Abstract
Mutant mouse models of neurodevelopmental disorders with intellectual disabilities provide useful translational research tools, especially in cases where robust cognitive deficits are reproducibly detected. However, motor, sensory, and/or health issues consequent to the mutation may introduce artifacts that preclude testing in some standard cognitive assays. Touchscreen learning and memory tasks in small operant chambers have the potential to circumvent these confounds. Here we employ touchscreen visual discrimination learning to evaluate performance in the maternally derived Ube3a mouse model of Angelman syndrome, the Ts65Dn trisomy mouse model of Down syndrome, and the Mecp2Bird mouse model of Rett syndrome. Significant deficits in acquisition of a two-choice visual discrimination task were detected in both Ube3a and Ts65Dn mice. Procedural control measures showed no genotype differences during pre-training phases or during acquisition. Mecp2 males did not survive long enough for touchscreen training, consistent with previous reports. Most Mecp2 females failed on pretraining criteria. Significant impairments on Morris water maze spatial learning were detected in both Ube3a and Ts65Dn, replicating previous findings. Abnormalities on rotarod in Ube3a, and on open field in Ts65Dn, replicating previous findings, may have contributed to the observed acquisition deficits and swim speed abnormalities during water maze performance. In contrast, these motor phenotypes do not appear to have affected touchscreen procedural abilities during pre-training or visual discrimination training. Our findings of slower touchscreen learning in two mouse models of neurodevelopmental disorders with intellectual disabilities indicate that operant tasks offer promising outcome measures for the preclinical discovery of effective pharmacological therapeutics.
Keywords: Ube3a, Angelman syndrome, Ts65Dn, Down syndrome, Mecp2 Rett syndrome, mice, cognitive, touchscreen, water maze, learning and memory, intellectual disabilities, neurodevelopmental disorders
Introduction
Expanding knowledge about genetic mutations that cause neurodevelopmental disorders has prompted the generation of mouse models with the syntenic mutation, for use in understanding biological mechanisms, and as translational tools to develop therapeutics (Capecchi, 2005; Geyer, 2008; Lynch et al., 2008; Khwaja and Sahin, 2011; Das and Reeves, 2011; Huang et al., 2011; Crawley, 2012; Kleschevnikov et al., 2012; Baudry et al., 2012; Kas et al., 2014; Vorstman et al., 2014; Hatami and Chesselet, 2015; Kazdoba et al., 2016a,b; Belichenko et al., 2016; Gogliotti et al., 2016; Duffney et al., 2016). For neurodevelopmental disorders in which the primary symptom is intellectual impairment, the corresponding mouse model will ideally display robust and highly reproducible cognitive deficits. Many excellent learning and memory tasks in current use (e.g. Fanselow and Poulos, 2005; Eichenbaum and Robitsek, 2009; da Silva et al., 2013; Seese et al., 2014; Grayson et al., 2015; Gyertyan) can be applied to evaluate cognitive abilities in mouse models of neurodevelopmental disorders with intellectual disabilities. One issue is that most of these tasks require motor skills and/or sensory abilities that may be impaired in the mutant mouse model. As examples, effective swimming and vision are necessary for Morris water maze procedures; normal pain perception and hearing are necessary for contextual and cued fear conditioning, and spontaneous exploratory locomotion is necessary for novel object recognition. Expanding the armamentarium of cognitive assays, particularly through approaches that avoid potential physical and procedural artifacts, could be helpful in evaluating cognitive deficits across a range of mouse models of intellectual disabilities. Here we focus on operant touchscreen learning and memory approaches that could circumvent many procedural confounds. The small operant chambers require minimum locomotion, the sensitive touchscreen requires minimal motor skills, enhanced visual images can be used for low-vision mice who are not completely blind, software programs can define the training schedules to vary the levels of cognitive challenge, and available tasks can interrogate specific brain regions and neuroanatomical circuitry (Bussey et al., 1997; Brigman and Rothblat 2008; Brigman et al., 2005, 2008; Talpos et al., 2010; Bussey et al., 2012; McTighue et al., 2013; Silverman et al., 2015; Yang et al., 2015; Leach et al., 2016; Nichols et al., 2017; Buscher et al., 2017).
We tested three mouse models of neurodevelopmental disorders with intellectual disabilities on a touchscreen visual discrimination task, in concert with other learning and memory tasks, along with measures of rotarod motor coordination and balance and open field exploratory locomotion. 1) Angelman syndrome is caused by a maternally-inherited deletion at chromosome 15q11-q13, in which the key mutation is in the UBE3A gene (Angelman, 1965; Williams, 2005; Maab et al., 2011), which codes for an E3 ubiquitin ligase. Ube3a heterozygous mice in which the mutation is similarly transmitted by the dam, originally generated by Jiang, Beaudet and co-workers (Jiang et al., 1998), display behavioral abnormalities including deficits in rotarod motor coordination and balance, balance beam walking, grip strength, reduced exploratory range, contextual fear conditioning, and water maze hidden platform acquisition (Jiang et al., 1998; Miura et al., 2002; van Woerden et al., 2007; Heck et al., 2008; Allensworth et al., 2011; Daily et al., 2011; Kaphzan et al., 2012; Baudry et al., 2012; Huang et al., 2013; Santini et al., 2015; Hethorn et al., 2015). 2) Down syndrome is caused by a triplication of chromosome 21, incorporating a third copy of approximately 300 genes (Holtzman and Epstein 1992; Hattori et al., 2000; Chapman et al., 2000; Gardiner et al., 2010; Dierssen, 2012; Kleschevnikov et al., 2012). Ts65Dn mice with partial trisomy of the syntenic genes on mouse chromosome 16, originally generated by Reeves, Davisson and co-workers (Reeves et al., 1995), display behavioral abnormalities including deficits in water maze hidden platform acquisition, novel object recognition and radial maze, and higher exploration in an open field and on an elevated plus-maze (Reeves et al., 1995; Coussons-Read and Crnic, 1996; Demas et al., 1996; Sago et al., 2000; Moran et al., 2002; Martinez-Cue et al., 2005; Costa et al., 2010; Das and Reeves, 2011; Braudeau et al., 2011; Cramer and Galdzicki, 2012; Velazquez et al., 2013; Smith et al., 2014; Gupta et al., 2016). 3) Rett syndrome is caused by a mutation in MECP2 on the X chromosome, which codes for the epigenetic methylation regulator methyl-CpG-binding protein (Amir et al., 1999; Lombardi et al., 2015). The Mecp2 mouse model of Rett syndrome, originally generated by Guy, Bird and co-workers, recapitulates aspects of the hand stereotypies and regression of motor functions that characterize Rett syndrome (Guy et al., 2001). The Bird Mecp2 heterozygous mice and several other subsequent Mecp2 mutant mouse models display poor survival, hindlimb clasping, tremor, and progressive motor deficits on rotarod and balance beam (Guy et al., 2001; Gemelli et al., 2006; Ricceri et al., 2008; Katz et al., 2012; Gadalla et al., 2014; Vogel Ciernia et al., 2017). Fear conditioning and novel object recognition have been feasible at a young age in some lines of Mecp2 mice, with cognitive deficits detected in some of the Mecp2 mutant mouse lines but not in others (Moretti et al., 2006; Pelka et al., 2006; Schaevitz et al., 2013; Vogel Ciernia et al., 2017).
Using a touchscreen operant visual discrimination task previously applied to mouse models of neuropsychiatric disorders, we examined properties of the learning curve and training parameters in male and female Ube3a, Ts65Dn, and Mecp2 mice and their respective wildtype littermates. To confirm previous reports, we tested for performance on water maze, rotarod, and open field activity. Results reveal impaired acquisition of touchscreen visual discrimination in Ube3a and Ts65Dn mice, with no detectable abnormalities on procedural components of the task. Water maze learning deficits in Ube3a and Ts65Dn mice, and rotarod deficits in Ube3a mice, were replicated. Early death in male Mecp2 mice, and motor decline in female Mecp2 mice, precluded touchscreen visual discrimination training. Our findings indicate promising applications of the forefront touchscreen technology in phenotyping cognitive deficits in some mouse models of neurodevelopmental disorders with intellectual disabilities, for use in the preclinical discovery of effective therapeutics.
Material and Methods
Mice
All studies were approved by the UC Davis Institutional Animal Care and Use Committee, using procedures consistent with the NIH Guide for the Care and Use of Laboratory Animals. Mice were purchased from The Jackson Laboratory and bred as described below. Housing cages were maintained in an AAALAC-approved temperature and humidity controlled vivarium on a conventional 12:12 light cycle, with lights on at 7 AM and behavioral testing conducted during the light phase of the circadian cycle. Behavioral testing followed the sequence: open field and rotarod at 8-12 weeks of age, Morris water maze at 9-14 weeks, touchscreen beginning at 12-16 weeks. Food restriction to reach ≥ 85% of free feeding body weight was initiated 1 week prior to touchscreen pre-training, to ensure motivation for food reinforcement at the start of pre-training. With the exception of food restriction for the touchscreen experiments, food and water were provided ad libitum. Both males and females of each genotype were tested in all behavioral tasks. As no evidence for sex differences was detected, data from males and females were combined in statistical analyses.
To generate subject mice for the Angelman syndrome model, heterozygous female Ube3a mice (JAX catalogue #016590) were mated with male C57BL/6J (JAX #000664). PCR genotyping of tailsnips was conducted using previously published primers (Jiang et al., 1998). To generate subject mice for the Down syndrome model, female Ts65Dn (JAX #005252), which do not harbor the retinal degeneration gene, were mated with male B6eiC3F1 (JAX #003647). PCR genotyping of tailsnips was conducted using previously published primers (Reinholdt et al., 2011). To generate subject mice for the Rett syndrome model, Mecp2 heterozygous females of the Bird line Mecp2tm1.1Bird/J (JAX #003890) were mated with male C57BL/6J (JAX #000664). Genotyped mice were kindly contributed by Dr. Annie Vogel Ciernia, UC Davis (Vogel Ciernia et al., 2017). Offspring were weaned at 21-25 days of age into cages of 3-4 mice of mixed genotypes, housed by sex. Since some of the females were sequestered for breeding, Ns typically contained more males than females, however data from males and females appeared to be similar on all behavioral assays.
Due to poor breeding and low litter yields for each of these lines of mutant mice, the number of subject mice per group was somewhat smaller than Ns usually employed for behavioral testing. Subsequent mortality issues caused Ns to decline across the sequence of behavioral assays in some cases. However, the final Ns employed in touchscreen testing were consistent with group sizes used for this assay by other laboratories, as cited above. For Ts65Dn and Ube3a, Ns for each behavioral assay are listed in the figure legend for each test. For Mecp2 mice, Ns at the start of touchscreen testing were 15 female WT, 20 male WT, 11 female heterozygotes, 19 male heterozygotes. Males either died or lost considerable body weight during food restriction, precluding their use in touchscreen testing.
Behavioral assays
Open field
Open field exploratory activity was tested using an automated VersaMax Animal Activity Monitoring System (Accuscan, Omnitech Electronics Inc., Columbus, Ohio) under 40 lux illumination. Photocells in the horizontal x and y panels, and in the z vertical panel, detected beam breaks. Dedicated software calculated total distance traveled, horizontal activity, vertical activity, and center distance. Center distance represents locomotor activity in the central zone of the arena. Open field parameters were collected in 5 minute time bins and summed for the full session length of 60 minutes.
Rotarod
Rotarod motor coordination and balance was tested using an Ugo-Basile accelerating rotarod, (Stoelting Co., Wood Dale, Illinois). Revolutions per minute (rpm) were set at an initial value of 4 rpm, increasing progressively to a maximum of 40 rpm across 5 minutes. Test sessions consisted of 3 trials, with 60 seconds between each trial. Latency to fall was recorded for each trial.
Morris water maze
Morris water maze spatial learning and memory was tested using a 120 cm circular pool, filled with water (24-25°C) containing Crayola liquid non-toxic white paint. External cues for distal spatial navigation included a prominent sink, computer, water temperature regulator with hose, a large black X on one wall, and a yellow paper lantern hung from the ceiling. Platform locations and start locations were pseudorandomized. Trials were videorecorded and scored by automated software (Noldus Ethovision, Wageningen, The Netherlands) for measures including latency to find the hidden platform, total distance traveled, and swim speed. Mice were trained in the hidden platform version of the Morris water maze using methods consistent with standards in the literature. Each subject mouse was given 4 consecutive trials per day until the WT control group reached the latency criterion of 15 seconds or less to reach the hidden platform. Mice were allowed to remain on the platform for ~15 seconds after each trial. After the fourth daily trial, each mouse was placed under an infrared heating lamp to help restore body temperature. Probe trial analysis, to confirm that learning the hidden platform location was accomplished using distal environmental room cues, was conducted 3 hours after the last training trial. Each subject mouse was given a 60 second probe trial. Time spent in each of the four pool quadrants, and number of crossings over the former platform location versus the three analogous imaginary platform locations in the other quadrants, were automatically scored by the Noldus videotracking software.
Touchscreen operant visual discrimination learning
Touchscreen operant visual discrimination learning was tested using a Bussey-Saksida touchscreen system (Campden Instruments/Lafayette Instrument Company, Lafayette, Indiana). Operant chambers were trapezoidal in shape, to enhance the focus of attention on the touchscreen apparatus. Touchscreens at the front of the chambers were fitted with a Plexiglas two-hole mask. Each hole measured 7 cm × 7 cm. Selected visual images generated by the software were displayed on each mask. Image pairs used were a large white X and an = sign, of equal luminance. At the rear of the chambers, a food magazine delivered 20 µl aliquots of liquid per reinforcement (Ensure strawberry milkshake diluted 1:1 with dH20), through plastic tubing, regulated by a peristaltic pump controlled by the touchscreen software.
Touchscreen pre-training
To begin the training procedure, mice first received one day of habituation to the touchscreen chamber and autotraining to the food reward. During habituation, the food magazine was initially filled with reinforcer and signaled with a small LED light located directly above the food magazine. Each nosepoke into the food magazine initiated a new trial, which consisted of a fixed 10 second ITI and delivery of another 20 µl of reinforcement/reward. Following habituation, mice received fixed-ratio (FR-1) training, where each touch to either image on the touchscreen was rewarded. Image location was randomized between the left and right mask locations. The first day of FR-1 training had no punishment for touching blank image locations. On subsequent days, blank touches were punished by a 20 second timeout. Following correct or incorrect trials, there was an ITI of 20 seconds. After each session, the number of correct touches and the number of incorrect/blank touches were recorded and used to calculate percent correct performance using the following formula: correct touches ÷ (correct touches + incorrect/blank touches). Training continued until a performance criterion of ≥ 80% was reached for 2 days. After reaching the pre-training criterion, the subject mouse was moved to pairwise discrimination training.
Touchscreen pairwise discrimination training
Contingency for reward changed during this phase from an FR-1 to a pairwise discrimination rule. Briefly, a touch to the rewarded image (S+) was reinforced while a touch to the unrewarded image (S-) was punished with a 20 second timeout. Incorrect trials led to correction trials, where the trial was repeated until the S+ was successfully chosen. Training continued until a performance criterion of ≥80% was reached for 2 days, with the correction trials counted toward % correct performance.
Statistical analysis
Data were analyzed with GraphPad Prism version 6.07. Open field parameters were compared between genotypes using Two Way Repeated Measures Analysis of Variance (ANOVA) for time bin analyses of each parameter, and unpaired two tailed Student’s t-tests for the full 60 minute session score analyses of each parameter. Rotarod data were analyzed with Two-factor ANOVA using genotype as a between subjects factor and trial as a within subjects factor. Water maze acquisition parameters were evaluated with a Two Way Repeated Measures ANOVA followed by Bonferroni post-hoc analyses in cases of significant ANOVA F values. Water maze probe trial data were evaluated with One Way ANOVA within genotype, followed by post-hoc Dunnett’s test comparing all other locations to the target location. Touchscreen visual discrimination acquisition data were evaluated by Mantel-Cox analysis for survival curves, and by unpaired Student’s t-test for days to criterion and screen touches.
Results
Touchscreen learning impairments in Ube3a mice (Figure 1)
Figure 1.
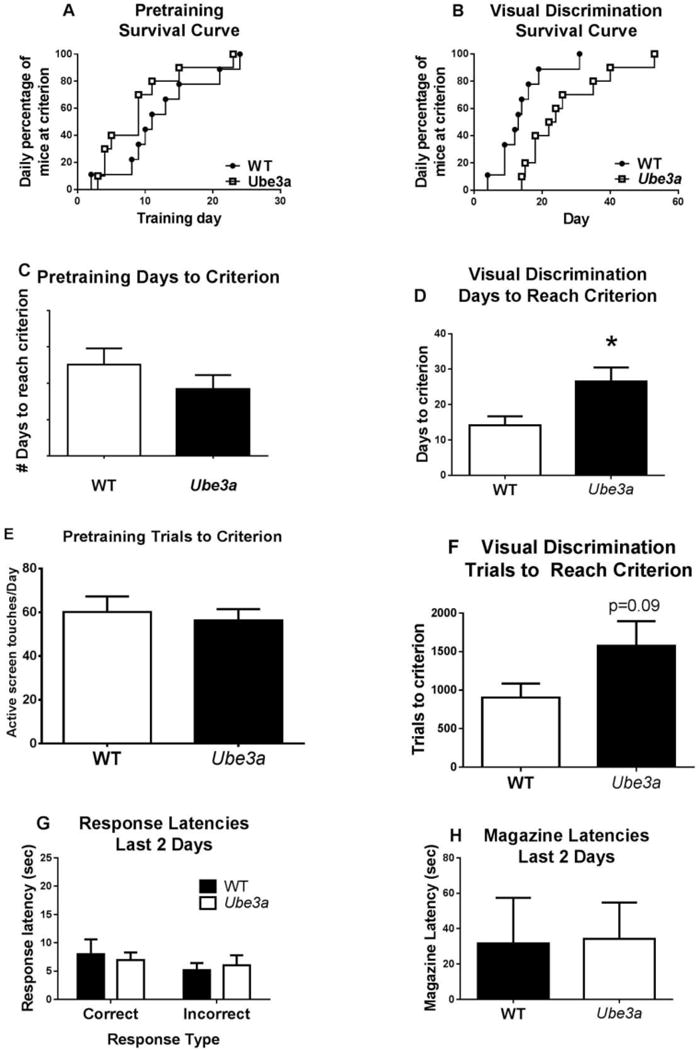
The Ube3a mouse model of Angelman syndrome, in which the heterozygous mutation is maternally derived, displayed deficits on acquisition of a pairwise 2-choice visual discrimination operant touchscreen task. A) Pretraining survival curve. Number of days to reach criterion indicated no gross differences in the rate of procedural learning. B) Visual discrimination survival curve indicated that Ube3a mutant mice required more days of training to reach the criterion of ≥ 80% correct, in learning the discrimination between the X image and the equal sign = image on the touchscreen, as compared to wildtype littermates (WT). C) WT and Ube3a required similar numbers of days to reach criterion on the pretraining phase, indicating similar procedural abilities. D) Ube3a required more days to reach criterion than WT on the visual discrimination task, indicating a slower learning curve. E) During pretraining, the number of trials to reach criterion (active screen touches per day, representing trials completed), was similar between WT and Ube3a. F) During visual discrimination learning, the number of trials to reach criterion (active screen touches) showed a trend for slower acquisition in Ube3a than WT. G) Response latencies during the last two training days indicated that physical movements were similar between genotypes, since a similar amount of time elapsed between image onset and selection of image choice. H) Latencies to reach the reinforcement magazine during the last two training days indicated that motivation for food reward was similar between genotypes, and corroborated generally normal motor function. Results indicate that Ube3a mice can learn the procedural components of an operant discrimination task, but episodic learning of specific image pairs was acquired more slowly by Ube3a than by WT. N=9 WT, 10 Ube3a, *p < .05.
Ube3a mice displayed significant deficits on acquisition of the touchscreen pairwise discrimination learning task, as compared to their WT littermate controls. Pretraining consisted of rule-learning about reward and punished contingencies, using illuminated and blank instead of unique symbols. Pretraining showed no genotype differences in survival curves when analyzed for individual mouse performance each day using the Mantel-Cox test (X2(1) = 1.102, p = 0.29) or by Student’s t-test of number of days to reach criterion (t17=1.134p = 0.27). Visual discrimination learning, illustrated by survival curves using Mantel-Cox analysis, revealed that Ube3a mutant mice took significantly more days to reach the criterion of at least 80% correct responses, as compared to WT mice: X2(1) = 6.50, p = 0.011. Visual discrimination learning t-test analysis of number of days to reach criterion revealed similarly significant slower acquisition (t17 = 2.56, p = 0.02). In terms of number of daily training trials (active screen touches) to reach criterion, genotypes showed no difference during pretraining. During visual discrimination training, t-test analysis of the number of training trials (active screen touches) revealed a non-significant trend for Ube3a requiring more trials to reach criterion (t17=1.78, p = 0.09). Thus, significantly more training days, and a strong trend toward more training trials, were required for Ube3a mice to reach criterion, consistent with an interpretation of cognitive impairment. Because of the discrepancy between significance when comparing days or active screen touches to criterion, and to address motor abnormalities in Ube3a mice, we analyzed response speed (latency to reach the correct or incorrect location and magazine latency) on the first two days and last two days of pairwise discrimination testing. Response speed was similar on correct latency, incorrect latency, and magazine latency for both WT and Ube3a mice, indicating no gross or overt motor phenotypes that may have contributed to the observed learning impairments. These findings indicate that pairwise discrimination learning was impaired in Ube3a mice, while the procedural rule was learned similarly by WT and Ube3a, and no motor issues appeared to interfere with the conduct of the procedures.
Normal open field exploratory locomotion in Ube3a mice (Figure 2)
Figure 2.
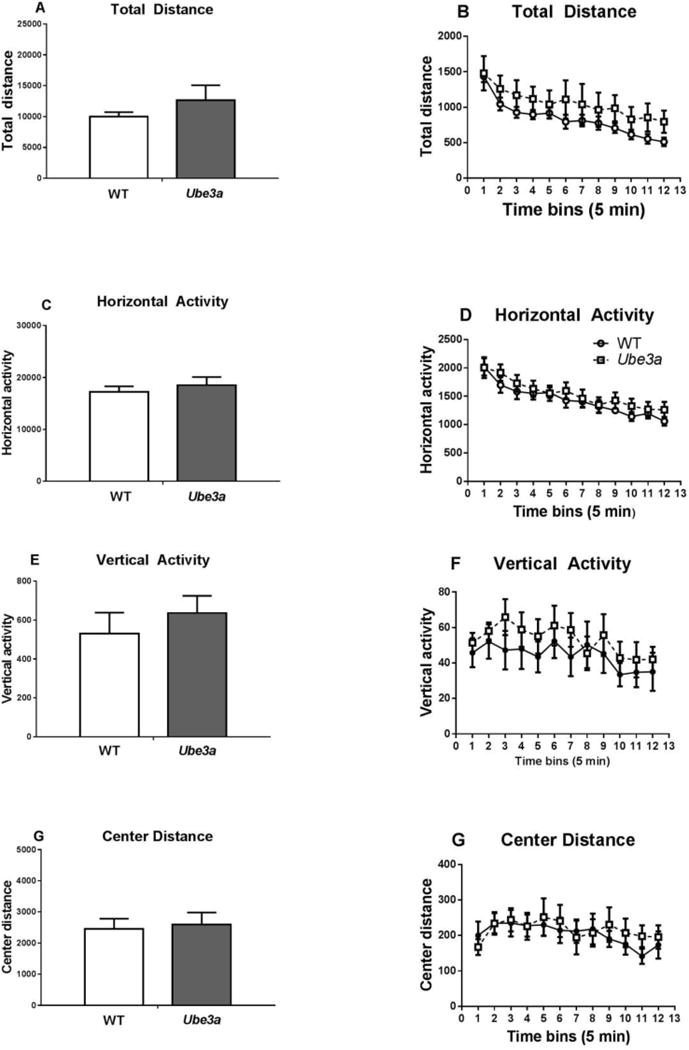
Ube3a mice showed normal exploratory locomotion. Ube3a and WT were tested for motor activity in automated Accuscan open fields. No genotype differences were detected on parameters of A,B) total distance, C,D) horizontal activity, E,F) vertical activity, G,H) center time as measured in 5 minute time bins (A,C,E,G) or as totaled across the 60 minute test session (B,D,F,H). N = 9 WT, 10 Ube3a.
No significant genotype differences were detected on open field activity: Total distance traveled t17 = 0.995, p = 0.33; Horizontal activity t17 = 0.669, p = 0.51; Vertical activity (t17 = 0.764, p = 0.46); Center distance (t17 = 0.280, p = 0.78).
Impaired rotarod performance in Ube3a (Figure 3)
Figure 3.
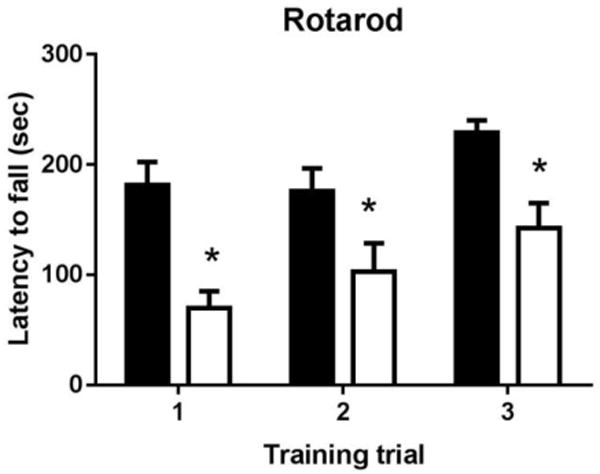
Ube3a mice displayed deficits on rotarod motor coordination and balance. Each test consisted of 5 minutes on an Ugo Basile mouse rotarod, accelerating from 4 to 40 rpm, with latency to fall as the dependent variable. Each subject mouse was given three trials within one day, to evaluate motor coordination and balance. Latency to fall from the rotarod was shorter in Ube3a as compared to WT on the first test trial, indicating impaired motor coordination and balance, consistent with previous reports. Latency to fall was similarly shorter in Ube3a on subsequent test trials, although a trend for improved performance across the three test trials indicated that some motor learning may have occurred. N = 12 WT, 12 Ube3a, *p < 0.001.
Rotarod testing revealed a significant motor impairment in maternally-derived Ube3a heterozygotes. Analysis of training trials confirmed a significant learning effect across the three rotarod training trials (F2,44 = 8.38, p < 0.001). Two-factor ANOVA revealed a significant effect of genotype (F1,22 = 16.9, p < 0.001). Bonferroni-corrected post-hoc tests revealed a significantly shorter latency to fall in Ube3a mice on each training trial (p<0.05). No genotype by trial interaction was detected (F2,44 = 0.791, NS).
Impaired Morris water maze performance in Ube3a (Figure 4)
Figure 4.
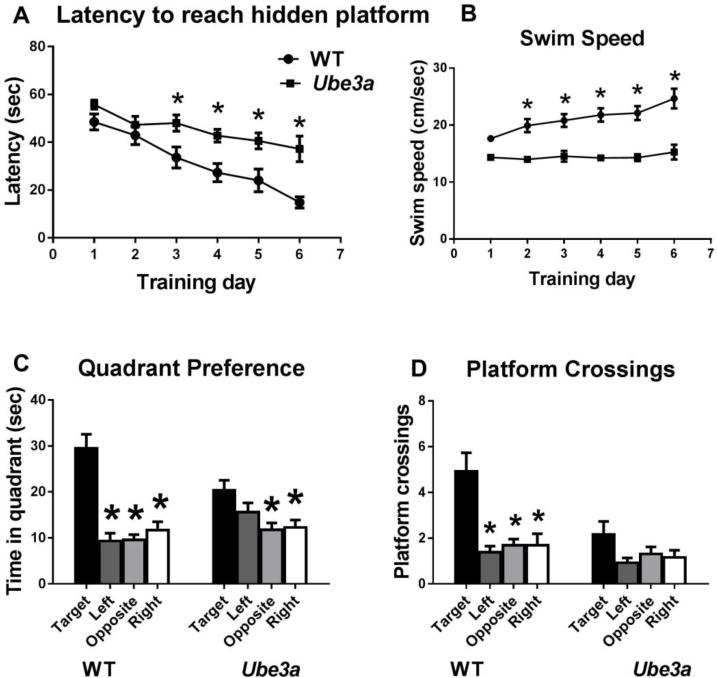
Ube3a mice displayed impaired Morris water maze performance. A) Latency to reach the hidden platform, measured by Noldus videotracking, with 4 trials per day for 6 training days, showed that WT attained the learning criterion of 15 seconds or less to reach the hidden platform. Ube3a did not reach criterion, and displayed significantly longer latencies as compared to WT. B) Swim speed was significantly faster in WT as compared to Ube3a. C) Selective search in the previously trained quadrant as compared to other three quadrants was significant for both WT and Ube3a. D) Probe trial crossings over the previous platform location as compared to the other three imaginary platform locations were significant for WT but not for Ube3a. N=13 WT, 13 Ube3a, *p < 0.05.
Ube3a mice failed to reach criterion on acquisition of the hidden platform location in the water maze. A significant effect of genotype was detected on latency to find the hidden platform (F1,24 = 25.8, p < 0.001). A significant effect of training day was detected (F5,120 = 14.4, p < 0.05), with no genotype by day interaction (F5,120 = 1.76, NS). Distance traveled showed no effect of genotype (F1,24 = 0.00922, NS), a significant effect of training day (F5,120 = 14.9, p < 0.001), and a trend for a genotype x day interaction (F5,120 = 1.99, p = 0.085). Swim speed revealed a significant genotype effect (F1,24 = 58.3, p < 0.05), a significant training day effect (F5,120 =4.46, p < 0.05), and a significant genotype by day interaction (F5,120 = 2.71, p < 0.05). Thus, Ube3a mice demonstrated slower acquisition of the location of the hidden platform, along with slower swim speeds as compared to WT which showed increasing swim speeds across training trials. Probe trial analysis for WT revealed a significant selective quadrant search in the previously trained target quadrant as compared to other quadrants of the pool (F3,36 = 16.6, p<0.0001), and more crossings over the previous platform location than corresponding locations in other quadrants of the pool (F3,36 = 9.94, p < 0.001), confirming spatial learning using distal room cues. Probe trial analysis for Ube3a showed a significant effect of searching in the previously trained target quadrant (F3,36 =3.51, p < 0.05), indicating some learning using distal spatial cues, however the Ube3a group did not display significantly more crossings over the previous platform location, (F3,36 = 2.24, NS), indicating some impairment in using distal spatial cues to solve the task.
Touchscreen learning impairments in Ts65Dn mice (Figure 5)
Figure 5.
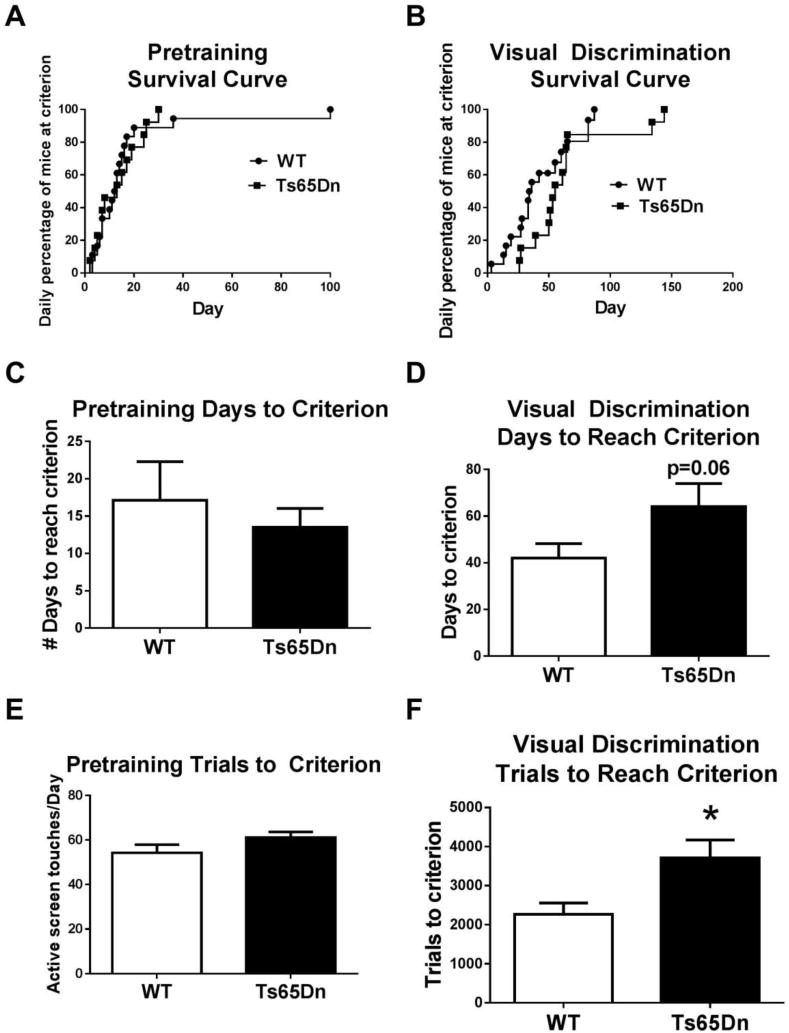
The Ts65Dn trisomy model of Down syndrome displayed performance impairments on acquisition of a touchscreen pairwise visual discrimination task. A) Pretraining survival curves were similar in WT and Ts65Dn, indicating no gross differences in procedural learning. B) Visual discrimination survival curves indicated a trend toward delayed acquisition of the image pairs by Ts65Dn mice. More Ts65Dn than WT required extra days of training to reach the criterion of ≥ 80% correct responses in learning the discrimination between the X and = touchscreen images. C) WT and Ts65Dn required similar number of days to reach criterion on the pretraining phase, indicated similar procedural abilities. D) Ts65Dn showed a strong trend for more days to reach criterion than WT on the visual discrimination task, indicating a slower learning curve. E) During pretraining, the number of trials to reach criterion (active screen touches) did not differ between WT and Ts65Dn. F) During visual discrimination learning, the number of trials to reach criterion (active screen touches) was significantly higher in Ts65Dn than WT. Results indicate that Ts65Dn mice can learn the operant discrimination task, but learn more slowly than WT. N=17 WT, 13 Ts65Dn, *p < .05.
Ts65Dn mice displayed a deficit on acquisition of the touchscreen pairwise discrimination learning task, as compared to their WT littermate controls. Mantel-Cox analysis for individual mouse performance each day indicated similar number of days to criterion (X2(1)=1.62, p=0.203). Total number of training days to reach criterion showed a strong trend for slower learning in Ts65Dn (t28 =1.97, p = 0.059). Significantly more training trials were required for Ts65Dn to reach criterion (t28 = 2.70, p < 0.02). Trials per day analysis (Figure 5F) revealed no significant difference in rates of responding between strains (t28 =1.43, NS).
Higher open field exploratory locomotion in Ts65Dn as compared to WT littermates (Figure 6)
Figure 6.
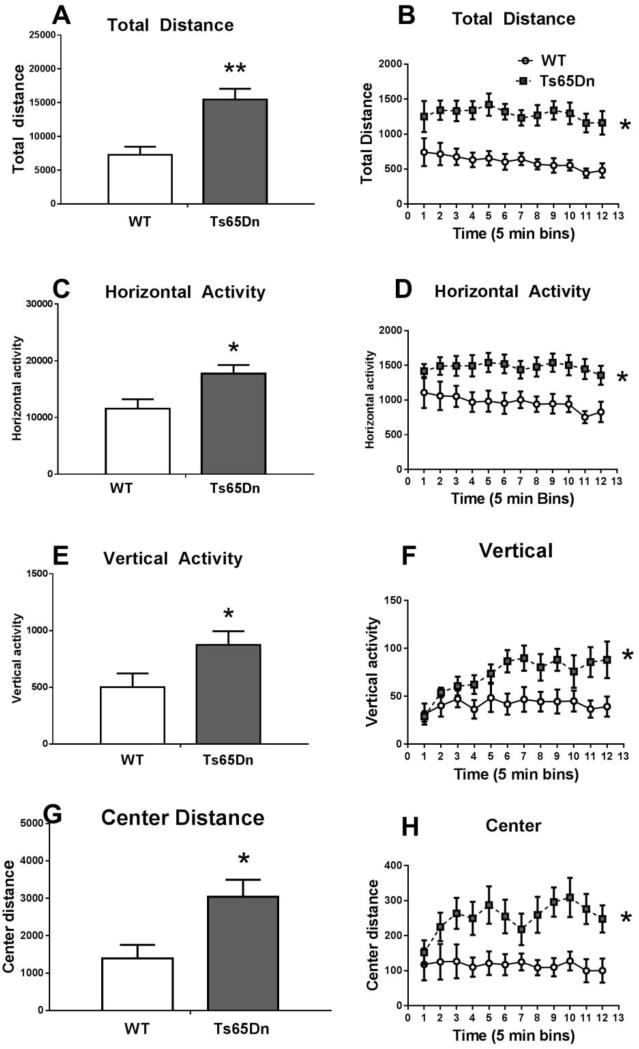
Higher exploratory locomotion in Ts65Dn mice. Ts65Dn and WT were tested for motor activity during a 60 minute session in automated Accuscan open fields. Significantly higher scores were detected in the Ts65Dn group on parameters of A,B) total distance, C,D) horizontal activity, E,F) vertical activity, G,H) center distance, as totaled across the 60 minute test session (A,C,E,G), and as measured in 5 minute time bins (B,D,F,H). N=8 WT, 10 Ts65Dn, *p < .0.05, **p < 0.01.
Ts65Dn mice displayed significantly higher exploratory activity than WT mice on parameters of total distance traveled (t16 = 3.95, p < 0.01), horizontal activity (t16 = 2.73, p < 0.05), vertical activity (t16 = 2.15, p < 0.05) and center distance (t16 = 2.72, p < 0.05).
Impaired Morris water maze performance in Ts65Dn (Figure 7)
Figure 7.
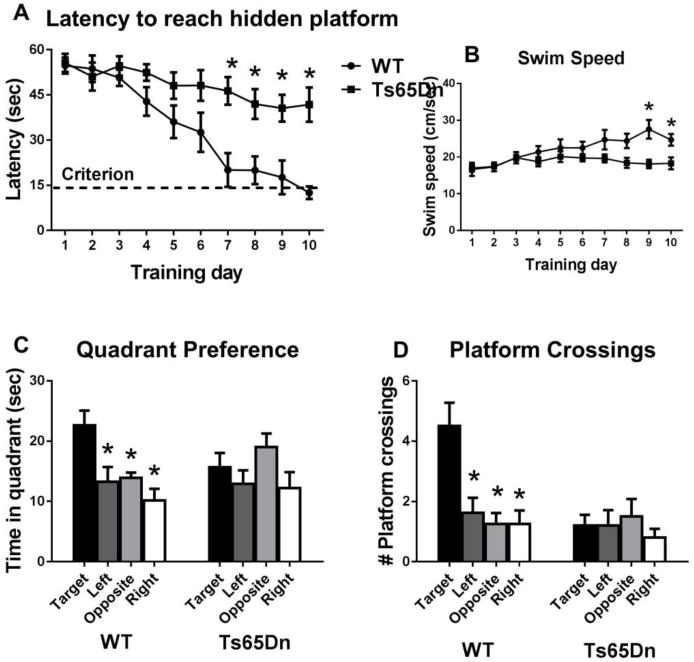
Ts65Dn mice displayed impaired Morris water maze performance. A) Latency to reach the hidden platform, measured by Noldus videotracking, with 4 trials per day for 10 training days, showed that WT attained the learning criterion of 15 seconds or less to reach the hidden platform (dashed line), while Ts65Dn did not reach criterion and showed significantly longer latencies than WT on days 7, 8, 9 and 10. B) Swim speed was slightly higher in Ts65Dn than WT on the last two training days. C) Probe trial selective quadrant search in the previously trained quadrant as compared to the other three quadrants was significant for WT but not for Ts65Dn. D) Probe trial crossings over the previous platform location as compared to the other three imaginary platform locations was significant for WT but not for Ts65Dn. N=8 WT, 10 Ts65Dn, *p < 0.05.
Ts65Dn mice failed to reach criterion on acquisition of the hidden platform location in the Morris water maze. A significant effect of genotype was seen on latency to find the hidden platform (F1,16 = 9.93, p < 0.05). A significant effect of training day was detected (F9,144 = 18.5, p < 0.05), with a significant genotype by day interaction (F1,16 = 5.04, p < 0.05). Significant differences in distance traveled were observed between genotypes (F1,16 = 6.79, p < 0.05) and day (F9,144 = 12.9, p < 0.05), and there was a significant genotype by day interaction (F9,144 = 2.66, p < 0.05). Swim speed showed a significant genotype effect (F1,16 = 4.47, p = 0.05), a significant day effect (F9,144 = 6.44, p < 0.050), and a significant genotype by day interaction (F9,144 = 4.36, p < 0.05), indicating somewhat slower swimming by Ts65Dn during the last two training days. Probe trial analysis revealed that WT spent significantly more time in the previously trained quadrant (F3,21 = 5.21, p < 0.05), and significantly more crossings over the previously trained platform location (F3,21 = 7.57, p < 0.05). In contrast, Ts65Dn did not display selective quadrant search (F3,27 = 1.27, NS) nor significantly more crossings over the previously trained platform location (F3,27 = 0.937, NS), indicating failure to use distal spatial cues to solve the task.
Touchscreen training in Mecp2 mice
Motor declines in Mecp2 mice were observed on open field and rotarod motor assays, as described in Vogel Ciernia et al., 2016. Due to significant and progressive motor impairments, Morris water maze was not attempted on these animals. Pre-training with mild food restriction was attempted in male Mecp2 mice, however morbidity and lethality occurred by approximately 10 weeks of age. Female Mecp2 mice completed pre-training in only a few cases. Of the 11 Mecp2 heterozygous females obtained after extensive breeding, 4 did not ingest the liquid reinforcement and did not complete pre-training; 2 consumed some of the liquid reinforcement but did not reach the pre-training criterion; and 5 females reached criterion on pre-training. Given the early lethality of male Mecp2 mice, the difficulty of breeding and maintaining the Mecp2 female heterozygotes through the adult weeks required for touchscreen training and testing, and the low percentages of females reaching criterion on the pre-training phase, further touchscreen experiments were not pursued with this mouse model of Rett syndrome in the present studies.
Discussion
Translational usefulness of genetic mouse models depends on the strength of the phenotypes relevant to the human disease. Developing effective therapeutics for a neurodevelopmental disorder characterized by intellectual disabilities requires sufficiently robust and replicable cognitive deficits in the mouse model. Learning and memory deficits detected in multiple tasks will increase confidence in the strength of the mutant mouse model. Here we identify new cognitive deficits on a 2-choice visual discrimination assay in genetic mouse models of Angelman and Down syndromes, using forefront operant touchscreen technology. Our findings indicate that Angelman mice with the conventional Ube3a heterozygous mutation and Down mice with the conventional Ts65Dn trisomy learn touchscreen visual discrimination at a significantly slower rate. These impaired learning curves offer new translational outcome measures to evaluate pharmacological interventions.
Our replication of deficits on water maze hidden platform acquisition in both Ube3a and Ts65Dn mice reinforces evidence in the literature that spatial learning is impaired in genetic mouse models of Angelman and Down syndrome (Reeves et al., 1995; Moran et al., 2002; Miura et al., 2002; Martínez-Cué et al., 2002; Daily et al., 2012; Huang et al., 2013; Kleschevnikov et al., 2015). Rotarod motor coordination and balance were significantly impaired in Ube3a mice, consistent with previous reports (Huang et al., 2013; Bruinsma et al., 2015). However, open field exploratory locomotion scores were not significantly different between Ube3a and WT littermate controls. It is important to note that motor disabilities detected in a genetic mouse model can introduce artifacts that may directly affect performance on cognitive tasks such as water maze. Since relatively minimal locomotion, coordination and balance are required for touchscreen procedures, it appears that the cognitive deficits detected on visual discrimination learning were independent of the rotarod phenotype in this mouse model. In addition, latencies for correct and incorrect screen touches, latency to retrieve liquid food reinforcers, and pre-training days to reach criterion were similar inUbe3a and WT mice, further supporting the interpretation that motor impairments were not the major factor underlying the touchscreen acquisition deficit. In contrast, water maze acquisition of the hidden platform location may have been impacted by the significantly slower swim speeds in Ube3a as compared to WT.
Ts65Dn mice similarly took significantly more training trials than their WT littermates to reach criterion on the touchscreen visual discrimination task. Open field scores were higher in Ts65Dn than WT on parameters of total distance, horizontal activity, vertical activity, and center distance, consistent with previous reports of elevated exploration and/or locomotion (Coussons-Read and Crnic, 1996; Faizi et al., 2011). It seems unlikely that the higher open field activity was responsible for the touchscreen learning deficit in Ts65Dn, since scores on pre-training showed no genotype differences. Latencies for correct and incorrect screen touches and latencies to retrieve the liquid food reinforcers were also similar between genotypes. Morris water maze acquisition was profoundly impaired in Ts65Dn mice. Swim speeds did not differ between Ts65Dn and WT during the first six training days. Swim speeds were somewhat lower in Ts65Dn than WT during the last training days, when the learning curve differences were most pronounced. This could indicate some contribution of swimming impairment to the performance scores, but more likely was due to increased swim speed in WTs as they progressively acquired the hidden platform location. More sensitive measures of swimming abilities in both Ts65Dn and Ube3a would be useful to determine the extent to which slower swimming contributed to apparent cognitive impairments. Another interpretation is that slower swimming during the last training days was a motivational consequence of repeated failures to locate the hidden platform.
Mecp2Bird mice are well known for early death and progressive loss in motor functions (Guy et al., 2001; Gemelli et al., 2006; Ricceri et al., 2008; Katz et al., 2012; Gadalla et al., 2014; Vogel Ciernia et al., 2017). We previously replicated findings of poor survival in male heterozygous Mecp2 mice, and age-dependent rotarod and open field deficits in female heterozygous Mecp2 mice, and extended the motor profile of Mecp2Bird female mice with new findings of balance beam deficits, gait abnormalities, and developmental milestone delays in female heterozygous Mecp2 mice (Vogel Cierna 2017). These significant motor disabilities precluded our testing this group of Mecp2 females on water maze learning, which requires a high level of swimming ability. Given the minimal motor demands within the small touchscreen chamber, we postulated that motor dysfunctions in Mecp2 mice may not introduce a major artifact in operant learning. In the present study, pre-training was attempted in male Mecp2 mice. None of the male heterozygous Mecp2 mice lived long enough to complete pre-training. Female Mecp2 heterozygotes were able to conduct the pre-training procedures to some extent. In many cases, however, the food reward was not retrieved or not completely consumed. Few female Mecp2 successfully completing pretraining. Future studies with larger breeding colonies, to generate considerably more female Mecp2 mice, may enable touchscreen learning to be pursued in this mouse model of Rett syndrome.
Touchscreen operant tests have several conceptual and technical advantages. These include flexibility in programming tasks that vary in level of difficulty, opportunities to conduct longitudinal cognitive testing across the lifespan, engagement of defined sensory and motor functions, and the ability to interrogate distinct neuroanatomical substrates. The touchscreen visual discrimination assay sensitively detected learning deficits in both the Ube3a mouse model of Angelman syndrome and the Ts65Dn mouse model of Down syndrome. This forefront operant technology may be particularly advantageous for rodent models with mild to moderate motor deficits, which could impact performance on procedural components of other cognitive assays with greater motor demands. Robust acquisition deficits in touchscreen assays offer new preclinical outcome measures for discovering effective therapeutics for neurodevelopmental disorders with intellectual disabilities such as Angelman and Down syndromes.
Acknowledgments
We are grateful to our collaborators Drs. Gary Lynch, Christine Gall, and Julie Lauterborn, University of California Irvine, for their conceptual input to the hypotheses underlying our cognitive testing of Ube3a, Ts65Dn, and Mecp2 mutant mouse models of intellectual disabilities. We thank Dr. Annie Vogel Ciernia, UC Davis, for generously contributing the genotyped Mecp2 and WT mice, University of California Davis undergraduate student Kamela Sison for technical assistance in conducting open field and rotarod testing, and UC Davis undergraduate student Stephen White for technical assistance in aspects of touchscreen testing. We are grateful to Heather Boyle for excellent assistance with animal care, including maintenance of the sensitive breeding colonies of the three mutant lines of mice. This work was supported by National Institutes of Health grants R01NS085709 and U54HD079125 to JNC.
Footnotes
The authors declare no conflicts of interest.
References
- Allensworth M, Saha A, Reiter LT, Heck DH. Normal social seeking behavior, hypoactivity and reduced exploratory range in a mouse model of Angelman syndrome. BMC Genetics. 2011;12:7. doi: 10.1186/1471-2156-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genetics. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Angelman H. Puppet children: a report on three cases. Developmental Medicine and Child Neurology. 1965;7:681–688. doi: 10.1111/j.1469-8749.2008.03035.x. [DOI] [PubMed] [Google Scholar]
- Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, Gall C, Bi X. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiology of Disease. 2012;47:210–215. doi: 10.1016/j.nbd.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko PV, Madani R, Rey-Bellet L, Pihlgren M, Becker A, Plassard A, Vuillermot S, Giriens V, Nosheny RL, Kleschevnikov AM, Valletta JS, Bengtsson SK, Linke GR, Maloney MT, Hickman DT, Reis P, Granet A, Mlaki D, Lopez-Deber MP, Do L, Singhal N, Masliah E, Pearn ML, Pfeifer A, Muhs A, Mobley WC. An anti-β-amyloid vaccine for treating cognitive deficits in a mouse model of down syndrome. PLoS One. 2016;11(3):e0152471. doi: 10.1371/journal.pone.0152471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braudeau J, Delatour B, Duchon A, Pereira PL, Dauphinot L, de Chaumont F, Olivo-Marin JC, Dodd RH, Herault Y, Potier MC. Specific targeting of the GABA-A receptor alpha5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. Journal of Psychopharmacology. 2011;25:1030–1042. doi: 10.1177/0269881111405366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Bussey TJ, Saksida LM, Rothblat LA. Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behavioral Neuroscience. 2005;119:839–42. doi: 10.1037/0735-7044.119.3.839. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learning and Memory. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behavioural Brain Research. 2008;187:405–410. doi: 10.1016/j.bbr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bruinsma CF, Schonewille M, Gao Z, Aronica EM, Judson MC, Philpot BD, Hoebeek FE, van Woerden GM, De Zeeuw CI, Elgersma Y. Dissociation of locomotor and cerebellar deficits in a murine Angelman syndrome model. Journal of Clinical Investigation. 2015;125:4305–4315. doi: 10.1172/JCI83541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscher N, van Dorsselaer P, Steckler T, Talpos JC. Evaluating aged mice in three touchscreen tests that differ in visual demands: Impaired cognitive function and impaired visual abilities. Behavioural Brain Research. 2017;S0166-4328(17):30889–6. doi: 10.1016/j.bbr.2017.06.053. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behavioral Neuroscience. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nature Reviews Genetics. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Hesketh LJ. Behavioral phenotype of individuals with Down syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:84–95. doi: 10.1002/1098-2779(2000)6:2<84::AID-MRDD2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Schmidt C, Davisson MT. Behavioral validation of the Ts65Dn mouse model for Down syndrome of a genetic background free of the retinal degeneration mutation Pde6b(rd1) Behavioural Brain Research. 2010;206:52–62. doi: 10.1016/j.bbr.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Crnic LS. Behavioral assessment of the Ts65Dn mouse, a model for Down syndrome: altered behavior in the elevated plus maze and open field. Behavior Genetics. 1996;26:7–13. doi: 10.1007/BF02361154. [DOI] [PubMed] [Google Scholar]
- Cramer N, Galdzicki Z. From abnormal hippocampal synaptic plasticity in down syndrome mouse models to cognitive disability in down syndrome. Neural Plasticity. 2012;2012(101542):1–12. doi: 10.1155/2012/101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues in Clinical Neuroscience. 2012;14:293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily JL, Nash K, Jinwal U, Golde T, Rogers J, Peters MM, Burdine RD, Dickey C, Banko JL, Weeber EJ. Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome. PLoS One. 2011;6:e27221. doi: 10.1371/journal.pone.0027221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva BM, Bast T, Morris RG. Spatial memory: behavioral determinants of persistence in the watermaze delayed matching-to-place task. Learning and Memory. 2013;21:28–36. doi: 10.1101/lm.032169.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, Reeves RH. The use of mouse models to understand and improve cognitive deficits in Down syndrome. Disease Models and Mechanisms. 2011;4:596–606. doi: 10.1242/dmm.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ, Krueger BK, Yarowsky PJ. Spatial memory deficits in segmental trisomic Ts65Dn mice. Behavioural Brain Research. 1996;82:85–92. doi: 10.1016/s0166-4328(97)81111-4. [DOI] [PubMed] [Google Scholar]
- Dierssen M. Down syndrome: the brain in trisomic mode. Nature Reviews Neuroscience. 2012;13:844–858. doi: 10.1038/nrn3314. [DOI] [PubMed] [Google Scholar]
- Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, Ma K, Dietz DM, Kajiwara Y, Buxbaum JD, Yan Z. Autism-like deficits in Shank3-deficient mice are rescued by targeting actin regulators. Cell Reports. 2016;11:1400–1413. doi: 10.1016/j.celrep.2015.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Robitsek RJ. Olfactory memory: a bridge between humans and animals in models of cognitive aging. Annals of the New York Academy of Sciences. 2009;1170:658–663. doi: 10.1111/j.1749-6632.2009.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizi M, Bader PL, Tun C, Encarnacion A, Kleschevnikov A, Belichenko P, Saw N, Priestley M, Tsien RW, Mobley WC, Shamloo M. Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: activation of β1-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiology of Disease. 2011;43:397–413. doi: 10.1016/j.nbd.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Gadalla KK, Ross PD, Riddell JS, Bailey ME, Cobb SR. Gait analysis in a Mecp2 knockout mouse model of Rett syndrome reveals early-onset and progressive motor deficits. PLoS One. 2014;9(11):e112889. doi: 10.1371/journal.pone.0112889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K, Herault Y, Lott IT, Antonarakis SE, Reeves RH, Dierssen M. Down syndrome: from understanding the neurobiology to therapy. The Journal of Neuroscience. 2010;30:14943–14945. doi: 10.1523/JNEUROSCI.3728-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biological Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Gertyan I. Cognitive “omics”: Pattern-based validation of potential drug targets. Trends in Pharmacological Sciences. 2017;38:113–128. doi: 10.1016/j.tips.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Developing translational animal models for symptoms of schizophrenia or bipolar mania. Neurotoxicity Research. 2008;14:71–78. doi: 10.1007/BF03033576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Rook JM, Ghoshal A, Zamorano R, Malosh C, Stauffer SR, Bridges TM, Bartolome JM, Daniels JS, Jones CK, Lindsley CW, Conn PJ, Niswender CM. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Human Molecular Genetics. 2016;25:1990–2004. doi: 10.1093/hmg/ddw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson B, Leger M, Piercy C, Adamson L, Harte M, Neill JC. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behavioural Brain Research. 2015;285:176–193. doi: 10.1016/j.bbr.2014.10.025. [DOI] [PubMed] [Google Scholar]
- Gupta M, Dhanasekaran AR, Gardiner KJ. Mouse models of Down syndrome: gene content and consequences. Mammalian Genome. 2016;27:538–555. doi: 10.1007/s00335-016-9661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genetics. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hatami A, Chesselet MF. Transgenic rodent models to study alpha-synuclein pathogenesis, with a focus on cognitive deficits. Current Topics in Behavioral Neuroscience. 2015;22:303–330. doi: 10.1007/7854_2014_355. [DOI] [PubMed] [Google Scholar]
- Hattori M, Fujiyama A, Taylor TD, Watanabe H, Yada T, Park HS, Toyoda A, Ishii K, Totoki Y, Choi DK, Groner Y, Soeda E, Ohki M, Takagi T, Sakaki Y, Taudien S, Blechschmidt K, Polley A, Menzel U, Delabar J, Kumpf K, Lehmann R, Patterson D, Reichwald K, Rump A, Schillhabel M, Schudy A, Zimmermann W, Rosenthal A, Kudoh J, Schibuya K, Kawasaki K, Asakawa S, Shintani A, Sasaki T, Nagamine K, Mitsuyama S, Antonarakis SE, Minoshima S, Shimizu N, Nordsiek G, Hornischer K, Brant P, Scharfe M, Schon O, Desario A, Reichelt J, Kauer G, Blocker H, Ramser J, Beck A, Klages S, Hennig S, Riesselmann L, Dagand E, Haaf T, Wehrmeyer S, Borzym K, Gardiner K, Nizetic D, Francis F, Lehrach H, Reinhardt R, Yaspo ML, Chromosome 21 mapping and sequencing consortium The DNA sequence of human chromosome 21. Nature. 2000;405:311–319. doi: 10.1038/35012518. [DOI] [PubMed] [Google Scholar]
- Heck DH, Zhao Y, Roy S, LeDoux MS, Reiter LT. Analysis of cerebellar function in Ube3a-deficient mice reveals novel genotype-specific behaviors. Human Molecular Genetics. 2008;17:2181–2189. doi: 10.1093/hmg/ddn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hethorn WR, Ciarlone SL, Filonova I, Rogers JT, Aguirre D, Ramirez RA, Grieco JC, Peters MM, Gulick D, Anderson AE, Banko JL, Lussier AL, Weeber EJ. Reelin supplementation recovers synaptic plasticity and cognitive deficits in a mouse model for Angelman syndrome. European Journal of Neuroscience. 2015;41:1372–1380. doi: 10.1111/ejn.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Epstein CJ. The molecular genetics of Down syndrome. Molecular and Genetic Medicine. 1992;2:105–120. doi: 10.1016/b978-0-12-462002-5.50009-1. [DOI] [PubMed] [Google Scholar]
- Huang HS, Allen JA, Mabb AM, King IF, Miriyala J, Taylor-Blake B, Sciaky N, Dutton JW, Jr, Lee HM, Chen X, Jin J, Bridges AS, Zylka MJ, Roth BL, Philpot BD. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature. 2011;481:185–189. doi: 10.1038/nature10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Burns AJ, Nonneman RJ, Baker LK, Riddick NV, Nikolova VD, Riday TT, Yashiro K, Philpot BD, Moy SS. Behavioral deficits in an Angelman syndrome model: effects of genetic background and age. Behavioural Brain Research. 2013;243:79–90. doi: 10.1016/j.bbr.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Kaphzan H, Hernandez P, Jung JI, Cowansage KK, Deinhardt K, Chao MV, Abel T, Klann E. Reversal of impaired hippocampal long-term potentiation and contextual fear memory deficits in Angelman syndrome model mice by ErbB inhibitors. Biological Psychiatry. 2012;72:182–190. doi: 10.1016/j.biopsych.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, Glennon JC, Buitelaar J, Ey E, Biemans B, Crawley J, Ring RH, Lajonchere C, Esclassan F, Talpos J, Noldus LP, Burbach JP, Steckler T. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: current status and future perspectives. Psychopharmacology. 2014;231:1125–1146. doi: 10.1007/s00213-013-3268-5. [DOI] [PubMed] [Google Scholar]
- Katz DM, Berger-Sweeney JE, Eubanks JH, Justice MJ, Neul JL, Pozzo-Miller L, Blue ME, Christian D, Crawley JN, Giustetto M, Guy J, Howell CJ, Kron M, Nelson SB, Samaco RC, Schaevitz LR, St Hillaire-Clarke C, Young JL, Zoghbi HY, Mamounas LA. Preclinical research in Rett syndrome: setting the foundation for translational success. Disease Models and Mechanisms. 2012;5:733–745. doi: 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdoba TM, Leach TL, Crawley JN. Behavioral phenotypes of genetic mouse models of autism. Genes, Brain and Behavior. 2016a;15:7–26. doi: 10.1111/gbb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdobs TM, Leach PT, Yang M, Silverman JL, Solomon M, Crawley JN. Translational mouse models of autism: Advancing toward pharmacological therapeutics. Current Topics in Behavioral Neuroscience. 2016b;28:1–52. doi: 10.1007/7854_2015_5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja OS, Sahin M. Translational research: Rett syndrome and tuberous sclerosis complex. Current Opinion in Pediatrics. 2011;23:633–639. doi: 10.1097/MOP.0b013e32834c9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, Mobley WC. Deficits in cognition and synaptic plasticity in a mouse model of Down syndrome ameliorated by GABAB receptor antagonists. The Journal of Neuroscience. 2012;32:9217–9227. doi: 10.1523/JNEUROSCI.1673-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach PT, Hayes J, Pride M, Silverman JL, Crawley JN. Normal performance of Fmr1 mice on a touchscreen delayed non-matching to position working memory task. eNeuro. 2016;0143–15 doi: 10.1523/ENEURO.0143-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi LM, Baker SA, Zoghbi HY. MECP2 disorders: from the clinic to mice and back. Journal of Clinical Investigation. 2015;125:2914–2923. doi: 10.1172/JCI78167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. European Journal of Pharmacology. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabb AM, Judson MC, Zylka MJ, Philpot BD. Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends in Neuroscience. 2011;34:293–303. doi: 10.1016/j.tins.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cué C, Rueda N, García E, Davisson MT, Schmidt C, Flórez J. Behavioral, cognitive and biochemical responses to different environmental conditions in male Ts65Dn mice, a model of Down syndrome. Behavioural Brain Research. 2005;163:174–185. doi: 10.1016/j.bbr.2005.04.016. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One. 2013;8:e62189. doi: 10.1371/journal.pone.0062189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Kishino T, Li E, Webber H, Dikkes P, Holmes GL, Wagstaff J. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiology of Disease. 2002;9:149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- Moran TH, Capone GT, Knipp S, Davisson MT, Reeves RH, Gearhart JD. The effects of piracetam on cognitive performance in a mouse model of Down's syndrome. Physiology and Behavior. 2002;77:403–409. doi: 10.1016/s0031-9384(02)00873-9. [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. The Journal of Neuroscience. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JN, Hagan KL, Floyd CL. Evaluation of touchscreen chambers to assess cognition in adult mice: Effect of training and mild traumatic brain injury. J Neurotrauma. 2017;10 doi: 10.1089/neu.2017.4998. 1089/neu.2017.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PP. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nature Genetics. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Reinholdt LG, Ding Y, Gilbert GJ, Czechanski A, Solzak JP, Roper RJ, Johnson MT, Donahue LR, Lutz C, Davisson MT. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mammalian Genome. 2011;22:685–691. doi: 10.1007/s00335-011-9357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behavioural Pharmacology. 2008;19:501–517. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]
- Sago H, Carlson EJ, Smith DJ, Rubin EM, Crnic LS, Huang TT, Epstein CJ. Genetic dissection of region associated with behavioral abnormalities in mouse models for Down syndrome. Pediatric Research. 2000;48:606–613. doi: 10.1203/00006450-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Santini E, Turner KL, Ramaraj AB, Murphy MP, Klann E, Kaphzan H. Mitochondrial superoxide contributes to hippocampal synaptic dysfunction and memory deficits in Angelman syndrome model mice. The Journal of Neuroscience. 2015;35:16213–16220. doi: 10.1523/JNEUROSCI.2246-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaevitz LR, Gómez NB, Zhen DP, Berger-Sweeney JE. MeCP2 R168X male and female mutant mice exhibit Rett-like behavioral deficits. Genes Brain and Behavior. 2013;12:732–740. doi: 10.1111/gbb.12070. [DOI] [PubMed] [Google Scholar]
- Seese RR, Wang K, Yao YQ, Lynch G, Gall CM. Spaced training rescues memory and ERK1/2 signaling in fragile X syndrome model mice. Proceedings of the National Academy of Sciences USA. 2014;111:16907–16912. doi: 10.1073/pnas.1413335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Gastrell PT, Karras MN, Solomon M, Crawley JN. Cognitive abilities on transitive inference using a novel touchscreen technology for mice. Cerebral Cortex. 2015;25:1133–1142. doi: 10.1093/cercor/bht293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GK, Kesner RP, Korenberg JR. Dentate gyrus mediates cognitive function in the Ts65Dn/DnJ mouse model of Down syndrome. Hippocampus. 2014;24:354–362. doi: 10.1002/hipo.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiology of Learning and Memory. 2010;94:341–352. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nature Neuroscience. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Velazquez R, Ash JA, Powers BE, Kelley CM, Strawderman M, Luscher ZI, Ginsberg SD, Mufson EJ, Strupp BJ. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiology of Disease. 2013;58:92–101. doi: 10.1016/j.nbd.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Ciernia A, Pride MC, Durbin-Johnson B, Noronha A, Chang A, Yasui DH, Crawley JN, LaSalle JM. Early motor phenotype detection in a female mouse model of Rett syndrome is improved by cross-fostering. Human Molecular Genetics. 2017;26:1839–1854. doi: 10.1093/hmg/ddx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JA, Spooren W, Persico AM, Collier DA, Aigner S, Jagasia R, Glennon JC, Buitelaar JK. Using genetic findings in autism for the development of new pharmaceutical compounds. Psychopharmacology. 2014;231:1063–1078. doi: 10.1007/s00213-013-3334-z. [DOI] [PubMed] [Google Scholar]
- Williams CA. Neurological aspects of the Angelman syndrome. Brain Development. 2005;27:88–94. doi: 10.1016/j.braindev.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Yang M, Lewis FC, Sarvi MS, Foley GM, Crawley JN. 16p11.2 deletion mice display cognitive deficits in touchscreen learning and novelty recognition tasks. Learning and Memory. 2015;22:622–632. doi: 10.1101/lm.039602.115. [DOI] [PMC free article] [PubMed] [Google Scholar]


