Abstract
Dietary methionine restriction (MR) is implemented using a semi-purified diet that reduces methionine by ~80% and eliminates dietary cysteine. Within hours of its introduction, dietary MR initiates coordinated series of transcriptional programs and physiological responses that include increased energy intake and expenditure, decreased adiposity, enhanced insulin sensitivity, and reduction in circulating and tissue lipids. Significant progress has been made in cataloguing the physiological responses to MR in males but not females, but identities of the sensing and communication networks that orchestrate these responses remain poorly understood. Recent work has implicated hepatic FGF21 as an important mediator of MR, but it is clear that other mechanisms are also involved. The goal of this review is to explore the temporal and spatial organization of the responses to dietary MR as a model for understanding how nutrient sensing systems function to integrate complex transcriptional, physiological, and behavioral responses to changes in dietary composition.
Keywords: amino acid sensing, obesity, lipid metabolism, insulin sensitivity, FGF21
1. Introduction
All life forms sense and respond to environmental cues by engaging coordinated homeostatic responses that function to enhance survival of the organism. In higher organisms, variation in the macronutrient makeup (e.g., carbohydrate, lipid, protein) of the diet requires tissue-specific adaptations to ongoing changes in the fuel sources and molecular building blocks that make up each meal. Simpson and Raubenheimer (Simpson and Raubenheimer, 1997) used an integrative modeling approach to develop a Geometric Framework to evaluate the effects of dietary macronutrients on response variables such as nutrient selection, body composition, and longevity. The authors originally tested the Geometric Framework in experiments where lifespan or fecundity were endpoints in insect species given ad libitum access to 28 different diets varying in their protein to carbohydrate ratio. The responses to all 28 diets were summarized by plotting the protein consumed per day on the X axis, the carbohydrate consumed per day on the Y axis, and the biological response (e.g., longevity, fecundity, etc.) to each nutritional combination as a heat map in the Z-plane (Raubenheimer and Simpson, 1997; Piper et al., 2011). The effectiveness of this approach in describing biological responses to nutritional complexity led to its application in examining how varying dietary macronutrient composition affected ingestive behavior, and how animals prioritize macronutrient intake when given a choice (Simpson & Raubenheimer, 1997; Simpson and Raubenheimer, 2005; Piper et al., 2011; Wilder et al., 2012). It was found that lowering the percentage of protein in the diet causes a concomitant increase in energy intake to maintain constant protein intake. This leveraging of carbohydrate and fat intake that occurs with dilution of dietary protein represents the conceptual basis for the Protein Leverage hypothesis (Simpson & Raubenheimer, 2005). Recent studies of dietary protein dilution show that in addition to the hyperphagia predicted by the Protein Leverage hypothesis, low protein diets also produce an increase in EE of sufficient magnitude to limit fat deposition (Laeger et al., 2016; Laeger et al., 2014b; Morrison and Berthoud, 2007; Solon-Biet et al., 2015).
These findings support an emerging consensus that dietary protein intake is monitored through nutrient sensing mechanisms that detect changes in the essential amino acid (EAA) composition of the consumed protein (Simpson & Raubenheimer, 1997; Morrison et al., 2012; Gosby et al., 2011; Bosse and Dixon, 2012). Studies with semi-purified diets that restrict single EAAs within a defined narrow range show that such diets mirror the effects of low protein diets on ingestive behavior, energy expenditure, insulin sensitivity, and lipid metabolism (Hasek et al., 2010; Plaisance et al., 2010; Hasek et al., 2013; Stone et al., 2014; Forney et al., 2017; Wanders et al., 2015b). Collectively, these studies provide compelling support for the existence of real-time EAA sensing systems linked to translational mechanisms that function together to detect changes in dietary EAAs and affect a highly integrated and beneficial set of adaptive responses. The goal of the present review is to explore recent findings on how these sensing and effector systems are organized, with special emphasis on how endocrine and neuroendocrine mechanisms may function to provide the communication networks that integrate the overall response.
It should be noted that our work, and to our knowledge all work reported to date on dietary methionine restriction, has been conducted in male rodents. However, recent work with low protein diets reported sex differences in the hormonal and metabolic responses to dietary protein dilution (Larson et al., 2017). Given the many similar responses to methionine restriction and protein dilution, it will be important in future preclinical studies to extend work on dietary methionine restriction to female subjects and identify any gender-specific responses in this model.
2. The role of amino acid sensing in maintenance of protein nutrition
2.1 Essential amino acids are the measured indices of protein nutrition
A subgroup of amino acids that make up proteins cannot be synthesized endogenously and must be provided in the diet (e.g. EAAs). Therefore, the ability to detect and respond to dietary EAA deficiencies is an indispensable survival mechanism. Although a number of molecular and cellular EAA sensing mechanisms have been identified (Anthony et al., 2001; Anthony et al., 2004; Kimball et al., 2004; Wek et al., 2006; Zhang et al., 2002; Hao et al., 2005; Deval et al., 2009; Maurin et al., 2005), recent studies suggest that multiple sensing mechanisms are involved in responding to low protein or EAA-restricted diets (Laeger et al., 2016; Wanders et al., 2016). The well documented in vivo responses to perturbation of dietary EAA composition make a compelling case that EAAs play the dominant role as mediators of the effects of dietary protein restriction on metabolism and energy balance (Du et al., 2012; Cheng et al., 2011; Han et al., 2012; Cheng et al., 2010; Guo and Cavener, 2007; Hasek et al., 2010; Plaisance et al., 2010). Thus, the primary experimental model examined in this review involves the responses of rats or mice to ad libitum consumption of semi-purified diets with defined restrictions of specific EAAs, with special emphasis on methionine.
2.2 Experimental models – dietary methionine restriction
The focus of this review is an experimental diet that restricts dietary methionine from normal levels of 0.86% to 0.17% while also eliminating dietary cysteine. The diet was originally described by Orentreich and colleagues (Orentreich et al., 1993; Richie, Jr. et al., 1994), who documented its ability to increase mean and maximal life span in rats. Their findings have been extended to other species (Lee et al., 2014; Sun et al., 2009; Johnson and Johnson, 2014; Miller et al., 2005), and a unique but common feature of these studies is that the life extending properties of the MR diet do not require food restriction (Orentreich et al., 1993; Richie, Jr. et al., 1994; Malloy et al., 2006). The short-term physiological responses to dietary MR have come into sharper focus over the last two decades because the diet produces improvements in essentially all biomarkers of metabolic health and extends healthspan (Malloy et al., 2006; Malloy et al., 2013; Perrone et al., 2012b; Perrone et al., 2012a; Perrone et al., 2008; Perrone et al., 2009; Hasek et al., 2010; Plaisance et al., 2010; Zimmerman et al., 2003; Lee et al., 2014; Sun et al., 2009; Miller et al., 2005; Lees et al., 2014; Ghosh et al., 2017; Wanders et al., 2013). The most prominent physiological responses to MR are increased insulin sensitivity and coordinated increases in energy intake and expenditure (Hasek et al., 2010; Stone et al., 2014; Wanders et al., 2015a). The proportionately larger effect of the diet on EE limits ongoing fat deposition by increasing the proportion of energy intake required for maintenance of existing tissue (Hasek et al., 2010; Wanders et al., 2015a). When the MR effect on EE is integrated over time, it effectively limits the normal age-associated expansion of adipose tissue. Dietary MR also initiates a transcriptional program in liver that coordinately down-regulates lipogenic gene expression and produces a corresponding reduction in the capacity of the liver to synthesize and export lipid (Hasek et al., 2013). In adipose tissue, dietary MR induces a depot-specific increase in lipogenic and oxidative genes that increase the capacity of these fat depots to synthesize and oxidize fatty acids (Hasek et al., 2013; Patil et al., 2015).
The initial emphasis of many studies has been to document the effects of the MR diet on specific metabolic endpoints, while more recent studies have been directed towards understanding how the reductions in methionine are being sensed and how the sensing systems are linked to specific physiological responses. Most work on EAA restriction has focused on methionine, but it will be important in future studies to extend this work to other EAAs and determine whether the beneficial responses are specific to methionine or can be reproduced to varying degrees by restricting other EAAs.
2.3 Experimental models – dietary EAA deprivation
Diets devoid of single EAAs such as leucine or threonine have also received significant attention (Blais et al., 2003; Gietzen et al., 2004; Leung and Rogers, 1971; Koehnle et al., 2003) because they produce a well-documented, albeit counterproductive series of responses including food aversion, increased EE, rapid loss of body weight (BW) and adiposity, and ultimately death (Maurin et al., 2005; Hao et al., 2005; Guo & Cavener, 2007; Anthony et al., 2004; Cheng et al., 2011; Cheng et al., 2010). In contrast, the responses to dietary methionine or leucine restriction (Wanders et al., 2015b) are fundamentally different from EAA deprivation because they do not produce harmful health effects. The primary basis for this difference is the opposing effects of the two diets on energy intake. Whereas EAA restriction produces hyperphagia (Wanders et al., 2015b; Hasek et al., 2010; Malloy et al., 2006), leucine or threonine deprivation produce food aversion and a cumulative decrease in energy intake (Guo & Cavener, 2007; Anthony et al., 2004). Interestingly, both EAA restriction and EAA deprivation produce significant increases in EE (Hasek et al., 2010; Plaisance et al., 2010; Cheng et al., 2010; Cheng et al., 2011). However, the strong anorexigenic response to EAA deprivation, combined with increased EE, produces a profound negative energy balance and unsustainable weight loss (Cheng et al., 2010; Cheng et al., 2011; Guo & Cavener, 2007; Ross-Inta et al., 2009). The mechanistic basis for this difference between EAA restriction and EAA deprivation represents a critical gap in our understanding of how the requisite sensing systems detect and mediate these opposing responses.
2.4. EAA-specific versus concentration-dependent effects of limiting dietary EAAs
The differential responses to EAA restriction versus EAA deprivation may stem from their respective impacts on circulating levels of the limited EAA, and this difference may dictate recruitment of the responses that are unique to each diet. For example, leucine deprivation produces a rapid 7-fold decrease in circulating leucine while threonine deprivation produces a 5-fold decrease in plasma threonine (Maurin et al., 2005). Studies with the 0.17% MR diet showed that it reduced plasma methionine by ~3-fold in rats (Perrone et al., 2012b; Elshorbagy et al., 2013; Elshorbagy et al., 2010). Given that restricting methionine to 0.17% produces hyperphagia while leucine deprivation produces food aversion, an important question is whether these opposing responses are EAA-specific or a function of the degree of EAA restriction. Support for the latter can be found in recent work showing that restricting dietary leucine to 0.17% increased food intake in a manner that paralleled the increase in energy intake produced by dietary MR (Wanders et al., 2015b). Thus, with leucine, the data suggest that it is the degree of restriction rather than the EAA being restricted that determines the effect on food intake. In contrast, when dietary methionine deprivation was compared to methionine restriction, we found that methionine deprivation reversed the hyperphagia produced by dietary MR, but did not cause the predicted food aversion produced by leucine deprivation (authors’ unpublished data). In fact, food consumption in control mice and methionine-deprived mice did not differ. Nevertheless, methionine deprivation produced rapid and profound weight loss despite their normal food consumption (authors’ unpublished data). Although the mechanisms are unclear, these findings suggest that the central sensing circuits linked to food aversion after leucine or threonine deprivation are not activated by methionine deprivation, and support the case for methionine-specific effects on food intake. Alternatively, when methionine or leucine restriction to 0.17% are considered, the EAAs may be increasing energy intake through a common mechanism. In fact, our recent work showed that dietary leucine restriction to 0.17% recapitulated many but not all of the physiological responses produced by dietary MR to 0.17% (Wanders et al., 2015b). In particular, dietary leucine restriction increased energy intake, increased EE, limited fat deposition, and improved glucose tolerance in a manner that was comparable between the diets (Wanders et al., 2015b). However, dietary MR reduced the capacity of the liver to conduct de novo lipogenesis and synthesize triglyceride by producing a coordinated downregulation of the rate-limiting lipogenic genes (Hasek et al., 2013). In contrast, dietary leucine restriction was without effect on hepatic lipogenic gene expression and had no effect on plasma or liver triglycerides (Wanders et al., 2015b). Considered together, these findings argue that dietary MR produces EAA-specific effects on hepatic lipid metabolism while the effects of leucine and methionine restriction on energy balance and insulin sensitivity share a common mechanism. In a more practical sense, protein restriction limits the intake of multiple EAAs in a cumulative manner that is dependent on the protein source in the meal. An important remaining question is whether the modest restriction of multiple EAAs that is produced during dietary protein restriction could alter the signaling threshold for several EAAs being sensed through a common mechanism. Stated another way, would a simultaneous restriction of leucine and methionine by 60% produce the same effect on energy balance obtained when either EAA is individually restricted by 85%? It seems clear that much additional work is needed to understand how the sensing systems that detect reductions in specific EAAs function both independently and collectively in the biological context of protein restriction.
Based on the well-documented metabolic responses to dietary MR, an important goal of our work is to develop therapeutic tools based on the sensing and signaling biology of dietary MR. We have found that concentrations of dietary methionine much below 0.17% fail to provide sufficient methionine to sustain growth, and the lower threshold seems to be concentrations below 0.12% (Forney et al., 2017). We have also investigated the upper threshold of dietary MR efficacy and found that 0.25% methionine is where the beneficial responses of methionine restriction can first be detected (Forney et al., 2017). For example, we found that restricting methionine from control levels (e.g., 0.86%) to 0.34% reduced plasma methionine from ~200 µM to ~100 µM (authors’ unpublished data), but had no discernible effect on any of the metabolic endpoints (Forney et al., 2017). The 0.17% MR diet produced an additional 2-fold reduction in plasma methionine to ~50 µM (authors’ unpublished data), which corresponds almost exactly to the 4-fold reduction in total methionine intake produced by the 0.17% MR diet (Forney et al., 2017). Viewed collectively, these findings illustrate that the beneficial metabolic effects of dietary MR are limited to a narrow, sharply-defined range of methionine intake. Our studies have shown that it is only within this narrow range of methionine restriction where hyperphagia occurs (Forney et al., 2017), suggesting that a methionine-sensing system is linked to a compensatory appetitive mechanism, but only when there is sufficient methionine in the diet to prevent weight loss through a compensatory increase in its consumption. Our studies support the operation of a similar compensatory hyperphagic mechanism when dietary leucine is restricted to 0.17% (Wanders et al., 2015b), but as noted above, more severe limitation of dietary leucine engages an aversive mechanism. Together these findings demonstrate that the EAA composition of the diet has a complex EAA-specific and concentration-dependent effect on the perceived palatability of the diet.
3. GCN2 and cellular mechanisms of essential amino acid sensing and signaling
At the cellular level, limiting the availability of EAAs limits charging of tRNAs with their corresponding AA, activating the ubiquitously expressed protein kinase, general control nondepressible 2 (GCN2). Subsequent phosphorylation of eukaryotic initiation factor 2α (eIF2α) by GCN2 limits ribosomal translation of most mRNAs (Deval et al., 2009; Palii et al., 2009; Shan et al., 2009) while selectively de-repressing the translation of genes containing specific upstream open reading frames. The combination of increased duration of ribosomal scanning and re-initiation efficiency (Palam et al., 2011) enhances recruitment of activating transcription factor 4 (ATF4) to promoter regions of additional transcription factors (CHOP, C/EBPβ, ATF3, ATF2) and regulatory proteins like asparagine synthetase (ASNS), Tribbles homolog 3 (TRB3), and the histone demethylase JMJD3 (Bunpo et al., 2009; Shan et al., 2012; Pan et al., 2003; Carraro et al., 2010; Jousse et al., 2007). ATF4 then heterodimerizes with both C/EBP family members and other ATFs, forming multimeric complexes that bind C/EBP-ATF response elements (CARE) that serve as amino acid response elements (AARE) (Chen et al., 2004; Pan et al., 2003). The ubiquitous expression of GCN2 emphasizes the high priority placed on defending against EAA deficiency through this mechanism, but the more difficult challenge has been to understand how GCN2 and other EAA sensing systems are anatomically organized to interpret subtle differences in the degree of EAA restriction and produce coordinated physiological responses that are matched to the specific EAA being reduced.
4. Central sensing of EAAs during dietary EAA restriction and deprivation
4.1. Central EAA sensing and the significance of GCN2
A model illustrating the potential anatomical sites contributing to EAA sensing during dietary MR or EAA deprivation is presented in Fig. 1, and based on the documented effects of each experimental diet in these locations, a logical case could be made that the reduction in circulating EAA produced by each diet is directly sensed and responded to in each tissue. In contrast, it is easy to envision how central sensing of EAAs could explain behavioral changes in food intake, and secondarily regulate tissue-specific responses by modulating the activity of the autonomic nervous system. For example, studies have shown that EAA-deficient diets activate GCN2 in the anterior piriform cortex (APC) and produce an aversive feeding response within 20–40 min (Maurin et al., 2005; Hao et al., 2005). Loss of function studies establish that the acute aversive response to EAA deprivation is mediated by GCN2 (Hao et al., 2005; Maurin et al., 2005). However, EAA deprivation also produces a chronic anorexigenic response that is fully intact in GCN2 null mice (Anthony et al., 2004; Guo & Cavener, 2007). Additionally, recent work has shown that the behavioral and physiological responses to dietary MR were fully intact in GCN2 null mice (Wanders et al., 2016). Collectively, these findings support the view that mechanisms additional to GCN2 are involved in EAA sensing during both dietary MR and EAA deprivation.
Figure 1.
A schematic model of the tissues and organs that participate in and respond to changes in dietary levels of EAAs. This organ-centric model proposes that each tissue senses changes in circulating EAAs directly and responds in a tissue-specific manner. Abbreviations include: anterior piriform cortex (APC), hypothalamus (hypo), dorsal vagal complex (DVC), brown adipose tissue (BAT), white adipose tissue (WAT), and essential amino acids (EAA).
It is logical that the hypothalamus would also be an important target during EAA limitation, as it receives axonal projections from the APC (Anthony and Gietzen, 2013; Gietzen and Aja, 2012) and responds directly to depletion and repletion of EAAs (Gietzen et al., 2007). Recent studies show that EAA deprivation alters anorexigenic neuropeptides in hypothalamic feeding centers (Blevins et al., 2003; Goto et al., 2010; Nakahara et al., 2012), and these centers are responsive to repletion with the limiting EAA (Goto et al., 2010). In addition, EAA deprivation activates sympathetic nervous system (SNS) outflow to brown and white adipose tissue, increasing lipid mobilization, oxidation, and uncoupled respiration (Cheng et al., 2010; Cheng et al., 2011). Cheng et al. (Cheng et al., 2011; Xia et al., 2012) propose that leucine deprivation activates the SNS by increasing hypothalamic corticotrophin releasing hormone expression in a S6K1-dependent manner, suggesting that the sensing of leucine deprivation is occurring directly in the hypothalamus. In contrast, the SNS activation produced by dietary MR or dietary protein restriction are linked to hepatic detection of EAA restriction and signaling to the hypothalamus through a secondary endocrine mechanism (Wanders et al., 2016; Wanders et al., 2017; Laeger et al., 2014a; Laeger et al., 2016). Thus, although substantial progress has been made, it seems clear that we are far from having an integrated picture of the EAA sensing and signaling mechanisms that function centrally and peripherally to produce the unique and overlapping responses produced by EAA deprivation versus EAA restriction.
5. Peripheral EAA sensing by GCN2 and noncanonical eIF2 kinases
5.1. The case for EAA sensing in the liver
When the coordinated changes in ingestive behavior and activation of the SNS produced by dietary MR are considered, it seems logical that central EAA sensing mechanisms would be involved. However, emerging evidence points to the importance of peripheral tissues where EAA sensing mechanisms are linked to the resulting responses through an endocrine mechanism (Stone et al., 2014). Given that the hepatic portal system perfuses the liver with blood draining directly from the digestive tract, the liver is ideally positioned to sense and respond to changes in dietary EAA composition. Blood plasma is filtered in the liver through the endothelial lining of sinusoids and the filtrate bathes the hepatocytes with interstitial fluid reflecting the composition of consumed meals. In the case of dietary MR, the hepatocytes immediately sense the 5-fold decrease in dietary methionine, the absence of cysteine, and respond by activating the transcriptional programs of the cytoprotective, integrated stress response (ISR) (Wanders et al., 2016). The eIF2 kinase, GCN2, mediates this response by phosphorylating the α-subunit of eIF2, which alters gene-specific translation to produce the ISR (Sood et al., 2000; Visweswaraiah et al., 2011). ATF4 is an important, preferentially translated target of this adaptive program, but ATF4 plays an additional role in glucose homeostasis (Seo et al., 2009; Yoshizawa et al., 2009) by activating transcription of the metabolic hormone, FGF21 (De Sousa-Coelho et al., 2012; Kilberg et al., 2009). More importantly, dietary MR produces a robust and persistent activation of transcription and release of hepatic FGF21 (Stone et al., 2014; Wanders et al., 2015a; Stone et al., 2015; Ables et al., 2012)(4,5,15,34). Pharmacological and transgenic approaches have shown that FGF21 is a powerful metabolic regulator in the context of glucose homeostasis, lipid metabolism, and energy balance (Coskun et al., 2008; Holland et al., 2013a; Kharitonenkov et al., 2005; Adams et al., 2012a; Xu et al., 2009b; Bookout et al., 2013; Xu et al., 2009a). However, the high levels of FGF21 produced by these approaches make it unclear whether the modest physiological increases in plasma FGF21 produced by dietary MR are sufficient to link dietary MR to its metabolic responses. Loss of function approaches have been used to address the relative importance of these individual signaling components (e.g., GCN2, FGF21) as mediators of the complex physiological responses to dietary MR, and will be addressed in subsequent sections.
5.2. Hepatic sensing of dietary MR by GCN2, PERK and CREBH
The known function and ubiquitous expression of GCN2 make it a prime candidate as an essential sensor and mediator of the biological effects of dietary MR. Based on comprehensive in vivo metabolic phenotyping and ex vivo analysis of harvested tissues, we found that all components of the metabolic phenotype produced by MR were fully intact in Gcn2−/− mice (Wanders et al., 2016). MR produced a comparable reduction in accrual of body weight and adiposity, and a comparable increase in energy intake and EE within each genotype (Wanders et al., 2016). In addition, dietary MR produced a comparable increase in hepatic FGF21 expression and release of FGF21 after 6 h, 6 d, and 14 weeks in both genotypes (Wanders et al., 2016), arguing that GCN2 is not an essential sensor of reduced methionine linked to induction of hepatic FGF21. We have previously shown that MR produces extensive remodeling and browning of inguinal white adipose tissue (IWAT) in conjunction with activation of thermogenic gene expression in brown adipose tissue (BAT) (Hasek et al., 2010; Plaisance et al., 2010; Hasek et al., 2013; Ghosh et al., 2014; Wanders et al., 2015a; Patil et al., 2015). We found no evidence that the transcriptional responses to dietary MR in IWAT, BAT, or liver were compromised in Gcn2−/− mice (Wanders et al., 2016). Additional major physiological responses to dietary MR include increased overall glucose utilization, enhanced suppression of hepatic glucose production by insulin, and increased insulin-dependent glucose uptake in peripheral tissues (Stone et al., 2014; Wanders et al., 2015a). Using hyperinsulinemic-euglycemic clamps to evaluate these responses, Wanders et al. (Wanders et al., 2016) showed that dietary MR produced comparable increases in all measures of in vivo insulin sensitivity in wild type (WT) and Gcn2−/− mice. Together, these studies establish that GCN2 is not an essential mediator of any of the metabolic responses to dietary MR.
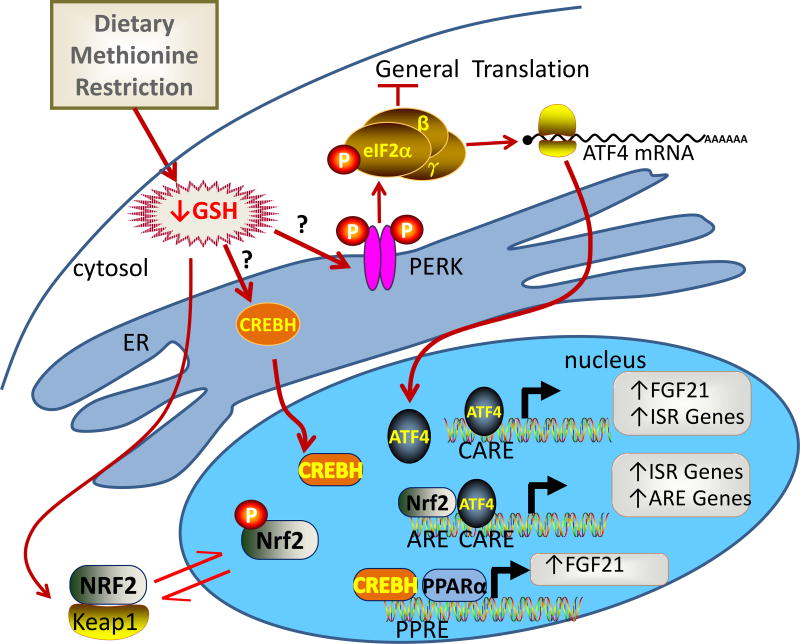
Our original focus on GCN2 stemmed from the fact that it phosphorylates eIF2α and activated eIF-2α produces the ISR (Sood et al., 2000; Visweswaraiah et al., 2011) by selectively increasing translation of ATF4. Our recent work established that phosphorylation and activation of hepatic eIF2α by dietary MR was uncompromised in the absence of GCN2, suggesting signaling through the eIF2 kinase, PKR-like Endoplasmic Reticulum Kinase (PERK) (Wanders et al., 2016). PERK is localized within the endoplasmic reticulum (ER) and while it is normally activated by ER stress, it can also be activated by reductions in glutathione (GSH) (Cullinan and Diehl, 2004; Harding et al., 2003). GSH is concentrated in the ER where it plays an indispensable role in disulfide bond formation and protein folding. PERK is normally activated when there is a mismatch between the protein folding demands placed on the ER and its capacity to process non-native disulfides (Donnelly et al., 2013). Activation of PERK by misfolded proteins is usually accompanied by activation of Inositol-requiring enzyme 1α (IRE1α) and activating transcription factor 6 (ATF6) (Donnelly et al., 2013), which initiate the transcriptional program of the unfolded protein response (UPR). IRE1α-dependent signaling leads to generation of spliced X-box-binding protein-1 (XBP1), which in concert with ATF6 activation, promotes expression of ER-localized chaperones that restore proper protein folding in the ER (Lee and Ozcan, 2014). Therefore, PERK-dependent activation of eIF2α lessens the requirement for GSH by slowing the flow of client proteins through the ER, but it also elicits the activation of the pro-survival transcription factor, nuclear factor-erythroid 2-related factor 2 (NRF2) (Cullinan & Diehl, 2004). Thus, co-activation of PERK and NRF2 link ER stress to a transcriptional program that increases production of GSH by increasing amino acid transport while simultaneously decreasing GSH utilization by inhibiting protein translation. We found that the absence of GCN2 did not compromise activation of eIF2α and PERK (Wanders et al., 2016), but an equally important question was whether MR was activating the UPR. To answer this question, we compared the induction of multiple transcriptional targets of ER Stress/UPR, NRF2 activation, and eIF2α/ATF4 activation in WT and GCN2−/− mice. Our strategy was to determine whether ER stress was upstream of PERK activation, whether PERK activation led to NRF2 activation, and whether MR activated the IRE1α and ATF6 components of the UPR. In Wanders et al. (Wanders et al., 2016) we showed that MR produced (1) equivalent induction of eIF2/ATF4 targets in both genotypes, targets of NRF2 were up- and down-regulated as predicted when NRF2 is activated, and (3) none of the targets indicative of ER stress/UPR were increased by MR in either genotype. Collectively, these data make the case that MR is activating the ISR and the NRF2-dependent antioxidant response program in liver via a GCN2-independent mechanism that does not involve ER stress. Our findings provide the first evidence of a nutrient-sensing system for MR that activates hepatic PERK through a non-canonical, GSH-dependent mechanism (Wanders et al., 2016).
To further explore whether the activation of PERK was mediated by the MR-dependent reduction in hepatic GSH, experiments were conducted in which 0.2% cysteine, the rate limiting substrate for formation of GSH, was added back to the MR diet. Data from these experiments showed that adding back cysteine to the MR diet (1) reversed the reduction in hepatic GSH produced by MR, (2) reversed the MR-induced increase in hepatic FGF21, (3) reversed the increase in EE produced by MR, (4) reversed the induction of hepatic NRF2-sensitive genes, and (5) reversed the MR-dependent activation of hepatic PERK and eIF2α (Wanders et al., 2016). These findings link the MR-induced reduction in hepatic GSH to many of the transcriptional and physiological responses produced by the diet. It will be important to confirm these findings in future loss of function studies that test the overall importance of PERK to MR’s transcriptional activation of hepatic FGF21 and the downstream biological effects of dietary MR.
A cadre of current studies make a compelling case that the liver is an essential site for sensing of dietary MR. These studies also show that the induction of hepatic FGF21 by dietary MR is a critical link to many of the biological responses produced by the MR diet. Therefore, understanding the mechanisms of transcriptional activation of FGF21 by MR is essential to understanding how the sensing process is linked to ATF4 recruitment to the FGF21 promoter. A potential alternative mechanism of transactivating the FGF21 promoter is provided by CREBH, a liver-specific form of CREB called CREBH that mediates fasting-dependent induction of FGF21 (Kim et al., 2014; Kim et al., 2015). CREBH is an ER membrane-anchored transcription factor that is released and activated by regulated intramembrane proteolysis (Zhang et al., 2006). Zhang et al. (Zhang et al., 2006; Zhang et al., 2012) showed that ER stress activates cleavage of CREBH so we hypothesized that the MR-dependent reduction in hepatic GSH that activates PERK might also produce activation of CREBH. This hypothesis will need to be pursued in future studies using CREBH−/− mice, but preliminary studies from our lab showed that the short-term induction of hepatic FGF21 by dietary MR was partially compromised in CREBH−/− mice (author’s unpublished data). Cell fractionation approaches can also be used to test for MR-dependent increases in proteolytic release of CREBH from the ER. Chromatin immunoprecipitation would provide a further approach to test for MR-dependent increases in recruitment of CREBH to PPRE sites in the FGF21 promoter. An important unresolved question is whether signaling through CREBH by the MR diet is essential to the chronic effects of the diet on hepatic FGF21 and its associated biological effects. A proposed model showing the GCN2-independent, GSH-dependent signaling through PERK to eIF2A/ATF4 and NRF2 and CREBH is presented in Fig. 2.
Figure 2.
Schematic model of proposed mechanisms of GCN2-independent signaling by dietary MR in the liver. The diet-induced decrease is GSH activates PERK, which is proposed to activate eIF2/ATF4, NRF2, and their corresponding ISR and NRF2 transcriptional programs. The decrease in GSH produces GSH-sensitive proteolytic activation of CREBH, which migrates to the nucleus and functions in tandem with NRF2 and ATF4 to activate FGF21 transcription. Abbreviations include: glutathione (GSH), PRK-like ER kinase (PERK), activating transcription factor 4 (ATF4), eukaryotic initiation factor 2 (eIF2α), cAMP response element binding protein H (CREBH), Nuclear factor (erythroid-derived 2)-like 2 (NRF2), Kelch-like ECH-associated protein 1 (Keap1), peroxisome proliferator activated receptor αk (PPARα), peroxisome proliferator response element (PPRE), antioxidant response element (ARE), CHOP amino acid response element (CARE), integrative stress response (ISR), endoplasmic reticulum (ER).
In previous work, we addressed the significance of the MR-induced reduction in hepatic GSH from the additional perspective of GSH’s role as an essential co-factor of GSH peroxidases (Stone et al., 2014). These peroxidases play a key role in regulating insulin signaling intensity because of their role in regulating the activity of the protein tyrosine phosphatase, phosphatase and tensin homolog (PTEN). PTEN regulates insulin signaling intensity by degrading PIP3 formed by PI3K after insulin receptor activation, but insulin also activates NADPH oxidases that temporarily inactivate PTEN by oxidizing the cysteine in their active sites (Lee et al., 2002; Meng et al., 2004; Goldstein et al., 2005; Loh et al., 2009). GSH-dependent peroxidases play a key role here by reducing the oxidized cysteine in the active site and shift PTEN from inactive back to its active state. Insulin-dependent formation of PIP3 activates protein kinase B (Akt) by activating phosphoinositide-dependent kinase (PDK), but because activated PTEN degrades PIP3 to inactive PIP2, we proposed that dietary MR amplifies insulin-dependent Akt phosphorylation in the liver by reducing GSH-dependent reactivation of PTEN. Support for both components of this hypothesis were obtained with in vivo and in vitro data (Stone et al., 2014). These findings provide an additional GSH-dependent mechanism through which dietary MR is affecting hepatic signaling, and make the case that methionine is unique in its role to influence glucose homeostasis when dietary methionine is limited.
6. Role of FGF21 as a mediator of the biological response to dietary MR
The most significant unanswered question in the field of dietary MR is understanding how the sensing of reduced dietary methionine is translated into the complex series of biochemical, physiological, and behavioral responses that are produced by the MR diet. A significant advance in the field came with the observation that within hours of its introduction, the MR diet produced a 5-fold increase in hepatic expression and release of FGF21 (Stone et al., 2014; Wanders et al., 2016). The responses attributable to FGF21 in the context of glucose homeostasis, lipid metabolism, and energy balance have been well documented (Coskun et al., 2008; Holland et al., 2013b; Kharitonenkov et al., 2005; Adams et al., 2012a; Xu et al., 2009b; Bookout et al., 2013; Xu et al., 2009a), but many of these studies used pharmacological manipulation of FGF21. However, a careful comparison reveals that many of the effects of FGF21 on energy balance, insulin sensitivity, and adipose tissue remodeling are fully reproduced by dietary MR (Ghosh et al., 2014; Hasek et al., 2010; Hasek et al., 2013; Stone et al., 2014; Wanders et al., 2015a). To test the hypothesis that FGF21 is an endocrine mediator of the biological responses to dietary MR, a comprehensive metabolic phenotyping approach was used to assess the responses to MR in mice lacking FGF21 (FGF21−/−) (Wanders et al., 2017).
6.1 Role of FGF21 in mediating effects of MR on energy balance
With respect to energy balance, the absence of FGF21 prevented the normal MR-induced increase in energy intake and EE in FGF21−/− mice. Surprisingly, consumption of the MR diet was reduced in FGF21−/− mice and this produced a loss of weight and adiposity that was comparable to the losses observed in WT mice on the MR diet (Wanders et al., 2017). Expressing energy intake per unit BW showed that the weight loss produced by the MR diet in FGF21−/− mice was exactly proportional to the reduction in consumption of the MR diet in these mice (Wanders et al., 2017). So in WT mice, dietary MR increased energy intake but it reduced BW and fat accumulation by increasing EE, whereas in Fgf21−/− mice, dietary MR had no effect on EE and reduced BW and adiposity by reducing food consumption (Wanders et al., 2017).
Although all the sites where FGF21 is acting remain ill-defined, it is well established that FGF21 acts centrally to increase EE through an increase in SNS outflow to adipose tissue (Holland et al., 2013a; Bookout et al., 2013; Owen et al., 2014; Douris et al., 2015). Previous studies have shown that dietary MR increases core temperature, EE, and thermogenic function in BAT and WAT through increases in SNS stimulation of adipose tissue (Hasek et al., 2010; Plaisance et al., 2010). The loss of this response in FGF21−/− mice makes a compelling case that the MR-dependent increase in hepatic FGF21 is the key event linking MR to its metabolic effects on EE (Wanders et al., 2017).
6.2 Role of FGF21 in mediating effects of dietary MR on insulin sensitivity
Consuming the MR diet for 8-10 weeks produces an average 2.5-fold increase in insulin-dependent glucose utilization (Stone et al., 2014; Wanders et al., 2015a; Wanders et al., 2016). The MR-dependent improvements in insulin sensitivity are thought to accrue in part from MR-induced reductions in BW and adiposity and in part from FGF21-dependent enhancement of insulin signaling in specific tissues. The study in FGF21−/− mice offered the opportunity to address this question because of the comparable reductions in BW and adiposity in WT and FGF21−/− mice on the MR diet. Hyperinsulinemic-euglycemic clamps of the two genotypes showed that MR produced a 3-fold increase in glucose infusion rate (GIF) in WT mice but only a 2-fold increase in GIF in FGF21−/− mice (Wanders et al., 2017). One interpretation is that the MR-induced loss of weight and increase in FGF21 account for two-thirds and one-third, respectively, in the improvements in insulin sensitivity produced by MR. Based on comparisons of insulin-dependent glucose uptake across multiple tissues, the heart and adipose tissue were the primary sites where MR was able to produce greater improvements in insulin sensitivity in WT compared to FGF21−/− mice (Wanders et al., 2017). These findings in adipose tissue are not surprising because of the well-documented direct glycemic effects of FGF21 in this tissue (Ding et al., 2012; Kharitonenkov et al., 2005; Adams et al., 2012b; Stone et al., 2014). However, the present study provides intriguing new evidence that the heart is an important target for the enhancement of insulin-dependent glucose uptake by dietary MR, and that FGF21 is required for this effect (Wanders et al., 2017). The heart makes a large contribution to whole-body glucose disposal in the mouse, so it seems likely that the MR-dependent increase in FGF21 is an important element of the diet’s effect on overall insulin-dependent glucose utilization. These findings are the first to show that physiologically-relevant alterations of FGF21 enhance insulin-dependent glucose uptake in the heart (Wanders et al., 2017).
6.3 Role of FGF21 in mediating transcriptional effects of dietary MR in adipose tissue
We have shown in previous work that dietary MR produces extensive remodeling of WAT and activation of thermogenic gene expression in BAT (Wanders et al., 2015a; Patil et al., 2015; Hasek et al., 2010; Plaisance et al., 2010). The MR diet also remodels WAT lipid metabolism by upregulating lipogenic gene expression in these tissues (Hasek et al., 2013). In alignment with the proposed role of FGF21 as acting centrally to increase sympathetic nervous system (SNS) activity and induce browning of WAT and thermogenesis in BAT (Owen et al., 2014; Douris et al., 2015), we hypothesized that the transcriptional induction of thermogenic genes in both tissues would be compromised in Fgf21−/− mice fed the MR diet. In a side by side comparison of WT and FGF21−/− mice, the absence of FGF21 prevented MR from increasing Ucp1, Bmp8b, Cidea, and Elovl3 mRNA in BAT from Fgf21−/− mice (Wanders et al., 2017). Similar results were obtained in IWAT where MR produced significant induction of Ucp1, Cox7a1, Cox8b, and Cidea mRNA in WT but not in Fgf21−/− mice. The MR diet was also ineffective in inducing thermogenic or lipogenic gene expression in IWAT of Fgf21−/− mice (Wanders et al., 2017). Collectively, these findings show that MR is ineffective in inducing thermogenic or lipogenic programs in BAT and WAT of Fgf21−/− mice, suggesting that MR uses FGF21 as an endocrine signal to affect these responses in WT mice (Wanders et al., 2017).
6.4 Role of FGF21 in the Effects of Dietary MR on Hepatic Lipogenic Genes
Dietary MR induces a transcriptional program that coordinately reduces expression of lipogenic genes in liver (Hasek et al., 2013). Our work supports the view that FGF21 is an essential mediator of MR effects in adipose tissue (Wanders et al., 2017), but it is unclear whether FGF21 is essential to the remodeling of hepatic lipid metabolism. However, given the direct role of dietary MR in activating ATF4- and NRF2-dependent transcriptional programs in the liver, we tested whether dietary MR would produce the expected reductions in lipogenic gene expression in liver of Fgf21−/− mice. Dietary MR produced a comparable reduction in expression of Scd1 mRNA and hepatic triglycerides in both genotypes, illustrating that FGF21 is not necessary for these responses to dietary MR (Wanders et al., 2017). In addition, the previously reported induction of NRF2-sensitive and ATF4-sensitive genes in the liver by dietary MR was uncompromised in Fgf21−/− mice, establishing that FGF21 is not the mediator of these effects of MR (Wanders et al., 2017). It seems likely that these responses may be direct effects of methionine on hepatic GSH metabolism and signaling.
7. Perspectives and Future Directions
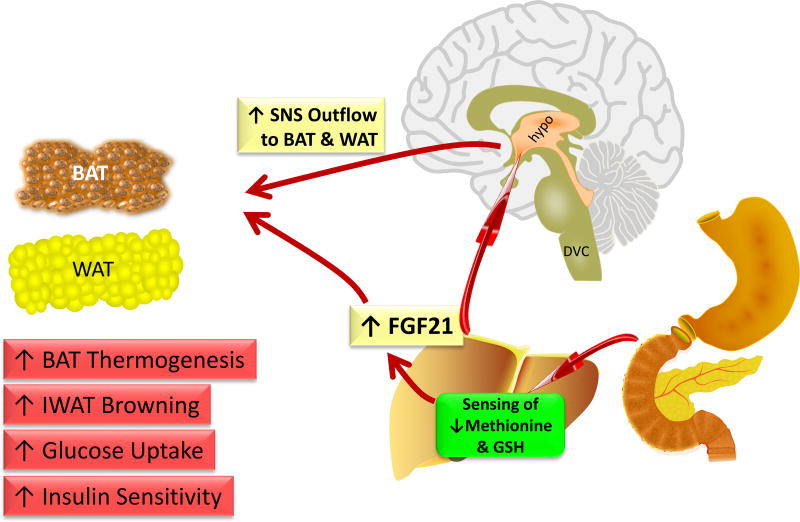
A conceptual model of the anatomical organization of the sensing and communication networks that are either known or proposed to be involved in coordinating the complex physiological responses to dietary MR are presented in Figure 3. Using a comprehensive series of experimental approaches, we have obtained compelling support for our hypothesis that dietary MR activates hepatic PERK and possibly CREBH through a GSH-sensitive mechanism, and that activated PERK and CREBH increase downstream signaling through eIF2α and NRF2 to produce coordinated transcriptional activation of hepatic FGF21 (Wanders et al., 2016). We propose that FGF21 is the critical mediator of all the physiological responses to MR except its effects on hepatic lipid metabolism. An important remaining objective is to identify the sites where FGF21 is acting to produce the components of the overall biological response to dietary MR. For example, are the documented effects of dietary MR on insulin sensitivity in adipose tissue the result of direct actions of FGF21 signaling in this tissue, or are they secondary to the FGF21-mediated remodeling of adipose tissue that results from FGF21-dependent increases in SNS outflow to adipose tissue. These questions will be addressed in future studies using a tissue-specific loss of function approach that alternatively and selectively deletes FGF21 signaling in adipose tissue or the hypothalamus. The specific cell types within the hypothalamus that sense FGF21 are not yet defined so future studies will be needed to precisely identify the population of responsive neurons within the hypothalamus. Success in these experiments will guide the development of targeting vectors and the corresponding loss of function models that will be needed to provide definitive in vivo identification of the neurons linking MR-dependent increases in FGF21 to SNS activation. An additional challenging aspect of these experiments will be the interdependence of the multiple components of the phenotype (e.g., adiposity, insulin sensitivity) and correctly mapping the loss of MR-dependent responses to the anatomical site where FGF21 is acting to produce them.
Figure 3.
Model of proposed anatomical organization of the sensing and signaling mechanisms which produce the coordinated biochemical, transcriptional, physiological, and behavioral responses to dietary methionine restriction. The model proposes that the liver senses the reduction in plasma methionine and the associated decrease in hepatic glutathione signals to activate hepatic transcription and release of FGF21. The model proposes that increased SNS outflow to adipose tissue is produced by FGF21 acting in the hypothalamus where it may also regulate appetitive behavior. FGF21 is also proposed to act directly in adipose tissue to affect remodeling of white adipose tissue and activate thermogenesis in brown adipose tissue. FGF21 also acts directly in adipose tissue to enhance insulin sensitivity and increase glucose uptake and utilization. The net result is increased energy expenditure and decreased deposition of fat in adipose tissue. Abbreviations include: hypothalamus (hypo), dorsal vagal complex (DVC), brown adipose tissue (BAT), white adipose tissue (WAT), sympathetic nervous system (SNS)
The liver is the key anatomical site for sensing of dietary methionine restriction
The liver responds to dietary methionine restriction by releasing FGF21
FGF21 mediates many of the metabolic effects of dietary methionine restriction
Acknowledgments
This work was supported in part by ADA 1-12-BS-58 (TWG), NIH 2RO1 DK-096311 (TWG), and the Mouse Metabolic Phenotyping Center Consortium NIH U24 DK-076169. This work made use of the Genomics and Cell Biology and Bioimaging core facilities supported by NIH P30-GM-118430 (TWG) and NIH P30 DK-072476.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No potential conflicts of interest relevant to this article were reported. TWG, LAF, KPS, and DW contributed to the writing and editing of the manuscript. LAF, KPS, and DW conducted the experiments described in the manuscript. TWG is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS ONE. 2012;7:e51357. doi: 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AC, Coskun T, Rovira AR, Schneider MA, Raches DW, Micanovic R, Bina HA, Dunbar JD, Kharitonenkov A. Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLoS ONE. 2012a;7:e38438. doi: 10.1371/journal.pone.0038438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol.Metab. 2012b;2:31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TG, Gietzen DW. Detection of amino acid deprivation in the central nervous system. Curr.Opin.Clin.Nutr.Metab Care. 2013;16:96–101. doi: 10.1097/MCO.0b013e32835b618b. [DOI] [PubMed] [Google Scholar]
- Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol.Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- Anthony TG, Reiter AK, Anthony JC, Kimball SR, Jefferson LS. Deficiency of dietary EAA preferentially inhibits mRNA translation of ribosomal proteins in liver of meal-fed rats. Am.J Physiol Endocrinol.Metab. 2001;281:E430–E439. doi: 10.1152/ajpendo.2001.281.3.E430. [DOI] [PubMed] [Google Scholar]
- Blais A, Huneau JF, Magrum LJ, Koehnle TJ, Sharp JW, Tome D, Gietzen DW. Threonine deprivation rapidly activates the system A amino acid transporter in primary cultures of rat neurons from the essential amino acid sensor in the anterior piriform cortex. J Nutr. 2003;133:2156–2164. doi: 10.1093/jn/133.7.2156. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Teh PS, Wang CX, Gietzen DW. Effects of amino acid deficiency on monoamines in the lateral hypothalamus (LH) in rats. Nutr.Neurosci. 2003;6:291–299. doi: 10.1080/10284150310001622248. [DOI] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat.Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse JD, Dixon BM. Dietary protein in weight management: a review proposing protein spread and change theories. Nutr.Metab (Lond) 2012;9:81. doi: 10.1186/1743-7075-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol.Chem. 2009;284:32742–32749. doi: 10.1074/jbc.M109.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro V, Maurin AC, Lambert-Langlais S, Averous J, Chaveroux C, Parry L, Jousse C, Ord D, Ord T, Fafournoux P, Bruhat A. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2alpha/ATF4 pathway. PLoS ONE. 2010;5:e15716. doi: 10.1371/journal.pone.0015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol.Chem. 2004;279:50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F, Guo F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59:17–25. doi: 10.2337/db09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhang Q, Meng Q, Xia T, Huang Z, Wang C, Liu B, Chen S, Xiao F, Du Y, Guo F. Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating sympathetic nervous system. Molecular Endocrinology. 2011;25:1624–1635. doi: 10.1210/me.2011-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol.Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem.J. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- Deval C, Chaveroux C, Maurin AC, Cherasse Y, Parry L, Carraro V, Milenkovic D, Ferrara M, Bruhat A, Jousse C, Fafournoux P. Amino acid limitation regulates the expression of genes involved in several specific biological processes through GCN2-dependent and GCN2-independent pathways. FEBS J. 2009;276:707–718. doi: 10.1111/j.1742-4658.2008.06818.x. [DOI] [PubMed] [Google Scholar]
- Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, Kliewer SA. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci. 2013;70:3493–3511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Stevanovic D, Fisher FM, Cisu TI, Chee MJ, Ly NN, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, Maratos-Flier E. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology. 2015;156:2470–2481. doi: 10.1210/en.2014-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Meng Q, Zhang Q, Guo F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids. 2012;43:725–734. doi: 10.1007/s00726-011-1123-8. [DOI] [PubMed] [Google Scholar]
- Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, Plummer JD, Orentreich DS, Orentreich N, Refsum H, Perrone CE. Effect of taurine and N-acetylcysteine on methionine restriction-mediated adiposity resistance. Metabolism. 2013;62:509–517. doi: 10.1016/j.metabol.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Elshorbagy AK, Valdivia-Garcia M, Refsum H, Smith AD, Mattocks DA, Perrone CE. Sulfur amino acids in methionine-restricted rats: Hyperhomocysteinemia. Nutrition. 2010;26:1201–1204. doi: 10.1016/j.nut.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Forney LA, Wanders D, Stone KP, Pierse A, Gettys TW. Concentration-dependent linkage of dietary methionine restriction to the components of its metabolic phenotype. Obesity (Silver Spring) 2017;25:730–738. doi: 10.1002/oby.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Forney LA, Wanders D, Stone KP, Gettys TW. An integrative analysis of tissue-specific transcriptomic and metabolomic responses to short-term dietary methionine restriction in mice. PLoS ONE. 2017;12:e0177513. doi: 10.1371/journal.pone.0177513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wanders D, Stone KP, Van NT, Cortez CC, Gettys TW. A systems biology analysis of the unique and overlapping transcriptional responses to caloric restriction and dietary methionine restriction in rats. FASEB J. 2014;28:2577–2590. doi: 10.1096/fj.14-249458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen DW, Aja SM. The brain's response to an essential amino acid-deficient diet and the circuitous route to a better meal. Mol.Neurobiol. 2012;46:332–348. doi: 10.1007/s12035-012-8283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annual Review of Nutrition. 2007;27:63–78. 63–78. doi: 10.1146/annurev.nutr.27.061406.093726. [DOI] [PubMed] [Google Scholar]
- Gietzen DW, Ross CM, Hao S, Sharp JW. Phosphorylation of eIF2alpha is involved in the signaling of indispensable amino acid deficiency in the anterior piriform cortex of the brain in rats. J Nutr. 2004;134:717–723. doi: 10.1093/jn/134.4.717. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosby AK, Conigrave AD, Lau NS, Iglesias MA, Hall RM, Jebb SA, Brand-Miller J, Caterson ID, Raubenheimer D, Simpson SJ. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS ONE. 2011;6:e25929. doi: 10.1371/journal.pone.0025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Nagao K, Bannai M, Takahashi M, Nakahara K, Kangawa K, Murakami N. Anorexia in rats caused by a valine-deficient diet is not ameliorated by systemic ghrelin treatment. Neuroscience. 2010;166:333–340. doi: 10.1016/j.neuroscience.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol.Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hasek BE, Boudreau A, Shin J, Feng D, Hulver M, Van N, Laque A, Stewart LK, Stone K, Wanders D, Ghosh S, Pessin JE, Gettys TW. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats. Diabetes. 2013;62:3362–3372. doi: 10.2337/db13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasek BE, Stewart LK, Henagan TM, Boudreau A, Lenard NR, Black C, Shin J, Huypens P, Malloy V, Plaisance EP, Krajcik RA, Orentreich N, Gettys TW. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2010;299:R728–R739. doi: 10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kuzminski CM, Bauer S, Wade M, Singhal E, Cheng C, Volk K, Kuo M, et al. An FGF21-Adiponectin-Ceramide Axis Controls Energy Expenditure and Insulin Action in Mice. Cell Metab. 2013a:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013b;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Johnson FB. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS ONE. 2014;9:e97729. doi: 10.1371/journal.pone.0097729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C, Deval C, Maurin AC, Parry L, Cherasse Y, Chaveroux C, Lefloch R, Lenormand P, Bruhat A, Fafournoux P. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol.Chem. 2007;282:15851–15861. doi: 10.1074/jbc.M611723200. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol.Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Mendez R, Chen X, Fang D, Zhang K. Lysine Acetylation of CREBH Regulates Fasting-Induced Hepatic Lipid Metabolism. Mol.Cell Biol. 2015;35:4121–4134. doi: 10.1128/MCB.00665-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Mendez R, Zheng Z, Chang L, Cai J, Zhang R, Zhang K. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor alpha to regulate metabolic hormone FGF21. Endocrinology. 2014;155:769–782. doi: 10.1210/en.2013-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S, Anthony TG, avener D, efferson LS. Nutrient signaling through mammalian GCN2. Topics in Current Genetics. 2004;7:113–130. [Google Scholar]
- Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr. 2003;133:2331–2335. doi: 10.1093/jn/133.7.2331. [DOI] [PubMed] [Google Scholar]
- Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, Gettys TW, Collier JJ, Munzberg H, Morrison CD. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 that Is Delayed by the Absence of GCN2. Cell Rep. 2016;16:707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014a;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Reed SD, Henagan TM, Fernandez DH, Taghavi M, Addington A, Munzberg H, Martin RJ, Hutson SM, Morrison CD. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am.J.Physiol Regul.Integr.Comp Physiol. 2014b;307:R310–R320. doi: 10.1152/ajpregu.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson KR, Russo KA, Fang Y, Mohajerani N, Goodson ML, Ryan KK. Sex Differences in the Hormonal and Metabolic Response to Dietary Protein Dilution. Endocrinology. 2017;158:3477–3487. doi: 10.1210/en.2017-00331. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat.Commun. 2014;5:3592–3603. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol.Chem. 2014;289:1203–1211. doi: 10.1074/jbc.R113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol.Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, Delibegovic M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13:817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung PM, Rogers QR. Importance of prepyriform cortex in food-intake response of rats to amino acids. Am J Physiol. 1971;221:929–935. doi: 10.1152/ajplegacy.1971.221.3.929. [DOI] [PubMed] [Google Scholar]
- Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5:305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Malloy VL, Perrone CE, Mattocks DA, Ables GP, Caliendo NS, Orentreich DS, Orentreich N. Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice. Metabolism. 2013;62:1651–1661. doi: 10.1016/j.metabol.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, Fafournoux P. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol.Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Berthoud HR. Neurobiology of nutrition and obesity. Nutr.Rev. 2007;65:517–534. doi: 10.1301/nr.2007.dec.517-534. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Reed SD, Henagan TM. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul.Integr.Comp Physiol. 2012;302:R917–R928. doi: 10.1152/ajpregu.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Takata S, Ishii A, Nagao K, Bannai M, Takahashi M, Murakami N. Somatostatin is involved in anorexia in mice fed a valine-deficient diet. Amino Acids. 2012;42:1397–1404. doi: 10.1007/s00726-011-0836-z. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 Acts Centrally to Induce Sympathetic Nerve Activity, Energy Expenditure, and Weight Loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol.Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids. 2009;37:79–88. doi: 10.1007/s00726-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Chen H, Siu F, Kilberg MS. Amino acid deprivation and endoplasmic reticulum stress induce expression of multiple activating transcription factor-3 mRNA species that, when overexpressed in HepG2 cells, modulate transcription by the human asparagine synthetase promoter. J Biol.Chem. 2003;278:38402–38412. doi: 10.1074/jbc.M304574200. [DOI] [PubMed] [Google Scholar]
- Patil Y, Dille KN, Burk DH, Cortez CC, Gettys TW. Cellular and molecular remodeling of inguinal adipose tissue mitochondria by dietary methionine restriction. J Nutr.Biochem. 2015;26:1235–1247. doi: 10.1016/j.jnutbio.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CE, Malloy VL, Orentreich DS, Orentreich N. Metabolic Adaptations to Methionine Restriction that Benefit Health and Lifespan in Rodents. Exp.Gerontol. 2012a;48:654–660. doi: 10.1016/j.exger.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Perrone CE, Mattocks DA, Hristopoulos G, Plummer JD, Krajcik RA, Orentreich N. Methionine restriction effects on 11β-HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J Lipid Res. 2008;49:12–23. doi: 10.1194/jlr.M700194-JLR200. [DOI] [PubMed] [Google Scholar]
- Perrone CE, Mattocks DA, Jarvis-Morar M, Plummer JD, Orentreich N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism. 2009;59:1000–1011. doi: 10.1016/j.metabol.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Perrone CE, Mattocks DA, Plummer JD, Chittur SV, Mohney R, Vignola K, Orentreich DS, Orentreich N. Genomic and Metabolic Responses to Methionine-Restricted and Methionine-Restricted, Cysteine-Supplemented Diets in Fischer 344 Rat Inguinal Adipose Tissue, Liver and Quadriceps Muscle. J Nutrigenet.Nutrigenomics. 2012b;5:132–157. doi: 10.1159/000339347. [DOI] [PubMed] [Google Scholar]
- Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisance EP, Henagan TM, Echlin H, Boudreau A, Hill KL, Lenard NR, Hasek BE, Orentreich N, Gettys TW. Role of β-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol. 2010;299:R740–R750. doi: 10.1152/ajpregu.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res.Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Ross-Inta CM, Zhang YF, Almendares A, Giulivi C. Threonine-deficient diets induced changes in hepatic bioenergetics. Am J Physiol Gastrointest.Liver Physiol. 2009;296:G1130–G1139. doi: 10.1152/ajpgi.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Fortuno ES, III, Suh JM, Stenesen D, Tang W, Parks EJ, Adams CM, Townes T, Graff JM. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58:2565–2573. doi: 10.2337/db09-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Fu L, Balasubramanian MN, Anthony TG, Kilberg MS. ATF4-dependent Regulation of the JMJD3 Gene during Amino Acid Deprivation Can be Rescued in Atf4-deficient Cells by Inhibition of Deacetylation. J Biol.Chem. 2012;287:36393–36403. doi: 10.1074/jbc.M112.399600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Ord D, Ord T, Kilberg MS. Elevated ATF4 expression, in the absence of other signals, is sufficient for transcriptional induction via CCAAT enhancer-binding protein-activating transcription factor response elements. J Biol.Chem. 2009;284:21241–21248. doi: 10.1074/jbc.M109.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite. 1997;28:201–213. doi: 10.1006/appe.1996.0077. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes.Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de CR, Simpson SJ, Le Couteur DG. Dietary Protein to Carbohydrate Ratio and Caloric Restriction: Comparing Metabolic Outcomes in Mice. Cell Rep. 2015;11:1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics. 2000;154:787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KP, Wanders D, Calderon LF, Spurgin SB, Scherer PE, Gettys TW. Compromised responses to dietary methionine restriction in adipose tissue but not liver of ob/ob mice. Obesity. 2015;23:1836–1844. doi: 10.1002/oby.21177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63:3721–3733. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J.Gerontol.A Biol.Sci.Med.Sci. 2009;64:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visweswaraiah J, Lageix S, Castilho BA, Izotova L, Kinzy TG, Hinnebusch AG, Sattlegger E. Evidence That Eukaryotic Translation Elongation Factor 1A (eEF1A) Binds the Gcn2 Protein C Terminus and Inhibits Gcn2 Activity. J Biol.Chem. 2011;286:36568–36579. doi: 10.1074/jbc.M111.248898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Burk DH, Cortez CC, Van NT, Stone KP, Baker M, Mendoza T, Mynatt RL, Gettys TW. UCP1 is an essential mediator of the effects of methionine restrictin on energy balance but not insulin sensitivity. FASEB J. 2015a;29:2603–2615. doi: 10.1096/fj.14-270348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Forney LA, Stone KP, Burk DH, Pierse A, Gettys TW. FGF21 Mediates the Thermogenic and Insulin-Sensitizing Effects of Dietary Methionine Restriction but not its Effects on Hepatic Lipid Metabolism. Diabetes. 2017;66:858–867. doi: 10.2337/db16-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Ghosh S, Stone K, Van NT, Gettys TW. Transcriptional impact of dietary methionine restriction on systemic inflammation: Relevance to biomarkers of metabolic disease during aging. Biofactors. 2013;40:13–26. doi: 10.1002/biof.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Stone KP, Dille KN, Simon J, Pierse A, Gettys TW. Metabolic responses to dietary leucine restriction involve remodeling of adipose tissue and enhanced hepatic insulin signaling. Biofactors. 2015b;41:391–402. doi: 10.1002/biof.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Stone KP, Forney LA, Cortez CC, Dille KN, Simon J, Xu M, Hotard EC, Nikonorova IA, Pettit AP, Anthony TG, Gettys TW. Role of GCN2-independent signaling through a non-canonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes. 2016;65:1499–1510. doi: 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem.Soc.Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wilder SM, Le Couteur DG, Simpson SJ. Diet mediates the relationship between longevity and reproduction in mammals. Age (Dordr) 2012;35:921–927. doi: 10.1007/s11357-011-9380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Cheng Y, Zhang Q, Xiao F, Liu B, Chen S, Guo F. S6K1 in the Central Nervous System Regulates Energy Expenditure via MC4R/CRH Pathways in Response to Deprivation of an Essential Amino Acid. Diabetes. 2012;61:2461–2471. doi: 10.2337/db11-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009a;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, Busby J, Hecht R, Li YS, Li Y, Lindberg R, Veniant MM. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am J Physiol Endocrinol.Metab. 2009b;297:E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin.Invest. 2009;119:2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang G, Zheng Z, Maddipati KR, Zhang X, Dyson G, Williams P, Duncan SA, Kaufman RJ, Zhang K. Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology. 2012;55:1070–1082. doi: 10.1002/hep.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol.Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp.Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]