Abstract
Sialic acid-binding immunoglobulin-like lectin (Siglec)-8 is a human cell surface protein expressed exclusively on eosinophils, mast cells, and basophils that, when engaged, induces eosinophil apoptosis and inhibits mast cell mediator release. This makes Siglec-8 a promising therapeutic target to treat diseases involving these cell types. However, pre-clinical studies of Siglec-8 targeting in vivo are lacking because this protein is only found in humans, apes, and some monkeys. Therefore, we have developed a mouse strain in which SIGLEC8 transcription is activated by Cre recombinase and have crossed this mouse with the eoCre mouse to achieve eosinophil-specific expression. We confirmed that Siglec-8 is expressed exclusively on the surface of mature eosinophils in multiple tissues at levels comparable to those on human blood eosinophils. Following ovalbumin sensitization and airway challenge, Siglec-8 knock-in mice generated a pattern of allergic lung inflammation indistinguishable from that of littermate controls, suggesting that Siglec-8 expression within the eosinophil compartment does not alter allergic eosinophilic inflammation. Using bone marrow from these mice, we demonstrated that, during maturation, Siglec-8 expression occurs well before the late eosinophil developmental marker CCR3, consistent with eoCre expression. Antibody ligation of the receptor induces Siglec-8 endocytosis and alters the phosphotyrosine profile of these cells, indicative of productive signaling. Finally, we demonstrated that mouse eosinophils expressing Siglec-8 undergo cell death when the receptor is engaged, further evidence that Siglec-8 is functional on these cells. These mice should prove useful to investigate Siglec-8 biology and targeting in vivo in a variety of eosinophilic disease models.
Keywords: Transgenic, knock-in, pre-clinical, Siglec-F, asthma, apoptosis
Introduction
Sialic acid-binding immunoglobulin-like lectins (siglecs) are a family of cell surface receptors found on leukocytes and a few other cell types [1, 2]. Among them is Siglec-8, a human cell surface protein expressed exclusively on eosinophils, mast cells, and basophils. When Siglec-8 is ligated by antibodies or sialylated ligands, it causes eosinophil apoptosis and the inhibition of mediator release from mast cells [3–7]. These features make Siglec-8 an attractive therapeutic target to treat diseases involving these cell types [8]. However, the fact that Siglec-8 is only found in certain primates has precluded pre-clinical studies of Siglec-8 targeting in vivo [9, 10]. Furthermore, while Siglec-F is considered to be the closest functional paralog to Siglec-8 [11], its biology, cellular expression patterns, and sialoside ligand binding characteristics differ substantially from Siglec-8 [5, 7, 8, 12, 13]. This suggests that in order to explore Siglec-8 biology in vivo in a preclinical model, novel animal strains are needed. Therefore, we have developed such a Siglec-8 knock-in mouse strain on the C57BL/6J background in which human Siglec-8 is exclusively and consistently expressed in the eosinophil compartment. In this report, we describe the methods used to generate this SIGLEC8Eo mouse strain and provide data from our initial characterization of Siglec-8 expression, function, and immune responses in these mice.
Materials and Methods
Mice
Knock-in mice (SIGLEC8LSL) were generated by homologous recombination on a C57BL/6 genetic background using a targeting construct (Figure 1) at the LSU Pennington Transgenic Facility. Details will be published elsewhere (Y. Wei, et al., “Creation of a novel strain of mice expressing Siglec-8 on their mast cells: expression and initial characterization,” manuscript in preparation). The targeting construct was built based on the pRosa26-DEST construct with cDNA encoding the human protein Siglec-8 preceded by a loxP site-flanked Neo/Kan resistance gene as a STOP cassette (LSL) and upstream CAG promoter [14]. These mice were bred with eoCre mice, which express Cre recombinase under the control of the eosinophil peroxidase (EPX) promoter [15] to remove the STOP cassette, thereby generating SIGLEC8Eo mice. Mouse studies were performed with the approval of the Institutional Animal Care and Use Committees of Northwestern University and Yale University.
Figure 1.
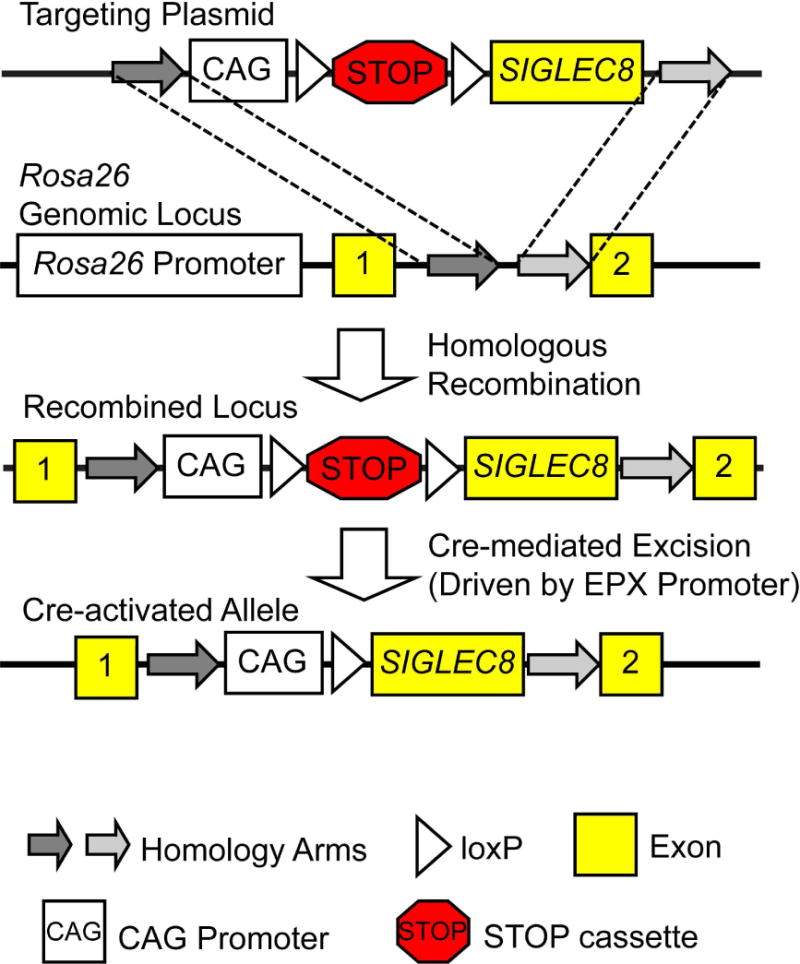
Generation of the SIGLEC8Eo mouse. The SIGLEC8 gene was introduced by homologous recombination into the Rosa26 locus with an upstream floxed STOP cassette (LSL). Following crossing this mouse with one expressing Cre recombinase under the control of the eosinophil peroxidase (EPX) promoter, Cre-mediated excision of the STOP cassette permitted Siglec-8 expression only in cells expressing EPX.
Flow cytometric analysis of surface phenotype and apoptosis
Gut lamina propria was isolated using the Lamina Propria Dissociation Kit from Miltenyi Biotec (Bergisch Gladbach, Germany) according to the manufacturer’s instructions, and the epithelial fraction was saved for flow cytometric analysis as well. Spleen and bone marrow were dissociated into single-cell suspensions prior to staining. Blood and peritoneal lavage fluid were collected. Cells were stained (antibody information is included in Table 1) and analyzed on an LSR II flow cytometer (BD Biosciences, San Jose, CA) using FlowJo v.10.0.8 (Tree Star, Ashland, OR). Endocytosis of Siglec-F and Siglec-8 was followed using flow cytometry by engaging and labeling the proteins with unlabeled antibodies and detecting the remaining surface-bound antibody after incubations of various durations at 37°C with labeled secondary antibodies. Isotype controls were used to determine staining background. Eosinophil cell death was detected using fluorophore-labeled annexin V (BD Biosciences) and DAPI (Thermo Fisher Scientific, Waltham, MA) following 18–24 h of culture with unlabeled anti-Siglec-8, anti-Siglec-F, or their isotype-matched control mAbs at 2.5 μg/ml.
Table 1.
Antibody clone, fluorophore conjugate, and source information.
| Antibody | Clone | Fluorophore Conjugation | Source |
|---|---|---|---|
| Anti-Siglec-8 (mouse IgG1) | 2C4 | Unconjugated or Alexa Fluor 647 | Produced in-house [3] |
| Mouse IgG1 isotype control | MOPC-21 | Unconjugated or Alexa Fluor 647 | Tonbo Biosciences, San Diego, CA |
| Anti-Siglec-F (rat IgG2b) | 9C7 | Unconjugated | Generously provided by James Paulson, The Scripps Research Institute (La Jolla, CA) [18] |
| Rat IgG2b isotype control | LTF-2 | Unconjugated | Tonbo Biosciences |
| Anti-mouse IgG1 secondary mAb | RMG1-1 | FITC | BioLegend, San Diego, CA |
| Anti-rat IgG secondary pAb | Polyclonal goat | PE | BD Pharmingen, San Jose, CA |
| Anti-CD45 | 30-F11 | PerCP | BD Pharmingen |
| Anti-Siglec-F | E50-2440 | PE | BD Pharmingen |
| Anti-CCR3 | 83103 | Alexa Fluor 647 or PE-Cy7 | BD Pharmingen |
| Anti-CD11b | M1/70 | APC-Cy7 or PE-CF594 | BD Pharmingen |
| Anti-TCRβ | H57-597 | FITC | BD Pharmingen |
| Anti-CD11c | HL3 | PE-CF594 | BD Pharmingen |
| Anti-Ly6G | 1A8 | Alexa Fluor 700 | BD Pharmingen |
| Anti-c-kit | 2B8 | FITC | BD Pharmingen |
| Anti-F4/80 | BM8 | eFluor 450 | eBioscience, San Diego, CA |
| Anti-B220 | RA3-6B2 | PE-Cy7 | eBioscience |
| Anti-CD49b | DX5 | eFluor 450 | eBioscience |
| Anti-NK1.1 | PK136 | FITC | eBioscience |
| Anti-FcεRIα | MAR-1 | PE-Cy7 | BioLegend |
| Anti-IL5Rα | T21 | BV421 | BD Biosciences |
| Anti-CD16/CD32 | 93 | APC-Cy7 | BioLegend |
Eosinophil differentiation from bone marrow precursors
Eosinophils were generated from bone marrow from SIGLEC8Eo mice using a protocol described by Dyer et al. [16]. Bone marrow-derived eosinophil (BMDE) development and viability was assessed by flow cytometry with antibodies against Siglec-8, Siglec-F, and CCR3 (BD Pharmingen, San Diego, CA) and DAPI (Thermo Fisher Scientific).
Phosphotyrosine western blot
Following antibody engagement of Siglec-8 or Siglec-F on BMDEs for 1 h at 37°C, global tyrosine phosphorylation changes were detected by western blot. Proteins were separated on 4-15% gradient mini-PROTEAN TGX precast gels and transferred to Immun-Blot PVDF membranes using the Trans-Blot SD semi-dry transfer cell (Bio-Rad, Hercules, CA). The 4G10 platinum anti-phosphotyrosine mAb (Millipore, Billerica, MA) was used at a 1:1000 dilution. Anti-β-actin (Thermo Scientific) was used to indicate equal loading. IRDye 680RD- or 800RD-conjugated secondary antibodies (LI-COR, Lincoln, NE) were used, and images and quantification were obtained using the Odyssey Imaging System (LI-COR).
ROS generation assay
Following antibody engagement of Siglec-8 or Siglec-F on BMDEs for the indicated durations at 37°C, total ROS production was determined using the ROS-ID Total ROS detection kit (Enzo Life Sciences, Farmingdale, NY) by flow cytometry. ROS levels are presented as a percentage of the levels observed with the positive control (pyocyanin).
OVA asthma model
SIGLEC8Eo mice and SIGLEC8LSL littermate controls were put through an ovalbumin (OVA)-induced asthma model. In brief, the mice were sensitized through intraperitoneal (i.p.) injections of 20 μg of OVA (Sigma, St. Louis, MO) in 4 mg alum adjuvant (Imject, Thermo Scientific) on days 0 and 5. Six days later, the mice were challenged with 1% OVA aerosol or HBSS vehicle alone using a Pulmo-Aide nebulizer (DeVilbiss Healthcare, Somerset, PA) for 20 minutes each day for 4 days. Four days after the final challenge, the mice were sacrificed, and bronchoalveolar lavage (BAL) fluid and tissues were obtained for analysis. Lung sections were fixed with 4% paraformaldehyde and stained with either hematoxylin and eosin or periodic acid-Schiff. Lung sections were imaged using an Olympus IX71 inverted microscope (Tokyo, Japan).
Statistical analysis
Data were analyzed and t-tests and Tukey multiple comparisons tests were performed using GraphPad Prism v6.0 (La Jolla, CA). Differences between means were considered statistically significant when p < 0.05.
Results
We introduced cDNA encoding the full-length form of Siglec-8 into the first intron of the Rosa26 genomic locus by homologous recombination to generate a mouse strain capable of expressing the human receptor Siglec-8 (Figure 1). Upstream of the SIGLEC8 gene we placed a loxP-flanked STOP cassette (LSL) that would prevent expression of this gene except in cells also expressing Cre recombinase. We have referred to this recombined locus as SIGLEC8LSL. Expression of Siglec-8 on eosinophil-lineage committed cells was achieved by crossing these mice with a previously published strain on the C57BL/6 background in which Cre recombinase expression is driven by the eosinophil peroxidase (EPX) promoter (eoCre) [15] to generate SIGLEC8Eo mice.
We first sought to confirm that Siglec-8 expression was restricted to eosinophils in mice possessing both of these recombinant loci. We examined cells in the blood, spleen, bone marrow, peritoneal lavage, and gut by flow cytometry. We confirmed that Siglec-8 was restricted to eosinophils (i.e., SSChi/CD45+/Siglec-F+ cells) and that it was expressed by all eosinophils examined in these tissues at levels similar to those seen on human eosinophils (Figure 2). In addition to eosinophils, alveolar macrophages and subtypes of gut epithelial cells also express Siglec-F in the mouse [17–19]. However, Siglec-F+ gut epithelial cells, as expected, do not express Siglec-8 (Figure 2). Moreover, by including markers for other leukocyte populations, we further found that there was no Siglec-8 expression in T cells, B cells, NK cells, mast cells, basophils, neutrophils, macrophages, or dendritic cells.
Figure 2.
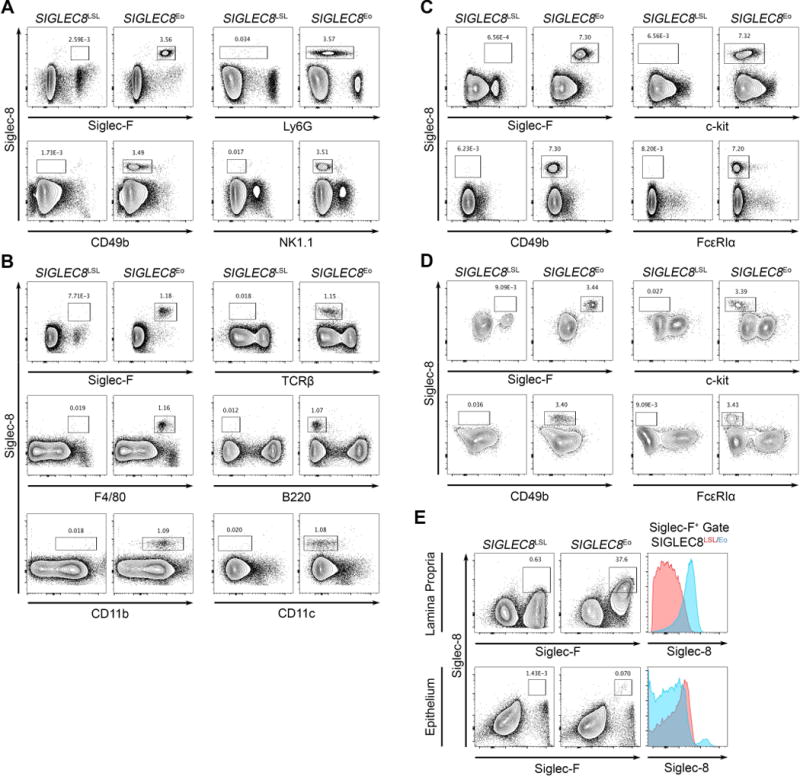
Siglec-8 is selectively expressed in eosinophils in the SIGLEC8Eo mouse but not in other leukocyte compartments. Tissues were collected from SIGLEC8Eo and SIGLEC8LSL mice lacking Cre expression. Cell populations were identified in peripheral blood (A), spleen (B), bone marrow (C), peritoneal lavage (D), and gut (E) by flow cytometry. Histograms of Siglec-8 expression on all Siglec-F+ cells in each gut tissue fraction are shown for each mouse strain. Data are representative of three mice per genotype. Cells are pre-gated on CD45+ single cells as demonstrated in Supp. Figure S1.
We next examined the timing of Siglec-8 expression during eosinophil development from bone marrow precursors. We differentiated eosinophils from tibial and femoral bone marrow of SIGLEC8Eo mice using a protocol developed by Dyer et al. [16]. This protocol involves the ex vivo differentiation of mouse eosinophils by culturing the bone marrow cells in medium containing mouse stem cell factor and Flt3 ligand for four days and thereafter culturing the cells in medium containing the mouse cytokine IL-5. These bone marrow-derived eosinophils (BMDEs) expressed Siglec-8 early in development with approximately the same kinetics as Siglec-F expression and well before the chemokine receptor and late differentiation marker, CCR3, all consistent with the EPX-driven expression of Siglec-8 (Figure 3A). To assess Siglec-8 expression during eosinophil development in the mouse, we harvested bone marrow and stained for markers of distinct eosinophil precursor populations. Siglec-8 was not found to be expressed at the developmental stage preceding Siglec-F expression or earlier but began to be expressed at the eosinophil progenitor stage, at which point the cells begin to express Siglec-F and become granular (Figure 3B), consistent with the in vitro differentiation results.
Figure 3.
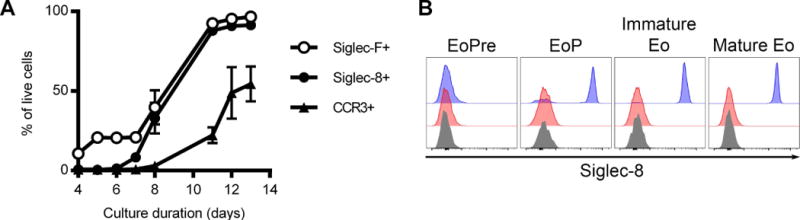
Siglec-8 is expressed in SIGLEC8Eo mouse eosinophils relatively early in hematopoietic development. (A) Mouse eosinophils were differentiated from bone marrow hematopoietic stem cells. Siglec-8, Siglec-F, and CCR3 expression was assessed throughout the differentiation of the eosinophils by flow cytometry in the live (DAPI-negative) cell population. Data represent the means and standard deviations from four independent experiments. (B) Bone marrow cells were analyzed for Siglec-8 expression based on the stage of eosinophil development. Siglec-8 levels on eosinophil pre-cursors (EoPre; SSClow CD11b+ CD16/CD32+ c-kitdim IL5Rα+ Siglec-F−), eosinophil progenitors (EoP; SSChigh CD11b+ CD16/CD32+ c-kitdim IL5Rα+ Siglec-F+), immature eosinophils (Immature Eo; SSChigh CD11b+ CD16/CD32+ c-kit− IL5Rαint Siglec-F+ CCR3−), and mature eosinophils (Mature Eo; SSChigh CD11b+ CD16/CD32+ c-kit− IL5Rαint Siglec-F+ CCR3+) from SIGLEC8LSL (red filled histograms) and SIGLEC8Eo (blue filled histograms) are shown. Fluorescence-minus-one controls for Siglec-8 are shown in gray filled histograms for each cell population. Data are representative of 5 SIGLEC8Eo mice.
Next, we engaged the Siglec-8 receptor on BMDEs using an anti-Siglec-8 mAb (clone 2C4) to establish whether Siglec-8 was capable of signaling on these cells. First, we compared general tyrosine phosphorylation and receptor endocytosis induced by this treatment with anti-Siglec-F (clone 9C7) or isotype control mAbs treatments. While anti-Siglec-F did not appreciably alter downstream tyrosine phosphorylation as determined by pan-phosphotyrosine blot and caused Siglec-F to be internalized slowly, Siglec-8 ligation induced distinct patterns of tyrosine phosphorylation and caused Siglec-8 to be endocytosed more rapidly, indicating that the receptor is capable of transducing a signal on these cells (Figure 4A and B). The presence of a secondary cross-linking antibody did not augment 2C4-induced signaling but did appear to induce 9C7-dependent tyrosine phosphorylation through Siglec-F (Figure 4A). Siglec-F antibody ligation did not induce ROS production in BMDEs, consistent with previous reports that Siglec-F signaling does not lead to ROS production in mouse eosinophils (Supp. Figure S2). However, Siglec-8 ligation on these cells also failed to induce ROS production within a 2-hour timeframe (Supp. Figure S2). To determine whether Siglec-8 expression on mouse eosinophils perturbs eosinophil number, survival, or tissue trafficking in the absence of any intervention with antibody or ligand for Siglec-8, we utilized a chicken ovalbumin (OVA)-driven model of allergic respiratory inflammation. SIGLEC8Eo mice and littermate controls were sensitized to OVA and challenged with nebulized OVA or, as a negative control, with nebulized HBSS vehicle. We harvested blood, bone marrow, and BAL fluid from the mice 96 hours after the last challenge. As expected, we found that eosinophils had migrated to the lungs as a result of the OVA challenges (Figure 5A). Consistent with the lack of eosinophil peroxidase expression in Siglec-F+ alveolar macrophages, there was no Siglec-8 expression observed in these cells (data not shown). In addition, there were trends towards increases of eosinophil levels in the blood and bone marrow, consistent with the effects of this allergen sensitization/challenge model (Figure 5B and C). However, there were no statistically significant differences between the SIGLEC8Eo mice and the littermate controls (Figure 5A–C), indicating that the presence of the human Siglec-8 receptor on these cells did not have an impact on eosinophil numbers or tissue homing. Additionally, histological staining of lung tissue demonstrated that lung inflammation and goblet cell hyperplasia were not appreciably affected by the expression of Siglec-8 on eosinophils (Figure 5D and E).
Figure 4.
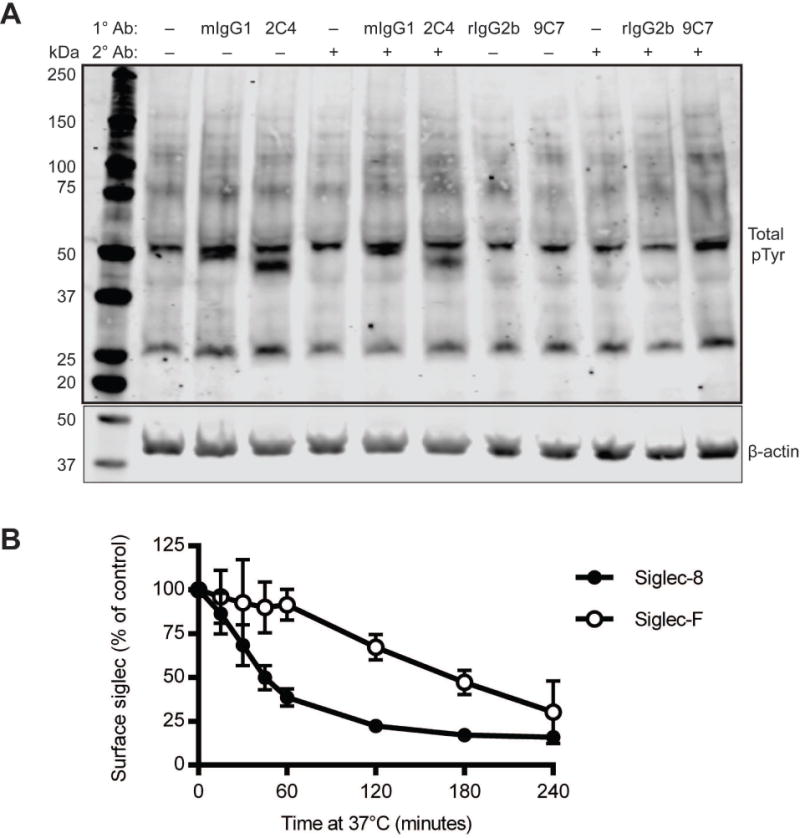
Siglec-8 signals and is internalized on SIGLEC8Eo mouse eosinophils. (A) BMDEs (d12 of differentiation or later) were treated with antibodies against Siglec-8 (2C4) or Siglec-F (9C7), isotype control mAbs (mouse IgG1 as a control for 2C4 or rat IgG2b as a control for 9C7), or left untreated (−) in the presence (+) or absence (−) of secondary (2°) crosslinking antibody against mIgG1 or rat IgG as appropriate. The cells were lysed after 1 h of antibody treatment, and global tyrosine phosphorylation changes were detected by western blot using a phosphotyrosine-specific antibody. Beta-actin was used as a loading control. pTyr, phosphotyrosine. (B) Endocytosis of Siglec-8 or Siglec-F was assessed by flow cytometry on BMDEs (d12 or later) by delayed secondary antibody staining as detailed in the Materials and Methods section. Results are representative (A) or represent the means and standard deviations (B) of at least 3 independent experiments.
Figure 5.
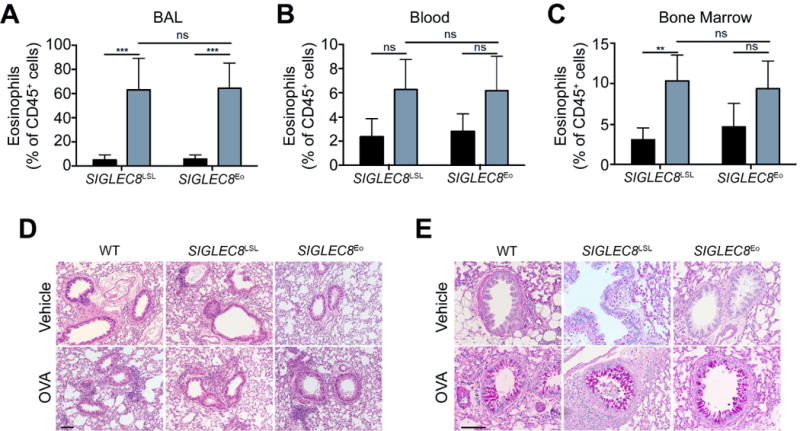
Eosinophils expand in the blood and bone marrow and are recruited to the lung equally well regardless of Siglec-8 expression in an OVA asthma model. Mice were either sensitized and challenged with chicken ovalbumin (OVA, gray filled bars) or mock sensitized and challenged (Vehicle, black filled bars). Eosinophil levels as a proportion of CD45+ cells in the BAL (A), blood (B), and bone marrow (C) are shown. (D) Lung sections were stained with hematoxylin and eosin and were imaged to visualize leukocyte infiltration. (E) Lung tissue was stained with periodic acid-Schiff and imaged to visualize goblet cell hyperplasia. Scale bars, 100 μm. ns, not statistically significant; **, p<0.01; ***, p<0.001. n = 4–5 mice per group.
Finally, we examined whether Siglec-8 engagement on mouse eosinophils could induce cell death as it does on human eosinophils. To do this, we isolated eosinophils from the blood and spleens of SIGLECEo mice. We cultured these cells overnight with antibodies to Siglec-8 or Siglec-F or with appropriate isotype controls before assessing the proportion of remaining eosinophils, siglec internalization, and eosinophil cell death by flow cytometry. We found that anti-Siglec-8 antibody treatment potently depleted eosinophils as a percentage of CD11b-expressing cells in the blood and spleen (Figure 6A and B and data not shown). In addition, nearly all of the remaining eosinophils in the samples treated with anti-Siglec-8 underwent cell death (Figure 6C and D). Anti-Siglec-F antibody treatment caused trends toward decreases in the proportion of remaining eosinophils and decreases in cell viability in the remaining eosinophils, but these effects were not statistically significant despite an apparent decrease in unbound surface Siglec-F caused by the antibody treatment (Figure 6A–D).
Figure 6.
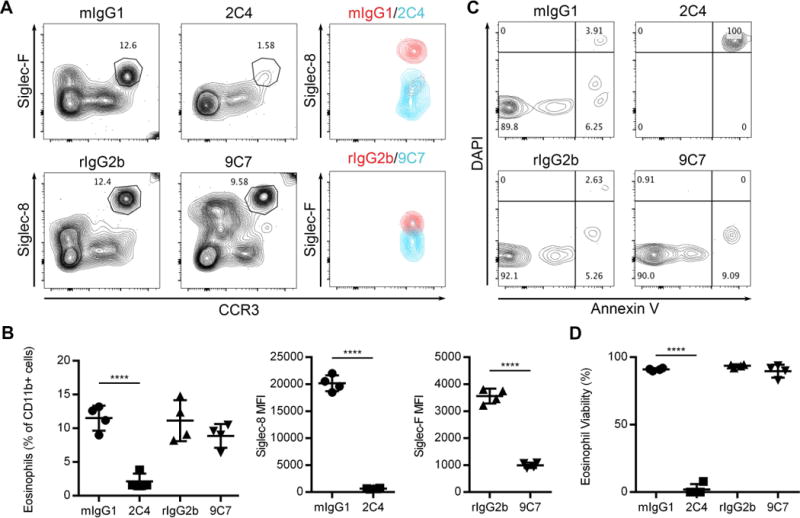
Antibody ligation of Siglec-8 on mouse eosinophils leads to cell death in vitro. Blood eosinophils were treated with anti-Siglec-8 mAb (2C4), anti-Siglec-F mAb (9C7), mouse IgG1 (mIgG1), or rat IgG2b (rIgG2b) isotype controls at 2.5 μg/ml for 18–24 hours prior to flow cytometric analysis. (A) Eosinophils as a percentage of CD11b+ cells was determined based on expression of CCR3 and the untargeted siglec (Siglec-F or Siglec-8). Within the eosinophil gate, expression of unbound surface Siglec-8 or Siglec-F was also determined by flow cytometry. (B) Eosinophils remaining after treatment is expressed as a proportion of CD11b+ cells. Loss of surface Siglec-8 and Siglec-F following targeting is presented as mean fluorescence intensity (MFI). (C) Cell viability within the eosinophil gates in (A) was determined by Annexin V and DAPI staining. The proportion of viable (Annexin V− DAPI−) eosinophils for each treatment is quantified in (D). Data are representative (A and C) or represent the means and standard deviations (B and D) of 4 biological replicates per treatment. ****, p<0.0001.
Discussion
We describe a novel mouse strain in which the human protein Siglec-8 was expressed consistently and exclusively in the eosinophil compartment. In the blood, bone marrow, spleen, peritoneal cavity, and gut lamina propria, Siglec-8 expression was observed on all eosinophils examined and only on eosinophils. That is, no Siglec-8 was detected on any other cell type, including other cell types that express Siglec-F, such as alveolar macrophages, specific subtypes of gut epithelial cells, or Siglec-F+ GMPs [17–19]. Both in vitro and in vivo, Siglec-8 is expressed relatively early in eosinophil development (d7 of culture or at the EoP stage) in this mouse strain, which is consistent with its induction by EPX promoter activity. During eosinophil development in this mouse strain, Siglec-8 expression coincides with the acquisition of granularity (SSChigh) and Siglec-F expression. The Siglec-8− SSClow Siglec-Flow/− developmental stage that otherwise expresses markers similar to the EoP stage appears to be that described by Johnston et al. as the eosinophil precursor (EoPre), which is how it has been denoted here [20]. The surface expression levels of Siglec-8 on eosinophils in this mouse strain appear similar to levels found on human eosinophils [21]. Furthermore, expression of Siglec-8 does not significantly affect Siglec-F levels on the mouse eosinophil cell surface. Expression of Siglec-8 in the eosinophil compartment exerts no apparent effect on baseline numbers of eosinophils or other leukocytes in this new strain nor on allergen-induced pulmonary responses. However, ex vivo engagement of Siglec-8 on mouse eosinophils from the blood or spleen of these mice potently induces cell death to a degree similar to that found with IL-5–primed human eosinophils, demonstrating the value of this mouse in evaluating the efficacy of targeting Siglec-8 in the context of various models of eosinophilic disease.
It is not yet clear whether Siglec-8 on the surface of mouse eosinophils leads to the activation of the same sets of kinases responsible for the pro-apoptotic effect in human eosinophils or whether it engages the same endocytic machinery, both of which have recently been described [21–23]. Given the weak pro-apoptotic effects of Siglec-F compared to the robust response to Siglec-8, it is tempting to speculate that these pathways will turn out to be distinct. Ultimately, the ability to cross this mouse with strains lacking particular signaling molecules, endocytic components, or cell death effectors may well provide additional insight into the mechanisms employed by Siglec-F and Siglec-8, and this will be the focus of future studies.
One explanation for the lack of effect of the introduction of Siglec-8 on eosinophil numbers in this mouse strain is that mice may lack glycan ligands for Siglec-8. Indeed, while Siglec-8 and Siglec-F share similar glycan binding profiles, Siglec-F recognizes certain branched structures not recognized by Siglec-8 [11, 13]. Furthermore, the endogenous ligands for each of these receptors appear to be distinct. Siglec-F recognizes glycans displayed on mouse Muc5b from upper airway mucosa [13], but Siglec-8 fails to recognize human MUC5B and does not recognize any mouse airways structures [24, 25]. Siglec-8 has an absolute requirement for seeing α2,3-linked, 6′-sulfated ligands while Siglec-F binding is more promiscuous and may not require sulfation [13, 26, 27]. In the absence of endogenous ligand, the engineered expression of Siglec-8 may exert no new biological effect in vivo on these cells. While this system may not lend itself to the study of Siglec-8 biology in response to endogenous signals and tissue-derived ligands, this new mouse strain is ideal to study the efficacy and consequences of targeting Siglec-8 with specific antibodies or ligand-based agents, such as nanoparticles decorated with Siglec-8-specific glycan or glycomimetic ligands, due to the lack of baseline differences between mice that express Siglec-8 and those that do not. Based on the distinct but overlapping binding preferences of Siglec-8 and Siglec-F, it may be possible to identify ligands for Siglec-8 that do not bind Siglec-F. However, in the absence of such a specific ligand, this mouse strain can be crossed with Siglec-F−/− mice in future studies to focus solely on the effect of ligand binding to Siglec-8 and to determine whether Siglec-8 can substitute for Siglec-F during inflammation and other eosinophil-associated responses. For example, Siglec-F−/− mice develop blood and lung eosinophilia following allergen sensitization and challenge [28, 29], but whether the substitution of Siglec-8 for Siglec-F will rescue this phenotype remains to be determined. Interestingly, targeting Siglec-F on mouse eosinophils failed to achieve the same effects as targeting Siglec-8 in this study. As mentioned above, ligation of Siglec-F results in a much weaker cell death effect on mouse eosinophils than Siglec-8 exerts on, in particular, IL-5–primed human eosinophils [30–32]. However, antibody ligation of Siglec-F can rapidly clear circulating eosinophils and reduces eosinophilic inflammation [31, 33, 34], and loss of endogenous sialoside ligands for Siglec-F exacerbates eosinophilic inflammation [35, 36]. This study used a distinct anti-Siglec-F mAb clone (9C7, rat IgG2b) [18] from studies that have examined the pro-apoptotic effect of Siglec-F and, in order to compare Siglec-F targeting more directly with Siglec-8 targeting, used a slightly lower, yet saturating concentration of anti-Siglec-F than other studies. It is possible that, due to increased efficacy, the provision of small amounts of Siglec-8 targeting agents will allow Siglec-8 to more than adequately compensate for the loss of Siglec-F. However, Siglec-F may primarily function in a manner distinct from Siglec-8, and in that event Siglec-8 may very well not substitute for Siglec-F in the mouse regardless of the targeting strategy employed. Finally, studies involving systemic Siglec-F mAb administration to mice may exert some of its eosinophil depleting effects via antibody dependent cytotoxicity rather than direct induction of cell death. Ongoing work should ultimately clarify these issues, and will also be focusing on expressing Siglec-8 in the mast cell compartment. These approaches will again utilize the SIGLEC8LSL mice but will instead cross them with mast cell-selective Cre mice to additionally study Siglec-8 targeting on mast cells, another key Siglec-8-expressing cell type in humans. Once both strains are established, they can be crossed to create a mouse that finally resembles their pattern of human Siglec-8 expression, namely on both the eosinophil and mast cell.
While Siglec-8 has been studied extensively in vitro using human eosinophils and mast cells, studying Siglec-8 as a target in the context of animal models of asthma or allergic inflammation in vivo has not been possible. This mouse strain in which Siglec-8 is expressed uniquely in the eosinophil compartment provides a critical tool for the study of Siglec-8 targeting in models of eosinophilic inflammation. In addition, the SIGLEC8LSL mouse will allow us to easily express Siglec-8 in other cell compartments through breeding with existing cell type-specific Cre mice and will furthermore allow us to make use of a complement of knockout mice to study other proteins that may play downstream roles in Siglec-8 signaling and function.
Supplementary Material
Summary.
We have developed a novel eosinophil-specific Siglec-8 knock-in mouse strain that facilitates the in vivo study of Siglec-8 targeting and biology.
Acknowledgments
The authors thank James Paulson (The Scripps Research Institute, La Jolla, CA) for generously providing anti-Siglec-F antibody and acknowledge the contributions of the staff members of the Center for Comparative Medicine of the Feinberg School of Medicine at Northwestern University and the Office of Animal Research Support at Yale University School of Medicine. This work was supported by the National Heart, Lung, and Blood Institute (P01HL107151 to Z.Z. and B.S.B.) and the National Institute of Allergy and Infectious Diseases (AI072265 to B.S.B.; T32AI083216 to J.A.O.).
B.S.B. has current or recent consulting or scientific advisory board arrangements with or has received honoraria from, Sanofi-Aventis, TEVA, GlaxoSmithKline, AstraZeneca and Allakos, and owns stock in Allakos and Glycomimetics. He receives publication-related royalty payments from Elsevier and UpToDate™ and is a co-inventor on existing Siglec-8-related patents and thus may be entitled to a share of royalties received by Johns Hopkins University on the potential sales of such products. B.S.B. is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy.
Abbreviations
- BAL
bronchoalveolar lavage
- BMDE
bone marrow-derived eosinophil
- CCR3
C-C motif chemokine receptor 3
- DAPI
4′,6-diamidino-2-phenylindole
- eoCre mice
Mice that express Cre recombinase under the control of the eosinophil peroxidase, (EPX) promoter
- EoP
eosinophil progenitors
- EoPre
eosinophil pre-cursors
- EPX
eosinophil peroxidase
- HBSS
Hank’s balanced salt solution
- LSL
loxP-flanked STOP cassette
- mAb
monoclonal antibody
- OVA
chicken egg ovalbumin
- Siglec
sialic acid-binding immunoglobulin-like lectin
- SIGLEC8Eo
Rosa26-STOP-SIGLEC8 crossed with the eoCre mouse
- SIGLEC8LSL
Rosa26-STOP-SIGLEC8 knock-in
Footnotes
Authorship
Conception and design: JAO, YW, JJL, ZZ, BSB; performed experiments: JAO, YW, DJC, LMV, YC, FZ, ZZ; analysis and interpretation: JAO, YW, DJC, LMV, ZZ, BSB; drafting the manuscript and contribution of important intellectual content: JAO, JJL, ZZ, BSB.
Conflict of Interest Disclosure
The terms of this arrangement are being managed by the Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies. The authors have no additional competing financial interests.
References
- 1.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–66. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol. 2015;135:598–608. doi: 10.1016/j.jaci.2014.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’Alessio K J, Holmes SD, Abrahamson JA, Erickson-Miller CL, Murdock PR, Tachimoto H, Schleimer RP, White JR. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105:1093–100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 4.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, Crocker PR. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–6. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 5.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–24. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson SA, Bovin N, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and pro-apoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis X. J Pharmacol Exp Ther. 2009;330:608–612. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochner BS. “Siglec”ting the allergic response for therapeutic targeting. Glycobiology. 2016;26:546–52. doi: 10.1093/glycob/cww024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327–36. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson SA, Herrmann H, Du J, Cox P, Haddadel B, Butler B, Crocker PR, Ackerman SJ, Valent P, Bochner BS. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil Siglec-8 expression. J Clin Immunol. 2011;31:1045–53. doi: 10.1007/s10875-011-9589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci U S A. 2004;101:13251–6. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–35. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 12.Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, Bochner BS. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS One. 2013;8:e68143. doi: 10.1371/journal.pone.0068143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, Zhu Z, Tiemeyer M, Bochner BS. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135:1329–40. doi: 10.1016/j.jaci.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohenstein P, Slight J, Ozdemir DD, Burn SF, Berry R, Hastie ND. High-efficiency Rosa26 knock-in vector construction for Cre-regulated overexpression and RNAi. Pathogenetics. 2008;1:3. doi: 10.1186/1755-8417-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle AD, Jacobsen EA, Ochkur SI, Willetts L, Shim K, Neely J, Kloeber J, Lesuer WE, Pero RS, Lacy P, Moqbel R, Lee NA, Lee JJ. Homologous recombination into the eosinophil peroxidase locus generates a strain of mice expressing Cre recombinase exclusively in eosinophils. J Leukoc Biol. 2013;94:17–24. doi: 10.1189/jlb.0213089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–9. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–30. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gicheva N, Macauley MS, Arlian BM, Paulson JC, Kawasaki N. Siglec-F is a novel intestinal M cell marker. Biochem Biophys Res Commun. 2016;479:1–4. doi: 10.1016/j.bbrc.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, Berdnikovs S, Bryce PJ. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J Immunol. 2016;197:3445–3453. doi: 10.4049/jimmunol.1600611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Sullivan JA, Carroll DJ, Cao Y, Salicru AN, Bochner BS. Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Sullivan JA, Carroll DJ, Cao Y, Bochner BS. Mechanisms of Siglec-8 endocytosis, reactive oxygen species (ROS) production and apoptosis by ligand binding in primary human eosinophils; Proceedings of the 31th Symposium of the Collegium Internationale Allergologicum. Pacini Editore Medicina; Pisa, Italy. In press. [Google Scholar]
- 23.Carroll DJ, O’Sullivan JA, Nix DB, Cao Y, Tiemeyer M, Bochner BS. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS, Kern RC, Schleimer RP, Schnaar RL. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 2015;135:799–810 e7. doi: 10.1016/j.jaci.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H, Gonzalez-Gil A, Wei Y, Fernandes SM, Porell RN, Vajn K, Paulson JC, Nycholat CM, Schnaar RL. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology. 2017;27:657–668. doi: 10.1093/glycob/cwx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patnode ML, Cheng CW, Chou CC, Singer MS, Elin MS, Uchimura K, Crocker PR, Khoo KH, Rosen SD. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J Biol Chem. 2013;288:26533–45. doi: 10.1074/jbc.M113.485409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Propster JM, Yang F, Rabbani S, Ernst B, Allain FH, Schubert M. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci U S A. 2016;113:E4170–9. doi: 10.1073/pnas.1602214113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, Doherty TA, Varki A, Broide DH. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–7. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, Bochner BS. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS One. 2013;8:e68143. doi: 10.1371/journal.pone.0068143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–63. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–41. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009;131:157–69. doi: 10.1016/j.clim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiwamoto T, Brummet ME, Wu F, Motari MG, Smith DF, Schnaar RL, Zhu Z, Bochner BS. Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J Allergy Clin Immunol. 2014;133:240–7. e1–3. doi: 10.1016/j.jaci.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzukawa M, Miller M, Rosenthal P, Cho JY, Doherty TA, Varki A, Broide D. Sialyltransferase ST3Gal-III regulates Siglec-F ligand formation and eosinophilic lung inflammation in mice. J Immunol. 2013;190:5939–48. doi: 10.4049/jimmunol.1203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


