Abstract
Although muscle mass influences strength in older adults, it is unclear whether low lean mass measured by dual-energy x-ray absorptiometry (DXA) is an independent risk factor hip fracture. Our objective was to determine the association between DXA lean mass and incident hip fracture risk among 1,978 women aged 50 years and older participating in the Framingham Study Original and Offspring cohorts. Leg and total body lean mass (kg) were assessed from whole body DXA scans collected in 1992–2001. Hip fracture follow-up extended from DXA assessment to the occurrence of fracture, death, drop-out, or end of follow-up in 2007. Cox proportional hazards regression was used to calculate hazard ratios (HR) and 95% confidence intervals (CI) estimating the relative risk of hip fracture associated with a 1 kg increase in baseline lean mass. Mean age was 66 years (range 50–93). Over a median of 8 years of follow-up, 99 hip fractures occurred. In models adjusted for age, height, study cohort and percent total body fat, neither leg (HR 1.11; 95% CI 0.94, 1.31) nor total body (HR 1.06; 95% CI 0.99, 1.13) lean mass were associated with hip fracture. After further adjustment for femoral neck bone mineral density, leg lean mass results were similar (HR 1.10; 95% CI 0.93, 1.30). In contrast, one kg greater total body lean mass was associated with 9% higher hip fracture risk. Our findings suggest that in women, lower lean mass measured by DXA is not a risk factor for hip fracture.
Keywords: lean mass, dual energy x-ray absorptiometry, hip fracture, prospective study, population-based cohort
Introduction
Low proximal femur bone mineral density (BMD) is perhaps the strongest predictor of hip fracture risk [1]. Nevertheless, it is estimated that nearly 60% of women and almost 80% of men with hip fractures have BMD above the osteoporotic range [2, 3], emphasizing the importance of non-BMD factors and the multifactorial etiology of hip fracture. Several non-BMD clinical risk factors have been identified, including increasing age, low body mass index (BMI) and history falls [4, 5], and as the number of coexisting factors aggregates within an individual, the risk for hip fracture increases significantly. Thus, it is of great interest to identify additional risk factors that will help improve the ability of clinicians to better predict which patients are most likely to fracture and should therefore be prioritized for treatment.
Age-related loss of skeletal muscle mass is associated with loss of both bone mass [6, 7] and muscle strength, an important determinant of falls and consequent hip fractures [8]. Muscle mass in the lower extremities may be of particular interest for hip fracture risk, yet it is seldom considered when assessing hip fracture risk in older patients. Dual energy x-ray absorptiometry (DXA) provides an estimate of total and leg lean mass [9], and it is the most commonly used method to estimate s muscle mass in large studies [10]. Despite its limitations, DXA derived measurement of lean mass is currently the most pragmatic option to estimate muscle mass. The measure of lean mass can be performed at the same time as BMD assessment with results read directly from the densitometer’s automated body composition report. Furthermore, muscle mass, which makes up the majority of the lean mass compartment is potentially modifiable as resistance exercise has been shown to be effective at increasing muscle mass, even among frail older adults [11–13]. Although DXA measures of lean mass in patients following a hip fracture have been described [14–16], the relation between DXA lean mass and incident hip fractures is not fully understood. Therefore, there is great clinical interest in confirming whether DXA derived measures of lean mass are independent predictors of hip fractures.
To date, only a few prospective population-based studies have examined the relationship between lean mass and fracture, and the results have been inconsistent [17–20]. Therefore, we investigated the association between DXA lean mass and incident hip fractures among adults aged 50 years and older participating in the Framingham Osteoporosis Study. We hypothesized that greater leg and total body lean mass would be associated with lower risk for hip fracture.
Materials and Methods
Study Participants
The participants for this study included members of the Original and Offspring cohorts of the Framingham Heart Study. The Original Cohort was initiated in 1948 with the purpose of determining risk factors for cardiovascular disease, and systematically recruited 5,209 men and women from a two-thirds sample of houses in the town of Framingham, MA [21]. The Offspring Cohort was begun in 1971 to examine familial clustering of cardiovascular disease [22], and originally included 5,124 adult children, and their spouses, of members of the Original Cohort. Participants in both cohorts have completed regular comprehensive physical examinations by trained physicians, along with clinical risk factor assessments (every two years for Original Cohort members, approximately every four years for Offspring Cohort members).
Participants eligible for the current study included 847 members of the Original Cohort and 2,918 members of the Offspring Cohort who had a valid whole body DXA scan obtained as part of the Framingham Osteoporosis Study in 1992 to 1995 (Original) and 1996 to 2001 (Offspring). Offspring members aged < 50 years (n=343) were excluded. Among men (n=1,444), only 27 hip fractures occurred over follow-up, and inspection of hip fracture survival curves for lean mass tertiles indicated non-proportional hazard functions over time, thereby limiting our ability to calculate robust relative risk estimates. Therefore, only 1,978 women were included in this analysis and followed for hip fracture from the date of DXA assessment until December 31, 2007.
Measures
Lean mass
Whole body DXA was performed using a Lunar DPX-L densitometer (Lunar Radiation Corp., Madison, WI) using the “Fast” mode for all participants. Methods and reproducibility have been described previously [23]. For both the leg (sum of both legs) and total body regions, lean mass (kg) was calculated by subtracting fat tissue mass from soft tissue mass in grams divided by 1000. Total body lean mass was unavailable for 166 (8%) women because their “trunk” region of interest soft tissue values were invalid due to incomplete penetration of the photon beam through the trunk region of the body to reach the detector
Hip fracture
Hip fractures were defined as non-pathological fractures of the proximal femur that occurred only with minimal trauma (fall from standing height or less) during follow up. Fractures were ascertained by a review of hospitalization and death records, and were reported by interview at each Framingham Study examination or by telephone interview for participants unable to attend an examination [24]. Hip fractures are among the most accurately self-reported fractures [25], and >90% of hip fractures in our study were confirmed by review of medical records, including radiographic and operative reports.
Covariates
We considered additional characteristics that are known to influence hip fracture risk and may be associated with lean mass as potential confounders: age, height, weight, history of hip fracture, current smoking, physical activity, bone mineral density (BMD), estrogen replacement therapy use in women, osteoporosis medication use (available in Offspring cohort only), and total body percent fat mass. Characteristics were collected at the same time lean mass was measured or at the examination closest to and preceding the measure of lean mass.
Height was measured to the nearest one-quarter inch using a stadiometer, and weight was measured to the nearest pound in light clothing without shoes. History of hip fracture (y/n) was the occurrence of any hip fracture prior to the lean mass measurement. Smoking status was ascertained by self-report and classified as current smoker versus non-smoker. Physical activity was measured using the validated physical activity scale for the elderly (PASE) score [26]. One of our hypothesized pathways for a lean mass-hip fracture relation was through an increased propensity for falls, and evidence suggests the association of physical activity with falls and fractures is u-shaped [27–30]. Therefore, in addition to considering PASE as a continuous variable, we created quartiles (Q1 to Q4) of PASE and specified physical activity as a three-level categorical variable (low=Q1, reference=Q2–Q3, high=Q4) in our analyses. Femoral neck BMD (g/cm2, LUNAR DPX-L) was assessed at the right femur, unless there was a history of fracture and/or hip joint replacement, in which case, the left side was scanned (coefficient of variation 1.7%) [31]. Estrogen replacement therapy was evaluated as current use (yes/no) of oral conjugated estrogen, patch, or cream. Osteoporosis medications (i.e., bisphosphonates, selective estrogen receptor modulators (SERMs), or calcitonin) were ascertained in the Offspring cohort only (current use of any osteoporosis medication versus none). In those participants with valid trunk region soft tissue data, total body percent fat mass, ascertained from the whole body DXA scans, was calculated as total body fat mass (kg) divided by the sum of total body lean mass, fat mass and bone mineral content (kg), multiplied by 100.
Statistical Analysis
To evaluate the associations of leg and total body lean mass with the subsequent occurrence of hip fracture, women were categorized into tertiles of leg and total lean mass, and age-adjusted Kaplan-Meier survival curves [32] were calculated to examine hip fracture rates over the follow-up period. Person-years of follow-up accrued from the time of lean mass assessment until the occurrence of hip fracture, or until censoring at date of death, date of last contact if prior to end of follow-up (drop-out), or the end of study follow-up on December 31, 2007. Cox proportional hazards regression was used to calculate hazard ratios (HR), and 95% confidence intervals (CI), to estimate the relative risk for hip fracture for a 1 kg increase in baseline lean mass and for tertiles of baseline lean mass. To assess the influence of potential confounders on the association between baseline lean mass and hip fracture, models were constructed in a step-wise fashion as follows: Model 1 was unadjusted; Model 2 adjusted for age, height, cohort (Original vs. Offspring) and total body percent fat, Model 3 additionally adjusted for femoral neck BMD; Model 4 further adjusted for history of hip fracture, smoking status, PASE score (modeled as a continuous variable and categorically in separate models), estrogen replacement therapy use, and osteoporosis medication use. We tested Cox regression models for violations of the proportional hazards assumption by including in the model terms for the interaction of each variable with time. No violations were detected. In a sensitivity analysis, we modeled lean mass as lean mass/height2 (kg/m2).
We conducted other sensitivity analyses to determine whether our results were influenced by missing data on physical activity and BMD, or by competing risk of death. Missing data on PASE (n=166) and BMD (n=48) were imputed 10 times using the Markov Chain Monte Carlo (MCMC) method for multiple imputation (SAS PROC MI). The final multivariable Cox regression model was calculated separately for each of the 10 imputed datasets, and results were subsequently combined (PROC MIANALYZE) to obtain a single valid summary hazard ratio with confidence interval [33, 34]. Additionally, all Cox regression models were repeated accounting for the competing risk of death using the SAS macro %pshreg developed by Kohl et al. [35], which fits the Fine and Gray proportional sub-distribution hazards model for survival data subject to competing risks [36]. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, N.C.).
Results
At baseline, mean age in the women was 66.9 years (range 50 to 93). Median hip fracture follow-up was 8.3 years, over which 99 fractures occurred. Women who experienced a hip fracture over follow up were older and less physically active, weighed less, and had lower BMD (Table 1). Over the follow-up period, there were more deaths than hip fractures, and the number of deaths decreased with increasing leg lean mass tertile (Table 2).
Table 1.
Baseline characteristics of Framingham Osteoporosis Study women by hip fracture status over follow-up
| Characteristica | Fracture | No fracture |
|---|---|---|
| N | 99 | 1,879 |
| Original Cohort (%) | 74 | 25 |
| Age (years) | 76.4 (6.5) | 66.4 (10.2) |
| Height (in) | 61.5 (2.6 ) | 62.8 (2.6) |
| Weight (lbs) | 136 (28) | 152 (31) |
| History of hip fracture (%) | 7 | 2 |
| Current smokers (%) | 12 | 12 |
| Physical Activity (PASE)b | 95 (46) | 124 (68) |
| Current osteoporosis medication use (%) | 3 | 3 |
| Femoral neck BMD (g/cm2)c | 0.66 (0.11) | 0.82 (0.14) |
| Current estrogen therapy use (%) | 7 | 25 |
| Total body percent fat mass (%)d | 37 | 41 |
| Leg lean mass (kg) | 11.3 (1.7) | 11.4 (1.6) |
| Total body lean mass (kg)d | 35.3 (4.3) | 35.9 (4.1) |
| Median follow-up (yrs) | 6.6 | 8.3 |
Mean ± standard deviation, unless otherwise noted.
N=166 (8%) missing
N=48 (2%) missing
N=166 (8%) missing
Table 2.
Number of hip fractures and deaths for tertiles of leg and total lean mass among women in the Framingham Osteoporosis Study (1992–2007)
| Leg lean mass tertile (Range, kg) | Total lean mass tertile (Range, kg) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 (6.40, 10.72) |
2 (10.73, 11.92) |
3 (11.93, 22.34) |
1 (23.67, 34.00) |
2 (34.01, 37.40) |
3 (37.41, 56.74) |
|
| N | 659 | 659 | 660 | 604 | 604 | 604 |
| No. of hip fractures (%) | 38 (6) | 35 (5) | 26 (4) | 42 (7) | 28 (5) | 27 (4) |
| No. of deaths prior to hip fracture (%) | 166 (25) | 147 (22) | 131 (20) | 178 (29) | 133 (22) | 106 (18) |
| Median follow-up (yrs) | 8.2 | 8.2 | 8.4 | 8.2 | 8.3 | 8.4 |
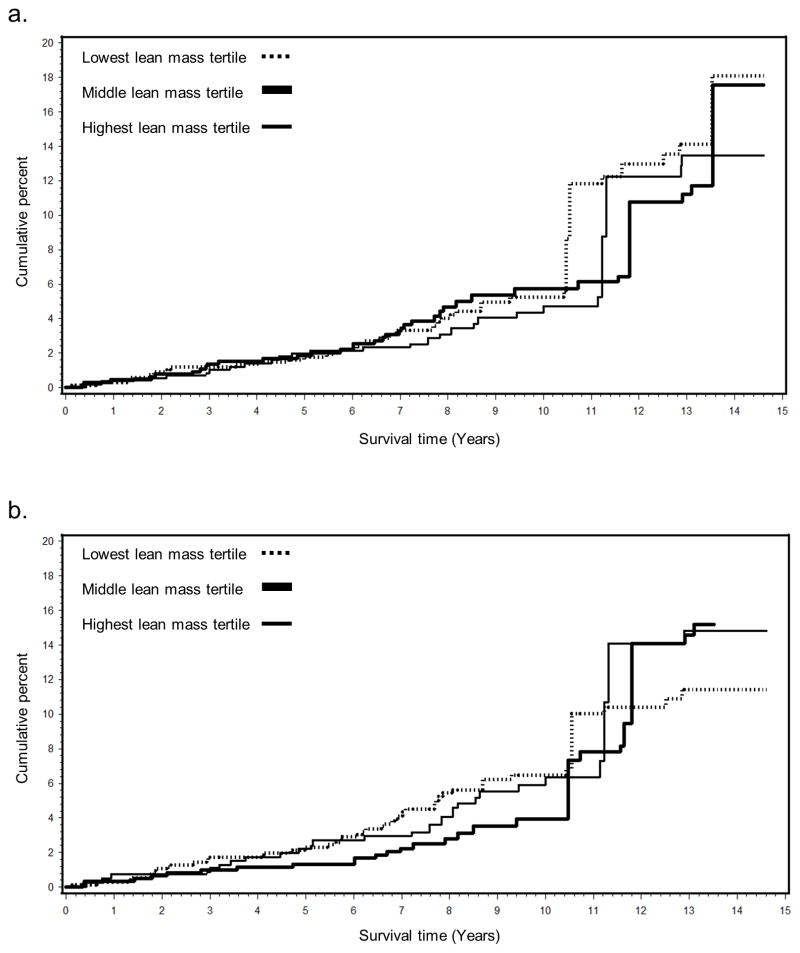
Age-adjusted Kaplan-Meier survival curves did not indicate a clear association between leg lean mass and hip fracture, until year 7 when the cumulative incidence in the lower 2 tertiles increased relative to the highest tertile (Figure 1). For total lean mass, the risk in the lowest and highest tertiles increased compared to the middle tertile starting around year 4. For both leg and total lean mass, after approximately 10 years of follow-up the survival curves demonstrated considerable overlap and no consistent association between lean mass and fracture.
Fig. 1.
Age-adjusted Kaplan-Meier curves for the cumulative incidence (%) of hip fractures for tertiles of leg (a) and total body (b) lean mass among Framingham Osteoporosis Study women
Leg lean mass and total body lean mass were not associated with hip fracture risk in unadjusted models or in models adjusting for age, height, cohort and percent fat (Table 3). After further adjustment for femoral neck BMD, there remained no association for leg lean mass, however for total lean mass there was a statistically significant positive association such that a 1 kg increase in baseline total lean mass was associated with 9% greater risk for hip fracture. Hazard ratios were similar following adjustment for history of hip fracture, smoking status, PASE score (continuous or categorical), and use of estrogen replacement or osteoporosis medications. Results of analyses with participants categorized into lean mass tertiles suggested similar associations with hip fracture, although there were no statistically significant associations (Table 4).
Table 3.
Hazard ratios (95% confidence interval) for hip fracture over 15 years of follow-up per 1 kg increase in baseline leg and total lean mass among women in the Framingham Osteoporosis Study (1992–2007)
| Model 1a | Model 2b | Model 3c | Model 4d | |
|---|---|---|---|---|
| Leg lean mass (n=1,978) | 0.95 (0.83, 1.08) | 1.11 (0.94, 1.31) | 1.10 (0.93, 1.30) | 1.18 (0.96, 1.44) |
| Total lean mass (n=1,812) | 0.96 (0.92, 1.02) | 1.06 (0.99, 1.13) | 1.09 (1.02, 1.18) | 1.12 (1.04. 1.22) |
Model 1 unadjusted
Model 2 includes age, height, cohort, % body fat
Model 3 additionally adjusts for femoral neck BMD
Model 4 additionally adjusts for history of hip fracture, smoking, physical activity, estrogen replacement use, osteoporosis medication use
Table 4.
Hazard ratios (95% confidence interval) for hip fracture over 15 years of follow-up for tertiles of baseline leg and total lean mass among women in the Framingham Osteoporosis Study (1992–2007)
| Model 1a | Model 2b | Model 3c | Model 4d | |
|---|---|---|---|---|
| Leg lean mass | ||||
| Tertile 1 (n=659) | 1.0 | 1.0 | 1.0 | 1.0 |
| Tertile 2 (n=659) | 0.89 (0.56, 1.41) | 1.08 (0.67, 1.56) | 1.32 (0.80, 2.18) | 1.40 (0.78, 2.51) |
| Tertile 3 (n=659) | 0.68 (0.42, 1.13) | 1.03 (0.57, 1.86) | 1.05 (0.57, 1.95) | 1.47 (0.72, 3.01) |
| Total lean mass (n=1,812) | ||||
| Tertile 1 (n=659) | 1.0 | 1.0 | 1.0 | 1.0 |
| Tertile 2 (n=659) | 0.68 (0.42, 1.10) | 1.04 (0.62, 1.73) | 1.27 (0.75, 2.15) | 1.42 (0.76, 2.64) |
| Tertile 3 (n=659) | 0.69 (0.42, 1.11) | 1.36 (0.75, 2.44) | 1.42 (0.76, 2.64) | 1.95 (0.95, 3.99) |
Model 1 unadjusted
Model 2 includes age, height, cohort, % body fat
Model 3 additionally adjusts for femoral neck BMD
Model 4 additionally adjusts for history of hip fracture, smoking, physical activity, estrogen replacement use, osteoporosis medication use
In sensitivity analyses, results were similar after imputation of missing data on PASE and BMD, after accounting for competing risk of death and after modeling lean mass as lean mass/height2 (data not shown).
Discussion
In our prospective study of community-dwelling women aged 50 years and older, leg lean mass measured by DXA was not associated with the risk for hip fracture after adjustment for several major risk factors for hip fracture, including age, body size, percent fat mass, physical activity and femoral neck BMD. Unexpectedly, results suggested that greater total lean mass may be associated with increased hip fracture risk after adjustment for BMD. All associations were consistent after accounting for the competing risk of mortality.
Few prior investigations have examined the prospective association between lean mass measured directly by DXA and the risk for incident hip fracture. In the EPIDOS study, a cohort study of 7,598 women aged 75 years and older living in five cities in France, Schott et al. found that total lean mass was not associated with hip fracture over 2 years of follow-up after adjustment for age, weight and study center [17]. A recent study examined the association of DXA appendicular (arms + legs), trunk and whole body lean mass with hip fracture among women participating in the Women’s Health Initiative Observational Study who were aged ≥65 years and classified as frail [20]. Results suggested that 1 unit higher appendicular lean mass normalized to height squared, was associated with 37% lower risk of hip fracture over a mean of 11.5 years of follow-up. There was, however, a strong positive association between lean mass and total hip BMD, and the observed relation between lean mass and hip fracture was attenuated after adjustment for BMD.
Among women in the Health Aging and Body Composition Study (Health ABC), Malkov et al. reported that lower baseline lean mass (leg, appendicular, appendicular normalized to height squared) was associated with increased risk of hip fracture over 13 years of follow-up in age-adjusted analyses, though further adjustment for BMD and BMI completely attenuated the association [18]. In this same study, lower lean mass was not associated with hip fracture in men when adjusting for age, yet was associated with decreased risk in models accounting for BMD and BMI. While our study did not include men, these findings were similar to our total lean mass results among women. It is unclear why greater lean mass may be associated with increased hip fracture risk. It may be that individuals with greater lean mass are more physically active, or are more likely to engage in more “risky” activities, both of which could increase the opportunity to experience a fall resulting in a hip fracture. While we did adjust for physical activity, the self-reported PASE score may not adequately quantify the amount and type of activity experienced over follow-up. Malkov et al. posited that their observed detrimental effect of higher lean mass might be because the protection against falling afforded by larger thigh muscles may be offset by their exerting greater mechanical loads on the hip during a fall. They also point out that a similar phenomenon has been observed for BMI, where the protective effect of high BMI for hip fracture is reversed once BMD is accounted for [37]. Additional research on how the characteristics of tissues surrounding the hip influence loads on the femur during a fall may help elucidate the roles of muscle and fat in the occurrence of hip fractures.
Alternatively, the consistent lack of association, and occasional counterintuitive association, between DXA-based lean mass and hip fracture observed across the existing body of literature may be due to the limitations of DXA in quantifying skeletal muscle mass. DXA lean mass is not a direct measure of muscle mass [10]. While lean mass from DXA excludes the bone and fat compartments of body tissue, it does include other non-skeletal muscle soft-tissue organs (e.g. liver). Although we did examine the legs region specifically, which is primarily muscle tissue, DXA lean mass also includes non-contractile connective and fibrotic tissue. Lean mass is also limited in its ability to characterize the functional ability of muscle, particularly in longitudinal studies. A recent review by Manini and Clark pointed out that measures of muscle mass, and lean mass via DXA in particular, are weak predictors of future functional outcomes [38]. This can likely be attributed to the age-related decrease in strength outpacing the concomitant decrease in lean mass [39]. The discordance between strength and mass is likely attributed to changes in factors that influence muscle function but are not captured by lean mass, including impairments in excitation-contraction coupling and the neuromuscular junction [40]. Additionally, increased infiltration of fat into skeletal muscle with aging appears to be an important contributor to age-related declines in muscle strength [41]. Among combined men and women in the Health ABC cohort, greater leg muscle attenuation measured by CT (reflecting increased fatty infiltration) was associated with higher risk for hip fracture, independent of BMD [42]. In fact, when muscle cross-sectional area, a surrogate for muscle mass, and strength were included in the same regression model, attenuation was the only parameter that remained significantly associated with hip fracture risk. This suggests a potentially larger role for fat infiltration of muscle, compared to lean mass, in the etiology of hip fracture. Further studies are needed to determine the measures of muscle health that are most relevant for predicting fracture risk, and whether these may differ for men and women.
When interpreting the results of this study, it is important to recognize some important limitations. Only baseline data on lean mass and important covariates such as physical activity were used in this analysis, thus we were unable to assess age-related changes in body composition that likely have an important impact on hip fracture risk. Our goal in this study, however, was to investigate if a baseline measure of DXA lean mass may be useful clinically as an important predictor of hip fracture risk. Results from analyses of total lean mass, and from analyses of leg lean mass that adjusted for total body percent fat, may not be wholly representative of all women in our cohort since these analyses excluded those with invalid trunk soft tissue measures, who tended to have higher mean BMI (37 vs. 26 kg/m2). Nevertheless, these analyses included women ranging in weight from 72 to 310 pounds, thereby including some of the heaviest women in our cohort. The PASE score has limitations as a measure of physical activity since it was developed specifically for adults aged 65 years or older [43], and self-reported measures can either under- or over-estimate actual physical activity levels [44]. Nevertheless, the mean age of our study population was 66 years, including adults up to age 93, and the PASE score has been validated against direct measures of physical activity such as doubly-labeled water [26] and accelerometry [45]. Our study included only women since the low number of fractures did now allow us to obtain valid estimates of risk among men in the Framingham cohorts. There was loss due to death in our study of older adults, though when we accounted for the competing risk of death our results were not affected. Finally, we are limited in our ability to generalize our results to other racial groups since the Framingham Study is comprised primarily of Caucasian men and women.
Despite the aforementioned limitations, our study has several strengths. This is the longest prospective study (up to 15 years) to date of the relation between lean mass as measured by DXA and hip fracture in community-dwelling women. Our cohort included women across a wide age range (50 years and older) and did not exclude participants based on baseline frailty or mobility status. The Framingham Study cohorts are well-characterized. The use of whole body scans allowed us to examine not only total body lean mass, but also leg lean mass, which may be a more direct determinant of fracture risk given its importance for lower-extremity function [46, 47]. Our fracture information is of high quality, having been confirmed primarily by medical records and x-ray reports from hospitals, physicians’ offices, nursing homes, and home health care services. Finally, information was available for several important covariates, allowing us to account for the major risk factors for hip fracture.
In conclusion, our findings suggest that greater absolute leg lean mass measured by DXA, the most commonly used method for assessment of muscle mass, is not protective against hip fracture risk in women over 50 years of age. Contrary to our hypothesis, our results indicate that greater total body lean mass may be associated with increased risk of hip fracture. Considering our results along with similar counterintuitive and multiple null findings from previous studies, the association of lean mass measured by DXA with hip fracture risk remains uncertain. Alternative, more direct assessment methods are needed if muscle mass is to be considered when attempting to identify individuals who may be at increased risk for hip fracture.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging of the National Institutes of Health under award number R01AR/AG41398 and the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. HHSN268201500001I). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors DPK is the guarantor. DPK, LAC, and MTH designed the study. RRM prepared the first draft of the paper. RRM, DPK, SDB, KEB, and MTH contributed to the data collection. RRM, KEB and XZ were responsible for statistical analysis of the data. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Compliance with Ethical Standards
Conflict of interest Douglas P. Kiel has received grants from Eli Lilly, Amgen, Merck Sharp & Dohme, and has served on scientific advisory boards for Eli Lilly, Amgen, Novartis, and Merck Sharp & Dohme. Sarah D. Berry has received grant funding from Amgen. Robert R. McLean, Kerry E. Broe, Xiaochun Zhang, L. Adrienne Cupples, and Marian T. Hannan declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This study was approved by the institutional review board at Hebrew SeniorLife and written informed consent was obtained for all participants included in the study.
References
- 1.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen ND, Eisman JA, Center JR, Nguyen TV. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 2007;92:955–962. doi: 10.1210/jc.2006-1476. [DOI] [PubMed] [Google Scholar]
- 3.Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, Hochberg MC, Vogt MT, Orwoll ES Study of Osteoporotic Fractures Research G. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005;90:2787–2793. doi: 10.1210/jc.2004-1568. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Cawthon PM, Peters KE, Cummings SR, Ensrud KE, Bauer DC, Taylor BC, Shikany JM, Hoffman AR, Lane NE, Kado DM, Stefanick ML, Orwoll ES Osteoporotic Fractures in Men Study Research G. Risk Factors for Hip Fracture in Older Men: The Osteoporotic Fractures in Men Study (MrOS) J Bone Miner Res. 2016;31:1810–1819. doi: 10.1002/jbmr.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor DN, Melton LJ, Khosla S, Crowson CS, O’Connor MK, Riggs BL. Relative influence of physical activity, muscle mass and strength on bone density. Osteoporos Int. 2000;11:944–952. doi: 10.1007/s001980070033. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Atkinson EJ, Riggs BL, Melton LJ., 3rd Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857–863. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 8.Tinetti ME, Doucette JT, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43:1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Wang Z, Lohman T, Heymsfield SB, Outwater E, Nicholas JS, Bassford T, LaCroix A, Sherrill D, Punyanitya M, Wu G, Going S. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007;137:2775–2780. doi: 10.1093/jn/137.12.2775. [DOI] [PubMed] [Google Scholar]
- 10.Cawthon PM. Assessment of Lean Mass and Physical Performance in Sarcopenia. J Clin Densitom. 2015;18:467–471. doi: 10.1016/j.jocd.2015.05.063. [DOI] [PubMed] [Google Scholar]
- 11.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 12.Kukuljan S, Nowson CA, Sanders K, Daly RM. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol. 2009;107:1864–1873. doi: 10.1152/japplphysiol.00392.2009. [DOI] [PubMed] [Google Scholar]
- 13.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Harris TB, Fox KM, Hawkes W, Hebel JR, Yahiro JY, Michael R, Zimmerman SI, Magaziner J. Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci. 2000;55:M434–440. doi: 10.1093/gerona/55.8.m434. [DOI] [PubMed] [Google Scholar]
- 15.Fox KM, Magaziner J, Hawkes WG, Yu-Yahiro J, Hebel JR, Zimmerman SI, Holder L, Michael R. Loss of bone density and lean body mass after hip fracture. Osteoporos Int. 2000;11:31–35. doi: 10.1007/s001980050003. [DOI] [PubMed] [Google Scholar]
- 16.Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J. Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci. 2005;60:80–84. doi: 10.1093/gerona/60.1.80. [DOI] [PubMed] [Google Scholar]
- 17.Schott AM, Cormier C, Hans D, Favier F, Hausherr E, Dargent-Molina P, Delmas PD, Ribot C, Sebert JL, Breart G, Meunier PJ. How hip and whole-body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporos Int. 1998;8:247–254. doi: 10.1007/s001980050061. [DOI] [PubMed] [Google Scholar]
- 18.Malkov S, Cawthon PM, Peters KW, Cauley JA, Murphy RA, Visser M, Wilson JP, Harris T, Satterfield S, Cummings S, Shepherd JA, Health ABCS. Hip Fractures Risk in Older Men and Women Associated With DXA-Derived Measures of Thigh Subcutaneous Fat Thickness, Cross-Sectional Muscle Area, and Muscle Density. J Bone Miner Res. 2015;30:1414–1421. doi: 10.1002/jbmr.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett-Connor E, Lee CG, Hoffman AR, Nevitt M, Stefanick ML, Lane NE, Ensrud KE, Cummings SR, Orwoll ES. Evaluation of the Usefulness of Consensus Definitions of Sarcopenia in Older Men: Results from the Observational Osteoporotic Fractures in Men Cohort Study. J Am Geriatr Soc. 2015;63:2247–2259. doi: 10.1111/jgs.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaslavsky O, Li W, Going S, Datta M, Snetselaar L, Zelber-Sagi S. Association between body composition and hip fractures in older women with physical frailty. Geriatr Gerontol Int. 2016 doi: 10.1111/ggi.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawber TR, Meadors GF, Moore FEJ. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 24.Kiel DP, Felson DT, Anderson JJ, Wilson PW, Moskowitz MA. Hip fracture and the use of estrogens in postmenopausal women. The Framingham Study. N Engl J Med. 1987;317:1169–1174. doi: 10.1056/NEJM198711053171901. [DOI] [PubMed] [Google Scholar]
- 25.Nevitt MC, Cummings SR, Browner WS, Seeley DG, Cauley JA, Vogt TM, Black DM. The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol. 1992;135:490–499. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- 26.Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. 1997;50:541–546. doi: 10.1016/s0895-4356(97)00010-3. [DOI] [PubMed] [Google Scholar]
- 27.Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:1129–1136. doi: 10.1093/oxfordjournals.aje.a008690. [DOI] [PubMed] [Google Scholar]
- 28.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 29.O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–354. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 30.Moayyeri A, Besson H, Luben RN, Wareham NJ, Khaw KT. The association between physical activity in different domains of life and risk of osteoporotic fractures. Bone. 2010;47:693–700. doi: 10.1016/j.bone.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 32.Cupples LA, Gagnon DR, Ramaswamy R, D’Agostino RB. Age-adjusted survival curves with application in the Framingham Study. Stat Med. 1995;14:1731–1744. doi: 10.1002/sim.4780141603. [DOI] [PubMed] [Google Scholar]
- 33.Rubin D. Multiple Imputation for Non Response in Surveys. John Wiley & Sons; New York: 1987. [Google Scholar]
- 34.Rubin DB. Multiple Imputation After 18+ Years. Journal of the American Statistical Association. 1996;91:473–489. [Google Scholar]
- 35.Kohl M, Plischke M, Leffondre K, Heinze G. PSHREG: a SAS macro for proportional and nonproportional subdistribution hazards regression. Computer methods and programs in biomedicine. 2015;118:218–233. doi: 10.1016/j.cmpb.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 37.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: An epidemiologic perspective. J Bone Miner Res. 2012;27:1–10. doi: 10.1002/jbmr.1486. [DOI] [PubMed] [Google Scholar]
- 38.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 40.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 41.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 42.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 44.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. The international journal of behavioral nutrition and physical activity. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. The Journal of sports medicine and physical fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 46.Bernardi M, Rosponi A, Castellano V, Rodio A, Traballesi M, Delussu AS, Marchetti M. Determinants of sit-to-stand capability in the motor impaired elderly. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2004;14:401–410. doi: 10.1016/j.jelekin.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 2012;46:555–558. doi: 10.1002/mus.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]