Abstract
The synaptic vesicle glycoprotein 2C (SV2C) is an undercharacterized protein with enriched expression in phylogenetically old brain regions. Its precise role within the brain is unclear, though various lines of evidence suggest that SV2C is involved in the function of synaptic vesicles through the regulation of vesicular trafficking, calcium-induced exocytosis, or synaptotagmin function. SV2C has been linked to multiple neurological disorders, including Parkinson’s disease and psychiatric conditions. SV2C is expressed in various cell types—primarily dopaminergic, GABAergic, and cholinergic cells. In mice, it is most highly expressed in nuclei within the basal ganglia, though it is unknown if this pattern of expression is consistent across species. Here, we use a custom SV2C-specific antiserum to describe localization within the brain of mouse, nonhuman primate, and human, including cell-type localization. We found that the immunoreactivity with this antiserum is consistent with previously-published antibodies, and confirmed localization of SV2C in the basal ganglia of rodent, rhesus macaque, and human. We observed strongest expression of SV2C in the substantia nigra, ventral tegmental area, dorsal striatum, pallidum, and nucleus accumbens of each species. Further, we demonstrate colocalization between SV2C and markers of dopaminergic, GABAergic, and cholinergic neurons within these brain regions. SV2C has been increasingly linked to dopamine and basal ganglia function. These antisera will be an important resource moving forward in our understanding of the role of SV2C in vesicle dynamics and neurological disease.
1. INTRODUCTION
The synaptic vesicle glycoprotein 2C (SV2C) is one of three proteins within the SV2 family, which also includes SV2A and SV2B. SV2B is expressed at moderate levels throughout the nervous system, and particularly within the retina. SV2A is the most widely expressed and most extensively characterized of this family of proteins. SV2A is present throughout the nervous and endocrine systems and, in fact, antibodies for SV2A are often used as molecular markers for axon terminals (Bajjalieh et al. 1994). Additionally, as it is the molecular target for the commonly used antiepileptic drug levetiracetam, SV2A is particularly relevant to the epilepsy research community (Lynch et al. 2004). Dysregulation of SV2A expression leads to seizures in mice and humans (Douaud et al. 2011; Feng et al. 2009; Gorter et al. 2006; Nowack et al. 2011; Ohno et al. 2009; Serajee and Huq 2015; Shi et al. 2015; van Vliet et al. 2009; Wang et al. 2014). SV2A is known to regulate neuronal excitability, synaptotagmin trafficking, calcium sensitivity, and vesicular mobilization (Chang et al. 2009; Crowder et al. 1999; Custer et al. 2006; de Toledo et al. 1993; Iezzi et al. 2005; Janz et al. 1999a; Lazzell et al. 2004; Schivell et al. 2005; Wan et al. 2010; Xu and Bajjalieh 2001; Yao et al. 2010). The functions of the individual members of the SV2 family of proteins are thought to be similar, though not interchangeable, as any two isoforms do not compensate for the loss of any one isoform.
SV2C is distinguished from SV2A and SV2B by its enriched expression within the basal ganglia. Previous rodent studies have demonstrated that SV2C is most highly expressed in the substantia nigra (pars compacta, SNc; pars reticulata, SNr), ventral tegmental area (VTA), caudoputamen (CPu), nucleus accumbens (NAc), globus pallidus (GP) and ventral pallidum (VP), with minimal expression in cortical regions (Dardou et al. 2010; Janz and Sudhof 1999b). This expression pattern is consistent with midbrain dopamine somata and striatal terminal regions; indeed, previous reports have estimated that SV2C localizes to approximately 75% of midbrain dopamine neurons and their striatal terminals. SV2C also appears to have a high degree of localization to GABAergic regions of the basal ganglia, and is present in about 30% of cholinergic interneurons within the CPu (Dardou et al. 2010). SV2C has been increasingly linked to dopamine-related disorders, including Parkinson’s disease (PD) (Hill-Burns et al. 2012; Dardou et al. 2013; Dunn et al. 2017; Altmann et al. 2016) and psychiatric conditions (Ramsey et al. 2013). In particular, polymorphisms upstream of the SV2C gene mediate the neuroprotective effect of smoking, response to L-DOPA, as well as response to atypical antipsychotics.
Antibodies previously used to characterize SV2C localization are not commercially available (Dardou et al. 2010; Janz and Sudhof 1999b), and other commercially-available SV2C antibodies have not been well-characterized in their usage in immunoblotting or immunohistochemistry. Thus, we designed and optimized two specific rabbit-anti-SV2C sera corresponding to both mouse and human SV2C, respectively. We have previously used both the human SV2C (hSV2C) and mouse SV2C (mSV2C) antisera to (1) characterize protein localization with both fluorescent and 3-3′-diaminiobenzidine (DAB) immunohistochemistry, (2) quantify protein expression with immunoblotting from brain homogenate and tissue culture lysate, and (3) investigate protein complexes with immunoprecipitation from brain homogenate and tissue culture lysate. Additionally, these SV2C antisera do not recognize either SV2A or SV2B (Dunn et al. 2017).
Here, we provide further characterization of these antibodies and demonstrate the utility of both the mSV2C and hSV2C antibodies to demonstrate SV2C expression in mouse, rhesus macaque, and human tissue using immunohistochemistry and immunoblotting. In intact postmortem tissue, SV2C localizes to terminals and somata in mouse, macaque, and human brain. In accordance with previous reports, we found that in mice, SV2C is expressed primarily in the basal ganglia and colocalizes with markers of dopaminergic, GABAergic, and cholinergic neurons. Similarly, SV2C expression in the macaque brain is consistent with expression patterns in mice, with the greatest expression in the midbrain, pallidum, and striatum. In humans, SV2C immunoreactivity is present throughout the dorsal striatum including in GABAergic medium spiny neurons (MSNs) and/or interneurons, as well as in the substantia nigra and VTA.
While SV2C expression is largely consistent across species, there is some variability in the degree of immunoreactivity in certain cell types. Given the growing importance of SV2C to PD in particular, a greater understanding of SV2C expression within the basal ganglia and elsewhere in the brain across species will be important in future studies hoping to translate rodent studies to human disease relevance.
2. RESULTS
2.1. Validation of SV2C antibodies
We performed immunoblotting and immunohistochemistry on positive and negative controls. For immunoblotting, we evaluated immunoreactivity in (1) WT and SV2C-KO mouse striatal homogenates, (2) HEK293 cells (human-derived) and PC12 cells (rat-derived) transfected to express human SV2C, and (3) N2A cells (mouse-derived), which endogenously express SV2C (Fig. 1). HEK293 and PC12 cells were transfected with a pcDNA3.1 DNA vector containing either human SV2C or no additional DNA construct (“Vector”) (Fig. 1A). We sought to compare immunoreactivity between the mSV2C and hSV2C antibodies in recognizing the converse species’ protein. Human and mouse SV2C have 97% sequence identity, and the antigen sequences chosen for the immunizing peptide are >88% similar between mouse and human. Therefore, we expected some cross-reactivity between the antisera. Indeed, we observed similar immunoreactivity in mouse tissue homogenate (Fig. 1A) and in mouse-derived (N2A) cell lysate with the mSV2CpAb and the hSV2CpAb (Fig. 1B). Similarly, the hSV2C expressed in transfected HEK293 and PC12 cells was recognized by both hSV2CpAb and mSV2CpAb (Fig. 1A). As expected, with both the hSV2CpAb and mSV2CpAb we observed a loss of SV2C-specific immunoreactivity in the SV2C-KO striatal homogenates as compared to WT (Fig. 1A), as well as a reduction in immunoreactivity following SV2C-knockdown with shRNA in N2A cell lysates (Fig. 1B).
Figure 1. Validation of the polyclonal SV2C antibodies with immunoblotting.
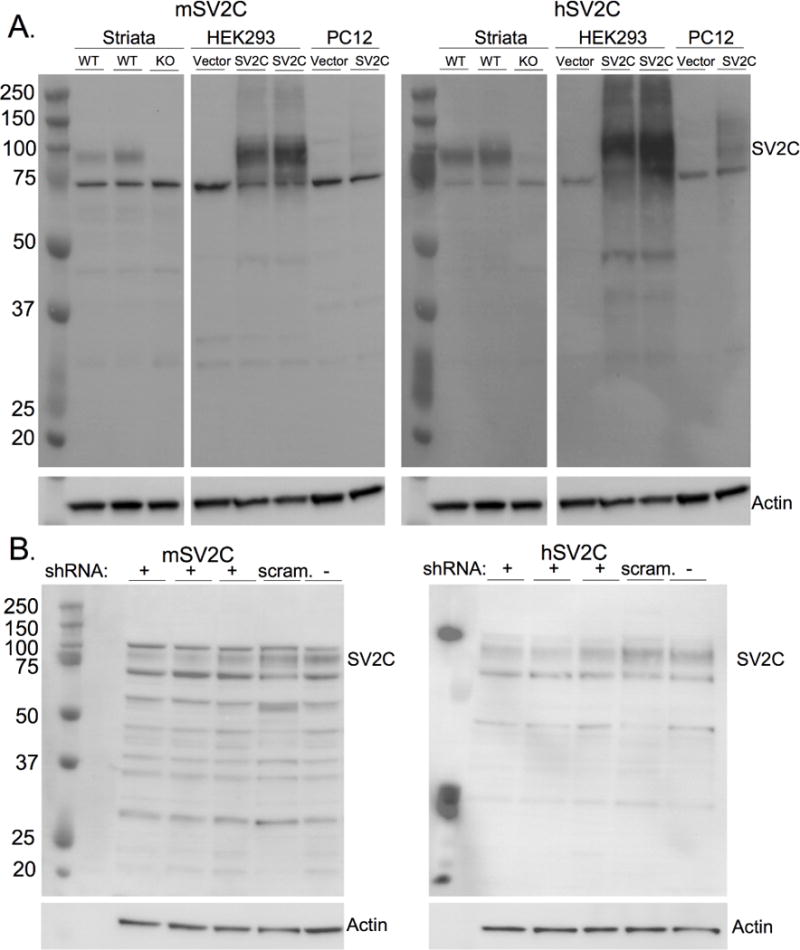
(A) Both the polyclonal human SV2C antibody (hSV2CpAb) and mouse SV2C antibody (mSV2CpAb) recognize human SV2C in transfected cell lines (HEK293 and PC12, neither of which endogenously express SV2C) and in endogenously-expressed mouse SV2C in mouse striatum homogenates and N2A cell lysates (B). Partial knockdown of endogenous SV2C using SV2C-targeted shRNA results in reduced immunoreactivity as compared to scrambled shRNA (“scram”) or control lysate with no shRNA transfection. The specific band is at the expected molecular weight of 90kD. Immunoreactivity at the specific band is eliminated in striatal homogenates from mice lacking SV2C (SV2C-KO). The unidentified nonspecific band at ~70kD remains in KO tissue and in untransfected cell lysate.
The mSV2CpAb shows strong immunoreactivity in WT striatal mouse homogenates and in hSV2C-transfected HEK293 cells. Lighter immunoreactivity was observed in hSV2C-transfected PC12 cells, possibly due to lower transfection efficiency. The hSV2CpAb shows strong immunoreactivity around the expected molecular weight of SV2C (~90kD) in the transfected but not the mock-transfected HEK293 cell lysates, with less immunoreactivity in transfected PC12 cells. There are nonspecific bands in both the vector- and SV2C-transfected lysates from all species-derived cells, as well as in mouse striatal homogenates. These nonspecific bands, particularly the darkest nonspecific band at ~70kD, are generally lighter when using the hSV2C antibody and are of unknown origin but may be expected as polyclonal antibodies generally have higher heterogeneity and nonspecific background reactivity compared to monoclonal antibodies (Lipman et al. 2005).
2.2. SV2C localization in mouse
We found SV2C expression in a variety of brain regions within the basal ganglia consistent with previously-published rodent studies. In particular, SV2C was localized to the neuropil of the CPu, VP, GP, and SNr (Fig. 2A, 3), and the cell bodies of the VTA and the SNc (Fig. 3). We previously published delineation of the VP, demonstrating high expression of SV2C in the VP (Stout et al. 2016). Outside of the basal ganglia, we observed light SV2C immunoreactivity in the polymorphic layer of the dentate gyrus in the hippocampus (HC) (Fig. 2A). There is also light staining for SV2C in the cortex (Ctx). In SV2C-KO tissue, we see a general ablation of SV2C immunoreactivity throughout the brain (Fig. 2A).
Figure 2. SV2C localization within the mouse brain.
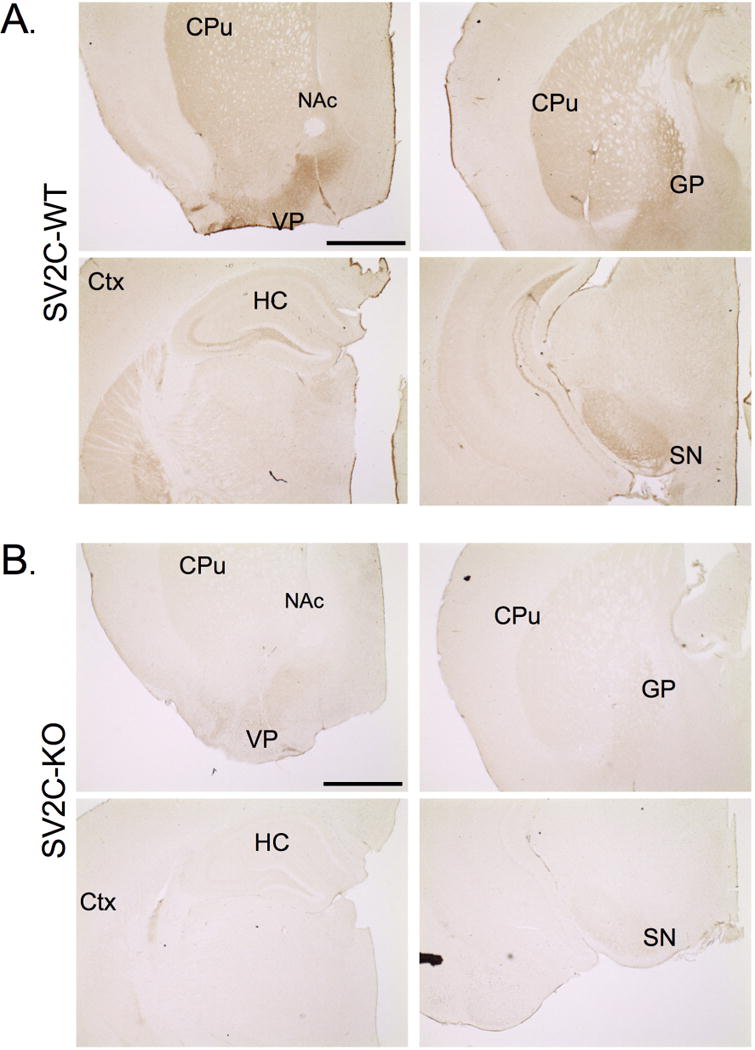
(A) SV2C is expressed at high levels in the dorsal striatum (CPu), the ventral pallidum (VP), and the globus pallidus (GP), as well as in the cortex (Ctx) and hippocampus (HC). (B) Immunoreactivity of the mouse SV2C polyclonal antibody is ablated in tissue from SV2C-KO animals in all brain regions. Scale bar = 500µm
Figure 3. SV2C colocalizes with the dopamine marker TH in the mouse basal ganglia.
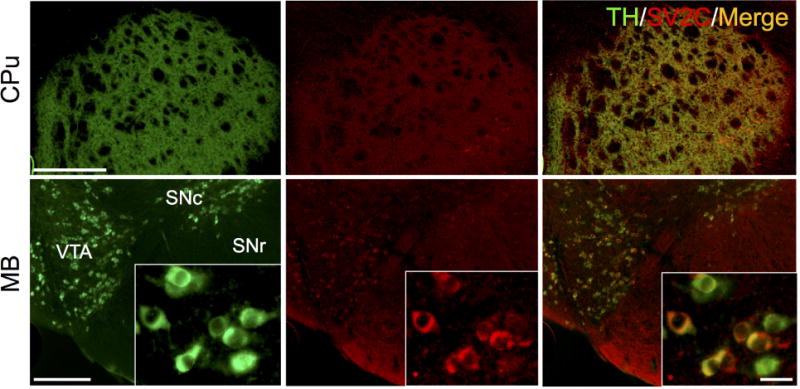
SV2C is expressed in dopamine terminal fields in the caudoputamen (CPu), as well as in dopaminergic neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc). Scale bars = 500 µm (low magnification), 20 µm (insets)
Within the basal ganglia, SV2C shows a similar pattern of expression as dopaminergic markers such as tyrosine hydroxylase (TH). SV2C and TH are highly colocalized in the dopamine terminal regions of the CPu, as well as in dopaminergic cell bodies in the VTA and SNc (Fig. 3). Previous reports have indicated that SV2C is localized to striatal GABAergic neurons, with SV2C preferentially localizing to GABA cells over glutamatergic cells (Dardou et al. 2010; Gronborg et al. 2010). We found that SV2C is highly expressed in GABAergic regions of the basal ganglia, including terminal regions of the GP, VP and midbrain (MB) and fibers in the SNr (Fig. 4A). We also evaluated whether the GABAergic marker VGAT is altered following genetic deletion of SV2C. We found no difference in expression level of VGAT in SV2C-KO animals compared to their WT littermates (WT: 464±66.1 AU, KO: 472±57.8 AU, P = 0.93, N = 10-12). Similarly, we did not see differences in pattern of expression of VGAT within the striatum of SV2C-KO animals (Fig. 4B).
Figure 4. SV2C expression in GABAergic regions of the mouse basal ganglia.
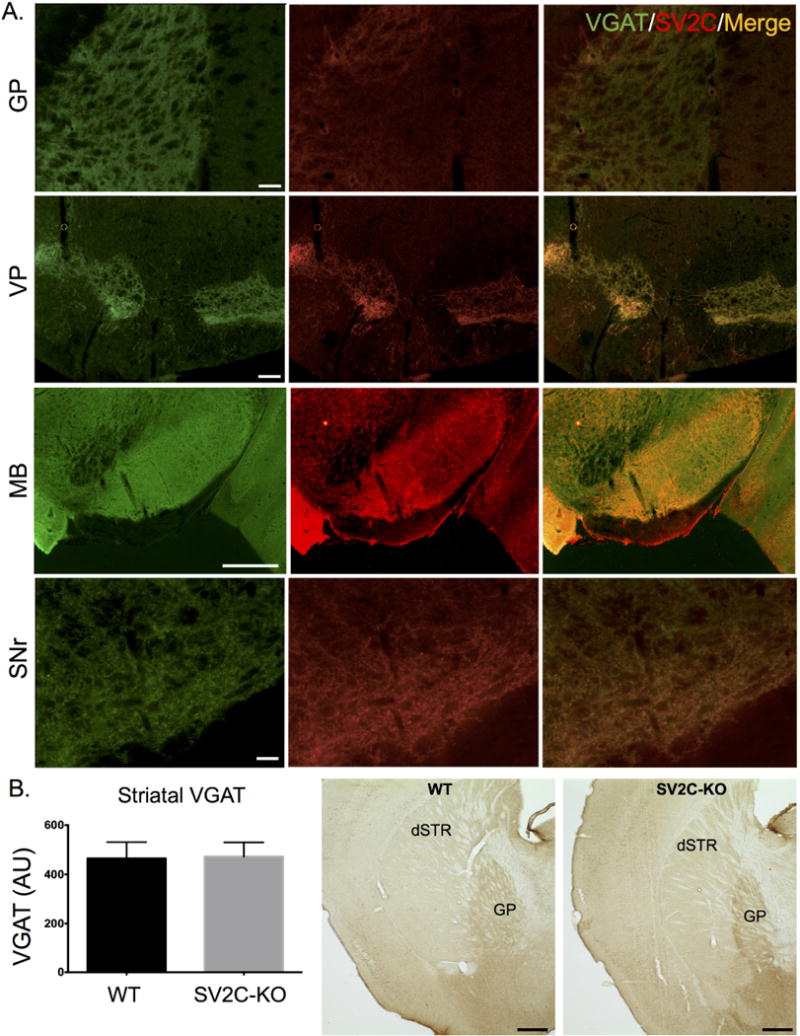
(A) SV2C and vesicular GABA transporter (VGAT) immunoreactivity is highly localized in the neuropil of GABAergic nuclei within the basal ganglia including the globus palldius (GP), ventral pallidum (VP), midbrain (MB) and substantia nigra pars reticulata (SNr). (B) Striatal VGAT expression is unchanged in SV2C-KO animals. Scale bars = 500 µm (MB), 100 µm for all others
Given previous indications that SV2C may be a mediator of the neuroprotective and neurochemical effects of nicotine (Hill-Burns et al. 2016; Dunn et al. 2017), as well as a previous record showing colocalization between SV2C and markers for acetylcholine (Dardou et al. 2010), we also examined SV2C expression in cholinergic cells. We found limited co-expression of SV2C and markers of cholinergic cells, choline acetyltransferase (ChAT) and the choline transporter (hCHT), indicating that SV2C is present in some striatal cholinergic cells (Fig. 5). We additionally evaluated potential alterations to the striatal cholinergic system following genetic ablation of SV2C. We did not observe any alterations to expression patterns of striatal ChAT, though we observed a slight but statistically insignificant increase in striatal VAchT expression levels in SV2C-KO animals compared to WT (WT: 2906±305 AU; KO: 3520±414, N = 3, P = 0.3) (Fig 5B).
Figure 5. SV2C localization in cholinergic cells within the mouse basal ganglia.
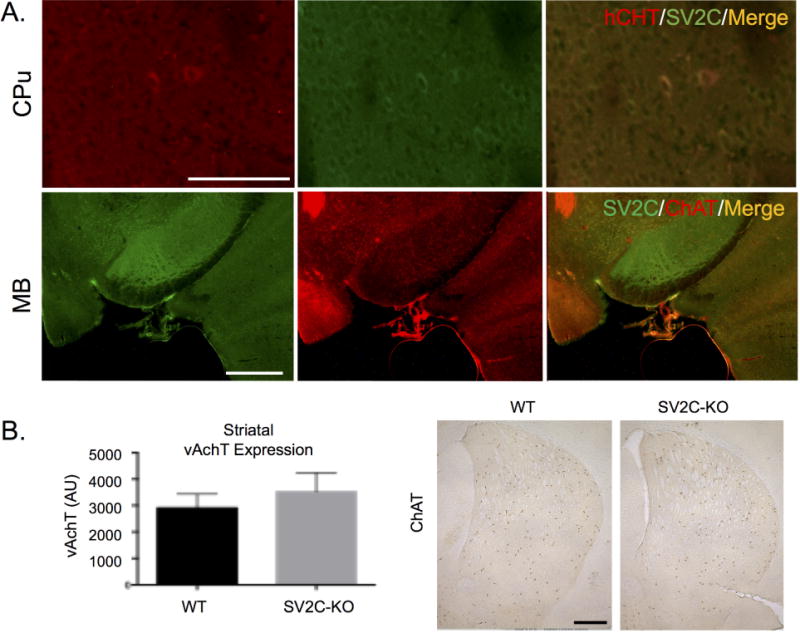
(A) SV2C partially colocalizes with markers for acetylcholine (choline acetyltransferase, ChAT, and the choline transporter, hCHT) in the midbrain (MB) and caudoputamen (CPu). (B) Striatal expression of acetylcholine markers is unchanged following genetic ablation of SV2C. Scale bars = 500 µm (A, MB; B), 100 µm (A, CPu).
2.3. SV2C localization in macaque
SV2C followed a similar pattern of expression in rhesus macaques as has been reported in rodents. Specifically, SV2C is strongly expressed in the basal ganglia, particularly the caudate nucleus (Cd), putamen (Pu), NAc, GP, SNc, and SNr, with some cortical immunoreactivity (Fig. 6A–E). The expression pattern of SV2C in macaques, as in mice, is consistent with a neuropil distribution throughout the basal ganglia. SV2C appears to localize to some cell bodies within the midbrain (both the SNc and VTA, see Fig. 6F–G) and neuropil throughout the striatum, pallidum, and SNr. Additionally, there is slightly lighter SV2C immunoreactivity in the cortex and thalamus (Thal) of the macaque brains.
Figure 6. SV2C is expressed in the basal ganglia of nonhuman primates.
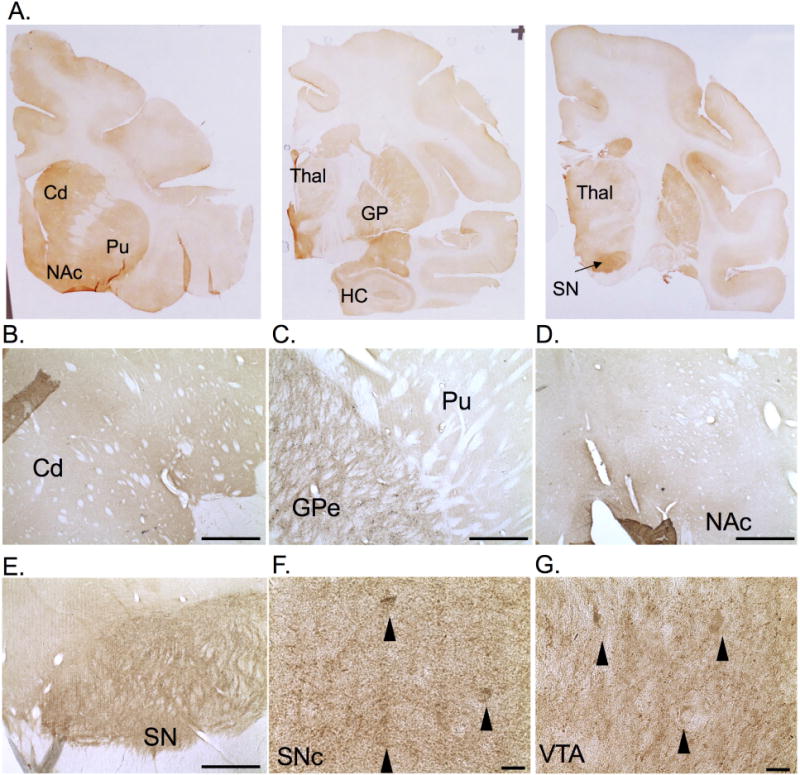
(A–D) SV2C is expressed in the caudate nucleus (Cd), the putamen (Pu), globus pallidus (GP), nucleus accumbens (NAc), thalamus (Thal), and cortex of rhesus macaques. SV2C expression is also observed in the neuropil of the midbrain (E), with possible expression in the cell bodies (black arrows) of the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA) (F–G). Scale bars = 1 mm (B–E), 20 µm (F–G)
2.4. SV2C localization in human
SV2C expression in humans follows a similar, though not identical pattern of expression as compared to rodents and nonhuman primates. Similar to rodents and nonhuman primates, SV2C expression in human tissue was present throughout the basal ganglia (Fig. 7). SV2C is expressed in the SNr and SNc, as well as in terminal and cell body regions of the striatum. In contrast to unstained tissue, SV2C immunoreactivity is diffusely expressed within the SN (Fig. 7A–B). There appears to be some SV2C immunoreactivity in neuromelanin-containing cells of the SNc, as contrasted to a mock-stained (no primary antibody) control SNc section where only neuromelanin is visible in the cell bodies. SV2C immunoreactivity appeared greater in the putamen than in the caudate nucleus, particularly diffusely in the neuropil (Fig. 7D–E). SV2C is strongly expressed in cell bodies throughout the striatum, including in the caudate nucleus, putamen and globus pallidus (Fig. 7D–F). In the striatum, SV2C colocalizes with VGAT, indicating expression of SV2C within GABAergic striatal medium spiny neurons, the principle cell type in the striatum, and/or GABAergic interneurons (Fig. 7G).
Figure 7. SV2C is localized to the basal ganglia in the human brain.
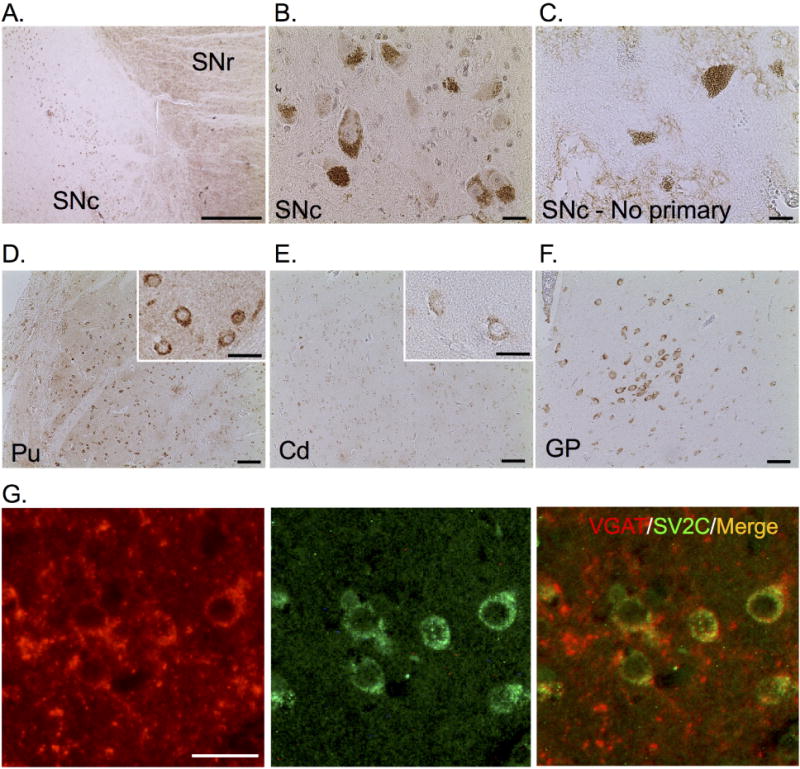
(A) SV2C is expressed in the substantia nigra pars compacta (SNc) and pars reticulata (SNr). (B) We observed light SV2C immunoreactivity in the somata of neuromelanin-containing SNc cells, which is contrasted to the lack of immunoreactivity in an unstained SNc section. Neuromelanin is visible in the unstained tissue (C). (D) SV2C is expressed in the somata and neuropil of the putamen (Pu) and, to a lesser extent, the caudate nucleus (Cd, E). We also observed immunoreactivity in the somata within the globus pallidus (GP, F). (G) SV2C is highly colocalized with the vesicular GABA transporter (VGAT) within the putamen. Scale bars = 500 µm (A, D–F), 20 µm (C–B, D–E insets, G).
3. DISCUSSION
SV2C is a vesicular protein with a distinct basal ganglia expression pattern. Previous immunological investigations into its localization focused exclusively on the rodent brain. Given the limited availability of well-validated SV2C antibodies, we designed and optimized two distinct polyclonal SV2C antisera recognizing both mouse and primate SV2C. Further clarification of the expression pattern of SV2C, particularly with respect to similarities across species, should be considered when interpreting future experimental results and their translatability to human relevance, and this pair of novel antibodies for SV2C will be important tools in these studies.
3.1. Specificity of the SV2C antibody
We previously demonstrated that the hSV2CpAb is specific to SV2C and does not recognize other proteins within the SV2 family (SV2A, SV2B) (Dunn et al. 2017). Here, we further demonstrate that these antibodies recognize SV2C by immunoblot of cell culture lysates and striatal homogenates, as well as by immunohistochemistry of mouse tissue. SV2C protein expression patterns were consistent with previous investigations, and we observed a loss of SV2C immunoreactivity throughout the brain of SV2C-KO animals that corresponded to an expected lack of SV2C protein (Fig. 1–2). This antiserum produces a relatively strong nonspecific band corresponding to an unknown antigen at approximately 70kD when immunoblotting denatured protein samples (Fig. 1). This nonspecific binding does not appear to substantially interfere with specific immunohistochemical staining, as the immunoreactivity is low in stained SV2C-KO tissue sections (Fig. 2B). Across several experiments in our laboratory, we have observed a loss of SV2C immunoreactivity in immunoblotting and/or immunohistochemistry in tissue from at least 50 SV2C-KO animals. Similarly, we have repeatedly overexpressed SV2C in vitro and confirmed SV2C immunoreactivity in subsequent cell lysates in over a dozen experiments. Anecdotally, SV2C immunoreactivity in immunoblots is highly consistent, though SV2C immunoreactivity in immunohistochemistry can be more variable depending on tissue fixation, antibody aliquot freeze-thaw history, and other factors.
3.2. Anatomical and subcellular distribution of SV2C in mice
SV2 is frequently used as a marker for synaptic terminals, and SV2A and SV2B are generally not expressed in the cell body (Bajjalieh et al. 1994; Stockburger et al. 2015). There is some evidence for mitochondrial SV2 expression (Stockburger et al. 2015). SV2C expression specifically varies from the observed terminal-only expression pattern of the other SV2s, and based on previous reports, we expected to detect SV2C expression in synaptic terminals and cell bodies. In tissue sections, SV2C was expressed in a diffuse pattern throughout various nuclei in the basal ganglia, suggesting localization to synaptic terminals of dopaminergic and GABAergic neurons. We also observed localization of SV2C to the somata of various cell types, including midbrain dopamine neurons, and striatal GABAergic and cholinergic cells. The contrast of subcellular and cell-type specific distribution of SV2C versus what has been reported for SV2A and SV2B suggests a distinct role of SV2C within these cells. The observation that GABAergic markers are unaltered in SV2C-KO animals suggests that no compensatory changes occur in response to a lack of SV2C within the GABA system; however, closer analysis may interrogate potential changes to GABA cell activity or function.
3.3. SV2C and cholinergic cells
Similar to previous investigations into the localization of SV2C, we found that SV2C localizes sparsely to cholinergic cells in the mouse striatum. The observation that the cholinergic transporters vAchT and ChAT are unaltered in SV2C-KO animals suggests that genetic deletion of SV2C does not induce changes to basal ganglia cholinergic pathways; however, closer investigation into potential alterations in cholinergic signaling may be valuable.
3.4. SV2C distribution in macaque brain
We evaluated SV2C distribution throughout the rhesus macaque brain using the hSV2C antibody. As in mice, we observed a basal-ganglia enriched expression pattern of SV2C in the macaque brain. We observed strong SV2C expression in the macaque midbrain and striatum, as well as some additional immunoreactivity in the hippocampus, cortex and subcortical regions such as the thalamus (Fig. 6).
The areas of highest expression in mice and primates were the SN, the VTA, the Cd and Pu (or CPu), the GP and the VP. There was some SV2C expression in the cortex of all species. The similar expression patterns of SV2C across species suggests a preserved function and role for SV2C in these brain regions. SV2C was previously found to regulate dopamine expression and basal ganglia function in mice, and because SV2C expression is in similar brain regions, it is likely that SV2C also regulates dopamine release in primates. This provides support to the translatability and human relevance of experimental results of SV2C function in mice.
3.5. Key differences of SV2C expression in humans versus other species
Immunohistochemistry in human brain tissue, particularly aged cases, presents several human-specific staining problems. In particular, human tissue is susceptible to nonspecific antibody binding to various cellular components and antigen masking through formalin-based fixation (Sun et al. 2010; Buchwalow et al. 2011; Alelu-Paz et al. 2008) and inconsistent postmortem intervals prior to fixation. Nonspecific binding may be largely prevented with proper blocking solutions, and antigen-retrieval steps may enhance specific antibody binding. To combat these issues, we optimized antigen retrieval using a hot citric acid buffer incubation, and performed each wash and blocking step in Triton-X100 to minimize nonspecific antibody binding. To ensure that we were not observing nonspecific secondary antibody binding, we also performed staining without the presence of primary antibody. These precautions allow us to evaluate the observed immunoreactivity as specific antibody binding in human tissue, though some inherent limitations of working with human tissue may still be present.
SV2C immunoreactivity was observed in GABAergic cell bodies throughout the striatum of humans (Fig. 7D–F). This contrasts with what we and others have observed in rodent tissue, and suggests an elevated importance of SV2C in GABA transmission in humans. However, the SV2C antibody strongly labels the SNr in all species examined. Given that the main GABAergic input to the SNr are the MSNs from the dorsal striatum, it is possible that only the relative expression in the somata versus the synaptic terminals is the primary difference. Overall, data from the present experiments strongly suggest consistent SV2C expression across species. Nonetheless, it will be important to consider the possible differences in the contribution of SV2C in neurotransmission in humans, particularly in GABA cells, when interpreting mouse data.
Overall, we demonstrate that SV2C expression is relatively consistent throughout various species, with a basal-ganglia enriched distribution. We introduce two distinct SV2C antisera that can be used in a variety of immunological methods in various species. These data will be useful tools in future investigations into the function of SV2C and its relevance in associated neurological diseases.
4. EXPERIMENTAL PROCEDURES
4.1. Antibodies
Polyclonal SV2C antibodies were designed by our lab and custom-produced by Covance as described previously (Dunn et al. 2017). Immunizing peptides corresponding to amino acids 97-114 of SV2C (mouse SV2C: STNQGKDSIVSVGQPKG; human SV2C: SMNQAKDSIVSVGQPKG) were mcKMH-conjugated and injected into rabbits. Each antibody recognizes SV2C in mouse, nonhuman primate and human tissue (Fig. 1), though only the mouse SV2C antiserum (mSV2CpAb) was used for immunohistochemistry in mouse tissue, and only human SV2C antiserum (hSV2CpAb) was used for immunohistochemistry in human and nonhuman primate tissue in these studies.
Antibodies against TH (cat. AB152) were purchased through Millipore (Caudle et al. 2007; Dunn et al. 2017; Lohr et al. 2014). Antibody against VGAT (cat. 131-011) was purchased through Synaptic Systems (Tafoya et al. 2006). Antibody against ChAT was purchased through Chemicon (cat. AB144P) (Abdi et al. 2015), and antibody against vAchT was purchased through Novus Biologicals (cat. NB100-91347). Monoclonal hCHT antibody (clone 62-2E8) was generously provided by the Emory Center for Neurodegenerative Diseases (Ferguson et al. 2003).
Secondary antibodies were purchased through Jackson ImmunoResearch (biotinylated, HRP-conjugated) or ThermoFisher (AlexaFluor; fluorescent). Category numbers are as follows. Biotinylated (DAB immunohistochemistry): goat-anti mouse (cat. 115-065-003), goat-anti rabbit (cat. 111-065-144); fluorescent (immunofluorescence): goat-anti mouse 594 (cat. A11005), goat-anti rabbit 488 (cat. A11034), goat-anti rabbit 594 (A11012); HRP-conjugated (immunoblots): goat-anti rabbit (111-035-003), goat-anti mouse (115-035-003).
4.2. Animals
Adult male wildtype C57BL/6J mice were used for all mouse immunohistochemistry (age 4-10mos). SV2C-KO animals were generated as described previously (Dunn et al. 2017; Skarnes et al. 2011). Briefly, animals were generated using the EUCOMM “knockout first allele” construct. These animals contained a lacZ/neomycin resistance cassette flanked by FRT sites inserted into the Sv2c gene. Animals were crossed with a line globally expressing Flp-recombinase to excise the cassette, resulting in a line of mice containing a floxed exon 2 of the Sv2c gene. These mice were then crossed with a line containing a Nestin-driven Cre-recombinase in order to achieve a pan-neuronal knockout of SV2C. Saline-treated control male rhesus macaques from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment experiments were used for all macaque immunohistochemistry.
4.3. Tissue culture
HEK293 cells were cultured according to standard protocols and maintained in DMEM with 10% fetal bovine serum (FBS) + 1% penicillin/streptomycin. N2A cells were cultured in 1:1 DMEM:EMEM and 10% FBS with 1% penicillin/streptomycin. PC12 cells were cultured in RPMI medium with 10% FBS and 1% penicillin/streptomycin. Transfections of SV2C in a pcDNA3.1 vector were performed with Lipofectamine 2000 (Invitrogen) according to manufacturer’s protocols. shRNA constructs were transfected using a nucleofector (Amaxa) according to standard protocols. Cells were harvested 24 hours post-transfection, lysed in RIPA buffer and total protein extraction was achieved through differential centrifugation (Dunn et al. 2017).
4.4. Western blotting
Western blots were performed as previously described (Dunn et al. 2017). Briefly, samples were homogenized and underwent differential centrifugation to achieve a crude protein extraction. Samples were run through SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked with 7.5% nonfat dry milk and incubated in primary antibody (SV2C, 1:2,500; actin, 1:5,000) overnight at 4°C with gentle agitation. Secondary antibodies (HRP-conjugated, 1:5,000) were incubated at room temperature for 1 hour. Signal was amplified using chemiluminescence (Thermo) and visualized using a BioRad UV imager.
4.5. Immunohistochemistry
4.5.1. Immunohistochemistry in mouse tissue
Mice were either transcardially perfused with 4% paraformaldehyde or sacrificed by rapid decapitation. Brains were removed and placed in 4% paraformaldehyde for 24 hours (perfused) or seven days (drop-fixed) days at 4°C for post-fixation. Brains were then transferred to sucrose and allowed to equilibrate for an additional 48 hours, at which point they were frozen and sectioned to 40µm. Sections were stored in cryoprotectant at −20°C until staining. For immunohistochemistry, sections were rinsed with 1X PBS containing 0.02% Triton X-100 (PBST). Sections underwent antigen retrieval using Citra Antigen Retrieval Buffer (Biogenex) for one hour at 70°C. Endogenous peroxidase activity was quenched with a 12-minute incubation in 3% hydrogen peroxide at room temperature. Nonspecific antibody binding was blocked using a 3% Normal Horse Serum (NHS) in PBST solution for one hour at room temperature. Sections incubated in primary antibody solution (1:1,000 in PBST for all antibodies, except SV2C, which was used at a concentration of 1:2,500) overnight at 4°C with gentle agitation, followed by incubation in secondary antibody (1:1,000 for biotinylated; 1:800 for fluorescent) for one hour at room temperature.
For biotinylated secondaries, the signal was amplified using an avidin-biotin complex (VectaStain Elite, Vector Laboratories) for one hour at room temperature. Biotinylated secondary antibodies were visualized using a 3-3′-diaminobenzidine (SIGMAFAST DAB tablets, Sigma) reaction for approximately 1 minute; reaction was quenched with PBST. Sections were mounted to charged slides, dehydrated in ethanol, cleared with xylenes and coverslipped.
For immunofluorescence, autofluorescence was quenched with a 7-minute incubation in a 0.1% Sudan Black B solution in 70% ethanol at room temperature. Slides were treated with hard-set mounting medium (Vector Laboratories) and coverslipped.
4.5.2. Immunohistochemistry in rhesus macaque tissue
We performed immunohistochemistry in rhesus macaque tissue using a protocol modified to optimize staining in nonhuman primate tissue. Vibratome-cut sections (60µm) from five macaques underwent antigen retrieval using a low-pH glycine buffer (0.5M glycine, pH 2.0) for one hour at room temperature. Endogenous peroxide activity was quenched with a 10% hydrogen peroxide solution for 20 minutes at room temperature. Nonspecific antibody binding was blocked using a solution of 1% NHS and 1% bovine serum albumin (BSA) in PBST. Sections were incubated in primary antibody solution (1:1,000) overnight at 4°C with gentle agitation. Sections were then incubated in biotinylated secondary antibody (1:200) for 1 hour at room temperature. Secondary antibodies were visualized as described above for mouse tissue.
4.5.3. Immunohistochemistry in human tissue
Paraffin-embedded sections (8µm) were obtained through the Alzheimer’s Disease Research Center Brain Bank at Emory University. Tissue from seven aged, non-demented controls (5 male, 2 female) were observed, ranging in age from 61-88 years. Slides were deparaffinized using xylenes and rehydrated in decreasing concentrations of ethanol in water. Sections underwent antigen retrieval using a hot citric acid buffer at 95°C for 1 hour. Endogenous peroxidase activity was quenched with a 12-minute incubation in 3% hydrogen peroxide at room temperature. Nonspecific antibody binding was blocked using 3% NHS in PBST for one hour at room temperature. Sections were incubated in primary antibody (1:1,000 in PBST) overnight at 4°C with gentle agitation. Sections were then incubated in secondary antibody solutions (1:1,000 biotinylated or 1:800 fluorescent). Secondary antibodies were visualized as described above for mouse tissue.
4.5.4. Micrograph visualization
Immunohistochemistry was visualized using a Zeiss AX10 microscope equipped with a MicroBrightField camera. Micrographs were captured using StereoInvestigator software. Full-field images of macaque sections were captured using an 8-megapixel camera equipped on an iPhone 5s (Apple). Micrographs directly comparing staining between groups (e.g. SV2C-WT vs. KO) were visualized with identical microscope settings.
Highlights for “Immunochemical analysis of the synaptic vesicle glycoprotein 2C (SV2C) in mouse, macaque, and human basal ganglia”.
SV2C is expressed in distinct nuclei within the brains of mouse, macaque, and human
Dopaminergic and GABAergic cells have enriched expression of SV2C, with a high degree of colocalization between SV2C and markers for both dopamine and GABA.
Similar expression of SV2C across species supports the translational relevance of rodent studies into the function of the protein and its role in human disease.
Acknowledgments
The authors would like to thank Drs. Marla Gearing, Yoland Smith, and Kalynda Gonzales Stokes for their technical advice and consultation. This work was supported by NIEHS R01ES023839, NIEHS P30ES019776 and the Lewis Dickey Memorial Fund to G.W.M.; NINDS F31NS089242 to A.R.D.; NIDA F31DA037652 to K.A.S., and the Neuropathology Core of the Emory Neuroscience NINDS Core Facilities (NIH P30NS055077).
A list of abbreviations used in this manuscript
- AU
arbitrary units
- BSA
bovine serum albumin
- Cd
caudate nucleus
- ChAT
choline acetyltransferase
- CPu
caudoputamen
- Ctx
cortex
- DMEM
Dulbecco’s modified Eagle’s medium
- EMEM
Eagle’s minimum essential medium
- GP(i/e)
globus palldius (internal/external segment)
- HC
hippocampus
- hCHT
choline transporter
- HEK293
human embryonic kidney cells 293
- MB
midbrain
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MSN
medium spiny neuron
- N2A
neuro-2A cells
- NAc
nucleus accumbens
- NHS
normal horse serum
- PBST
phosphate buffered saline with Triton-X100
- PC12
pheochromocytoma 12 cells
- PD
Parkinson’s disease
- Pu
putamen
- RIPA
radioimmunoprecipitation assay buffer
- SN(c/r)
substantia nigra pars compacta/reticulata
- SV2(A/B/C)
synaptic vesicle glycoprotein 2(A/B/C)
- SV2C-KO
SV2C-knockout
- (h/m)SV2CpAb
(human/mouse) SV2C polyclonal antibody
- TH
tyrosine hydroxylase
- Thal
thalamus
- VGAT
vesicular GABA transporter
- VP
ventral pallidum
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J, Magill PJ. Prototypic and Arkypallidal Neurons in the Dopamine-Intact External Globus Pallidus. J Neurosci. 2015;35:6667–88. doi: 10.1523/JNEUROSCI.4662-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alelu-Paz R0, Iturrieta-Zuazo I, Byne W, Haroutunian V, Garcia-Villanueva M, Rabano A, Garcia-Amado M, Prensa L, Gimenez-Amaya JM. A new antigen retrieval technique for human brain tissue. PLoS One. 2008;3:e3378. doi: 10.1371/journal.pone.0003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann V, Schumacher-Schuh AF, Rieck M, Callegari-Jacques SM, Rieder CRM, Hutz MH. Influence of genetic, biological and pharmacological factors on levodopa dose in Parkinson’s disease. Pharmacogenomics J. 2016;17:481–8. doi: 10.2217/pgs.15.183. [DOI] [PubMed] [Google Scholar]
- Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neuro. 1994;14:5223–35. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalow I, Samoilova V, Boecker W, Tiemann M. Non-specific binding of antibodies in immunohistochemistry: fallacies and facts. Sci Rep. 2011;1:28. doi: 10.1038/srep00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–48. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Sudhof TC. SV2 renders primed synaptic vesicles competent for Ca2+ -induced exocytosis. J Neurosci. 2009;29:960–7. doi: 10.1523/JNEUROSCI.4521-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Nat Acad Sci. 1999;96:15286–73. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci. 2006;26:1303–13. doi: 10.1523/JNEUROSCI.2699-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardou D, Dassesse D, Cuvelier L, Deprez T, De Ryck M, Schiffmann SN. Distribution of SV2C mRNA and protein expression in the mouse brain with a particular emphasis on the basal ganglia system. Brain Res. 2010;1367:130–45. doi: 10.1016/j.brainres.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Dardou D Monlezun, Foerch0 S, P, Courade JP, Cuvelier L, De Ryck M, Schiffmann SN. A role for SV2C in basal ganglia functions. Brain Res. 2013;1507:61–73. doi: 10.1016/j.brainres.2013.02.041. [DOI] [PubMed] [Google Scholar]
- de Toledo GA, Fernández-Chacón R, Fernández JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–8. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Douaud M, Feve K, Pituello F, Gourichon D, Boitard S, Leguern E, Coquerelle G, Vieaud A, Batini C, Naquet R, Vignal A, Tixier-Boichard M, Pitel F. Epilepsy caused by an abnormal alternative splicing with dosage effect of the SV2A gene in a chicken model. PLoS One. 2011;6:e26932. doi: 10.1371/journal.pone.0026932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AR, Stout KA, Ozawa M, Lohr KM, Hoffman CA, Bernstein AI, Li Y, Wang M, Sgobio C, Sastry N, Cai H, Caudle MW, Miller GW. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson’s disease. Proc Nat Acad Sci. 2017;114:E2253–E62. doi: 10.1073/pnas.1616892114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Xiao F, Lu Y, Huang Z, Yuan J, Xiao Z, Xi Z, Wang X. Down-regulation of synaptic vesicle protein 2A in the anterior temporal neocortex of patients with intractable epilepsy. J Mol Neuro. 2009;39:354–59. doi: 10.1007/s12031-009-9288-2. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23:9697–709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Breit T, Rauwerda H, Lopes da Silva FH, Wadman WJ. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci. 2006;26:11083–110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronborg M, Pavlos NJ, Brunk I, Chua JJ, Munster-Wandowski A, Riedel D, Ahnert-Hilger G, Urlaub H, Jahn R. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci. 2010;30:2–12. doi: 10.1523/JNEUROSCI.4074-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns EM, Singh N, Ganguly P, Hamza TH, Montimurro J, Kay DM, Yearout D, Sheehan P, Frodey K, McLear JA, Feany MB, Hanes SD, Wolfgang WJ, Zabetian CP, Factor SA, Payami H. A genetic basis for the variable effect of smoking/nicotine on Parkinson’s disease. Pharmacogenomics J. 2012;13:530–7. doi: 10.1038/tpj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns EM, Ross OA, Wissemann WT, Soto-Ortolaza AI, Zareparsi S, Siuda J, Lynch T, Wszolek ZK, Silburn PA, Mellick GD, Ritz B, Scherzer CR, Zabetian CP, Factor SA, Breheny PJ, Payami H. Identification of genetic modifiers of age-at-onset for familial Parkinson’s disease. Hum Mol Gen. 2016;25:3849–62. doi: 10.1093/hmg/ddw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi M, Theander S, Janz R, Loze C, Wollheim CB. SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Science. 2005;118:5647–60. doi: 10.1242/jcs.02658. [DOI] [PubMed] [Google Scholar]
- Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999a;24:1003–16. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Janz R, Sudhöf TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience. 1999b;94:1279–90. doi: 10.1016/s0306-4522(99)00370-x. [DOI] [PubMed] [Google Scholar]
- Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem. 2004;279:52124–31. doi: 10.1074/jbc.M407502200. [DOI] [PubMed] [Google Scholar]
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications and information resources. ILAR Journal. 2005;46:258–68. doi: 10.1093/ilar.46.3.258. [DOI] [PubMed] [Google Scholar]
- Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, Wang M, Li Y, Fan X, Hess EJ, Yi H, Vecchio LM, Goldstein DS, Guillot TS, Salahpour A, Miller GW. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. PNAS. 2014;111:9977–82. doi: 10.1073/pnas.1402134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Nat Acad Sci. 2004;101:9861–6. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, Levey AI. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol. 1997;41:530–9. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Nowack A, Malarkey EB, Jia Y, Bleckert A, Hill J, Bajjalieh SM. Levetiracetam reverses synaptic deficits produced by overexpression of SV2A. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Ishihara S, Terada R, Kikuta M, Sofue N, Kawai Y, Serikawa T, Sasa M. Preferential increase in the hippocampal synaptic vesicle protein 2A (SV2A) by pentylenetetrazole kindling. Biochem Biophys Res Commun. 2009;390:415–20. doi: 10.1016/j.bbrc.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Ramsey TL, Liu Q, Massey BW, Brennan MD. Genotypic variation in the SV2C gene impacts response to atypical antipsychotics the CATIE study. Schizophr Res. 2013;149:21–5. doi: 10.1016/j.schres.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM. SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci. 2005;29:56–64. doi: 10.1016/j.mcn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Huq AM. Homozygous Mutation in Synaptic Vesicle Glycoprotein 2A Gene Results in Intractable Epilepsy, Involuntary Movements, Microcephaly, and Developmental and Growth Retardation. Pediatr Neurol. 2015;52:642–6 e1. doi: 10.1016/j.pediatrneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhou F, Wang LK, Wu GF. Synaptic vesicle protein 2A decreases in amygdaloid kindling pharmacoresistant epileptic rats. Journal of Huazhon University of Science and Technology. 2015;35:716–22. doi: 10.1007/s11596-015-1496-0. [DOI] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–42. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockburger C, Miano D, Baeumlisberger M, Pallas T, Arrey TN, Karas M, Friedland K, Muller WE. A Mitochondrial Role of SV2a Protein in Aging and Alzheimer’s Disease: Studies with Levetiracetam. J Alzheimers Dis. 2015;50:201–15. doi: 10.3233/JAD-150687. [DOI] [PubMed] [Google Scholar]
- Stout KA, Dunn AR, Lohr KM, Alter SP, Cliburn RA, Guillot TS, Miller GW. Selective enhancement of dopamine release in the ventral pallidum of methamphetamine-sensitized mice. ACS Chem Neurosci. 2016;7:1364–73. doi: 10.1021/acschemneuro.6b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Liu M, Bing G. Improving the specificity of immunological detection in aged human brain samples. Int J Physiol Pathophysiol Pharmacol. 2010;2:29–35. [PMC free article] [PubMed] [Google Scholar]
- Tafoya LCR, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and Function of SNAP-25 as a Universal SNARE Component in GABAergic Neurons. J Neurosci. 2006;26:7826–38. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia. 2009;50:422–33. doi: 10.1111/j.1528-1167.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, Heidelberger R. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron. 2010;66:884–95. doi: 10.1016/j.neuron.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shi J, Wu G, Zhou F, Hong Z. Hippocampal low-frequency stimulation increased SV2A expression and inhibited the seizure degree in pharmacoresistant amygdala-kindling epileptic rats. Epilepsy Res. 2014;108:1483–91. doi: 10.1016/j.eplepsyres.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Xu T, Bajjalieh SM. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol. 2001;3:691–8. doi: 10.1038/35087000. [DOI] [PubMed] [Google Scholar]
- Yao J, Nowack A, Kensel-Hammes P, Gardner RG, Bajjalieh SM. Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci. 2010;30:5569–78. doi: 10.1523/JNEUROSCI.4781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


