Abstract
Uncertainty is often associated with subjective distress and a potentiated anxiety response. Occurrence uncertainty, or the inability to predict if a threat will occur, has rarely been compared experimentally with temporal uncertainty, or the inability to predict when a threat will occur. The current study aimed to: 1) directly compare the anxiogenic effects of anticipating these two types of uncertain threat, as indexed by the eye-blink startle response, and 2) assess the relationship between startle response to occurrence and temporal uncertainty and individual differences in self-reported intolerance of uncertainty and anxiety. The findings indicated that anticipation during occurrence uncertainty elicited a larger startle response than anticipating a certain threat, but anticipation during temporal uncertainty was superior at potentiating startle blink overall. Additional analyses of the effects of order and habituation further highlighted temporal uncertainty’s superiority in eliciting greater startle responding. This suggests that while uncertainty is physiologically anxiety provoking, some level of certainty that the threat will occur enhances the robustness of the physiological anxiety response. However, self-reported anxiety was equivalent for temporal and occurrence uncertainty, suggesting that while defensive responding may be more affected by temporal uncertainty people perceive both types of uncertainty as anxiogenic. Individual differences in the intolerance of uncertainty and other anxiety measures were not related to anticipatory startle responsivity during any of the conditions.
1. Introduction
Uncertainty is the inability to determine a situation’s outcome or to predict the valence, intensity, likelihood, or type of future stimulus (Carleton, 2012). Anticipating uncertain potential threat and adjusting cognitively (e.g., planning a means of response) and physiologically (e.g., fight or flight response) can be beneficial for the individual, especially when the threat is imminent and likely to occur (Barlow, 2002; Suárez, Bennett, Goldstein, & Barlow, 2009). In contrast, extreme anxiety is thought to be developed and maintained by excessive anticipatory processes in the face of uncertainty (Grupe & Nitschke, 2013). For example, panic attacks that occur unpredictably tend to substantially increase worry about if and when a panic attack will occur, leading to chronic anxious apprehension (Craske, Glover, & DeCola, 1995). The tendency of anxious individuals to maladaptively anticipate uncertain threat or negative outcomes has received substantial attention, with mounting evidence suggesting that uncertainty is associated with heightened physiological reactivity and increased recruitment of brain regions that support the expression of arousal and negative affect, such as fronto-limbic circuits (Grillon, Baas, Lissek, Smith, & Milstein, 2004; Grillon et al., 2006; Grillon et al., 2008; Grupe & Nitschke, 2013; Nelson & Shankman, 2011; Sarinopoulos et al., 2010; Shankman et al., 2014; Williams et al., 2014). Depending upon the manipulation the terms uncertainty and unpredictability are both used in the extant literature (Grupe & Nitschke, 2013; Lake & LaBar, 2011). While our task manipulates predictability of threat, we also use the term uncertainty in this paper to highlight our central construct of interest, the anxious anticipation evoked by unpredictability (Grupe & Nitschke, 2013).
One of the most common paradigms used to measure response to uncertainty is the NPU task, which compares threat/fear responses in temporally predictable threat (P), temporally unpredictable threat (U) and no threat (N) conditions (Grillon et al., 2004). Typically, the defensive startle blink response is enhanced during the unpredictable conditions (i.e., anxiety-potentiated startle), and during the predictable threat conditions (i.e., fear-potentiated startle; (Schmitz & Grillon, 2012). NPU studies of non-anxious subjects have found an increased startle response to temporally uncertain aversive events compared to certain threat and safe conditions. The NPU paradigm has also effectively identified heightened startle response to uncertainty in various anxiety-related disorders, including panic disorder, posttraumatic stress disorder, and childhood anxiety disorders (Grillon et al., 2008; Grillon et al., 2009; Williams et al., 2014). Recent evidence also indicates that the NPU task also has good psychometric properties (Kaye, Bradford, & Curtin, 2016). Thus, the NPU paradigm appears to be a useful tool for measuring response to uncertainty in both anxious and non-anxious populations.
Although there has been a significant amount of research examining uncertainty, there is still a need to better characterize the anxiogenic impact of various types of uncertainty, such as the likelihood, timing, or intensity of potential threat. The majority of studies that have measured uncertainty with a task manipulation, such as the NPU task, have manipulated temporal uncertainty (TU), or the inability to predict when an aversive stimulus will occur (Grillon et al., 2004; Grillon et al., 2008; Lissek et al., 2005; Nelson & Shankman, 2011; Shankman, Robison-Andrew, Nelson, Altman, & Campbell, 2011). In contrast, relatively little is known about other aspects of uncertain threat, such as uncertainty regarding the intensity or likelihood of the threat occurring. Establishing the relative aversiveness of these various forms of uncertainty would inform understanding of the role each plays in the elicitation of physiological and subjective anxiety, as well as the development of both adaptive and maladaptive anxiety. A number of studies have experimentally manipulated the intensity and frequency of threat (Bradford, Magruder, Korhumel, & Curtin, 2014; Chin, Nelson, Jackson, & Hajcak, 2016; Dunsmoor, Bandettini, & Knight, 2007; Dunsmoor, Bandettini, & Knight, 2008; Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005; Marlin, Sullivan, Berk, & Miller, 1979; Monat, Averill, & Lazarus, 1972; Shankman et al., 2011; Williams et al., 2014). Shankman and colleagues (2011) found that temporal uncertainty and intensity uncertainty produced significantly larger startle responses than when the aversive stimulus was predictable in both conditions. Interestingly, this effect was stronger in the temporal uncertainty condition, highlighting the affective impact of temporally uncertain threat. Focusing on the uncertainty of the frequency or likelihood of the aversive stimulus, Chin and colleagues (2016) found greater startle magnitudes during high (75%) reinforcement or more certain, compared to low (50%) reinforcement conditions in an associative learning study assessing response to uncertainty. However, self-reported intolerance of uncertainty was positively correlated with increased startle magnitudes only during the 50% reinforcement, or more uncertain, condition. These findings suggest that among individuals who are less tolerant of uncertainty, not knowing if a threat will occur provokes increases in anxiety. Overall, by examining the likelihood, probability, and reinforcement rate of threat, these studies provide some insight into the nuanced nature of uncertainty and anxiety and highlight the possible relevance of occurrence uncertainty (OU), the inability to determine if (i.e., the likelihood) an aversive stimulus will occur, for eliciting anxious states (Bradford, Starr, Shackman, & Curtin, 2015; Chin et al., 2016; Davies & Craske, 2015; Dunsmoor et al., 2007; Dunsmoor et al., 2008; Hsu et al., 2005; Monat et al., 1972; Williams et al., 2014).
Aside from the Chin and colleagues (2016) study, however, occurrence uncertainty has rarely been studied in humans; therefore, it is not yet well established how impactful occurrence uncertainty is for eliciting state anticipatory anxiety, nor what role it plays in the development of chronic and clinical anxiety. Although temporal uncertainty and occurrence uncertainty are similar in the underlying assumption that threat is more salient when it is not predictable, further research is needed to determine whether temporal and occurrence uncertainty are equally anxiety-provoking. To the best of our knowledge, only two studies to date have directly compared temporal and occurrence uncertainty experimentally (Davies & Craske, 2015; Monat et al., 1972). Davies and Craske (2015) manipulated both temporal and occurrence uncertainty and found that startle blinks were largest in the most certain (100% reinforcement, temporally predictable) and most uncertain (50% reinforcement, temporally unpredictable) conditions. Notably, this task differed from the typical NPU in a few ways. First, the temporal predictability manipulation was a between groups design; one group received only temporally predictable shocks and the other only temporally unpredictable shocks. As a result of this unique between subjects design the temporally unpredictable group did not receive a certain threat condition. Therefore, the authors were unable to directly compare either type of uncertain threat with certain threat. Their design also used a much longer anticipation period (30–60 s) than the typical NPU task (6–10 s; Grillon et al., 2004; Grillon et al., 2006; Grillon et al., 2009; Moberg & Curtin, 2009; Nelson & Shankman, 2011). Thus, we sought to directly compare temporal uncertainty and occurrence uncertainty with certain threat (and safety) in a within subjects design using anticipation period timing more similar to typical NPU designs.
Monat and colleagues’ design also differed the standard NPU in two key ways. First, they used a relatively long anticipation period and found that, depending on the modality used and when anxiety was assessed, there were marked differences in the potency of temporal and occurrence uncertainty. For example, although they found that temporal uncertainty elicited a larger anxiety response (skin conductance and relax-tension ratings) than occurrence uncertainty early in their task’s anticipation period, occurrence uncertainty produced a larger anxiety response (skin conductance, heart rate change, and relax-tension ratings) later in the anticipation period. While understanding responses to enduring periods of uncertainty is important, this design also complicates interpretation because sensitivity to uncertainty may vary depending on how distant or imminent the threat is likely to be. Second, their design was confounded by the fact that in the temporal uncertainty conditions participants always received the shock after three minutes transpired, which was the same time that the certain and occurrence uncertainty shocks were delivered. Thus, the temporal uncertainty became somewhat certain after the participant experienced repeated trials. Our study was designed to eliminate this confound by varying the time of delivery of the shock in the temporal uncertainty condition.
In sum, because of uncertainty's potential role in the development of anxiety problems, there is a clear need to address this gap in the literature and further clarify what types of uncertainty are most anxiety provoking (Carleton, Collimore, & Asmundson, 2010; Carleton et al., 2012; Dugas, Buhr, & Ladouceur, 2004; Grupe & Nitschke, 2013).
1.1 The Current Study
The current study directly compared anxiety elicited by temporal and occurrence uncertainty by measuring the magnitude of the startle blink response and self-reported anxiety while participants were under threat of potential shock in four brief anticipation conditions: 1) temporal uncertainty, or uncertainty as to when a potential threat would occur, 2) occurrence uncertainty, or uncertainty as to if a potential threat would occur, 3) certain threat, or absolute certainty a threat would occur at a given time, and 4) safe, in which no aversive stimulus occurred. Replicating and extending prior work, we predicted that the startle response during the anticipation period would be significantly potentiated during uncertain conditions (occurrence and temporal) compared to predictable threat and safe conditions, and that the startle response would be significantly larger during the anticipation period for certain threat compared to safe trials (Grillon et al., 2004; Grillon et al., 2006; Grillon et al., 2008; Shankman et al., 2011). Based on findings from Chin and colleagues (2016) that more certain (75% reinforcement) threat elicited larger startle responses compared to less certain (50% reinforcement) threat, we predicted that temporal uncertainty would elicit more anticipatory anxiety (i.e., larger startle response) than occurrence uncertainty. We predicted that self-reported anxiety during the different conditions would show the same pattern, greatest during temporal uncertainty, followed by occurrence uncertainty, certain threat, and safety. We also measured relationships between anticipatory anxiety during uncertain (occurrence and temporal) and certain threat with self-reported intolerance of uncertainty, state anxiety, trait anxiety, negative affect, and worry.
In addition to our primary research question comparing the anxiogenic effects of anticipation of temporal and occurrence uncertainty, we examined whether physiological anxiety carries over into the safe period following threat, and whether this differs as a function of type of uncertain threat. To do so, we altered the standard NPU task to isolate the anticipation and inter-trial interval (ITI) periods. In most studies using the NPU paradigm the aversive stimulus during unpredictable threat is explicitly unpaired from cue stimuli and can occur at any time, whereas during predictable threat the aversive stimulus will never occur during the ITI (Grillon, 2002; Grillon et al., 2004; Grillon et al., 2008; Nelson & Shankman, 2011; Shankman et al., 2011). In the current study the aversive stimulus only occurred at the end of an anticipation period and never during the ITI. This allowed us to test physiological anxiety elicited both by anticipation of uncertain threat, whether that anxiety carries over into the safe ITI periods in these conditions, and whether any carryover differs as a function of type of uncertainty.
2. Method
2.1 Participants
Fifty-one undergraduate students from the University of Wisconsin-Milwaukee completed the study for course extra credit and a $15 Amazon gift card. Participants were at least 18 years old, proficient in English, and had no visual or hearing impairments (corrected vision was acceptable). Data were collected from 51 participants with a final sample size of 42 (30 female; Mage = 20.8, SDage = 0.71). Four participants were dropped due to data collection errors, two due to file corruption, and three were classified as non-responders for the startle eyeblink response (Bradford et al., 2015). All participants provided written informed consent. Prior to conducting the study, a power analysis was conducted to determine the required sample size. Assuming a small to moderate effect size (partial η2 = 0.37) from Nelson and Shankman’s study (2011), a power of 0.95 and alpha of 0.05 yielded a required sample size of 31. A post-hoc power analysis with a total sample size of 42 and a small effect size (partial η2 = .29) yielded a power of 0.94.
2.2 Materials and Procedure
2.2.1 Procedure
Prior to the start of the task, participants underwent a shock threshold work-up to determine the level of electrical stimulation to be used throughout the task and completed a startle habituation procedure. Next, participants completed two runs of four blocks of the certain-uncertain threat task. Each block contained trials from one condition: certainty (C); occurrence uncertainty (OU); temporal uncertainty (TU); or safe (S). Physiological defensive responding (i.e., physiological anxiety) was measured as the magnitude of eyeblink responses to acoustic startle probes during the anticipation and ITI periods. At the end of each block, participants rated their level of subjective anxiety. After finishing the task participants completed questionnaires assessing intolerance of uncertainty, anxiety, and worry.
2.2.2 Shock Administration
Participants first completed a shock work-up to identify an individually titrated painful, but tolerable, level of electrical stimulation (i.e., a shock) to be used throughout the experiment. Shocks were delivered using Psychlab’s SHK1 Pain Stimulation Shocker (Contact Precision Instruments, Cambridge, MA). Stimulation was delivered via two sensors placed approximately two inches above the right ankle (using double sided tape and conductive gel). Participants were told that they would receive a mild electric shock and would be asked to rate it from 1 to 10, 1 being “didn’t feel anything,” and 10 being “painful, but tolerable.” The goal was to work up to a level that the participant subjectively rated as a 10: “painful, but tolerable.” Once that shock level was established, shock was set at that level for the duration of task; the participant could increase or decrease the level at any point in the study if they became too uncomfortable or habituated to the shock. One participant increased their shock level from 100 to 120 units (1.96mA to 2.35mA) during a break because they habituated to the shock. Their data was included in analyses. All other participants maintained their initial shock level.
2.2.3 Startle Habituation
Once the shock work-up was completed, participants then began a startle habituation procedure. Consistent with past research, this process was used to habituate the participants to the startle probes to prevent biased (excessively large) startle responses in the first few trials of the task (Blumenthal et al., 2005). Two sensors were applied under the left eye to measure the startle response. This response was measured while participants were presented nine 50ms 102dB white noise startles through Bose noise cancelling headphones. There was an 8–12 s ITI between each startle probe. Immediately after startle habituation participants began the task.
2.2.4 Occurrence Uncertainty vs. Temporal Unpredictability Task
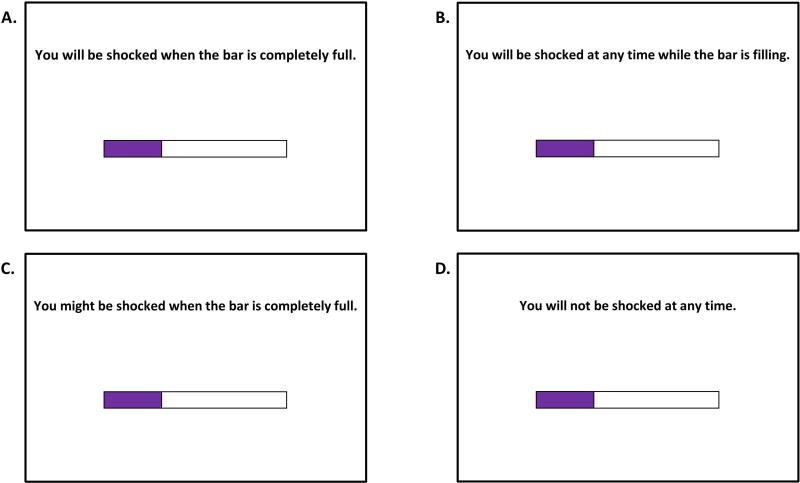
To create an anticipation period prior to delivery of the electrical stimulation (or lack thereof), participants viewed “loading bars” that slowly filled over the course of the anticipation period. These loading bars and associated instructions specified whether the trial was safe or there was a threat of shock during or at the end of the anticipation period. The task was blocked by condition, and the start of each trial featured a cue slide that explicitly stated which of the four conditions the participant was currently completing. The condition cue was replaced by a fixation cross after the anticipation period ended. Each trial’s anticipation period consisted of a loading bar that filled over the course of up to 10 s (Figure 1). The four conditions included: 1) certain threat (C), with a shock always occurring at the end of the loading bar (10s); 2) temporal uncertainty (TU), with a shock always occurring at a random time between 2s and 10s during the loading bar’s random filling (i.e., the bar “jumped” from spot to spot continuously); 3) occurrence uncertainty (OU), with a 50% chance of a shock occurring at the end of the loading bar (10s); and 4) safe (S), with no shocks throughout or at the end of the loading bar anticipation period (10s).
Figure 1. Anticipation Period Loading Bars.
An example of the four task condition anticipation periods while “loading bars” are filling. A.) Certainty (C). Participants were shocked when the bar is completely filled (100% purple). B.) Temporal uncertainty (TU). Participants were always shocked (100%), but the shock could occur at any time while the bar is filling. C.) Occurrence uncertainty (OU). Participants may or may not have been shocked (50% shock rate) when the bar completely filled. D.) Safe (S). Participants were not shocked at any point during the trial. Shocks were never presented during the ITI for any condition.
The task included eight blocks, each of which contained five trials of a single condition, for a total of 40 trials, 10 per condition. Similar to the Schmitz & Grillon (2012) NPU task, we used two counterbalanced run orders: TU-S-C-OU-OU-C-S-TU and OU-C-S-TU-TU-S-C-OU. There was a five-minute break in between the first and second half of the blocks (after four blocks). Each trial lasted for a maximum of 35 seconds and contained one startle probe during the loading bar anticipation period and one during the ITI, which varied between 9s and 24.5s. There were a total of 12 startle probes per condition, six during the bar, and six during the ITI, for a total of 48 startle probes overall (Table 1). Each startle probe was timed in such a way that there would always be at least 10s between startle probes and at least 10s following the aversive event (i.e., shock) to avoid the possibility of participants desensitizing to the startle probe (Grillon et al., 2008). Shock was delivered in each of the temporal uncertainty (TU) and certain (C) trials and in 50% of the occurrence uncertainty (OU) trials, and no shocks were delivered during the safe (S) trials. Thus, there was a maximum of 15 shocks per run and 25 shocks overall (Table 1). Shocks were never delivered during the intertrial interval (ITI) of any condition.
Table 1.
Task Conditions
| Condition | Bar Length | Trials | Shocks | Startles |
|---|---|---|---|---|
| Certainty (C) | 10s | 10 | 10 | 12 |
| Temporal Uncertainty (TU) | 2s–10s | 10 | 10 | 12 |
| Occurrence Uncertainty (OU) | 10s | 10 | 5 | 12 |
| Safe (S) | 10s | 10 | 0 | 12 |
|
| ||||
| Total | 40 | 25 | 48 | |
The four task conditions and number of trials, shocks, and startle probes are listed: C: certain shock, certain time; TU: certain shock, uncertain time; OU: uncertain shock, certain time; and S: no shock (safe).
2.2.5 Eye-blink Startle Collection
Peak raw startle eyeblink amplitudes were the primary dependent variable, consistent with previous startle eyeblink investigations (Bradford et al., 2015; Grillon et al., 2004; Grillon et al., 2009; Nelson & Shankman, 2011; Nelson, Hajcak, & Shankman, 2015). Startle response data were measured using a BioNomadix® 2Ch EMG Receiver (Biopac Systems, Goleta, CA) from two 4-mm Ag/AgCl sensors placed below the left eye, over the orbicularis muscle. One sensor was placed 1 cm below the pupil and the other 1 cm towards the outer canthi of the left eye. The ground sensor was placed in the center of the forehead. Blinks were recorded and processed using Biopac’s Acqknowledge software according to guidelines presented by Blumenthal and colleagues (2005). Eyeblink startle EMG was filtered online using a 5–500 Hz bandpass filter, filtered offline using a 28 Hz high-pass filter (4th order Butterworth), rectified, and filtered offline using a 30 Hz low-pass filter (4th order Butterworth). Peak amplitudes were measured in the 20–200 ms time window following the white noise startle probe. Trials were rejected if there was greater than a ±40µV deflection in the 50ms baseline period. Blinks were visually inspected and were removed from analyses if the startle response did not begin and end within the 20–200 ms time window. An average of 5.61 (SD=0.46) startle responses per condition were used for analysis. The number of trials dropped during the ITI period significantly varied by condition, F(3, 123) = 3.872, p < .02, ηp2 = 0.086, such that more OU trials were dropped compared to C trials, p < .05. Rejected trials during the anticipation period did not vary by condition. Participants were determined to be non-responders (N = 3) if mean startle response was below 5µV (Bradford et al., 2015). Raw startle blink amplitudes are reported. The findings were not different for T-scores so those are not presented here.
2.2.6 Subjective Ratings
At the end of each block, participants completed short subjective ratings to gauge their emotions during the task. They were asked to rate on a scale of one to seven how anxious they felt during the preceding block of trials and how aversive the electrical stimulation felt during that block. These subjective ratings were used as a manipulation check to determine if the electrical stimulation was perceived as aversive, consistent with previous research using an NPU paradigm (Nelson & Shankman, 2011).
2.2.7 Intolerance of Uncertainty
The Intolerance of Uncertainty Scale (IUS; Buhr & Dugas, 2002; Freeston, Rhéaume, Letarte, Dugas, & Ladouceur, 1994) was used to measure participants’ level of intolerance of uncertain threats, situations, and outcomes with a total score and two factors validated by Sexton & Dugas (2009): “uncertainty has negative behavioral and self-referent implications” and “uncertainty is unfair and spoils everything.” The IUS has good test-retest reliability at a five-week interval (r = 0.74), excellent internal consistency (α = 0.94), and good internal and external validity with measures of anxiety, depression, and worry (Buhr & Dugas, 2002). Our sample’s IUS total scores ranged from 31 to 96 (M = 63.31, SD = 17.15). The 12-item version of the IUS (Carleton, Norton, & Asmundson, 2007), which includes the Prospective IU and Inhibitory IU factors, was also used in order to fully capture individual differences in intolerance of uncertainty. The 12-item version of the IUS has excellent internal consistency (α = 0.91), and good internal and external validity with measures of anxiety, depression, and worry (Carleton et al., 2007). Our sample’s IUS-12 total scores ranged from 13 to 45 (M = 28.38, SD = 8.02).
2.2.8 Anxiety, Worry, and Negative Affect
The trait scale of the State-Trait Anxiety Index (STAI-T) was used to measure participants’ level of trait anxiety (Spielberger, 1970), with scores that ranged from 20 to 64 (M = 39.76, SD = 8.85). The STAI-T has good test-retest reliability (r = 0.76–0.84), excellent internal consistency (α = 0.86–0.92), and adequate internal and external validity (Barnes, Harp, & Jung, 2002; Spielberger, 1970). The Penn State Worry Questionnaire (PSWQ) was administered to measure participants’ trait worry, and has been shown to have excellent test-retest reliability (r = 0.74–0.93), excellent internal consistency (α = 0.86–0.95), and good internal and external validity (Brown, Antony, & Barlow, 1992; Meyer, Miller, Metzger, & Borkovec, 1990; Molina & Borkovec, 1994). Our sample’s PSWQ scores ranged from 23 to 69 (M = 48.52, SD = 11.57). The negative affect subscale of the Positive and Negative Affect Schedule (PANAS-NA) was used as a measure of negative affect (Watson, Clark, & Tellegen, 1988), with scores that ranged from 10 to 36 (M = 13.88, SD = 4.95) in our sample. The PANAS-NA has good test-retest reliability (r = 0.84–0.87), good internal consistency (α = 0.85), and good internal and external validity with measures of depression, anxiety, and stress (Crawford & Henry, 2004; Watson et al., 1988).
2.2.9 Data Analysis
To understand whether the startle responsivity during uncertain conditions differed from that during certain and safe conditions, and which uncertain condition was more anxiety-provoking, we conducted a one-way repeated measures ANOVA with Condition as a factor (TU, OU, C, S) on startle responses during the anticipation period. An ANOVA of the same design was also calculated for the ITI period. We also computed threat potentiation difference scores (TU-S, OU-S, C-S) and conducted a one-way ANOVA comparing threat potentiation for the three threat conditions during the anticipation period. For the self-report data we calculated a one-way repeated measures ANOVA with Condition as a factor (TU, OU, C, S) for the end-of-block subjective anxiety ratings. A second identical ANOVA was conducted for ratings of shock aversiveness. All follow-up comparisons for each ANOVA were Bonferroni corrected, such that reported p-values are products of the raw p-value and the number of comparisons (as implemented in SPSS; Bland & Altman, 1995).
In order to understand individual differences in responsivity to uncertain threat, bivariate correlations were computed for the relationship between intolerance of uncertainty (IUS), trait anxiety (STAI-T), worry (PSWQ), negative affect (PANAS-NA) and the anticipation startle response during certain and uncertain conditions.
3. Results
3.1 Self-reported Anxiety and Shock Aversiveness
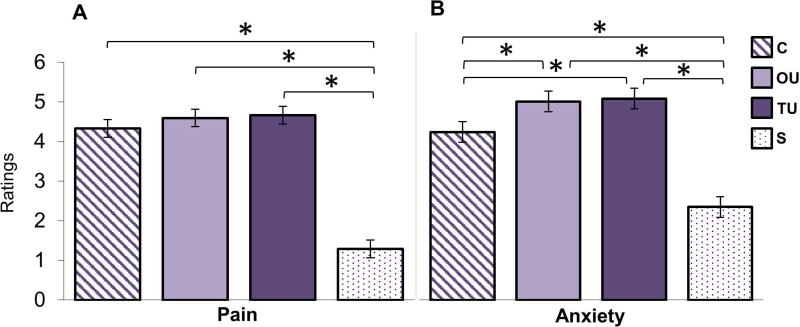
Repeated measures ANOVAs were conducted to determine whether the shock manipulation influenced self-reported anxiety and shock aversiveness (pain). For self-reported anxiety there was a main effect of condition, F(3, 120) = 67.41, p < .0001, ηp2 = 0.628. Pairwise comparisons revealed that participants rated feeling more anxious in the TU and OU conditions (no difference between these conditions) than in the S condition and C condition, which in turn was rated as more anxiety provoking than the S condition, ps < .001 (Figure 2). Results also indicated a main effect of condition on shock-related self-reported shock aversiveness, F(3, 120) = 167.46, p < .0001, ηp2 = 0.807. Participants reported significantly less shock-related pain in the S condition compared to all threat conditions, ps < .0001 (Figure 2). There were no significant differences among the threat conditions, ps > .18.
Figure 2. Subjective Ratings of Shock Aversiveness (Pain) and Anxiety.
A) Shock pain ratings recorded at the end of each block. B) Anxiety ratings recorded at the end of each block. Comparisons were Bonferroni-corrected. From left to right: certain (C), occurrence uncertainty (OU), temporal uncertainty (TU), and safe (S). * = p < .0001.
3.2 Eyeblink Startle Response: Anticipation Period
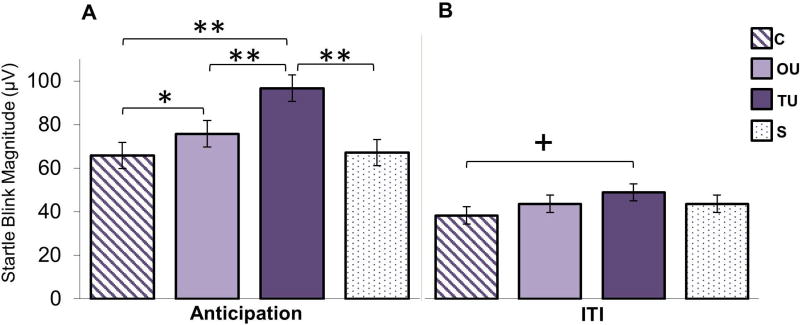
A within-subjects ANOVA was conducted to examine the effect of condition during the bar anticipation period. Results revealed a main effect of condition, F(3, 123) = 16.34, p < .0001, ηp2 = 0.285. Pairwise comparisons indicated that startle responses during the TU anticipation period were significantly larger than those for the OU, C, and S conditions, ps < .0001 (Figure 3). Startle responses were also larger during the OU anticipation period compared to the C, p < .012, but not S anticipation periods, p = .44. Startle response did not differ for C and S anticipation periods, p > .99.
Figure 3. Startle Blink Magnitudes.
A) Raw startle blink amplitudes during the anticipation period (while “loading bars” are filling). B) Raw startle blink amplitudes during the ITI period (always safe). Comparisons were Bonferroni-corrected. From left to right: certain (C), occurrence uncertainty (OU), temporal uncertainty (TU), and safe (S). * = p < .05; ** = p < .0001; + = p = .051.
In a second ANOVA we examined whether startle response potentiation (threat minus safe) differed between the three threat anticipation conditions. There was a main effect of condition, F(2, 82) = 21.99, p < .0001, ηp2 = 0.349. As with the raw amplitude findings, startle response potentiation (threat minus safe) during the TU anticipation period was significantly larger than during the OU and C conditions, ps < .002. Startle response potentiation during the OU anticipation period was significantly greater than the C anticipation period, p <.005.
3.3 Eyeblink Startle Response: Inter-trial Intervals
An ANOVA comparing the four conditions was conducted for the ITI period to examine potential physiological anxiety-related carry-over effects into the always-safe inter-trial interval. There was a significant main effect of condition, F(3, 123) = 3.10, p < .05, ηp2 = 0.070. Post-hoc tests indicated that there was a trend toward a greater startle response during the TU block ITIs, compared to during C block ITIs, p = .051 (Figure 3). However, this trend failed to survive correction for multiple comparison. No other conditions were different from each other after correction, ps > .20.
3.4 Habituation and Order Effect Startle Analyses: Anticipation Period
The omnibus ANOVA revealed a surprising null finding, the lack of greater startle response during the anticipation period for certain threat compared to safety. We examined whether this may be due to an order effect. To test this we ran a Condition (TU, OU, C, S) × Order (2 orders) × Half (first, second) mixed model ANOVA on startle blink magnitudes from the anticipation period (i.e., order 1, half 1; order 1, half 2; order 2, half 1; and order 2, half 2). As expected, based on the initial ANOVA that did not incorporate order or half as factors, we found a main effect for condition, F(3, 114) = 16.62, p < .001, ηp2 = 0.304. Post-hoc tests indicate that the initial pattern of findings remains: startle responses during TU were larger than those during OU, C, and S, ps < .01, startle responses during OU were larger than startle during C, p < .01, but not S, p = .085, and C startle was not larger than S startle, p > .05. There was also a main effect of half, F(1, 38) = 56.45, p < .001, ηp2 = 0.598, such that blinks across all conditions were larger during the first than second half of the task, p < .001. There was no main effect of order, F(1, 38) = 0.44, p > .51, ηp2 = 0.011.
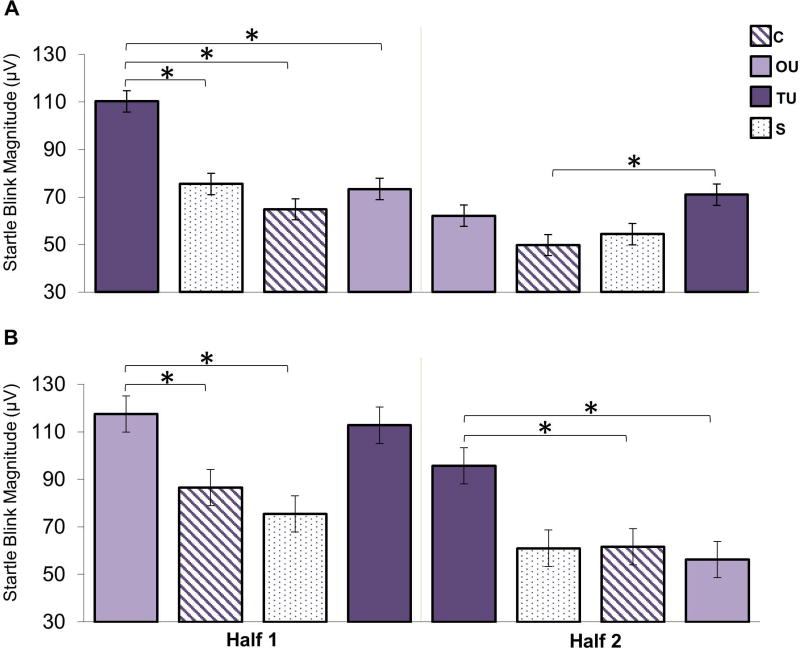
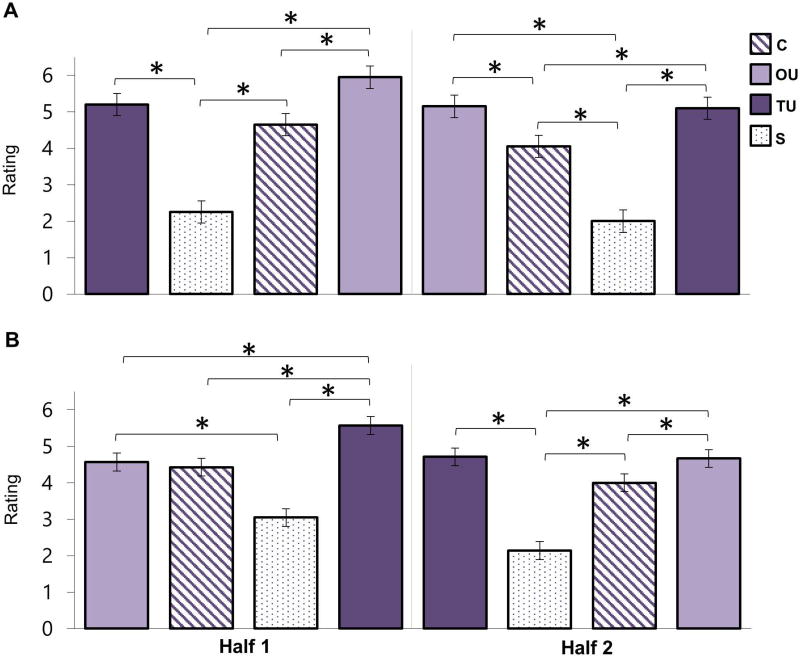
In addition to these main effects, there was also a significant 3-way interaction between condition, half, and order, F(3, 114) = 8.06, p < .001, ηp2 = 0.175. This interaction indicated that the main effect of condition we observed in the initial ANOVA (not collapsed across order and half) may be due to the order of conditions and habituation across the task. To decompose the 3-way interaction, four ANOVAs examining the main effect of condition were conducted for each half (first, second) of both orders. Each of these four ANOVAs yielded a main effect of condition, Fs > 3.89, ps < .04; however, the pattern differed depending upon the order of the conditions (order) and time during the task (half). We first present the results for each of the four ANOVAs, then briefly summarize the key patterns that emerged when considered jointly.
The main effect of condition in the first half of order 1 (TU-S-C-OU), F(3, 54) = 10.298, p < .001, ηp2 = 0.364, was due to larger startle responses during the TU condition than all other conditions, ps < .02 (Figure 4a). The main effect of condition for the second half of order 1 (OU-C-S-TU), F(3, 60) = 3.889, p < .05, ηp2 = 0.163, was driven by significantly larger startle blinks during TU compared to C, p < .005 (Figure 4a), but not OU or S ps > .15. Moving to order 2, the condition main effect for the first half (OU-C-S-TU), F(3, 60) = 6.662, p < .005, ηp2 = 0.250, indicated that startle response during the OU condition was significantly larger than that during the C and S conditions, ps < .05 (Figure 4b). While mathematically TU was larger than C and S, these comparisons did not reach statistical significance (ps > .07). TU and OU did not differ, p > .99. Lastly, the main effect of condition for the second half of order 2 (TU-S-C-OU), F(3, 60) = 9.266, p < .005, ηp2 = 0.317, was due to significantly larger startle responses during TU than OU and C, ps < .05, and approaching significance over the S condition, p = .053 (Figure 4b).
Figure 4. Anticipation Period Startle: Examination of Order and Habituation Effects.
A) Raw startle blink amplitudes during the anticipation period (while “loading bars” are filling) of Order 1. B) Raw startle blink amplitudes during the anticipation period of Order 2. The blocks are presented, in order, to show habituation within and across each order as a way to understand the significant 3-way interaction between condition, half, and order. Comparisons were Bonferroni-corrected. Key: certain (C), occurrence uncertainty (OU), temporal uncertainty (TU), and safe (S). * = p < .05.
Considered in aggregate, three key patterns emerge from these four ANOVAs. First, we continued to find that certain threat (C) was not significantly greater than safe conditions (S), ps > .65, regardless of order or half. Thus, the lack of startle potentiation for certain threat vs. safety was not due to order or habituation effects. Second, across order and time temporal uncertainty (TU) generally produced the most robust potentiation of startle. With the exception of one Order × Half combination (order 2, first half), TU startle responses were significantly larger in general, (although not larger than all other conditions in every ANOVA, see next paragraph). This further supports the strongly anxiogenic properties of TU. Lastly, in contrast to temporal uncertainty, occurrence uncertainty (OU) only led to potentiation of startle compared to C and S in one of the four ANOVAS, during the first half of Order 2, ps < .05. In this order, OU was the first condition presented (OU-C-S-TU). Thus, the initial finding that OU elicited greater startle blink than certain threat and safety may have been due to an order effect.
3.5 Habituation and Order Effect Startle Analyses: Inter-trial Interval
A Condition (TU, OU, C, S) × Order (2 orders) × Half (first, second) mixed model ANOVA on startle blink magnitudes from the ITI period was run to examine habituation across blocks and any potential condition order effects during the ITI period. Results indicated that there was a main effect of condition, F(3, 120) = 3.288, p < .05, ηp2 = 0.076, and half, F(1, 40) = 26.83, p < .001, ηp2 = 0.401, such that startle during TU was greater than during C, p < .05 and startle during the first half was greater than during the second half of the task, p < .001. There were no significant interactions, ps > .25.
3.6 Habituation and Order Effect Analyses for Self-Reported Anxiety
In light of the significant interaction of Condition × Order × Half for the anticipation period startle blink data, we were also curious as to whether the subjective anxiety ratings may also be impacted by the effects of order and time. To test this we ran an identical Condition (TU, OU, C, S) × Order (2 orders) × Half (first, second) mixed model ANOVA on self-reported anxiety. The test revealed a main effect for condition, F(3, 117) = 72.38, p < .001, ηp2 = 0.650. The pattern of findings was identical to that from the ANOVA collapsed across order and half, such that self-reported anxiety during TU was greater than anxiety during C and S, ps < .001, anxiety during OU was greater than during C and S, ps < .001, anxiety during C was greater than during S, p < .001, and anxiety during TU and OU did not differ, p > .14. There was also a main effect of half, F(1, 39) = 11.46, p < .005, ηp2 = 0.227, such that anxiety ratings across all conditions were higher during the first than second half of the task, p < .005. There was no main effect of order, F(1, 39) = 0.19, p > .66, ηp2 = 0.005. All follow-up comparisons were Bonferroni corrected.
In addition to these main effects, there was also a significant interaction between condition and order, F(3, 117) = 3.62, p < .03, ηp2 = 0.085, and a 3-way interaction between condition, half, and order, F(3, 117) = 3.56, p < .03, ηp2 = 0.084. To deconstruct the 3-way interaction four ANOVAs examining the main effect of condition were conducted for each half of both orders (i.e., order 1, half 1; order 1, half 2; order 2, half 1; and order 2, half 2) (Figure 5). All four ANOVAs yielded a main effect of condition, Fs > 15.93, ps < .001. While the existence of the Condition × Order × Half interaction does indicate some order and time effects, in general across all four ANOVAs, subjective anxiety was highest for both types of uncertainty, followed by certain threat, and all threat conditions tended to elicit more anxiety than safety. In all four ANOVAs, anxiety ratings during both types of uncertain threat (TU and OU) were significantly larger than S, ps < .005. Certain threat anxiety ratings were also larger than S in all of the order-half combinations, ps < .001, except order 2, half 1 (OU-C-S-TU), in which it did not reach statistical significance, p = .076. Anxiety ratings during OU were also larger than during C in all order-half combinations, ps < .05, except order 2, half 1 (OU-C-S-TU), p > .99. In comparison, TU anxiety ratings were significantly larger than those during C in order 1, half 2 (OU-C-S-TU), and order 2, half 1 (OU-C-S-TU), ps < .02, but failed to reach statistical significance in the other two combinations, ps > .14. Lastly, TU ratings were significantly larger than OU ratings in only order 2, half 1 (OU-C-S-TU), p < .01. All follow-up comparisons were Bonferroni corrected.
Figure 5. Anxiety Ratings: Examination of Order and Habituation Effects.
Anxiety ratings recorded at the end of each block of Order 1 (A) and Order 2 (B). The blocks are presented, in order, to show habituation within and across each order as a way to understand the significant 3-way interaction between condition, half, and order. Comparisons were Bonferroni-corrected. Key: certain (C), occurrence uncertainty (OU), temporal uncertainty (TU), and safe (S). * = p < .05.
3.7 Relationships Between Anticipation Period Startle Responding and Self-Report Indices
There were no significant correlations between startle response for any condition (aggregated across half and each half separately) with self-reported intolerance of uncertainty, including the total score and the two factors from both the 27-item version and 12-item version of the IUS, ps > .19. There were also no significant correlations between intolerance of uncertainty and startle response potentiation (threat minus safe difference scores) for any of the threat conditions, ps > .73. Finally, there were no significant correlations between measures of anxiety (STAI-T), worry (PSWQ), and negative affect (PANAS-NA) with startle response or threat-minus-safe startle potentiation for any condition, ps > .05.
4. Discussion
The current study examined the anxiogenic effects of the anticipation of occurrence uncertainty and temporal uncertainty. We directly compared temporal uncertainty to occurrence uncertainty, finding that temporal uncertainty was superior in eliciting anxiety-potentiated startle responsivity. The findings support the extant literature indicating that uncertainty associated with not knowing when an aversive event will occur (temporal uncertainty) is a particularly potent elicitor of physiological anxiety (Grillon et al., 2004; Grillon, 2008; Grillon et al., 2008; Grillon et al., 2009; Nelson & Shankman, 2011; Shankman et al., 2011). Our results further reinforce the anxiogenic properties of uncertainty more broadly. Although startle responsivity during occurrence uncertainty was smaller than that for temporal uncertainty and not significantly larger than that for the safe condition, it was significantly larger than when anticipating certain threat. However, order effect analyses revealed that occurrence uncertainty startle responsivity was only larger than certain threat when it was the first condition of the task. This suggests that occurrence uncertainty is not robust to order effects and is more susceptible to habituation across the task. However, participants report experiencing both temporal and occurrence uncertainty as equally anxiety provoking, and more anxiety provoking than certain threat. Thus, occurrence uncertainty, an understudied type of uncertainty, also appears to be an important aspect of uncertainty relevant for evoking anxiety. Somewhat unexpectedly, we found that anticipatory startle response during certain and safe conditions was not different (although subjective ratings of the two conditions did differ). Although these findings were not anticipated, this lack of difference was consistent for both orders and across the duration of the task. Finally, we did not find strong evidence for the carryover of physiological anxiety after the end of the anticipation period in any condition, nor did we find that startle blink during anticipation of uncertain threat was associated with self-reported intolerance of uncertainty, trait anxiety, worry, and negative affect.
4.1 Disentangling Temporal and Occurrence Uncertainty
To the best of our knowledge, there has only been two other studies that directly compared occurrence uncertainty and temporal uncertainty, also doing so with non-clinical student samples (Davies & Craske, 2015; Monat et al., 1972). Davies and Craske found that the conditions with the most certainty (100% reinforcement, temporally predictable) and the most uncertainty (50% reinforcement, temporally unpredictable) elicited the largest startle responses. Unlike their study, we did not include a combined temporal and occurrence uncertainty condition (50% reinforcement, temporally unpredictable), so we were unable to test whether this combined uncertainty also elicited larger startle responding in a within groups design. However, it should be noted that startle responses during their temporal uncertainty condition (100% reinforcement, temporally unpredictable) did not differ from the combined temporal and occurrence uncertainty condition, thus it is possible that temporal uncertainty may be the key element driving defensive responding. Results from Monat and colleagues’ study showed that early in their task’s anticipation period (first 60 seconds), temporal uncertainty produced a larger, sustained anxiety-related response (measured by skin conductance and retrospective relax-tension ratings) than occurrence uncertainty, as well as certain and safe conditions. In contrast, they found that just prior to the end of the anticipation period (last 60 seconds) occurrence uncertainty produced a larger anxiety-related response than temporal uncertainty (measured by skin conductance, heart rate change, and relax-tension ratings), supporting our assertion that although temporal uncertainty produces a robust sustained anxiety response, occurrence uncertainty also elicits anxiety.
Our finding that defensive startle blink responding is enhanced in uncertain conditions in which the threat will definitely occur (as is the case for TU) is consistent with findings that temporal and spatial proximity (i.e., increased likelihood/certainty) trigger a more potent anxiety response. Indeed, previous literature examining the time course of physiological anticipatory anxiety has shown that during anticipation of an aversive stimulus, threat potentiation of startle blink is not evident until just prior to the onset of the threat; in other words, the physiological anticipatory anxiety dramatically increases as the threat becomes more temporally certain to occur (Grillon, Ameli, Merikangas, Woods, & Davis, 1993; Grillon, Ameli, Goddard, Woods, & Davis, 1994). Non-human animal research has also supported the assertion that a defensive response pattern is governed by the physical distance of the threat, such that anxiety (e.g., defensive freezing) is initially produced in the presence of distal threat, and significantly increases as the threat becomes more imminent (Blanchard, Yudko, Rodgers, & Blanchard, 1993; Bolles & Fanselow, 1980; Davis, Walker, Miles, & Grillon, 2010; Fanselow, 1986). Thus, enhanced signaling that a threat is likely to occur, yet temporally unpredictable, and is increasingly imminent as time passes, (e.g., temporally uncertain shock) may elicit defensive responding more strongly than introducing uncertainty about whether it will occur, particularly in non-anxious individuals.
Interestingly, participants’ self-reported anxiety at the end of each block indicated that anticipating both types of uncertain shock felt equally anxiety-provoking, and more anxiety-provoking than anticipating certain threat. Thus, our results suggest that uncertain timing of threat may be especially anxiogenic for robust “primitive” physiological defensive responding, while both types of uncertain threat are experienced subjectively the same. Although a lack of coherence in self-report and physiological responding may seem unusual, the evidence for consistency among multi-method measures of emotion is actually mixed (Grillon et al., 2009; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005). Moreover, this inconsistency of self-report and startle responsivity is common among NPU and uncertainty-related studies (Chin et al., 2016; Grillon, Levenson, & Pine, 2007; Grillon et al., 2008; Grillon et al., 2009; Shankman et al., 2011).
Four possible methodological considerations should be taken into account. First, an alternate interpretation of the distinction between temporal uncertainty and occurrence uncertainty is that the significantly larger startle responsivity in the temporal uncertainty condition is merely the result of more aversive shocks being delivered in that condition, i.e., shock sensitivity (Chin et al., 2016; Davis, 1989). However, it does not seem that a greater number of shocks is always associated with greater startle blink responding. In fact, Bradford and colleagues (2014) showed that having more shocks (15) in a 100% probability condition produced less anticipatory startle responsivity than having fewer shocks (3) during a 20% probability condition with the number of trials held constant. These results, coupled with previous findings suggesting occurrence uncertainty and temporal uncertainty produce unique physiological and self-reported anxiety-related responses (Davies & Craske, 2015; Monat et al., 1972), suggest that the observed difference in startle responsivity between these two types of uncertainty is not due to the difference in the quantity of shocks.
Second, our analyses indicate that the difference between temporal and occurrence uncertainty is in part explained by the order and time during the task when each uncertain condition was presented. Order and time effects clearly influenced startle responding to occurrence uncertainty. In contrast, startle response during temporal uncertainty was relatively robust regardless of when it was presented during the task. Therefore, it is clear that although occurrence uncertainty is susceptible to timing and order effects, startle responsivity during the anticipation of temporally uncertain threat is more reliable.
Third, the visual uniqueness of the temporal uncertainty loading bar (i.e., “jumping” around unpredictably instead of gradually) may make it difficult to determine whether the TU results are due to this or the temporally uncertain threat itself. There is evidence that motion induces bradycardia, suggesting increased attention to movement (Simons, Detenber, Roedema, & Reiss, 1999). However, increased attention is associated with inhibition of startle (Bradley, Cuthbert, & Lang, 1993; Bradley, Codispoti, & Lang, 2006), suggesting that the greater startle blink response to TU occurred despite potential attention-related dampening of startle blink.
Lastly, as noted previously we did not find the typically observed enhancement of startle blink to certain threat compared to safe conditions. Although our design is consistent with the standard NPU task in terms of the duration of the cue period, number of startles, and timing of startles, our design differs from the standard NPU task in another significant way. Our task has more uncertain trials (occurrence uncertainty plus temporal uncertainty) compared to certain and safe trials than typical NPU studies, which tend to have either an equal number of trials across conditions (Chin et al., 2015; Gorka, Nelson, & Shankman, 2013; Gorka, Lieberman, Phan, & Shankman, 2016; Gorka et al., 2017; Nelson et al., 2014; Nelson & Hajcak, 2017; Nelson & Shankman, 2011; Nelson et al., 2015; Schmitz & Grillon, 2012; Shankman et al., 2011) or more safe trials compared to certain and uncertain trials (Ballard et al., 2014; Grillon et al., 2004; Grillon et al., 2006; Grillon et al., 2009; Grillon et al., 2016; Kaye et al., 2016; Moberg & Curtin, 2009). The increased uncertainty and reduced safety across our task may have contributed to the failure to observe a difference in startle between certain threat and safe conditions.
4.2 Individual Differences in Intolerance to Uncertainty and Anticipation of Different Types of Uncertain Threat
Individual differences in the intolerance of uncertainty may be relevant for the way an individual differentially responds to temporal uncertainty and occurrence uncertainty. However, the findings thus far are mixed. For example, in the Chin and colleagues (2016) study, intolerance of uncertainty was positively correlated with startle response during the more uncertain condition (similar to our occurrence uncertainty), rather than the less uncertain condition. This suggests that the more intolerant a person is of uncertainty, the stronger their anxiety-potentiated startle response is to uncertainty regarding the likelihood a threat will occur. In contrast, Nelson & Shankman (2011) found that intolerance of uncertainty was negatively correlated with startle response during the uncertain condition, and perceived control mediated this effect. However, in our study individual differences in the intolerance of uncertainty did not differentially affect how an individual physiologically responded to uncertainty regarding the likelihood or timing of threat. Indeed, intolerance of uncertainty, and related constructs of anxiety, worry, and negative affect, did not correlate with startle responsivity in any of the uncertain or certain threat conditions. Our sample showed sufficient variability on these individual difference measures, suggesting that restriction of range was not a factor. It is also possible that we did not observe associations with anxiety-related individual differences because of the nature of this modified NPU task. The short, isolated anticipation periods may not provide enough time for any physiological nuances reflecting mounting intolerance of uncertainty to emerge. More research is needed to understand how individual differences in self-reported intolerance of uncertainty may influence differential anxious anticipation responsivity in the face of different types of uncertainty.
4.3 Carry-over of Anxiety
In a secondary analysis, we did not find much evidence across conditions for the carryover of threat-related startle blink-measured anxiety into the ITI period, when the task was safe. Startle responsivity during the temporal uncertainty condition was greater than that during the certain threat condition; however, there were no other significant simple comparisons. Although there may have been a small carry-over effect for the temporal uncertainty condition (relative to certain threat), the overall findings suggest that our task isolated the ITI period from the anticipation and made it effectively safe. It should be noted, however, that startle blink amplitudes were assessed fairly late in the ITI (at least 10s, if a shock occurred) to prevent shocks from corrupting the startle response. Overall, fear-potentiated startle to uncertain threat therefore may only reflect transient anxiety, dissipating once the individual either experiences the threat or knows they are in a safe environment and thus minimizing the potential for carry-over effects (Grillon et al., 1993; Mobbs et al., 2007; Shankman et al., 2011).
4.4 Conclusion
The anticipation of uncertain threat is a strong elicitor of acute anxiety. Temporal uncertainty resulted in greater anticipatory anxiety than certain threat, consistent with the large body of research that uncertain threat tends to elicit anxiety (Bradford et al., 2014; Grillon et al., 2004; Grillon et al., 2008; Grupe & Nitschke, 2013; Nelson & Shankman, 2011; Nelson et al., 2015; Schmitz & Grillon, 2012; Shankman et al., 2011). Our results further highlight the potency of the anticipation of potential threat, and underscores its importance as a key component in the elicitation of anxiety (Bitsios, Philpott, Langley, Bradshaw, & Szabadi, 1999; Grupe & Nitschke, 2013; Monat et al., 1972; Sarinopoulos et al., 2010). Our results also suggest that although the uncertainty regarding the occurrence of a threatening stimulus is subjectively perceived as more anxiogenic than predictable threat, a temporally unpredictable, but certain-to-occur threat elicits more potent physiological anticipatory anxiety (i.e., startle blink responding), at least when threat is imminent.
Additional research is needed to further characterize the aspects of uncertainty that are most relevant for the experience of anxiety, both subjectively and physiologically. Future investigations should focus on the anticipation period as a means for manipulating the expectation about a future stimulus, such as the length of the anticipation period (Hefner, Moberg, Hachiya, & Curtin, 2013), the time at which anxiety is measured, or the impact of participant control over the degree of uncertainty of the threat. For example, examining anxiety-relevant responses (i.e., startle amplitude) at differing temporal distances from the threat would be important to disentangle fear from anxiety, a distinction that may be relevant for uncertainty research (Davis et al., 2010). Our findings also highlight the importance of examining the order of uncertain conditions in threat-of-shock studies. Uncertain threat may be a delicate construct that is especially susceptible to changes in timing, order, and other methodological changes. Uncertain threat may also be experienced differently depending on the type (e.g., TU vs. OU) and the measurement used (e.g., startle response vs. retrospective anxiety ratings).
Overall, the findings of the current study provide insight into how varying aspects of uncertainty may be related to anticipatory anxiety, and highlight the particularly potent anxiogenic impact of temporal uncertainty. Ultimately, such work will continue to provide more information about uncertainty’s role in the anticipation of threat and further support its potential role in the development and maintenance of anxiety (Barlow, 2000; Foa, Zinbarg, & Rothbaum, 1992; Mineka & Kihlstrom, 1978).
Acknowledgments
This work was supported by a National Institute of Mental Health grant to Larson (R01 MH106574). We thank Ashley Huggins and Walker Pedersen for their comments on the manuscript. We also thank Daniel Bradford and Jesse Kaye for data processing consultation. Any findings, opinions, conclusions, and recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NIH.
Footnotes
Portions of the current data were presented at the 2016 Society for Psychophysiological Research convention in Minneapolis, MN.
References
- Ballard ED, Ionescu DF, Voort JLV, Slonena EE, Franco-Chaves JA, Zarate CA, Grillon C. Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. Journal of affective disorders. 2014;162:34–38. doi: 10.1016/j.jad.2014.03.027. https://doi.org/10.1016/j.jad.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55(11):1247–1263. doi: 10.1037//0003-066x.55.11.1247. https://doi.org/10.1037/0003-066X.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. 2. New York: Guilford; 2002. [Google Scholar]
- Barnes LL, Harp D, Jung WS. Reliability generalization of scores on the spielberger state-trait anxiety inventory. Educational and Psychological Measurement. 2002;62(4):603–618. https://doi.org/10.1177/0013164402062004005. [Google Scholar]
- Bitsios P, Philpott A, Langley RW, Bradshaw CM, Szabadi E. Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. Journal of Psychopharmacology. 1999;13(3):226–234. doi: 10.1177/026988119901300303. https://doi.org/10.1177/026988119901300303. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Rodgers RJ, Blanchard DC. Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behavioural Brain Research. 1993;58(1):155–165. doi: 10.1016/0166-4328(93)90100-5. https://doi.org/10.1016/0166-4328(93)90100-5. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310(6973):170. doi: 10.1136/bmj.310.6973.170. https://doi.org/10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. https://doi.org/10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behavioral and Brain Sciences. 1980;3(02):291–301. [Google Scholar]
- Bradford DE, Starr MJ, Shackman AJ, Curtin JJ. Empirically based comparisons of the reliability and validity of common quantification approaches for eyeblink startle potentiation in humans. Psychophysiology. 2015;52(12):1669–1681. doi: 10.1111/psyp.12545. https://doi.org/10.1111/psyp.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Magruder KP, Korhumel RA, Curtin JJ. Using the threat probability task to assess anxiety and fear during uncertain and certain threat. Journal of Visualized Experiments. 2014;91:51905. doi: 10.3791/51905. https://doi.org/10.3791/51905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43(5):486–497. doi: 10.1111/j.1469-8986.2006.00412.x. https://doi.org/10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: Attention and emotion in startle modification. Psychophysiology. 1993;30(5):541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. https://doi.org/10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Brown TA, Antony MM, Barlow DH. Psychometric properties of the penn state worry questionnaire in a clinical anxiety disorders sample. Behaviour Research and Therapy. 1992;30(1):33–37. doi: 10.1016/0005-7967(92)90093-v. https://doi.org/10.1016/0005-7967(92)90093-V. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. The intolerance of uncertainty scale: Psychometric properties of the english version. Behaviour Research and Therapy. 2002;40(8):931–945. doi: 10.1016/s0005-7967(01)00092-4. https://doi.org/10.1016/S0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- Carleton RN. The intolerance of uncertainty construct in the context of anxiety disorders: Theoretical and practical perspectives. Expert Review of Neurotherapeutics. 2012;12(8):937–947. doi: 10.1586/ern.12.82. https://doi.org/10.1586/ern.12.82. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Collimore KC, Asmundson GJ. “It's not just the judgements—It's that I don’t know”: Intolerance of uncertainty as a predictor of social anxiety. Journal of Anxiety Disorders. 2010;24(2):189–195. doi: 10.1016/j.janxdis.2009.10.007. https://doi.org/10.1016/j.janxdis.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, Asmundson GJ. Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. Journal of Anxiety Disorders. 2012;26(3):468–479. doi: 10.1016/j.janxdis.2012.01.011. https://doi.org/10.1016/j.janxdis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Norton MPJ, Asmundson GJ. Fearing the unknown: A short version of the Intolerance of Uncertainty Scale. Journal of anxiety disorders. 2007;21(1):105–117. doi: 10.1016/j.janxdis.2006.03.014. https://doi.org/10.1016/j.janxdis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Chin B, Nelson BD, Jackson F, Hajcak G. Intolerance of uncertainty and startle potentiation in in relation to different threat reinforcement rates. International Journal of Psychophysiology. 2016;99:79–84. doi: 10.1016/j.ijpsycho.2015.11.006. https://doi.org/10.1016/j.ijpsycho.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Craske MG, Glover D, DeCola J. Predicted versus unpredicted panic attacks: Acute versus general distress. Journal of Abnormal Psychology. 1995;104(1):214. doi: 10.1037//0021-843x.104.1.214. https://doi.org/10.1037/0021-843X.104.1.214. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43(3):245–265. doi: 10.1348/0144665031752934. https://doi.org/10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Davies CD, Craske MG. Psychophysiological responses to unpredictable threat: Effects of cue and temporal unpredictability. Emotion. 2015;15(2):195. doi: 10.1037/emo0000038. https://doi.org/10.1037/emo0000038. [DOI] [PubMed] [Google Scholar]
- Davis M. Sensitization of the acoustic startle reflex by footshock. Behavioral Neuroscience. 1989;103(3):495–503. https://doi.org/10.1037/0735-7044.103.3.495. [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. https://doi.org/10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas MJ, Buhr K, Ladouceur R. The role of intolerance of uncertainty in etiology and maintenance. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized anxiety disorder: Advances in research and practice. New York, NY, US: Guilford Press; 2004. pp. 143–163. [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during pavlovian fear conditioning. Behavioral Neuroscience. 2007;121(4):635–642. doi: 10.1037/0735-7044.121.4.635. https://doi.org/10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during pavlovian conditioning. NeuroImage. 2008;40(2):811–817. doi: 10.1016/j.neuroimage.2007.11.042. https://doi.org/10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning and Motivation. 1986;17(1):16–39. https://doi.org/10.1016/0023-9690(86)90018-4. [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: An animal model. Psychological Bulletin. 1992;112(2):218–238. doi: 10.1037/0033-2909.112.2.218. https://doi.org/10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Freeston MH, Rhéaume J, Letarte H, Dugas MJ, Ladouceur R. Why do people worry? Personality and Individual Differences. 1994;17(6):791–802. https://doi.org/10.1016/0191-8869(94)90048-5. [Google Scholar]
- Gorka SM, Lieberman L, Klumpp H, Kinney KL, Kennedy AE, Ajilore O, Langenecker S. Reactivity to unpredictable threat as a treatment target for fear-based anxiety disorders. Psychological Medicine. 2017:1–11. doi: 10.1017/S0033291717000964. https://doi.org/10.1017/S0033291717000964. [DOI] [PubMed]
- Gorka SM, Lieberman L, Phan KL, Shankman SA. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug and alcohol dependence. 2016;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. https://doi.org/10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and alcohol dependence. 2013;132(1):216–222. doi: 10.1016/j.drugalcdep.2013.02.003. https://doi.org/10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52(10):958–975. doi: 10.1016/s0006-3223(02)01665-7. https://doi.org/10.1016/S0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C. Greater sustained anxiety but not phasic fear in women compared to men. Emotion. 2008;8(3):410. doi: 10.1037/1528-3542.8.3.410. https://doi.org/10.1037/1528-3542.8.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry. 1994;35(7):431–439. doi: 10.1016/0006-3223(94)90040-x. https://doi.org/10.1016/0006-3223(94)90040-X. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Merikangas KR, Woods SW, Davis M. Measuring the time course of anticipatory anxiety using the fear-potentiated startle reflex. Psychophysiology. 1993;30(4):340–346. doi: 10.1111/j.1469-8986.1993.tb02055.x. https://doi.org/10.1111/j.1469-8986.1993.tb02055.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60(7):760–766. doi: 10.1016/j.biopsych.2005.11.027. https://doi.org/10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118(5):916–924. doi: 10.1037/0735-7044.118.5.916. https://doi.org/10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: A fear-potentiated startle study. Neuropsychopharmacology. 2007;32(1):225–231. doi: 10.1038/sj.npp.1301204. https://doi.org/10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165(7):898–904. doi: 10.1176/appi.ajp.2007.07101581. https://doi.org/10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, O’Connell K, Lieberman L, Alvarez G, Geraci M, Pine DS, Ernst M. Distinct responses to predictable and unpredictable threat in anxiety pathologies: effect of panic attack. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;2(7):575–581. doi: 10.1016/j.bpsc.2016.08.005. https://doi.org/10.1016/j.bpsc.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66(1):47–53. doi: 10.1016/j.biopsych.2008.12.028. https://doi.org/10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. https://doi.org/10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, Curtin JJ. Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology. 2013;122(3):756–769. doi: 10.1037/a0033407. https://doi.org/10.1037/a0033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. https://doi.org/10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology. 2016;53(8):1241–1255. doi: 10.1111/psyp.12663. https://doi.org/10.1111/psyp.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JI, LaBar KS. Unpredictability and uncertainty in anxiety: a new direction for emotional timing research. Frontiers in Integrative Neuroscience. 2011;5(55):1–4. doi: 10.3389/fnint.2011.00055. https://doi.org/10.3389/fnint.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Baas JM, Pine DS, Orme K, Dvir S, Nugent M, Grillon C. Airpuff startle probes: An efficacious and less aversive alternative to white-noise. Biological Psychology. 2005;68(3):283–297. doi: 10.1016/j.biopsycho.2004.07.007. https://doi.org/10.1016/j.biopsycho.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Marlin NA, Sullivan JM, Berk AM, Miller RR. Preference for information about intensity of signaled tailshock. Learning and Motivation. 1979;10(1):85–97. https://doi.org/10.1016/0023-9690(79)90052-3. [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. https://doi.org/10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behaviour Research and Therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. https://doi.org/10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: A new perspective on experimental neurosis. Journal of Abnormal Psychology. 1978;87(2):256–271. doi: 10.1037//0021-843x.87.2.256. https://doi.org/10.1037/0021-843X.87.2.256. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology. 2009;118(2):335–347. doi: 10.1037/a0015636. https://doi.org/10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Frith CD. When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–1083. doi: 10.1126/science.1144298. https://doi.org/10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Wiley series in clinical psychology. Worrying: Perspectives on theory, assessment and treatment. Oxford, England: John Wiley; 1994. pp. 265–283. [Google Scholar]
- Monat A, Averill JR, Lazarus RS. Anticipatory stress and coping reactions under various conditions of uncertainty. Journal of Personality and Social Psychology. 1972;24(2):237–253. doi: 10.1037/h0033297. https://doi.org/10.1037/h0033297. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Bishop JR, Sarapas C, Kittles RA, Shankman SA. Asians demonstrate reduced sensitivity to unpredictable threat: a preliminary startle investigation using genetic ancestry in a multiethnic sample. Emotion. 2014;14(3):615–623. doi: 10.1037/a0035776. https://doi.org/10.1037/a0035776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G. Defensive motivation and attention in anticipation of different types of predictable and unpredictable threat: A startle and event-related potential investigation. Psychophysiology. 2017;54(8):1180–1194. doi: 10.1111/psyp.12869. https://doi.org/10.1111/psyp.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G, Shankman SA. Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology. 2015;52(7):887–894. doi: 10.1111/psyp.12418. https://doi.org/10.1111/psyp.12418. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology. 2011;81(2):107–115. doi: 10.1016/j.ijpsycho.2011.05.003. https://doi.org/10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex. 2010;20(4):929–940. doi: 10.1093/cercor/bhp155. https://doi.org/10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7(3):527–532. doi: 10.1038/nprot.2012.001. https://doi.org/10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton KA, Dugas MJ. Defining distinct negative beliefs about uncertainty: Validating the factor structure of the Intolerance of Uncertainty Scale. Psychological Assessment. 2009;21(2):176–186. doi: 10.1037/a0015827. https://doi.org/10.1037/a0015827. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Robison-Andrew EJ, Nelson BD, Altman SE, Campbell ML. Effects of predictability of shock timing and intensity on aversive responses. International Journal of Psychophysiology. 2011;80(2):112–118. doi: 10.1016/j.ijpsycho.2011.02.008. https://doi.org/10.1016/j.ijpsycho.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O'Daly O. Anterior insula responds to temporally unpredictable aversiveness: An fMRI study. Neuroreport. 2014;25(8):596–600. doi: 10.1097/WNR.0000000000000144. https://doi.org/10.1097/WNR.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RF, Detenber BH, Roedema TM, Reiss JE. Emotion processing in three systems: The medium and the message. Psychophysiology. 1999;36(5):619–627. [PubMed] [Google Scholar]
- Spielberger CD, editor. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Suárez L, Bennett S, Goldstein C, Barlow DH. Understanding anxiety disorders from a “triple vulnerability” framework. Oxford Handbook of Anxiety and Related Disorders. 2009:153–172. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. https://doi.org/10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams LE, Oler JA, Fox AS, McFarlin DR, Rogers GM, Jesson MA, Kalin NH. Fear of the unknown: Uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology. 2014;40:1428–1435. doi: 10.1038/npp.2014.328. https://doi.org/10.1038/npp.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]