–409. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsley MI. Structure and composition of pulmonary arteries, capillaries, and veins. Compr Physiol. 2012;2(1):675–709. doi: 10.1002/cphy.c100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash YS, Kong H. Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2015;308(11):L1125–35. doi: 10.1152/ajplung.00154.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AM, Suarez Velandia MM, Vitali S, Colas RA, Norris PC, et al. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight. 2016;1(8) doi: 10.1172/jci.insight.86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L344–57. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luque T, Melo E, Garreta E, Cortiella J, Nichols J, Farre R, Navajas D. Local micromechanical properties of decellularized lung scaffolds measured with atomic force microscopy. Acta Biomater. 2013;9(6):6852–9. doi: 10.1016/j.actbio.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Melo E, Cardenes N, Garreta E, Luque T, Rojas M, Navajas D, Farre R. Inhomogeneity of local stiffness in the extracellular matrix scaffold of fibrotic mouse lungs. J Mech Behav Biomed Mater. 2014;37:186–95. doi: 10.1016/j.jmbbm.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50(1) doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- 24.Calle EA, Hill RC, Leiby KL, Le AV, Gard AL, Madri JA, Hansen KC, Niklason LE. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater. 2016;46:91–100. doi: 10.1016/j.actbio.2016.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the "omics" era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11(4):M111014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186(9):866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiller HB, Fernandez IE, Burgstaller G, Schaab C, Scheltema RA, Schwarzmayr T, Strom TM, Eickelberg O, Mann M. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol. 2015;11(7):819. doi: 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124(4):1622–35. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayorca-Guiliani AE, Madsen CD, Cox TR, Horton ER, Venning FA, Erler JT. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat Med. 2017;23(7):890–8. doi: 10.1038/nm.4352. [DOI] [PubMed] [Google Scholar]
- 31.Ge Q, Chen L, Jaffar J, Argraves WS, Twal WO, Hansbro P, Black JL, Burgess JK, Oliver B. Fibulin1C peptide induces cell attachment and extracellular matrix deposition in lung fibroblasts. Sci Rep. 2015;5(9496) doi: 10.1038/srep09496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffar J, Unger S, Corte TJ, Keller M, Wolters PJ, Richeldi L, Cerri S, Prele CM, Hansbro PM, Argraves WS, et al. Fibulin-1 predicts disease progression in patients with idiopathic pulmonary fibrosis. Chest. 2014;146(4):1055–63. doi: 10.1378/chest.13-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Cooley MA, Jarnicki AG, Hsu AC, Nair PM, Haw TJ, Fricker M, Gellatly SL, Kim RY, Inman MD, et al. Fibulin-1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight. 2016;1(9) doi: 10.1172/jci.insight.86380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Cooley MA, Nair PM, Donovan C, Hsu AC, Jarnicki AG, Haw TJ, Hansbro NG, Ge Q, Brown AC, et al. Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c. J Pathol. 2017 doi: 10.1002/path.4979. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, White ES, Varga J. FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci Transl Med. 2014;6(232):232ra50. doi: 10.1126/scitranslmed.3008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(6):638–45. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berman JS, Serlin D, Li X, Whitley G, Hayes J, Rishikof DC, Ricupero DA, Liaw L, Goetschkes M, O'Regan AW. Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1311–8. doi: 10.1152/ajplung.00394.2003. [DOI] [PubMed] [Google Scholar]
- 38.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2(9):e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1046–56. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37(5):1119–27. doi: 10.1183/09031936.00059810. [DOI] [PubMed] [Google Scholar]
- 41.Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc. 2008;5(3):305–10. doi: 10.1513/pats.200710-160DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun H, Zhu Y, Pan H, Chen X, Balestrini JL, Lam TT, Kanyo JE, Eichmann A, Gulati M, Fares WH, et al. Netrin-1 Regulates Fibrocyte Accumulation in the Decellularized Fibrotic Sclerodermatous Lung Microenvironment and in Bleomycin-Induced Pulmonary Fibrosis. Arthritis Rheumatol. 2016;68(5):1251–61. doi: 10.1002/art.39575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Nebreda LI, Rogel MR, Eisenberg JL, Hamill KJ, Soberanes S, Nigdelioglu R, Chi M, Cho T, Radigan KA, Ridge KM, et al. Lung-specific loss of alpha3 laminin worsens bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;52(4):503–12. doi: 10.1165/rcmb.2014-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547(7662):179–84. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, Lakshmanan M, Tergaonkar V, Lim CT, Hong W. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017;18(10):2464–79. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 46.Gilpin SE, Li Q, Evangelista-Leite D, Ren X, Reinhardt DP, Frey BL, Ott HC. Fibrillin-2 and Tenascin-C bridge the age gap in lung epithelial regeneration. Biomaterials. 2017;140:212–9. doi: 10.1016/j.biomaterials.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis--FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372(13):1189–91. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 48.Brown AC, Fiore VF, Sulchek TA, Barker TH. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J Pathol. 2013;229(1):25–35. doi: 10.1002/path.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190(4):693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp. 2011;(54) doi: 10.3791/2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caravan P, Yang Y, Zachariah R, Schmitt A, Mino-Kenudson M, Chen HH, Sosnovik DE, Dai G, Fuchs BC, Lanuti M. Molecular magnetic resonance imaging of pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 2013;49(6):1120–6. doi: 10.1165/rcmb.2013-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desogere P, Tapias LF, Hariri LP, Rotile NJ, Rietz TA, Probst CK, Blasi F, Day H, Mino-Kenudson M, Weinreb P, et al. Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci Transl Med. 2017;9(384) doi: 10.1126/scitranslmed.aaf4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waghorn PA, Jones CM, Rotile NJ, Koerner SK, Ferreira DS, Chen HH, Probst CK, Tager AM, Caravan P. Molecular Magnetic Resonance Imaging of Lung Fibrogenesis with an Oxyamine-Based Probe. Angew Chem Int Ed Engl. 2017;56(33):9825–8. doi: 10.1002/anie.201704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen HH, Waghorn PA, Wei L, Tapias LF, Schu Hle DT, Rotile NJ, Jones CM, Looby RJ, Zhao G, Elliott JM, et al. Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI Insight. 2017;2(11) doi: 10.1172/jci.insight.91506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai Y, Zhu L, Zhang F, Niu G, Lee S, Kimura S, Chen X. Noninvasive monitoring of pulmonary fibrosis by targeting matrix metalloproteinases (MMPs) Mol Pharm. 2013;10(6):2237–47. doi: 10.1021/mp300613x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Withana NP, Ma X, McGuire HM, Verdoes M, van der Linden WA, Ofori LO, Zhang R, Li H, Sanman LE, Wei K, et al. Non-invasive Imaging of Idiopathic Pulmonary Fibrosis Using Cathepsin Protease Probes. Sci Rep. 2016;6:19755. doi: 10.1038/srep19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubin JM, Horowitz JC, Sisson TH, Kim K, Ortiz LA, Hamilton JD. Ultrasound Strain Measurements for Evaluating Local Pulmonary Ventilation. Ultrasound Med Biol. 2016;42(11):2525–31. doi: 10.1016/j.ultrasmedbio.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Osborn T, Kalra S. A noninvasive ultrasound elastography technique for measuring surface waves on the lung. Ultrasonics. 2016;71:183–8. doi: 10.1016/j.ultras.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Osborn T, Zhou B, Meixner D, Kinnick RR, Bartholmai B, Greenleaf JF, Kalra S. Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64(9):1298–304. doi: 10.1109/TUFFC.2017.2707981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13(3):440–51e6. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mariappan YK, Glaser KJ, Levin DL, Vassallo R, Hubmayr RD, Mottram C, Ehman RL, McGee KP. Estimation of the absolute shear stiffness of human lung parenchyma using (1) H spin echo, echo planar MR elastography. J Magn Reson Imaging. 2014;40(5):1230–7. doi: 10.1002/jmri.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mariappan YK, Kolipaka A, Manduca A, Hubmayr RD, Ehman RL, Araoz P, McGee KP. Magnetic resonance elastography of the lung parenchyma in an in situ porcine model with a noninvasive mechanical driver: correlation of shear stiffness with trans-respiratory system pressures. Magn Reson Med. 2012;67(1):210–7. doi: 10.1002/mrm.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGee KP, Hubmayr RD, Levin D, Ehman RL. Feasibility of quantifying the mechanical properties of lung parenchyma in a small-animal model using (1)H magnetic resonance elastography (MRE) J Magn Reson Imaging. 2009;29(4):838–45. doi: 10.1002/jmri.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marinelli JP, Levin DL, Vassallo R, Carter RE, Hubmayr RD, Ehman RL, McGee KP. Quantitative assessment of lung stiffness in patients with interstitial lung disease using MR elastography. J Magn Reson Imaging. 2017;46(2):365–74. doi: 10.1002/jmri.25579. [DOI] [PubMed] [Google Scholar]
- 65.Southern BD, Grove LM, Rahaman SO, Abraham S, Scheraga RG, Niese KA, Sun H, Herzog EL, Liu F, Tschumperlin DJ, et al. Matrix-driven Myosin II Mediates the Pro-fibrotic Fibroblast Phenotype. J Biol Chem. 2016;291(12):6083–95. doi: 10.1074/jbc.M115.712380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47(3):340–8. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marinkovic A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2013;48(4):422–30. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123(3):1096–108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tschumperlin DJ. Matrix, mesenchyme, and mechanotransduction. Ann Am Thorac Soc. 2015;12(Suppl 1):S24–9. doi: 10.1513/AnnalsATS.201407-320MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119(1):213–24. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J, Wheeler SE, Velikoff M, Kleaveland KR, LaFemina MJ, Frank JA, Chapman HA, Christensen PJ, Kim KK. Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am J Pathol. 2013;183(5):1559–70. doi: 10.1016/j.ajpath.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tschumperlin DJ, Liu F, Tager AM. Biomechanical regulation of mesenchymal cell function. Curr Opin Rheumatol. 2013;25(1):92–100. doi: 10.1097/BOR.0b013e32835b13cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Z, Guo SS, Fassler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215(4):445–56. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 76.Chen H, Qu J, Huang X, Kurundkar A, Zhu L, Yang N, Venado A, Ding Q, Liu G, Antony VB, et al. Mechanosensing by the alpha6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat Commun. 2016;7:12564. doi: 10.1038/ncomms12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177(1):56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 78.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 79.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, Sheppard D. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7(288):288ra79. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Perez-Gonzalez C, Castro N, Zhu C, Trepat X, Roca-Cusachs P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol. 2016;18(5):540–8. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- 82.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13(4):383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schiller HB, Friedel CC, Boulegue C, Fassler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12(3):259–66. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jorgenson AJ, Choi KM, Sicard D, Smith KM, Hiemer SE, Varelas X, Tschumperlin DJ. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol. 2017;312(3):C277–C85. doi: 10.1152/ajpcell.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noguchi S, Saito A, Mikami Y, Urushiyama H, Horie M, Matsuzaki H, Takeshima H, Makita K, Miyashita N, Mitani A, et al. TAZ contributes to pulmonary fibrosis by activating profibrotic functions of lung fibroblasts. Sci Rep. 2017;7:42595. doi: 10.1038/srep42595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim T, Hwang D, Lee D, Kim JH, Kim SY, Lim DS. MRTF potentiates TEAD-YAP transcriptional activity causing metastasis. EMBO J. 2017;36(4):520–35. doi: 10.15252/embj.201695137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Speight P, Kofler M, Szaszi K, Kapus A. Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFbeta-regulated Smad3. Nat Commun. 2016;7:11642. doi: 10.1038/ncomms11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: From pathways to scaling relationships. J Cell Biol. 2017;216(2):305–15. doi: 10.1083/jcb.201610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alam SG, Zhang Q, Prasad N, Li Y, Chamala S, Kuchibhotla R, Kc B, Aggarwal V, Shrestha S, Jones AL, et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci Rep. 2016;6:38063. doi: 10.1038/srep38063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017 doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 91.Heo SJ, Thorpe SD, Driscoll TP, Duncan RL, Lee DA, Mauck RL. Biophysical Regulation of Chromatin Architecture Instills a Mechanical Memory in Mesenchymal Stem Cells. Sci Rep. 2015;5:16895. doi: 10.1038/srep16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le HQ, Ghatak S, Yeung CY, Tellkamp F, Gunschmann C, Dieterich C, Yeroslaviz A, Habermann B, Pombo A, Niessen CM, et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol. 2016;18(8):864–75. doi: 10.1038/ncb3387. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Chu JS, Kurpinski K, Li X, Bautista DM, Yang L, Sung KL, Li S. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys J. 2011;100(8):1902–9. doi: 10.1016/j.bpj.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126(9):3313–35. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim J, Kim YH, Kim J, Park DY, Bae H, Lee DH, Kim KH, Hong SP, Jang SP, Kubota Y, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017;127(9):3441–61. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426–36. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caliari SR, Perepelyuk M, Soulas EM, Lee GY, Wells RG, Burdick JA. Gradually softening hydrogels for modeling hepatic stellate cell behavior during fibrosis regression. Integr Biol (Camb) 2016;8(6):720–8. doi: 10.1039/c6ib00027d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol. 2009;297(2):H802–10. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol (1985) 2000;89(4):1645–55. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 101.Zhang R, Pan Y, Fanelli V, Wu S, Luo AA, Islam D, Han B, Mao P, Ghazarian M, Zeng W, et al. Mechanical Stress and the Induction of Lung Fibrosis via the Midkine Signaling Pathway. Am J Respir Crit Care Med. 2015;192(3):315–23. doi: 10.1164/rccm.201412-2326OC. [DOI] [PubMed] [Google Scholar]
- 102.Cabrera-Benitez NE, Laffey JG, Parotto M, Spieth PM, Villar J, Zhang H, Slutsky AS. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121(1):189–98. doi: 10.1097/ALN.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 104.Froese AR, Shimbori C, Bellaye PS, Inman M, Obex S, Fatima S, Jenkins G, Gauldie J, Ask K, Kolb M. Stretch-induced Activation of Transforming Growth Factor-beta1 in Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016;194(1):84–96. doi: 10.1164/rccm.201508-1638OC. [DOI] [PubMed] [Google Scholar]
- 105.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 106.Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, Lederer DJ, Martinez FJ, Noble PW, Song JW, et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med. 2017;5(1):22–32. doi: 10.1016/S2213-2600(16)30421-0. [DOI] [PubMed] [Google Scholar]
- 107.Ikenaga N, Peng ZW, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, Mikels-Vigdal A, Smith V, Schuppan D, Popov YV. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017 doi: 10.1136/gutjnl-2016-312473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu SB, Ikenaga N, Peng ZW, Sverdlov DY, Greenstein A, Smith V, Schuppan D, Popov Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016;30(4):1599–609. doi: 10.1096/fj.14-268425. [DOI] [PubMed] [Google Scholar]
- 109.Rosin NL, Sopel MJ, Falkenham A, Lee TD, Legare JF. Disruption of collagen homeostasis can reverse established age-related myocardial fibrosis. Am J Pathol. 2015;185(3):631–42. doi: 10.1016/j.ajpath.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 110.Yang J, Savvatis K, Kang JS, Fan P, Zhong H, Schwartz K, Barry V, Mikels-Vigdal A, Karpinski S, Kornyeyev D, et al. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat Commun. 2016;7:13710. doi: 10.1038/ncomms13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hutchinson JH, Rowbottom MW, Lonergan D, Darlington J, Prodanovich P, King CD, Evans JF, Bain G. Small Molecule Lysyl Oxidase-like 2 (LOXL2) Inhibitors: The Identification of an Inhibitor Selective for LOXL2 over LOX. ACS Med Chem Lett. 2017;8(4):423–7. doi: 10.1021/acsmedchemlett.7b00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(11):L709–21. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glasser SW, Hagood JS, Wong S, Taype CA, Madala SK, Hardie WD. Mechanisms of Lung Fibrosis Resolution. Am J Pathol. 2016;186(5):1066–77. doi: 10.1016/j.ajpath.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, Zhou Y, Wells RG, White ES, Tschumperlin DJ. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol. 2014;184(6):1643–51. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7(2):193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hendrix AY, Kheradmand F. The Role of Matrix Metalloproteinases in Development, Repair, and Destruction of the Lungs. Prog Mol Biol Transl Sci. 2017;148:1–29. doi: 10.1016/bs.pmbts.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 118.Madsen DH, Leonard D, Masedunskas A, Moyer A, Jurgensen HJ, Peters DE, Amornphimoltham P, Selvarj A, Yamada SS, Brenner DA, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. Journal of Cell Biology. 2013;202(6):951–66. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yahyouche A, Zhidao X, Czernuszka JT, Clover AJP. Macrophage-mediated degradation of crosslinked collagen scaffolds. Acta Biomaterialia. 2011;7(1):278–86. doi: 10.1016/j.actbio.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 120.Rohani MG, McMahan RS, Razumova MV, Hertz AL, Cieslewicz M, Pun SH, Regnier M, Wang Y, Birkland TP, Parks WC. MMP-10 Regulates Collagenolytic Activity of Alternatively Activated Resident Macrophages. J Invest Dermatol. 2015;135(10):2377–84. doi: 10.1038/jid.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119(12):3713–22. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214(8):2387–404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, Minowa Y, Fukushima K, Ebina I, Yoshioka Y, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541(7635):96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 124.Laub R, Huybrechtsgodin G, Peetersjoris C, Vaes G. Degradation of Collagen and Proteoglycan by Macrophages and Fibroblasts - Individual Potentialities of Each Cell Type and Cooperative Effects through the Activation of Fibroblasts by Macrophages. Biochim Biophys Acta. 1982;721(4):425–33. doi: 10.1016/0167-4889(82)90098-2. [DOI] [PubMed] [Google Scholar]
- 125.Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaisse JM. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. Journal of Biological Chemistry. 1998;273(48):32347–52. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 126.Buhling F, Rocken C, Brasch F, Hartig R, Yasuda Y, Saftig P, Bromme D, Welte T. Pivotal role of cathepsin K in lung fibrosis. Am J Pathol. 2004;164(6):2203–16. doi: 10.1016/S0002-9440(10)63777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rowe RG, Keena D, Sabeh F, Willis AL, Weiss SJ. Pulmonary fibroblasts mobilize the membrane-tethered matrix metalloprotease, MT1-MMP, to destructively remodel and invade interstitial type I collagen barriers. Am J Physiol Lung Cell Mol Physiol. 2011;301(5):L683–92. doi: 10.1152/ajplung.00187.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zigrino P, Brinckmann J, Niehoff A, Lu Y, Giebeler N, Eckes B, Kadler KE, Mauch C. Fibroblast-Derived MMP-14 Regulates Collagen Homeostasis in Adult Skin. J Invest Dermatol. 2016;136(8):1575–83. doi: 10.1016/j.jid.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gu Z, Liu F, Tonkova EA, Lee SY, Tschumperlin DJ, Brenner MB. Soft matrix is a natural stimulator for cellular invasiveness. Mol Biol Cell. 2014;25(4):457–69. doi: 10.1091/mbc.E13-05-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee H, Overall CM, McCulloch CA, Sodek J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol Biol Cell. 2006;17(11):4812–26. doi: 10.1091/mbc.E06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bundesmann MM, Wagner TE, Chow YH, Altemeier WA, Steinbach T, Schnapp LM. Role of urokinase plasminogen activator receptor-associated protein in mouse lung. Am J Respir Cell Mol Biol. 2012;46(2):233–9. doi: 10.1165/rcmb.2010-0485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, et al. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem. 2007;282(37):27037–45. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- 133.Madsen DH, Ingvarsen S, Jurgensen HJ, Melander MC, Kjoller L, Moyer A, Honore C, Madsen CA, Garred P, Burgdorf S, et al. The non-phagocytic route of collagen uptake: a distinct degradation pathway. J Biol Chem. 2011;286(30):26996–7010. doi: 10.1074/jbc.M110.208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee TH, McKleroy W, Khalifeh-Soltani A, Sakuma S, Lazarev S, Riento K, Nishimura SL, Nichols BJ, Atabai K. Functional genomic screen identifies novel mediators of collagen uptake. Molecular Biology of the Cell. 2014;25(5):583–93. doi: 10.1091/mbc.E13-07-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, Molyneaux PL, McKeever TM, Wells AU, Flynn A, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3(6):462–72. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 136.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506–19. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14(8):467–73. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 138.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517(7536):616–20. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147(3):525–38. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–36. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. The Journal of clinical investigation. 2011;121(7):2855–62. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chernaya O, Shinin V, Liu Y, Minshall RD. Behavioral heterogeneity of adult mouse lung epithelial progenitor cells. Stem Cells Dev. 2014;23(22):2744–57. doi: 10.1089/scd.2013.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tan Q, Choi KM, Sicard D, Tschumperlin DJ. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials. 2017;113:118–32. doi: 10.1016/j.biomaterials.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci U S A. 2014;111(35):E3641–9. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hegab AE, Arai D, Gao J, Kuroda A, Yasuda H, Ishii M, Naoki K, Soejima K, Betsuyaku T. Mimicking the niche of lung epithelial stem cells and characterization of several effectors of their in vitro behavior. Stem Cell Res. 2015;15(1):109–21. doi: 10.1016/j.scr.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 146.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107(4):1414–9. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor alpha expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol. 2012;47(4):517–27. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188(7):820–30. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li A, Ma S, Smith SM, Lee MK, Fischer A, Borok Z, Bellusci S, Li C, Minoo P. Mesodermal ALK5 controls lung myofibroblast versus lipofibroblast cell fate. BMC Biol. 2016;14:19. doi: 10.1186/s12915-016-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, Szibor M, Kosanovic D, Schwind F, Schermuly RT, et al. Two-Way Conversion between Lipogenic and Myogenic Fibroblastic Phenotypes Marks the Progression and Resolution of Lung Fibrosis. Cell Stem Cell. 2017;20(4):571. doi: 10.1016/j.stem.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 151.Ahlbrecht K, McGowan SE. In search of the elusive lipofibroblast in human lungs. Am J Physiol Lung Cell Mol Physiol. 2014;307(8):L605–8. doi: 10.1152/ajplung.00230.2014. [DOI] [PubMed] [Google Scholar]
- 152.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 153.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, et al. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell. 2017;170(6):1149–63. e12. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170(6):1134–48. e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]