Abstract
While offspring-to-parent living donor kidney transplantations may represent an ideal donor-recipient combination to optimize long-term transplant outcomes, the gender-specific long-term success of these transplants remains unclear. We hypothesize that allograft and recipient survival in offspring-to-parent living donor kidney transplantation differs between men and women due to donor-specific alloimmunization during pregnancy. We retrospectively analyzed long-term allograft and patient survival among men and women who received an offspring living donor kidney compared to those who received other haplotype-matched living donor kidneys. By multivariable Cox proportional hazards modeling of Organ Procurement and Transplantation Network data from 2001 to 2015, we found that both men and women who received offspring living donor kidneys had significantly increased mortality compared to recipients who received non-offspring living donor kidneys. While male recipients of any living donor kidney had greater risk of mortality and allograft failure compared to females, there was no significant difference in all-cause allograft failure or mortality in male versus female recipients of offspring living donor kidney transplantations. Our analysis demonstrated no significant interaction between recipient gender and donor offspring status. We conclude that non-offspring living donors should be considered whenever feasible for both men and women with multiple donor options.
INTRODUCTION
Living donor kidney transplantation (LDKT) provides the greatest opportunity to maximize long-term patient and graft survival (1–4). Although multiple factors contribute to prolonged graft survival for LDKT recipients, human leukocyte antigen (HLA) matching remains a very impactful determinant of long-term outcome (5–8). First-degree genetic relatives (i.e. siblings, parents, offspring) therefore represent a prevalent and accessible pool of well-matched kidneys (1,9) which may optimize long-term allograft outcomes. Among these donor types, offspring ostensibly represent the ideal donor group given the combined benefit of haplotype matching and younger donor age.
Although most LDKT recipients would benefit from transplantation with offspring donor kidneys, women with a history of pregnancy may be poorly served by this approach. Given that pregnancy is an immune sensitizing event, long-lived immune memory cells with specificity for offspring HLA may increase the risk of acute or chronic rejection and negate long-term benefit. While utilization of offspring donors for female candidates was limited in the past by fears surrounding the potential harm posed by pregnancy-induced memory T and B cells (10), offspring living donors (LDs) have been associated with excellent short-term outcomes (11). However, there is limited contemporary evidence evaluating longitudinal allograft and patient survival among maternal recipients of offspring LDKTs. Furthermore, existing data do not thoroughly assess if these kidneys perform as expected given their high degree of HLA matching and overall quality. Most studies which report on outcomes of living donor kidney transplants directly compare the performance of kidneys in female recipients with male recipients (11–13). In light of a number of studies demonstrating inferior transplant outcomes in male recipients of LDKTs (14–16), it is unclear whether men represent an appropriate control group for comparison of outcomes. The question therefore remains whether long-term outcomes meet expectations for female recipients of an offspring kidney.
Recent innovations in LDKT and an improving understanding of the immunology of pregnancy prompt re-consideration of the potential risks and long-term benefits associated with offspring-to-parent LDKT. First, paired exchange programs are now extremely well established (17–20). These programs provide the option of finding an alternative and potentially more desirable LD for any given LDKT candidate. Second, animal studies of maternal immune responses to the fetus during pregnancy suggest that graft-destructive memory T cells may not necessarily predominate the postpartum repertoire. Instead, emerging data suggest that the maternal repertoire consists of both regulatory and “dysfunctional” antigen-experienced populations that may permit the long-term survival of an allograft (21,22). While comparable studies in humans have yet to be performed, these animal data suggest that the postpartum repertoire may promote long-term graft survival instead of graft loss. However, there is little epidemiologic evidence to support either immunologic model, as most studies which compare offspring-to-parent recipient outcomes were performed in earlier eras of immunosuppression therapy and have not sufficiently addressed important confounders such as panel reactive antibody (PRA), degree of HLA matching, or relevant donor and recipient characteristics (11,12,23,24).
In this study, we aimed to determine whether offspring LDKTs were associated with optimal long-term outcomes, especially among female recipients with prior donor-specific alloimmunization during pregnancy. To this end, we compared outcomes of recipients of offspring LDKTs with non-offspring LDKTs after taking gender, degree of detectable sensitization, and HLA matching into careful consideration. The primary objective of this work was thus to determine whether offspring-to-parent transplants should be embraced or avoided in kidney transplantation.
METHODS
Data Source
We performed a retrospective analysis of national registry data collected by the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the OPTN. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The study was determined to be exempt category 4 status by the Institutional Review Board at the University of Pennsylvania (protocol #823223).
Subjects
The cohort was restricted to patients who were transplanted between January 1, 2001 and March 31, 2015. Patient follow up was through June 1, 2015. Only patients who were recipients of LDKTs and age 36 years or older (the youngest age of a recipient of an offspring LD kidney) were included in the study. The primary cohort was restricted to LD recipients with exactly 3 HLA matches with his/her donor (to represent the expected number of matches between a mother and her offspring), and with a maximum PRA of 0%. A secondary, modified cohort included recipients with a minimum of 3 HLA matches, as opposed to exactly 3 HLA matches. The modified cohort did not require a maximum PRA of 0% for inclusion.
Gender was assessed as an effect modifier for offspring donor status with regard to recipient outcomes. However, given differences in alloimmunization during pregnancy and concerns that males are not appropriate controls for females in this context, we were particularly interested, a priori, in determining gender-specific associations between offspring LDKTs and longitudinal outcomes, even if gender was not a significant effect modifier. As such, our primary analysis compared female recipients of offspring LDKTs to female recipients of non-offspring LDKTs. Sensitivity analyses in both cohorts evaluated outcomes among 1) all recipients of offspring LDKTs vs. all recipients of non-offspring LDKTs, 2) all male recipients of LDKTs vs. all female recipients of LDKTs, 3) male recipients of offspring LDKTs vs. female recipients of offspring LDKTs, and 4) male recipients of offspring LDKTs vs. male recipients of non-offspring LDKTs.
Outcomes and Covariates
The primary outcomes in the study were acute rejection at one year, all-cause allograft failure (a composite of allograft failure and mortality), and all-cause mortality. Death-censored allograft failure and allograft failure with death as a competing risk were also evaluated (see Supplement). Recipient characteristics included in the primary models were recipient age, African American race, dialysis vintage time (reported in years), diabetes, previous sensitization events, and body mass index. Locally weighted scatterplot smoothing was performed to determine a cut-point for recipient age with regard to mortality and allograft failure (25,26). Previous sensitization events included previous transplants or blood transfusions; previous pregnancy was not reliably documented in the dataset and was not able to be included. Donor characteristics included age, African American race, gender, body mass index, and cold ischemia time. Immunologic factors included ABO compatibility (identical, compatible, or incompatible), induction type (lymphocyte depleting agents such as anti-thymocyte globulin or alemtuzumab vs. non-depleting agents such as daclizumab or basilixumab vs. no induction), calcineurin inhibitor therapy (tacrolimus, cyclosporine, or neither), and CMV risk status (both recipient and donor negative, recipient positive, or recipient negative with positive donor). All analyses were also adjusted by year of transplantation. To account for dependence among observations within the same transplant center (given center-specific differences in recipient and donor selection), all analyses were clustered by transplant center using a robust sandwich estimator for calculation of the standard error.
Statistical Analyses
Statistical analyses were performed using STATA version 13.0 (Statacorp LP, College Station, TX) with 2-sided hypothesis testing and p-value of < 0.05 as the criteria for statistical significance. Descriptive statistics (median and proportion) were used to describe baseline donor and recipient clinical and demographic characteristics. Rank-sum test was used to compare continuous variables, and chi-square test was used to compare categorical and binary variables.
Multivariable logistic regression models were performed to assess the outcome of acute rejection at one year. Multivariable Cox proportional hazards models were performed to assess the outcomes of allograft failure and mortality. The Cox models were subsequently stratified by recipient factors that are known to have an important impact on the outcomes, including diabetes (26), African American race, and age (27).
We generated Kaplan Meier curves with log rank testing to assess for equality of survival distributions (28). For the multivariable regressions, we selected variables a priori that were known to be risk factors for the outcomes based on clinical judgment and previously published literature (29,30). The proportional hazards assumption was assessed via weighted versions of Kaplan-Meier curves using statistical testing and graphical displays based on the Schoenfeld and scaled Schoenfeld residuals (31).
Handling of Covariate Missingness
Most covariates included in the multivariate models were < 5% incomplete. Donor hypertension was highly missing (over 20%) and was omitted; understanding current policies with regard to live kidney donation (32), we anticipated that donor hypertension would have a very low prevalence among LDs (among those donors in whom it was reported, a diagnosis of hypertension was present in less than 2%). We performed complete case analysis to address any other missing data (33).
RESULTS
Recipient and Donor Characteristics
2,767 women met inclusion criteria for the primary analyses (see Figure 1), of whom 1,332 were recipients of offspring LDKTs, and 1,435 were recipients of non-offspring LDKTs. Recipients of offspring LDKTs were significantly older (median age 59 vs. 49 years, p<0.001), more likely to be African American race (28% vs. 11%, p<0.001), and were more likely to be diabetic (40% vs. 27%, p<0.001) compared to recipients of non-offspring LDKTs (see Table 1). Recipients of offspring kidneys were less likely to be CMV high-risk (recipient negative, donor positive) than recipients of non-offspring kidneys (7% vs. 10%, p<0.001); other immunologic characteristics, including ABO compatibility, induction immunosuppression, and calcineurin inhibitor immunosuppression were similar across the two groups. Recipients of offspring kidneys had a similar prevalence of total pre-transplant sensitization events to recipients of non-offspring kidneys (23% vs. 25%, p=0.286), but significantly lower prevalence of previous kidney transplant (4% vs. 9%, p<0.001). While recipients of offspring LDKTs had a higher body mass index (BMI) than recipients of non-offspring LDKTs, the difference in donor and recipient BMI was the same across the two groups (0.5 vs. 0.5, p=0.897). Offspring donors were significantly younger than non-offspring donors (median age 34 vs. 46 years, p<0.001), and were more likely to be male (40% vs. 34%, p<0.001).
Figure 1.
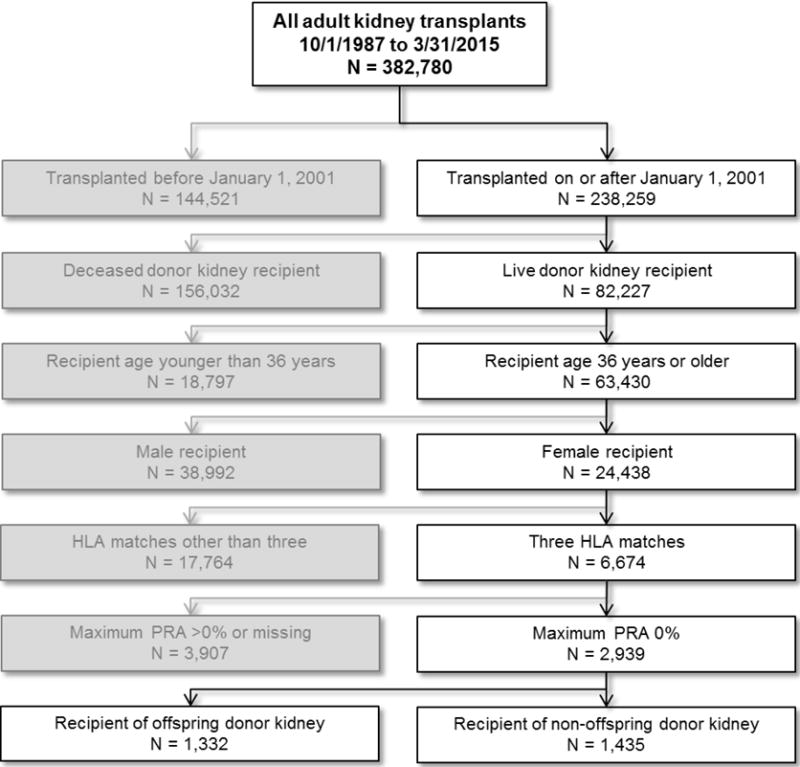
Primary cohort selection for evaluation of outcomes of recipients of offspring live donors
Table 1.
Recipient and Donor Characteristics Comparing Female Live Donor Recipients by Donor Relationship
| Offspring Donor n = 1,332 |
Non-Offspring Donor n = 1,435 |
P-value | |
|---|---|---|---|
| Recipient Characteristics | |||
| Median age, years (IQR) | 59 (53–65) | 49 (42–57) | <0.001 |
| African American race, N (%) | 369 (28%) | 154 (11%) | <0.001 |
| Median dialysis vintage, days (IQR) | 322 (0–714) | 168 (0–538) | <0.001 |
| Diabetic, N (%) | 524 (40%) | 378 (27%) | <0.001 |
| Cause of end stage renal disease, N (%) | <0.001 | ||
| Diabetes | 385 (29%) | 285 (20%) | |
| Hypertension | 369 (28%) | 209 (15%) | |
| Glomerular disease | 184 (14%) | 319 (22%) | |
| Cystic disease | 110 (8%) | 248 (17%) | |
| Other cause | 195 (15%) | 286 (20%) | |
| Missing | 88 (6%) | 87 (6%) | |
| Any pre-transplant sensitization events, N (%) | 275 (23%) | 324 (25%) | 0.286 |
| Previous kidney transplant, N (%) | 58 (4%) | 123 (9%) | <0.001 |
| Median body mass index, kg/m2 (IQR) | 27.9 (24.2–32.1) | 26.7 (22.7–31.6) | <0.001 |
| Donor Characteristics | |||
| Median age, years (IQR) | 34 (28–40) | 46 (38–53) | <0.001 |
| African American race, N (%) | 376 (28%) | 143 (10%) | <0.001 |
| Male gender, N (%) | 527 (40%) | 493 (34%) | <0.001 |
| Median cold ischemia time, hours (IQR) | 1 (1–2) | 1 (1–2) | 0.738 |
| Median body mass index, kg/m2 (IQR) | 27.1 (24.3–30.7) | 26.2 (23.5–29.5) | <0.001 |
| Median recipient minus donor body mass index (IQR) | 0.5 (−3.7–4.9) | 0.5 (−4.0–5.0) | 0.897 |
| Immunologic Characteristics | |||
| ABO blood type match level | 0.631 | ||
| Identical, N (%) | 1,053 (79%) | 1,117 (78%) | |
| Compatible, N (%) | 262 (20%) | 302 (21%) | |
| Incompatible, N (%) | 17 (1%) | 16 (1%) | |
| CMV risk status | <0.001 | ||
| Recipient positive, N (%) | 910 (75%) | 765 (62%) | |
| Donor and recipient negative, N (%) | 217 (18%) | 264 (21%) | |
| Recipient negative, donor positive, N (%) | 85 (7%) | 208 (17%) | |
| Induction immunosuppression | 0.799 | ||
| Depleting, N (%) | 630 (47%) | 662 (46%) | |
| Non-depleting, N (%) | 372 (28%) | 415 (29%) | |
| None, N (%) | 330 (25%) | 358 (25%) | |
| Calcineurin inhibitor immunosuppression | 0.078 | ||
| Tacrolimus, N (%) | 1,072 (81%) | 1,199 (84%) | |
| Cyclosporine, N (%) | 179 (13%) | 148 (10%) | |
| Both, N (%) | 3 (0%) | 2 (0%) | |
| Neither, N (%) | 78 (6%) | 86 (6%) | |
In analyses comparing the 1,332 female recipients of offspring LDKTs to 2,245 male recipients of offspring LDKTs (see Supplemental Table 1), the women were closer in age to the men (59 vs. 61 years, p<0.001), more likely to be African American race (28% vs. 16%, p<0.001), less likely to be diabetic (40% vs. 50%, p<0.001), and had a lower BMI (27.9 vs. 28.2 kg/m2, p=0.007). Male recipients had a significantly greater donor-recipient BMI differential (1.6 vs. 0.5 kg/m2, p<0.001), lower prevalence of sensitization events (23% vs. 20%, p=0.020), and the same prevalence of previous kidney transplantation (4%) compared to female recipients.
Multivariable Regression Models
Using multivariable logistic regression modeling, female recipients of offspring LDKTs had no difference in acute rejection at 1 year compared to female recipients of non-offspring kidneys (adjusted odds ratio 1.01, 95% confidence interval [CI] 0.68–1.51; see Table 2). In multivariable Cox proportional hazards models, female recipients of offspring kidneys had a significantly greater hazard of all-cause allograft failure (adjusted hazard ratio [aHR] 1.65, 95% CI 1.11–2.44; see Table 2 and Figure 2A) and mortality (aHR 1.37, 95% CI 1.02–1.86; see Table 2 and Figure 2B) compared to female recipients of non-offspring kidneys.
Table 2.
Multivariable Logistic Regression Model for Acute Rejection at One Year and Multivariable Cox Proportional Hazards Models for All-Cause Allograft Failure and Mortality Comparing Female Recipients of Offspring Live Donors vs. Female Recipients of Non-Offspring Live Donors, Clustered by Transplant Center
| Acute Rejection | Allograft Failure | Mortality | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Offspring Donor | 1.01 (0.68–1.51) | 0.949 | 1.65 (1.11–2.44) | 0.012 | 1.37 (1.02–1.86) | 0.040 |
| Recipient age category | ||||||
| <40 year | REF | REF | REF | |||
| 40–54 years | 1.08 (0.58–2.02) | 0.805 | 0.89 (0.53–1.48) | 0.645 | 1.08 (0.73–1.59) | 0.706 |
| ≥55 years | 0.88 (0.47–1.64) | 0.692 | 1.08 (0.61–1.91) | 0.787 | 1.92 (1.21–3.03) | 0.005 |
| Recipient African American race | 1.24 (0.75–2.05) | 0.394 | 1.12 (0.42–3.02) | 0.823 | 0.95 (0.44–2.06) | 0.904 |
| Dialysis vintage, years | 1.02 (0.94–1.10) | 0.689 | 1.11 (1.08–1.15) | <0.001 | 1.13 (1.08–1.18) | <0.001 |
| Recipient diabetes | 1.68 (1.30–2.18) | <0.001 | 1.94 (1.57–2.39) | <0.001 | ||
| Any previous sensitization event | 1.09 (0.66–1.79) | 0.734 | 1.16 (0.87–1.56) | 0.320 | ||
| Recipient body mass index | 0.99 (0.97–1.02) | 0.578 | ||||
| Donor age | 1.03 (1.01–1.04) | 0.005 | 1.02 (1.01–1.04) | 0.001 | ||
| Donor African American | 1.25 (0.42–3.67) | 0.689 | 1.17 (0.52–2.65) | 0.706 | ||
| ABO compatibility | ||||||
| Identical | REF | REF | ||||
| Compatible | 0.87 (0.50–1.50) | 0.614 | 1.02 (0.75–1.38) | 0.904 | ||
| Incompatible | 2.55 (0.91–7.13) | 0.075 | 1.10 (0.45–2.66) | 0.841 | ||
| Donor male gender | 1.00 (0.81–1.25) | 0.982 | 1.08 (0.90–1.30) | 0.425 | ||
| Induction type | ||||||
| None | REF | REF | ||||
| Depleting | 2.08 (1.05–4.11) | 0.035 | 1.09 (0.78–1.52) | 0.613 | ||
| Non-depleting | 1.09 (0.56–2.13) | 0.792 | 1.16 (0.83–1.60) | 0.383 | ||
| Calcineurin Inhibitor | ||||||
| Neither | REF | REF | ||||
| Tacrolimus | 0.92 (0.40–2.10) | 0.846 | 0.44 (0.25–0.77) | 0.004 | ||
| Cyclosporine | 1.18 (0.49–2.85) | 0.709 | 0.49 (0.27–0.89) | 0.018 | ||
| CMV risk status | ||||||
| Both negative | REF | |||||
| Recipient positive | 0.95 (0.70–1.28) | 0.745 | ||||
| Recipient negative, donor positive | 0.57 (0.34–0.94) | 0.029 | ||||
| Donor body mass index | 1.00 (0.98–1.02) | 0.755 | ||||
| Cold ischemia time | 0.99 (0.95–1.03) | 0.625 | ||||
| Transplant year | 0.92 (0.87–0.98) | 0.005 | 0.94 (0.90–0.98) | 0.002 | 0.95 (0.92–0.99) | 0.013 |
Figure 2A.
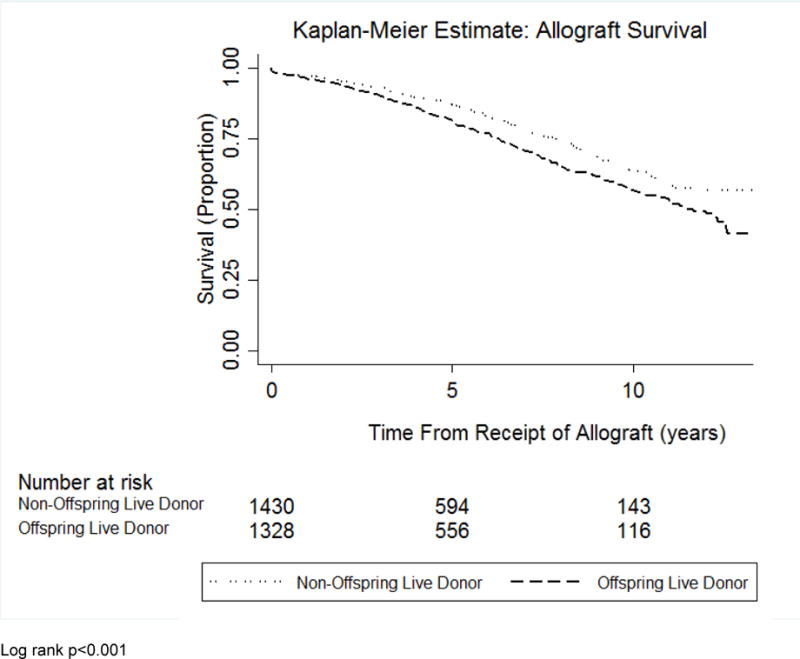
Kaplan-Meier curve evaluating allograft survival in female recipients of offspring donor kidneys versus female recipients of non-offspring live donor kidneys
Figure 2B.
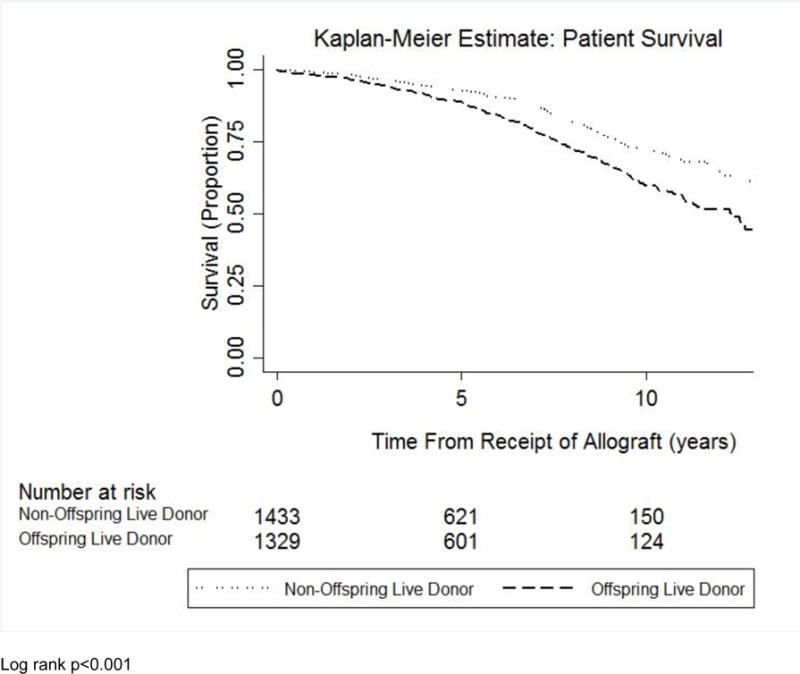
Kaplan-Meier curve evaluating patient survival in female recipients of offspring donor kidneys versus female recipients of non-offspring live donor kidneys
Multivariable Cox models for death-censored allograft failure and mortality as a competing risk demonstrated a trend toward increased risk among female recipients of offspring kidneys, but were under-powered to assess for a significant difference (see Supplemental Table 2). Secondary analyses using a modified, expanded cohort (comparing female recipients of offspring LDKTs to female recipients of non-offspring LDKTs with a minimum of 3 HLA matches and adjusting for PRA) demonstrated a significantly increased risk of death-censored allograft failure (aHR 1.27, 95% CI 1.04–1.56; see Supplemental Table 3) and allograft failure treating mortality as a competing risk (sub-aHR 1.24, 95% CI 1.01–1.53) among female recipients of offspring LDKTs.
In multivariable Cox proportional hazards models using the primary cohort inclusion criteria (recipient age ≥36, exactly 3 HLA matches, and maximum PRA 0%) and adjusting for gender instead of restricting to female recipients, recipients of offspring LDKTs had a significantly greater risk of all-cause allograft failure (aHR 1.35, 95% CI 1.08–1.68) and mortality (1.30, 95% CI 1.10–1.54) compared to recipients of non-offspring LDKTs (see Table 3). Male recipients of any LDKT, adjusting for offspring relationships status, had significantly greater hazard of all-cause allograft failure (aHR 1.23, 95% CI 1.08–1.39) and mortality (aHR 1.17, 95% CI 1.04–1.31) compared to female recipients of LDKTs. Male recipients of offspring LDKTs had no significant difference in all-cause allograft failure (aHR 1.19, 95% CI 0.99–1.44), but did have a significantly higher risk of mortality (aHR 1.25, 95% CI 1.07–1.47) compared to female recipients of offspring LDKTs. Similarly, compared to male recipients of non-offspring LDKTs, male recipients of offspring LDKTs had no significant difference in all-cause allograft failure (aHR 1.28, 95% CI 0.99–1.65), but showed a significantly higher risk of mortality (aHR 1.25, 95% CI 1.03–1.52).
Table 3.
Multivariable Cox Proportional Hazards Models for All-Cause Allograft Failure and Mortality, Clustered by Transplant Center: Sensitivity Analyses
| Allograft Failure* | Mortality† | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95%CI) | P-Value | |
| Primary Cohort‡ | ||||
| A. Recipients of Offspring Donors vs. Recipients of Non-Offspring Live Donors | 1.35 (1.08–1.68) | 0.007 | 1.30 (1.10–1.54) | 0.003 |
| B. Female Recipients of Offspring Donors vs. Female Recipients of Non-Offspring Live Donors (primary analysis) | 1.65 (1.11–2.44) | 0.012 | 1.37 (1.02–1.86) | 0.040 |
| C. Male Recipients of Live Donors vs. Female Recipients of Live Donors | 1.23 (1.08–1.39) | 0.002 | 1.17 (1.04–1.31) | 0.010 |
| D. Male Recipients of Offspring Donors vs. Female Recipients of Offspring Donors | 1.19 (0.99–1.44) | 0.070 | 1.25 (1.07–1.47) | 0.006 |
| E. Male Recipients of Offspring Donors vs. Male Recipients of Non-Offspring Live Donors | 1.28 (0.99–1.65) | 0.062 | 1.25 (1.03–1.52) | 0.023 |
|
| ||||
| Modified Cohort§ | ||||
| A. Recipients of Offspring Donors vs. Recipients of Non-Offspring Live Donors | 1.21 (1.07–1.37) | 0.002 | 1.55 (1.42–1.68) | <0.001 |
| B. Female Recipients of Offspring Donors vs. Female Recipients of Non-Offspring Live Donors | 1.66 (1.12–2.46) | 0.012 | 1.61 (1.42–1.84) | <0.001 |
| C. Male Recipients of Live Donors vs. Female Recipients of Live Donors | 1.10 (1.03–1.16) | 0.002 | 1.13 (1.07–1.18) | <0.001 |
| D. Male Recipients of Offspring Donors vs. Female Recipients of Offspring Donors | 1.14 (1.03–1.26) | 0.014 | 1.21 (1.14–1.29) | <0.001 |
| E. Male Recipients of Offspring Donors vs. Male Recipients of Non-Offspring Live Donors | 1.20 (1.00–1.45) | 0.056 | 1.50 (1.36–1.66) | <0.001 |
Allograft Failure models adjusted for recipient age, recipient race, dialysis vintage time, recipient diabetes status, prior sensitization events, recipient body mass index, donor age, donor race, donor gender, donor body mass index, cold ischemia time, ABO compatibility, induction immunosuppression, calcineurin inhibitor treatment, CMV risk status
Mortality models adjusted for recipient age, recipient race, dialysis vintage time, recipient diabetes status, donor age, donor race, and donor gender
Primary cohort inclusion criteria: Recipient age ≥36, transplanted on or after 2001, exactly 3 HLA matches, Maximum PRA 0%
Modified cohort inclusion criteria: Recipient age ≥36, transplanted on or after 2001, minimum of 3 HLA matches (adjusted for number of HLA mismatches and PRA)
The results were similar in the modified cohort (with inclusion criteria expanded to include recipients with a minimum of 3 HLA matches, adjusting for number of HLA matches and PRA), except male recipients of offspring LDKTs had a significantly increased hazard of all-cause allograft failure (aHR 1.14, 95% CI 1.03–1.26) and mortality (aHR 1.21, 95% CI 1.14–1.29) compared to female recipients of offspring LDKTs. Also, male recipients of offspring LDKTs had significantly higher risk of mortality (aHR 1.50, 95% CI 1.36–1.66), but not all-cause allograft failure (aHR 1.20, 95% CI 1.00–1.45), compared to male recipients of non-offspring LDKTs. There was no statistically significant interaction between recipient gender and offspring status with regard to allograft failure or mortality.
Stratified Analyses
Stratified Cox proportional hazards analyses evaluating all-cause allograft failure and mortality were performed using the modified cohort (comparing female recipients of offspring LDKTs to female recipients of non-offspring LDKTs with a minimum of 3 HLA matches) due to insufficient power in the primary cohort. The analyses demonstrated similar results across strata and compared to the primary analyses after stratifying by recipient diabetes status, recipient and donor African American race, recipient and donor age, cause of end stage renal disease, donor gender, and recipient/donor BMI mismatch. There was significant interaction between older donor age (≥40 years) and offspring status with regard to allograft failure and mortality (i.e. older donor age was associated with greater risk of allograft failure [HR 1.82, 95% CI 1.64–2.01] and mortality [HR 2.52, 95% CI 2.25–2.82] compared to younger donor age among recipients of offspring donors). There was no significant interaction between the other stratifying variables and donor offspring status with regard to all-cause allograft failure and mortality (see Table 4 and Supplemental Table 4).
Table 4.
Cox Proportional Hazards Models for All-Cause Allograft Failure and Mortality in Stratified Analyses Comparing Female Recipients of Offspring Donors vs. Female Recipients of Non-Offspring Donors in the Modified Cohort*, Clustered by Transplant Center
| N | Allograft Failure | Mortality | |||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95%CI) | P-Value | ||
|
Recipient Characteristics
| |||||
| Diabetics | 4,209 | 1.35 (1.20–1.53) | <0.001 | 1.41 (1.24–1.61) | <0.001 |
| Non-Diabetics | 9,405 | 1.38 (1.27–1.50) | <0.001 | 1.84 (1.64–2.05) | <0.001 |
|
| |||||
| African American | 2,472 | 1.26 (1.09–1.46) | 0.002 | 1.77 (1.43–2.20) | <0.001 |
| Non-African American | 11,344 | 1.40 (1.30–1.51) | <0.001 | 1.70 (1.55–1.86) | <0.001 |
|
| |||||
| Age <55 years | 7,525 | 1.31 (1.16–1.48) | <0.001 | 1.43 (1.23–1.65) | <0.001 |
| Age ≥55 years | 6,291 | 1.19 (1.07–1.33) | 0.002 | 1.20 (1.07–1.35) | 0.003 |
|
| |||||
| Recurrent cause of ESRD (diabetes or glomerular disease) | 5,828 | 1.52 (1.36–1.69) | <0.001 | 2.11 (1.78–2.51) | <0.001 |
| Non-recurrent cause of ESRD (hypertension or cystic disease) | 4,132 | 1.61 (1.41–1.85) | <0.001 | 1.76 (1.54–2.00) | <0.001 |
|
| |||||
| Transplanted before 2008 | 7,621 | 1.46 (1.35–1.58) | <0.001 | 1.75 (1.60–1.92) | <0.001 |
| Transplanted during or after 2008 | 6,195 | 1.24 (1.07–1.44) | 0.005 | 1.56 (1.31–1.85) | <0.001 |
|
| |||||
|
Donor Characteristics
| |||||
| Age < 40 years† | 6,729 | 1.41 (1.26–1.57) | <0.001 | 1.71 (1.48–1.97) | <0.001 |
| Age ≥ 40 years† | 7,087 | 1.82 (1.64–2.01) | <0.001 | 2.52 (2.25–2.82) | <0.001 |
|
| |||||
| Male | 5,281 | 1.45 (1.30–1.61) | <0.001 | 1.72 (1.51–1.96) | <0.001 |
| Female | 8,535 | 1.43 (1.30–1.57) | <0.001 | 1.75 (1.57–1.94) | <0.001 |
|
| |||||
| African American | 2,432 | 1.39 (1.29–1.50) | <0.001 | 1.83 (1.45–2.32) | <0.001 |
| Non-African American | 11,384 | 1.30 (1.11–1.52) | 0.001 | 1.69 (1.55–1.85) | <0.001 |
|
| |||||
| Recipient with larger BMI than donor | 6,509 | 1.34 (1.20–1.48) | <0.001 | 1.59 (1.42–1.79) | <0.001 |
| Recipient with the same or smaller BMI than donor | 6,278 | 1.53 (1.38–1.70) | <0.001 | 1.87 (1.65–2.13) | <0.001 |
Modified cohort inclusion criteria: Recipient age ≥36, transplanted on or after 2001, minimum of 3 HLA matches
Indicates significant interaction between the covariate and offspring status with regard to allograft failure and mortality
ESRD: End stage renal disease
DISCUSSION
Adult offspring remain a prevalent source of potential living donors for kidney transplant candidates (1,9). Although the utilization of offspring living donors has diminished in recent years in the United States (see Supplemental Figure 1), these donors may nevertheless represent the optimal choice to maximize long-term benefit in LDKT. However, it is unclear whether these donors are really the best option for women, who may have developed an immunologic memory response to the donor during exposure in prior pregnancy that ultimately threatens graft outcomes. Given that living donor kidney transplant candidates at many centers have access to alternative donors through the pipeline of paired exchange, we asked whether parents achieve the expected benefits of offspring living donors or should potentially be offered paired exchange as an alternative to optimize long-term outcomes. The primary goal of this study was to determine whether offspring donors perform up to expectations in individuals who have been previously exposed to the donor through the unique route of pregnancy.
In this study, we used a series of analytic strategies to compare the observed long-term outcomes of offspring-to-mother living donor kidney transplants against the expected outcomes among patient cohorts that were not immunologically exposed to the donor during pregnancy. In our primary analysis, female recipients that received a three antigen matched kidney in the absence of pregnancy immunization against their living donors defined the expected long-term patient and graft survival. We found that the risk of graft loss was significantly higher in women who received an offspring living donor kidney (i.e. mothers) compared to women who received a non-offspring kidney. This difference in graft survival expanded over 15 years of follow-up, and was greater among recipients of kidneys from older donors (age ≥40 years). Analysis of the modified cohort suggested that the difference in graft survival was not entirely attributable to differences in overall patient survival, as inferior graft survival persisted when we examined death censored graft survival or when death was treated as a competing risk (Supplemental Table 3).
Taken in isolation, the above results suggest that pregnancy immunization against the donor is detrimental to long-term graft survival. However, our analyses of offspring and non-offspring graft survival in men suggest an alternative interpretation. As noted in Table 3, all recipients of offspring living donors fared worse than recipients of non-offspring donors after adjusting for gender. Moreover, graft and patient survival were similar between mothers and fathers in both the primary and modified cohorts. Our analyses therefore collectively suggest that kidney transplants from offspring living donors do not provide the greatest long-term benefit to their recipients when compared to recipients who receive comparably well-matched kidneys. Nonetheless, male recipients had worse overall outcomes than female recipients across multiple sensitivity analyses, which has been demonstrated previously (14–16,34,35). Although there was no significant interaction between recipient gender and offspring status, these findings suggest that male recipients may not be an ideal control for female recipients, and further support that female recipients of offspring LDKTs had worse outcomes than expected compared to more fitting female controls. Furthermore, due to important gender-based differences in previous immunologic exposures (36,37), immune responses (38), and other unmeasured risk factors (34,39,40), males broadly make a poor control group when evaluating outcomes of kidney transplantation in women.
Given the premise of the study, we were surprised to find that recipients of any offspring donor fared worse no matter the gender of the recipient. We currently speculate that either genetic or shared environmental factors between donor and recipient dictate the inferior outcome of these grafts. This hypothesis is indirectly supported by the findings of other investigators who note higher rates of adverse allograft outcomes among recipients of kidneys from LDs who themselves go on to develop end stage renal disease (41). Indirect support for this hypothesis may also be provided from within our dataset, given the interaction between older donor age and offspring status, as well. However, while we had hoped that stratification by disease etiology would provide particularly useful insight into the biologic factors that contribute to inferior graft survival of offspring kidneys, we could find no interaction between disease etiology and offspring status. These epidemiologic, observational data therefore do not provide a biologic mechanism which explains why graft and patient survival are inferior among recipients of offspring LDKTs. Additional insights about the biologic process which diminishes offspring-to-parent outcomes may be gained through the study of paired exchange recipient outcomes, particularly the outcomes of recipients who received a haplotype-matched kidney originally intended for a parent.
The principal strengths of our study include 1) long duration of follow-up, 2) use of a large-scale, population-based, contemporary transplant cohort; 3) use of multiple sensitivity analyses to validate our interpretation of the dataset, and 4) use of a highly detailed national registry database, allowing for appropriate statistical control of multiple variables known to impact graft and patient survival. Our study particularly highlights the significant impact of donor and recipient gender, age, race, and HLA matching on long-term graft survival among LDKT recipients, which previous studies in this area have not thoroughly explored. We also took into careful account body size mismatch between the recipient and donor, which is emerging as an important factor in outcomes for recipients of deceased donor kidneys (42,43). Additionally, understanding that substantial center-specific variability exists with regard to donor and recipient selection criteria and the concerns related to the relationship between the donor and recipient, we used statistical techniques to account for clustering by transplant center.
Despite these strengths, our study also has a number of important limitations. As with any retrospective study, the analyses were susceptible to unmeasured confounding. Unmeasured confounders which we identified included previous number of pregnancies, which were not adequately captured in the dataset, donor hypertension, which was highly missing, and information on donor specific antibody and cardiovascular comorbidities. Regarding the absence of previous number of pregnancies in the dataset, we attempted to overcome this limitation by carefully controlling for sensitization in multiple other ways, including PRA (with our primary cohort being restricted only to patients with a maximum PRA of 0%), previous transfusion exposure, and prior transplant. We do not suspect that missing donor hypertension status influenced the results meaningfully, given that transplant centers generally have strict guidelines regarding LDKTs from LDs with hypertension, and those kidneys that are utilized tend to have no signs of end organ effects that would influence allograft outcomes (44). Regrettably, the lack of information on donor specific antibody in the dataset limits our ability to understand the degree to which any immunologic mechanisms contributed to long term graft loss. Similarly, insufficient data on cardiovascular comorbidities limit our ability to adequately adjust our outcome models. Furthermore, given that the OPTN database is a registry that relies on input from transplant centers and organ procurement organizations, it is prone to the possibility of inaccuracies which, in a cohort as select as this, could feasibly contribute considerable misinformation bias. Additionally, while we controlled for a multitude of critical confounders and covariates related to the relationship between donor type and recipient outcomes, we were inadequately powered to utilize more robust matching techniques to account for such issues as confounding by indication and selection bias.
In conclusion, we report that kidney transplants from offspring living donors appear to underperform transplants from comparably HLA-matched living donors, particularly among female recipients and recipients of kidneys from older donors. Altogether, our data suggest that offspring-to-parent transplantations represent an unfavorable pairing independent of recipient gender or prior immunologic exposure through pregnancy. While the decision to transplant any individual with any particular donor must take into account overall donor access and transplantation urgency, our results encourage the escalating utilization of paired kidney exchange whenever possible to avoid less favorable pairings such as offspring-to-parent transplantation while maintaining or improving HLA matching between donors and recipients. While this dataset was unable to delineate the biologic factors that contribute to diminished outcomes in recipients of offspring kidneys, our study nevertheless provides important information that will help guide selection of the optimal living donor for patients with multiple donor options. Additional work which helps define the long-term impact of donor relationship on recipient outcome will provide much needed information to help optimize living donor-recipient matching through any available vehicle.
Supplementary Material
Supplemental Table 1. Recipient and Donor Characteristics Comparing Recipients of Offspring Live Donor Kidneys by Recipient Gender
Supplemental Table 2. Multivariable Cox Proportional Hazards Models for Death-Censored Allograft Failure and Mortality as a Competing Risk for Allograft Failure, Comparing Female Recipients of Offspring Donors vs. Female Recipients of Non-Offspring Donors
Supplemental Table 3. Multivariable Cox Proportional Hazards Models for Death-Censored Allograft Failure Comparing Female Recipients of Offspring Donors vs. Female Recipients of Non-Offspring Donors in the Modified Cohort
Supplemental Table 4. Cox Proportional Hazards Models for All-Cause Allograft Failure and Mortality in Stratified Analyses Comparing Female Recipients of Offspring Live Donors vs. Female Recipients of Non-Offspring Live Donors in the Primary Cohort
Supplemental Figure 1. Secular trends of live donor transplantation by live donor type in UNOS from 2001–2014
Acknowledgments
This research was supported in part by the National Institutes of Health grant number K23-HL133843 (NHLBI, PI: Cohen). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the National Institutes of Health.
The data reported here have been supplied by the United Network of Organ Sharing as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- HLA
Human Leukocyte Antigen
- HR
Hazard ratio
- LD
Living donor
- LDKT
Living donor kidney transplantation
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel reactive antibody
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Organ Procurement and Transplantation Network: National Data. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed August 4, 2017.
- 2.Matas AJ, Gillingham KJ, Humar A, et al. 2202 kidney transplant recipients with 10 years of graft function: what happens next? Am J Transplant. 2008;8(11):2410–2419. doi: 10.1111/j.1600-6143.2008.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasaki M, Saito K, Nakagawa Y, et al. 20-year analysis of kidney transplantation: a single center in Japan. Transplant Proc. 2014;46(2):437–441. doi: 10.1016/j.transproceed.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 4.McCaughan JA, Courtney AE. The clinical course of kidney transplant recipients after 20 years of graft function. Am J Transplant. 2015;15(3):734–740. doi: 10.1111/ajt.13041. [DOI] [PubMed] [Google Scholar]
- 5.Susal C, Opelz G. Current role of human leukocyte antigen matching in kidney transplantation. Curr Opin Organ Transplant. 2013;18(4):438–444. doi: 10.1097/MOT.0b013e3283636ddf. [DOI] [PubMed] [Google Scholar]
- 6.Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014;27(1):19–27. doi: 10.1111/tri.12217. [DOI] [PubMed] [Google Scholar]
- 7.Roodnat JI, van Riemsdijk IC, Mulder PG, et al. The superior results of living-donor renal transplantation are not completely caused by selection or short cold ischemia time: a single-center, multivariate analysis. Transplantation. 2003;75(12):2014–2018. doi: 10.1097/01.TP.0000065176.06275.42. [DOI] [PubMed] [Google Scholar]
- 8.Milner J, Melcher ML, Lee B, et al. HLA Matching Trumps Donor Age: Donor-Recipient Pairing Characteristics That Impact Long-Term Success in Living Donor Kidney Transplantation in the Era of Paired Kidney Exchange. Transplant Direct. 2016;2(7):e85. doi: 10.1097/TXD.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberger B, Spragan D, Hashmi S, et al. Pregnancy-Induced Sensitization Promotes Sex Disparity in Living Donor Kidney Transplantation. Journal of the American Society of Nephrology: JASN. 2017 doi: 10.1681/ASN.2016101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouma GJ, van Caubergh P, van Bree SP, et al. Pregnancy can induce priming of cytotoxic T lymphocytes specific for paternal HLA antigens that is associated with antibody formation. Transplantation. 1996;15(62):672–678. doi: 10.1097/00007890-199609150-00023. [DOI] [PubMed] [Google Scholar]
- 11.Cohen EP, Rosendale JD, Bong CJ, Hariharan S. Benefit of child-to-parent kidney donation. Am J Transplant. 2003;3(7):865–872. doi: 10.1034/j.1600-6143.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 12.Fuggle SV, Allen JE, Johnson RJ, et al. Factors affecting graft and patient survival after live donor kidney transplantation in the UK. Transplantation. 2010;89(6):694–701. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 13.Axelrod DA, McCullough KP, Brewer ED, Becker BN, Segev DL, Rao PS. Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. Am J Transplant. 2010;10(4 Pt 2):987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 14.Redfield RR, Scalea JR, Zens TJ, et al. Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transpl Int. 2016;29(1):81–87. doi: 10.1111/tri.12696. [DOI] [PubMed] [Google Scholar]
- 15.Oien CM, Reisaeter AV, Leivestad T, Dekker FW, Line PD, Os I. Living donor kidney transplantation: the effects of donor age and gender on short- and long-term outcomes. Transplantation. 2007;83(5):600–606. doi: 10.1097/01.tp.0000255583.34329.dd. [DOI] [PubMed] [Google Scholar]
- 16.Andreoni KA, Forbes R, Andreoni RM, Phillips G, Stewart H, Ferris M. Age-related kidney transplant outcomes: health disparities amplified in adolescence. JAMA Intern Med. 2013;173(16):1524–1532. doi: 10.1001/jamainternmed.2013.8495. [DOI] [PubMed] [Google Scholar]
- 17.United Network for Organ Sharing: Kidney Paired Donation. https://www.unos.org/donation/kidney-paired-donation. Accessed August 4, 2017.
- 18.Cowan N, Gritsch HA, Nassiri N, Sinacore J, Veale J. Broken Chains and Reneging: A Review of 1748 Kidney Paired Donation Transplants. Am J Transplant. 2017 doi: 10.1111/ajt.14343. [DOI] [PubMed] [Google Scholar]
- 19.Leeser DB, Aull MJ, Afaneh C, et al. Living donor kidney paired donation transplantation: experience as a founding member center of the National Kidney Registry. Clin Transplant. 2012;26(3):E213–222. doi: 10.1111/j.1399-0012.2012.01606.x. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Registry Paired Exchange Results Quartlery Report as of March 31, 2017. Babylon, NY: 2017. http://www.kidneyregistry.org/pages/p410/NKR_Quarterly_Report_Q1_2017.php. [Google Scholar]
- 21.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton BM, Xu R, Wherry EJ, Porrett PM. Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J Leukoc Biol. 2017;101(4):975–987. doi: 10.1189/jlb.1A0316-135R. [DOI] [PubMed] [Google Scholar]
- 23.Miles CD, Schaubel DE, Liu D, Port FK, Rao PS. The role of donor-recipient relationship in long-term outcomes of living donor renal transplantation. Transplantation. 2008;85(10):1483–1488. doi: 10.1097/TP.0b013e3181705a0f. [DOI] [PubMed] [Google Scholar]
- 24.Mahanty HD, Cherikh WS, Chang GJ, Baxter-Lowe LA, Roberts JP. Influence of pretransplant pregnancy on survival of renal allografts from living donors. Transplantation. 2001;72(2):228–232. doi: 10.1097/00007890-200107270-00010. [DOI] [PubMed] [Google Scholar]
- 25.Goodall C. A survey of smoothing techniques. In: Long J, Fa JS, editors. Modern Methods of Data Analysis. Newbury Park, CA: Sage; 1990. pp. 126–176. [Google Scholar]
- 26.Cohen JB, Bloom RD, Reese PP, Porrett PM, Forde KA, Sawinski DL. National outcomes of kidney transplantation from deceased diabetic donors. Kidney international. 2015 doi: 10.1038/ki.2015.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shabir S, Halimi JM, Cherukuri A, et al. Predicting 5-year risk of kidney transplant failure: a prediction instrument using data available at 1 year posttransplantation. Am J Kidney Dis. 2014;63(4):643–651. doi: 10.1053/j.ajkd.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 29.Ashby VB, Leichtman AB, Rees MA, et al. A Kidney Graft Survival Calculator that Accounts for Mismatches in Age, Sex, HLA, and Body Size. Clin J Am Soc Nephrol. 2017 doi: 10.2215/CJN.09330916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massie AB, Leanza J, Fahmy LM, et al. A Risk Index for Living Donor Kidney Transplantation. Am J Transplant. 2016;16(7):2077–2084. doi: 10.1111/ajt.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health) New York: Springer; 2001. [Google Scholar]
- 32.Organ Procurement and Transplantation Network Policies. 14. Living Donation. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_14.2017. Accessed January 24, 2017.
- 33.White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Statistics in medicine. 2010;29(28):2920–2931. doi: 10.1002/sim.3944. [DOI] [PubMed] [Google Scholar]
- 34.Aufhauser DD, Jr, Wang Z, Murken DR, et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest. 2016;126(5):1968–1977. doi: 10.1172/JCI84712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gratwohl A, Döhler B, Stern M, Opelz G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. The Lancet. 2008;372(9632):49–53. doi: 10.1016/S0140-6736(08)60992-7. [DOI] [PubMed] [Google Scholar]
- 36.Lopes D, Barra T, Malheiro J, et al. Effect of Different Sensitization Events on HLA Alloimmunization in Kidney Transplantation Candidates. Transplant Proc. 2015;47(4):894–897. doi: 10.1016/j.transproceed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Honger G, Fornaro I, Granado C, Tiercy JM, Hosli I, Schaub S. Frequency and determinants of pregnancy-induced child-specific sensitization. Am J Transplant. 2013;13(3):746–753. doi: 10.1111/ajt.12048. [DOI] [PubMed] [Google Scholar]
- 38.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 39.Sanghavi M, Gulati M. Sex differences in the pathophysiology, treatment, and outcomes in IHD. Curr Atheroscler Rep. 2015;17(6):511. doi: 10.1007/s11883-015-0511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation. 2014;130(9):757–767. doi: 10.1161/CIRCULATIONAHA.114.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muzaale AD, Massie AB, Anjum S, et al. Recipient Outcomes Following Transplantation of Allografts From Live Kidney Donors Who Subsequently Developed End-Stage Renal Disease. Am J Transplant. 2016;16(12):3532–3539. doi: 10.1111/ajt.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller AJ, Kiberd BA, Alwayn IP, Odutayo A, Tennankore KK. Donor-Recipient Weight and Sex Mismatch and the Risk of Graft Loss in Renal Transplantation. Clin J Am Soc Nephrol. 2017;12(4):669–676. doi: 10.2215/CJN.07660716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen JB, Shults J, Goldberg DS, Abt PL, Sawinski DL, Reese PP. Kidney allograft offers: Predictors of turndown and the impact of late organ acceptance on allograft survival. Am J Transplant. 2017 doi: 10.1111/ajt.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend RR, Reese PP, Lim MA. Should living kidney donors with hypertension be considered for organ donation? Curr Opin Nephrol Hypertens. 2015;24(6):594–601. doi: 10.1097/MNH.0000000000000169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Recipient and Donor Characteristics Comparing Recipients of Offspring Live Donor Kidneys by Recipient Gender
Supplemental Table 2. Multivariable Cox Proportional Hazards Models for Death-Censored Allograft Failure and Mortality as a Competing Risk for Allograft Failure, Comparing Female Recipients of Offspring Donors vs. Female Recipients of Non-Offspring Donors
Supplemental Table 3. Multivariable Cox Proportional Hazards Models for Death-Censored Allograft Failure Comparing Female Recipients of Offspring Donors vs. Female Recipients of Non-Offspring Donors in the Modified Cohort
Supplemental Table 4. Cox Proportional Hazards Models for All-Cause Allograft Failure and Mortality in Stratified Analyses Comparing Female Recipients of Offspring Live Donors vs. Female Recipients of Non-Offspring Live Donors in the Primary Cohort
Supplemental Figure 1. Secular trends of live donor transplantation by live donor type in UNOS from 2001–2014


