Abstract
Eosinophils play homeostatic roles in different tissues and are found in several organs at a homeostatic baseline, though their tissue numbers increase significantly in development and disease. The morphological, phenotypical, and functional plasticity of recruited eosinophils are influenced by the dynamic tissue microenvironment changes between homeostatic, morphogenetic, and disease states. Activity of the epithelial-mesenchymal interface, extracellular matrix, hormonal inputs, metabolic state of the environment, as well as epithelial and mesenchymal-derived innate cytokines and growth factors all have the potential to regulate the attraction, retention, in situ hematopoiesis, phenotype, and function of eosinophils. This review examines the reciprocal relationship between eosinophils and such tissue factors, specifically addressing: 1) tissue microenvironments associated with the presence and activity of eosinophils; 2) non-immune tissue ligands regulatory for eosinophil accumulation, hematopoiesis, phenotype, and function (with an emphasis on the extracellular matrix and epithelial-mesenchymal interface); 3) the contribution of eosinophils to regulating tissue biology; 4) eosinophil phenotypic heterogeneity in different tissue microenvironments, classifying eosinophils as progenitors, steady state eosinophils, and Type 1 and 2 activated phenotypes. An appreciation of eosinophil regulation by non-immune tissue factors is necessary for completing the picture of eosinophil immune activation and understanding the functional contribution of these cells to development, homeostasis, and disease.
Keywords: eosinophils, epithelium, mesenchyme, extracellular matrix, phenotype, in situ hematopoiesis, development, homeostasis, asthma, metabolism
Eosinophil recruitment is a hallmark of allergic inflammation, thought to contribute to both the initiation and propagation of tissue damage and remodeling during allergic inflammation. However, it is important to realize that in return, changes in the tissue microenvironment itself may elicit recruitment as well as functional and phenotypic change in immune cells as part of the mechanism to return to tissue homeostatic condition. In agreement with this concept of immune cells as responders to changing tissue environments, studies employing eosinophil-deficient models now demonstrate that eosinophils have multiple novel roles in homeostasis. Eosinophils are found in several organs at normal baseline, assisting in normal tissue processes, and are also recruited to other sites during morphogenesis and repair. Recent studies demonstrate the existence of eosinophil subsets and plasticity in different tissue contexts, reinforcing the significance of change in the eosinophil’s immediate environment. Tissue remodeling events during normal morphogenesis and pathogenic remodeling in disease are remarkably similar, which may provide a key to understanding the fundamental nature of tissue programs necessitating eosinophils. Epithelial-derived cytokines and chemokines, increased epithelial shedding and turnover, the extracellular matrix, multiple mucosal ligands such as mucins and sialic acids, mesenchymal stem cell activity, and the metabolic state of the environment are all implicated in the regulation of the attraction, retention, in situ hematopoiesis, and function of eosinophils. This review will concisely summarize the current understanding of the two-way communication between tissue and eosinophils, discussing tissue ligands in the framework of biological processes occurring in different tissue states. This review will be divided into four parts: 1) tissue processes that eosinophils associate with in development, homeostasis, and disease; 2) tissue ligands with the potential to engage eosinophils; 3) the potential return contribution of eosinophils in shaping their tissue microenvironments; 4) tissue-based heterogeneity of eosinophil phenotypes and morphology.
Tissue microenvironments associated with increased eosinophil presence and activity
It is now being increasingly recognized that eosinophils populate various tissues in normal development, homeostasis, and disease and that they can occur in both immune and non-immune contexts. Moreover, the same organ may experience influxes of eosinophils in different tissue states, such as development, normal injury repair, or chronic disease. In most homeostatic adult tissues, the numbers of resident eosinophils range from very limited to non-existent. For instance, resident eosinophils in the healthy adult mouse lung average 1.5% of the total CD45+ hematopoietic cell population (1, 2), although the turnover rate and functional significance of such resident populations is not well understood. Eosinophil numbers increase significantly in the following types of tissue microenvironments: 1) at homeostatic sites where epithelial cell turnover and stem cell activity are high (small intestine, endometrial lining of the uterus, bone marrow, thymus); 2) during developmental/morphogenetic events (ductal differentiation of mammary glands (3), development of Peyer’s patches (4), postnatal lung development (5), beige fat biogenesis (6–8); 3) during processes of normal injury repair; 4) in diseases involving extensive tissue remodeling (helminthic parasite infections, acute lung injury (oxygen stress), fibrosis, cancers, allergic diseases of the gut, skin, and airway, and non-atopic diseases with a significant remodeling component (eosinophilic esophagitis, chronic rhinosinusitis, AERD)) and 5) endocrinopathies. For details of eosinophil activity in each of these particular scenarios, we would like to refer you to the excellent reviews by James Lee et al. (9, 10), Melo et al. (11), and Rosenberg et al. (12). Instead, we will focus on reviewing fundamental features of the tissue microenvironment common to all these situations and summarize the representative features of tissue physiological states associated with eosinophil presence and activity.
When comparing the different situations in which eosinophils are found, it becomes clear that the processes engaging eosinophils in health and disease are morphogenesis and regeneration, stem cell activity, and changes in the tissue metabolic state. One has to recognize that these three processes are intricately intertwined, as stem cells give rise to differentiated cells and metabolic adjustments are necessary to provide sufficient energy for regeneration processes. As initially postulated by James Lee in the LIAR hypothesis (9), eosinophils are intrinsically homeostatic cells that are regulators of Local Immunity And/or Remodeling/Repair in both health and disease, generally associated with sites characterized by bursts of cell proliferation and death. This notion fits well with an emerging view of Type 2 immunity having evolved as a restorative response to assist in tissue repair and remodeling, an arm of immune response with which eosinophils are associated (13, 14). For example, Huang et al. (15) provide supporting evidence suggesting that Th2 immune responses and homeostatic eosinophil activity may have evolved not to expel parasites but rather to limit inflammation, control tissue glucose uptake, and minimize potential damage to the host (15). Here, we will provide an in-depth view of these processes, focusing on overarching mechanisms associating with eosinophils in all types of the environment.
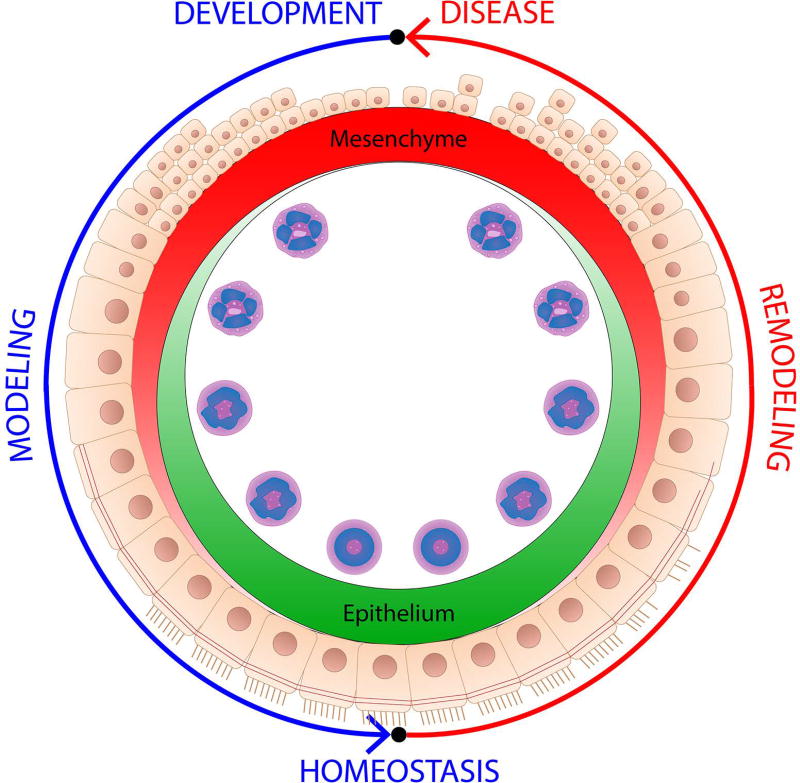
Upon careful review, the establishment of epithelial-mesenchymal communication emerges as one of the central themes in tissue morphogenesis associated with eosinophils. It also becomes remarkably apparent that the association between the epithelial-mesenchymal interface and eosinophils is conserved between normal morphogenesis (modeling) and disease (remodeling) (Figure 1), involving the same key players (activity of mesenchymal cells and growth factors, modification of the ECM) and an identical set of developmental pathways (WNT, Notch, Hippo, Smoothened, Hedgehog) responsible for epithelial patterning. For example, normal mammary gland development has been described as wound-like in both mice and humans (16, 17). Similarly, morphogenesis of the lung continues during postnatal development, characterized by the deposition of the provisional extracellular matrix, increased mesenchymal-epithelial communication, differentiation of mature stratified epithelium (18, 19), as well as processes associated with the loss of epithelial differentiation in chronic allergic disease (20, 21). The only apparent difference between normal and pathological states of the tissue undergoing remodeling is in heterochrony: timing of the onset, offset, and persistence of these signals. In normal development and injury repair, homeostatic modeling programs are perfectly timed, while in disease they are perpetuated, perhaps associated with a continuous attempt to return tissue to a homeostatic state.
Figure 1.
Eosinophils associate with transient epithelial differentiation (modeling) events in development and persistent (remodeling) loss of differentiation in allergic disease. The two processes are analogous. The only apparent difference between the normal and pathological states of tissue undergoing remodeling is in heterochrony: the timing of onset, offset, and persistence of these signals. In normal development and injury repair, homeostatic modeling programs are perfectly timed while in disease they are perpetuated, perhaps associated with a continuous attempt to return the tissue to its homeostatic state. Changes in eosinophil phenotype typically associate with morphogenetic changes in tissue environment.
In allergic disease, the notion of eosinophils as direct responders to changing states of the tissue environment is rarely exercised. In the subsequent sections of this review, we will address the two-way communication between eosinophils and tissue in homeostasis, development, and disease, with a focus on the tissue ligands acting on eosinophils as well as eosinophil products with an impact on tissue biological processes. Acknowledging this communication is a significant step towards understanding how tissue microenvironments shape eosinophil identity and function in disease and how these cells could be targeted therapeutically in highly heterogeneous allergic disease scenarios.
Tissue ligands regulatory for eosinophil accumulation, phenotype, and function
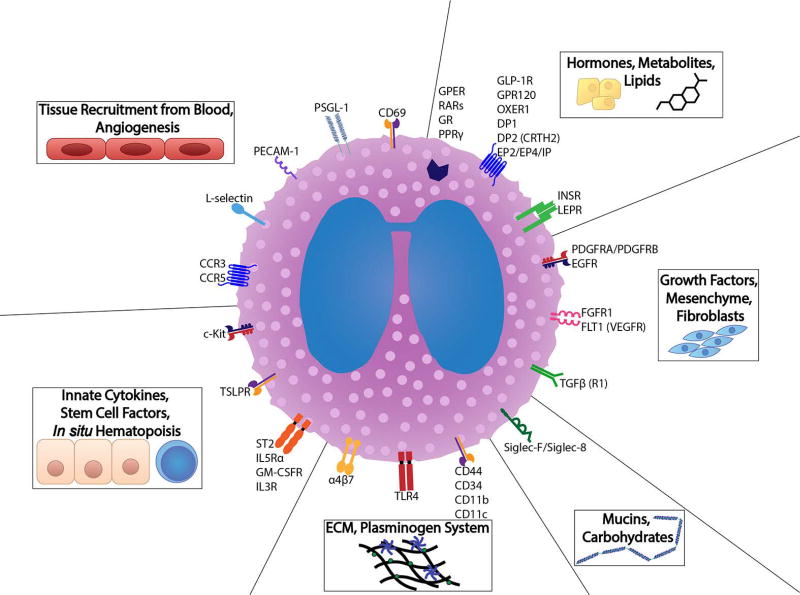
The coordinated behavior of multiple processes is necessary to achieve tissue homeostasis, such as well-tuned epithelial-mesenchymal communication, the controlled deposition of extracellular matrix, the differentiation of specialized cells, proteoglycan, aminoglycan, and mucin synthesis, proper regulatory input of hormones and metabolites, and balanced activity of the innate immune system. Here, we will review key tissue players and ligands that both represent these processes and are known to elicit an eosinophil response (summarized in Figures 2 and 3).
Figure 2.
Eosinophils are well equipped to interact with all components of their tissue microenvironment. Representative receptors are illustrated that correspond to the diverse tissue ligands and cellular processes encountered both by mature and immature eosinophils in the contexts of development, homeostasis, and disease.
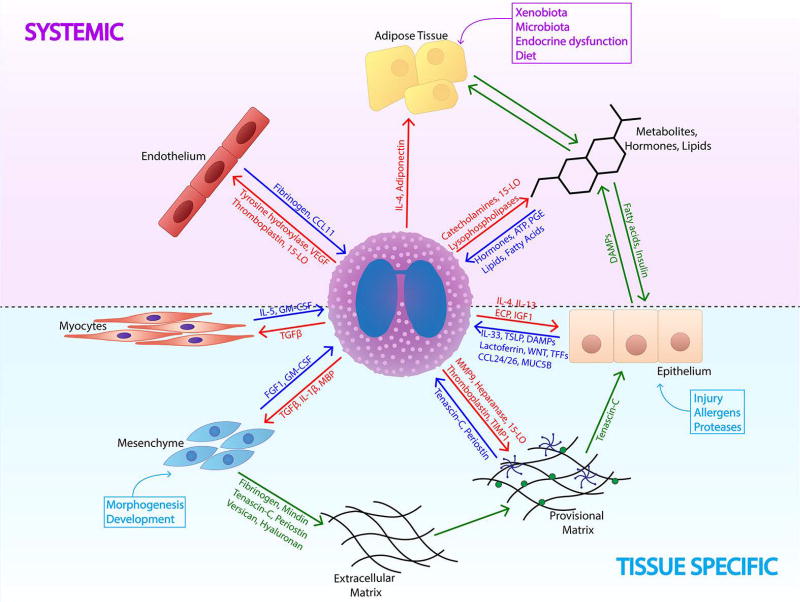
Figure 3.
Eosinophil in the context of its immediate non-immune tissue microenvironment. Eosinophils are both affected by and affect their tissue environments. Eosinophil-tissue interactions are governed both on the systemic level by changes in nutrient availability, hormones, lipids, and overall metabolism (purple) and by tissue-specific processes, such as activation of the epithelial-mesenchymal interface and ECM deposition (light blue). During such activation of tissue programs, eosinophils receive multiple tissue inputs such as alarmins, DAMPs, ECM molecules, lipids, hormones, and metabolites (dark blue arrows). They respond to these cues by releasing products (red arrows) in an effort to return the tissue to homeostatic conditions. Balanced communication of different tissue components and systemic factors (green arrows) is necessary to maintain peripheral tissue homeostasis.
Epithelial-mesenchymal interface
The LIAR hypothesis postulates that eosinophils are attracted to sites of high stem cell activity that accompany all tissue generation and regeneration events. The epithelial-mesenchymal transition (EMT) has recently been implicated in multiple allergic diseases as a mechanism causative of mucosal barrier disruption (20, 22, 23). In homeostatic conditions, EMT occurs as a mechanism of maintaining epithelial barrier integrity by potentiating the mesenchymal unit to create new layers of differentiated epithelial cells (18). At sites such as the intestine, epithelial shedding rates are high and mesenchymal-epithelial turnover is much higher. Interestingly, eosinophils frequent such sites in the small intestine and colon while being much less frequently found in organs such as the lung, where epithelial turnover is much slower. Chemokines, growth factors, and secreted ECM proteoglycans and morphogenetic ligands are all mesenchymal-secreted ligands that can engage eosinophils. Fibroblasts are frequently seen as central to eosinophil recruitment as they can be a prominent source of eotaxins and growth factors (24, 25). Activation of the Wnt (26), Hippo (27), Notch/Jagged (28) and Hedgehog (29) developmental pathways is necessary for epithelial development and patterning and is strongly associated with epithelial remodeling. Importantly, EMT-related morphogenetic ligands such as WNTs and Hedgehog proteins can be secreted by remodeling tissue and can directly engage eosinophils and other immune cells (29–31). Moreover, the mesenchymal compartment and myofibroblasts in particular have the ability to secrete multiple provisional ECM proteins with multiple regulatory effects on immune cells (32–34).
Extracellular matrix
The ECM is an intricate network of macromolecules that forms the 'scaffolding' of the airways and other tissues. This scaffolding not only acts as a mechanical support that plays a crucial role in the maintenance of airway function and structure but is also a dynamic and complex signaling network that has the potential to regulate the function of multiple structural and immune cells, including migration, proliferation, and differentiation via integrin and toll-like receptor engagement (35). The ECM is critical for guiding development and repair at the mesenchymal-epithelial interface (36). ECM composition is not uniform along the epithelial barrier; it is a dynamic structure exhibiting spatio-temporal heterogeneity depending on the needs of the surrounding tissue. The ECM changes dramatically during development and disease (37, 38). The transient deposition of the provisional, “immature” matrix, characterized by the secretion of proteoglycans such as tenascins, periostin, mindin, hyaluronan, and versican (39, 40), is critical for epithelial differentiation (41–43). This is exemplified by the unique deposition of Tenascin-C during development in areas undergoing active epithelial differentiation (41, 44, 45). Eosinophil numbers in the asthmatic airways correlate with ECM markers such as periostin and the thickening of the reticular basement membrane (RBM), characterized by the deposition of specific ECM proteins (46–48). The basement membrane thickness can range from 7 to 23 µm in subjects who have asthma, while it is only 4 to 5 µm in healthy individuals (49). Airway eosinophils in asthma exhibit a hyperadhesive phenotype towards provisional ECM and are well equipped to interact with its components via expression of specific integrins (CD11c, CD11b, beta 5 integrins) and TLRs (TLR4, in particular) (50–52). An interesting implication of the eosinophil-ECM association in disease is that an EMT-perpetuated imbalance between ECM component synthesis and degradation may represent a persistent tissue-based driver of eosinophilia. This idea remains to be tested. Another interesting implication of changing ECM dynamics in development and disease is a potential regulatory role for eosinophil bone marrow or in situ hematopoiesis. For example, the extracellular matrix establishes hematopoietic gradients in the stromal environment of murine bone marrow and is critical for sustaining hematopoiesis (53). Mice lacking TNC show reduced colony-forming capacity and hematopoietic cell production (54). Hyaluronic acid scaffolds are sufficient to maintain long-term cultures of CD34+ hematopoietic cells obtained from human cord blood (55). Periostin is highly expressed, specifically in fibroblasts that support hematopoiesis (56).
Mucosal-derived factors
The trafficking of eosinophils to mucosal tissue during homeostasis and disease is regulated by CCL11 and Th2 cytokines. Epithelial-derived eotaxins are fundamental signals that regulate eosinophil homing to the GI tract in mice (57). CCL11 is required for the baseline level of tissue eosinophils (58). Mice deficient in CCR3 or CCL11 have defective tissue homing of eosinophils to the lamina propria of the GI tract (59). In addition to CCL11, the Th2 cytokines IL-5 and IL-13 are also critical in sustaining the GI trafficking of eosinophils during homeostasis (60). Eosinophils are also regulated by the epithelial-derived innate cytokines TSLP and IL-33 that both directly activate eosinophils and promote their recruitment via Th2 response amplification. TSLP is an IL-2 family member that primes Th2 responses via the activation of dendritic cells (DCs) (61) and basophils (62). IL-33 is an IL-1 cytokine family member present in the nucleus of structural cells such as fibroblasts, epithelial cells, and endothelial cells and is released during inflammation and cellular distress (63). TSLP prevents the apoptosis of eosinophils by direct activation of the TSLPR present on eosinophils (64). Stolarski et al. (65) showed that IL-33/ST2 signaling activates murine airway eosinophils, and other work showed that the IL-33 stimulation of eosinophils induces marked gene expression and the release of chemokines and cytokines such as IL-4 and IL-13 (66, 67). IL-33 also increases the activity and survival of human eosinophils (68) and, in a murine adoptive transfer model system, provides a survival advantage that allows for greater pulmonary trafficking (69). Type 2 lymphoid cells (ILC2) control the local accumulation of mature eosinophils in peripheral tissues at baseline (70) and development (5). Among other mucosal factors, the glycoprotein lactoferrin secreted by glandular epithelial cells stimulates eosinophil activation (71). Surfactant protein SP-D (a C-type lectin) is a limiting factor for eosinophils in the airway (72). Similarly, the engagement of another lectin, Siglec-F in mice and Siglec-8 in humans, by engaging corresponding ligands (such as 6’-sulfo-sialyl Lewis X glycoproteins for both, and glycans on Muc5b for Siglec-F) activates a pro-apoptotic response, especially for human eosinophils (73, 74).
Metabolism and hormones
Local tissue processes are also governed by local and system-level changes in metabolism (conceptualized in Figure 3). There is mounting evidence that eosinophils play a crucial role in metabolic homeostasis and also respond to changes in metabolism. In mouse models of high-fat diet-induced obesity, eosinophils promote insulin sensitivity and are associated with better glucose responses, more lean body mass, and higher energy expenditures (75, 76). Wu et al. (75) first linked the recruitment of eosinophils to the beneficial process of fat beiging and the control of metabolism and glucose homeostasis. The authors showed that eosinophils are the main source of interleukin (IL)-4 in white adipose tissue (WAT), which was necessary for the induction of alternatively activated macrophages (AAMs). Eosinophils were also found to associate with insulin resistance and β-cell dysfunction in pre-diabetic subjects (77), as well as corticoadrenal insufficiency (78). Eosinophils are linked to the development of Type I diabetes and express high levels of myeloid alpha-defensins and myeloperoxidase in patients with type 1 diabetes mellitus (79). Remarkably, eosinophils express multiple hormone receptors, implicating that their activity in peripheral tissues may be subject to systemic-level regulation by the endocrine system. Human tissue eosinophils express leptin surface receptors, and leptin delays the apoptosis of mature eosinophils in vitro (80). Similarly, the estrogen receptor (GPER) is expressed on human eosinophils, which also inhibits eosinophil apoptosis (81). Human eosinophils also express retinoic acid receptors (RARs); retinoic acids are potent inhibitors of human eosinophil apoptosis (82) and upregulate eosinophil expression of CCR3 (83). Moreover, human eosinophils have been shown to express multiple prostaglandin receptors (84) and GPR120, a G protein-coupled receptor for long-chain fatty acids (85). The stimulation of eosinophils with a synthetic GPR120 agonist led to the increased expression of IL-4 and the inhibition of apoptosis. These findings suggest that eosinophils might function as nutrient sensors (85). Finally, there is a strong link between eosinophils and the arachidonic acid metabolism. 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE), a 5-lipoxygenase product, has powerful eosinophil chemotactic activity (86, 87). In addition, 5-oxo-ETE can induce the expression and secretion of matrix-metallopeptidase-9 and urokinase-type plasminogen activator receptor (88, 89), which results in the degradation of matrix components. Therefore, 5-oxo-ETE also promotes the infiltration of eosinophils into tissues. In turn, eosinophils residing in the colonic mucosa have been shown to mediate inflammation resolution by expressing 12/15 lipoxygenase products (resolvins and protectins) in experimental models of colitis and self-resolving acute peritonitis (90, 91).
What do eosinophils contribute back to their tissue environments?
As illustrated above, there are multiple tissue and metabolic factors that are known to modulate eosinophil phenotype and function. However, it is much less clear which processes and morphogenetic events depend on eosinophil presence in tissues, in part due to the complexity of in vivo environments and redundant contribution (frequently compensatory) by multiple cell types. In general, eosinophils are thought of as contributors to allergic inflammatory processes and tissue remodeling/fibrosis in asthma (92–96).
The development of constitutive eosinophil knockout mice (PHIL) and inducible eosinophil knockout mice (iPHIL) by Jamie Lee labs allowed us to better understand the tissue roles of eosinophils in development and disease, which in many cases produced surprising results. Contrary to the view of eosinophils as destructive effector cells, eosinophils were found to be necessary for the resolution of inflammation, tissue repair, and the return of lung tissue to homeostasis in a chronic model of asthma, as PHIL mice showed a lack of resolution (97). Interestingly, despite the strong association with the postnatal lung and mammary gland development, eosinophil-deficient mice developed normally with fully functional lungs and mammary ducts and exhibited an overall lack of the developmental phenotype (98). This is not entirely surprising, as many molecules involved in postnatal development (as opposed to embryonic) do not exhibit developmental phenotypes in knockout mice, among them important factors such as IL-33 and Tenascin-C (5, 99). However, by contrasting the gene expression profiles of PHIL mice with wild type controls, we were able to identify a more subtle phenotype, which consisted of the downregulation of mesenchymal genes (Nes, Smo, Vim) and changes in ECM genes (Spon2, Adam33) not obvious from a histological examination (unpublished observation). We hypothesize that a lack of eosinophils alters tissue ECM architecture and mesenchymal responses, which may lead to aberrant repair and resolution responses when tissue homeostasis is disrupted.
Eosinophils are well equipped to modify their immediate tissue environment. Eosinophils were originally shown to be active players in fibrosis by their capacity to store and release the most potent pro-fibrogenic factor transforming growth factor (TGFβ) (100, 101). TGFβ is generally considered anti-inflammatory; its levels are increased (33) in the bronchoalveolar lavage of patients with allergic asthma and correlates well with airway and parenchymal remodeling (102). When added to fibroblasts in vitro, eosinophils stimulate fibroblast proliferation, ECM synthesis, and lattice contraction mostly by the release of TGFβ and IL-1β in vitro (96, 103). Both in vitro and in vivo, eosinophils have been shown to promote airway remodeling by inducing myofibroblast differentiation and ECM deposition (50). Eosinophils can modulate myofibroblasts by the release of growth factors such as fibroblast growth factor (FGF2), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF) (93). VEGF expression has been demonstrated in asthmatic airways where, in addition to angiogenesis, it is thought to have a role in airway remodeling (104). TGFβ and eosinophils dramatically induce Tenascin-C mRNA and protein expression in nasal epithelial cells. The effect of eosinophils could be inhibited partly by a neutralizing antibody to TGFβ (105). Eosinophils store and release MMP-9, which is important for ECM modification and migration through the basement membrane (106). In fact, the main source of MMP-9 in asthmatic airways is believed to be eosinophils (107). Tenascin-C induces eosinophil MMP-9 expression directly and by collaboration with TGFβ (108). Many of the effects of IL-13 may be mediated by the metalloproteinases. IL-13 overexpression is mediated by TGFβ, and TGFβ activation is MMP-9 dependent. It is possible, therefore, that MMP-9 could be a key molecule proximal to the IL-13-mediated signaling pathway (109). In addition to classical pro-fibrotic factors, some eosinophil cationic proteins were found to be involved in fibrosis (110). Eosinophil cationic protein (ECP) stimulates TGFβ release by human lung fibroblasts (111). Eosinophil major basic protein (MBP) interacts in a synergistic fashion with rIL-1-α or TGFβ to augment fibroblast IL-6-type cytokine production (112). MBP can also activate the synthesis of remodeling factors by airway epithelial cells (113). Moreover, eosinophils contain heparanase, a pivotal enzyme for ECM degradation, and eosinophil MBP was identified as heparanase-inhibiting protein (114). TGFβ release by eosinophils plays another important role in asthma, as TGFβ1 has been shown to induce airway smooth muscle (ASM) cell growth and is a key mediator involved in the tissue remodeling of the asthmatic lung (115). ASM cells also have an effect on eosinophils by stimulating eosinophil differentiation. Both IL-5 and GM-CSF are suggested as the key factors produced by ASM cells that promote this process (116).
Eosinophils exhibit a remarkable functional association with the tissue plasminogen system. CCL11 was shown to promote eosinophil transmigration specifically via the activation of the plasminogen-plasmin system (117). A new study by Uderhardt et al. (118) shows that eosinophils contribute to intravascular thrombosis by exhibiting a strong endogenous thrombin-generation capacity. It relies on enzymatic generation and active provision of a procoagulant phospholipid surface enriched in 12/15-LO-derived hydroxyeicosatetraenoic acid-phosphatidylethanolamines (118). Eosinophils were also described to accumulate in human thrombi (119) and reported to serve as a major source of Tissue Factor (thromboplastin) (120). Importantly, the plasminogen system not only controls blood clotting but also is involved in epithelial remodeling. Hara et al. (121) showed that eosinophils play an inhibitory role on cell surface plasmin generation by bronchial epithelial cells by means of the up-regulation of PAI-1 expression induced by TGFβ. Therefore, the accumulation of eosinophils in bronchial walls may directly promote fibrin deposition and bronchial tissue repair/remodeling in asthma through this protease network (121).
Eosinophils directly interact with epithelial cells as well. Eosinophil-epithelial interactions significantly stimulate the secretion of MUC5AC, PDGF-AB, VEGF, TGF-β1, and IL-8 from cultured NCI-H292 epithelial cells (122). Neutralizing antibodies directed against amphiregulin, as well as pan-metalloproteinase inhibitor GM6001 inhibited the coculture-induced secretion of MUC5AC (122). Human eosinophils produce and release amphiregulin, which is typical for many Type 2 immune cells interacting with the epithelium (123).
Eosinophils are emerging as potentially critical regulators of metabolic homeostasis (85, 124–127). In murine models, eosinophils present in visceral adipose tissue secrete IL-4 (75) that causes subcutaneous white adipose tissue macrophages to polarize toward the alternatively activated phenotype (128). Eosinophils also promote catecholamine production via tyrosine hydroxylase (6), which causes the development of beige fat that ameliorates obesity-induced metabolic changes. Human eosinophils are an abundant source of 15-Lypoxygenase (129). Very recently, Withers et al. (130) identified eosinophils as a novel source of tissue adiponectin. They conclude that eosinophils play a key role in the regulation of the normal function of vasculature and perivascular adipose tissue.
Overall, eosinophil regulatory roles in the tissue appear to center on promoting the tissue-restorative activity of mesenchymal cells, facilitating ECM scaffold deposition (and possibly, subsequent removal) and maintenance of tissue and systemic metabolic homeostasis. Eosinophils may also directly promote epithelial differentiation via the release of amphiregulin and growth factors, as well as by improving insulin sensitivity necessary for the proper guidance of epithelial differentiation.
Shaping eosinophil phenotype and morphology in heterogeneous tissue contexts
Surprisingly, unlike most other immune system cell types, eosinophils are frequently described as terminally differentiated cells uniform in phenotype and function. However, this view of the eosinophil is rapidly changing. Multiple marker-, morphology-, and function-based eosinophil subtypes are now being identified in various immune and non-immune contexts. Upon review of the literature, it is becoming apparent that a few unifying themes determine eosinophil tissue phenotype: (1) Maturation. Eosinophils can be found in many organs and tissue contexts in different states along their differentiation continuum. There is plenty of evidence that eosinophils can traffic to tissues in immature states or even develop locally in the tissue via in situ hematopoiesis (131); (2) Organ location. For example, in steady state, intestinal resident eosinophils are phenotypically different from resident lung eosinophils (132–134); (3) Morphogenetic activity of the tissue. Regardless of organ location, eosinophils are shaped by the morphogenetic plasticity of their environment. The lung perfectly exemplifies this scenario. The lung is characterized by on-going airway morphogenesis during postnatal development, then achieves steady state in the adult homeostatic state, and undergoes active remodeling reminiscent of development in chronic allergic asthma. Eosinophils are found in very low numbers at steady state but those numbers significantly increase during development and disease. Moreover, developmental and disease eosinophils are phenotypically distinct from true resident cells in homeostasis (1, 2). Analogous to this, the homeostatic intestine undergoes active epithelial turnover, which also happens at a much slower rate in the steady state lung. In support of a tissue state-driven phenotype, intestinal resident eosinophils constitutively express CD11c, Ly6G, and CD44, which are only expressed in subsets of lung eosinophils during development and epithelial remodeling (132); (4) Location within tissue. In allergic murine models, eosinophils frequently exhibit bimodal and compartment-specific phenotypes (135). We previously demonstrated that upon their transition to the airway, eosinophils upregulate levels of Siglec-F and CD11c, which is not typical of interstitial eosinophils (1). Such phenotypic transition is also associated with changes in eosinophil morphology, where vacuolarization and high segmentation of the nucleus are typical of airway luminal eosinophils (1); (5) Immune tissue microenvironment. Immune drivers of the eosinophil phenotype are reviewed elsewhere. In general, Type 2 immunity aligns well with epithelial morphogenesis and repair/resolution, while Type 1 immune activation is characteristic of defense responses not involving extensive epithelial remodeling.
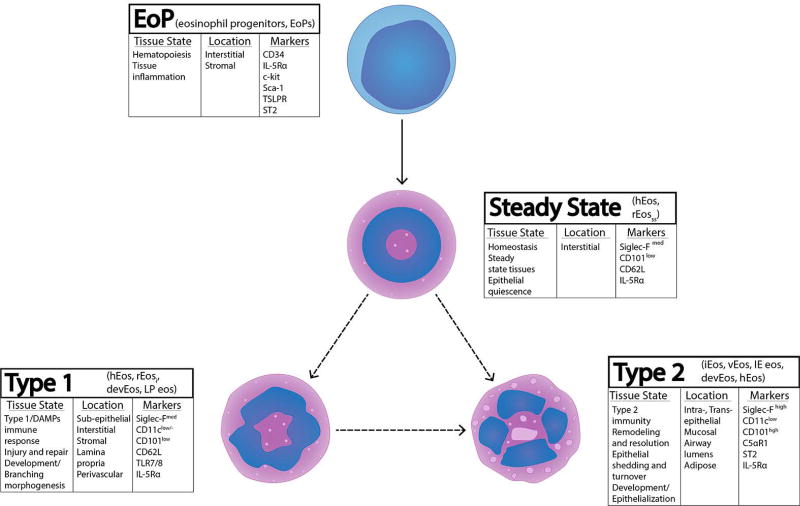
Along with the discovery of heterogeneous phenotypes and morphology in various biological scenarios, eosinophil classification terminology used in publications and conference talks is also becoming more complex. On one hand, multiple terms are given to eosinophil sub-phenotypes found in similar functional contexts. On the other hand, homeostatic eosinophils that could be described with an over-arching term (for instance, hEos) are represented by distinct homeostatic sub-phenotypes, depending on the tissue and functional process context. For example, the term “resident eosinophils” (rEos) could be referring to an eosinophil exhibiting one phenotype in the steady state lung but a completely different phenotype in the homeostatic intestine where there is high epithelial turnover. Likewise, the term “developmental eosinophils” (devEos) could refer to an eosinophil present in the lung during postnatal development that is clearly homeostatic in nature, but the eosinophil could phenotypically resemble an inflammatory eosinophil (rEosi and iEos) that is very different from the true resident eosinophils at steady state. Here, for the ease of interpretation, we will succinctly review the phenotyping literature in the framework of a tissue-based classification of eosinophils, which should also align with the immune-based classification, as specific immune responses associate with specific tissue processes. We will describe murine eosinophil sub-phenotypes as falling within one of these four tissue-based categories: (1) EoP. Immature eosinophils recruited as precursors or undergoing in situ hematopoiesis; (2) Steady state. True tissue residents in morphogenetically quiescent tissues; (3) Type 1. Typically interstitial (stromal in general) in acute inflammatory, innate defense, and transient morphogenetic contexts; (4) Type 2. Eosinophils associated with a Type 2 immune response, typically found in epithelial contexts (Figure 4).
Figure 4.
Tissue-based classification of murine eosinophils. EoP: Eosinophil Progenitors - immature eosinophils or committed precursors undergoing in situ hematopoiesis. Steady State: true resident eosinophils in morphogenetically quiescent tissues, featuring non-segmented “donut-shape” nuclear morphology and eosin staining. Type 1: interstitial/stromal eosinophils found in transient morphogenetic contexts and during Type 1 immune activation, featuring segmented nuclear morphology but lacking vacuolarization. Type 2: eosinophils associated with the epithelium and Type 2 immune environment, characterized by highly segmented nuclei and the presence of vacuoles.
EoP eosinophils
A growing body of evidence suggests that in situ hematopoiesis is a conserved mechanism of the innate immune system that significantly contributes to the development of immunity at mucosal sites (131). Interestingly, eosinophil progenitors can be found alongside mature cells during allergic inflammation (128). In mice, eosinophil progenitors (EoP) are typically described as expressing CD34, IL-5Rα, and Siglec-F, with weaker eosin staining (136). Recently, it was shown that eosinophil precursors and progenitors express ST2 and TSLPR, the receptors for IL-33 and TSLP, which determine IL-5Rα expression and regulate eosinophil progenitor homing and hematopoietic decisions (136, 137). CD34 eosinophil precursors also express functional TLR4 with the potential to influence hematopoietic decisions (138). Importantly, TLR4 stimulation of human HSCs yields higher numbers of TSLPR+ cells in asthmatics compared with healthy subjects (137, 139). It is still unknown to what extent in situ hematopoiesis contributes to the generation of eosinophils at tissue sites as opposed to the recruitment of eosinophils from bone marrow and circulation.
Steady state eosinophils
Resident eosinophils in true steady state can be found only in the parenchyma of homeostatic adult lung mouse tissue. These cells are characterized by having very limited numbers in the lung (1–2% of all CD45+ hematopoietic cells in murine lung) and “donut-shape” non-segmented nuclear morphology (1, 2). Phenotypically, in mouse lungs, these cells express intermediate levels of Siglec-F (1), low levels of CD101 and CD62L, and lack expression of CD11c or any other marker typically associated with tissue-activated status (2). Though recently characterized in the lung, it is not yet characterized whether eosinophils of this phenotype can be found in other organs with little morphogenetic activity. Although intestinal, uterine, and skin eosinophils are also resident and homeostatic, their phenotype and morphology is more characteristic of Type 1 activated eosinophils. This could be due to the fact that portions of the GI tract and uterus are more active in homeostasis, constantly undergoing active epithelial shedding and self-renewal.
Type 1 eosinophils
These eosinophils feature markers of steady state eosinophils (CD101low, CD62L, Siglec-Fmed), but exhibit segmented nuclear morphology more typical of activated eosinophils, although they lack the distinct vacuolarization of Type 2 eosinophils (1). In homeostasis, this phenotype and morphology is typical for postnatal mouse lung development (unpublished observations) and postnatal mammary gland development (140). In development, these cells can be found in the stroma surrounding epithelial cells during branching morphogenesis events (140). In adult murine homeostatic tissues, eosinophils with this morphology can be found in the stroma of the uterus at estrus under the influence of estrogen-regulated eotaxin synthesis (141) and in the lamina propria of the jejunum (58). In models of allergic inflammation, Type 1 eosinophils are seen as a minor population of eosinophils (described as rEosi by Mesnil et al.) (2). In a kinetic murine model of asthma, we demonstrated that the expansion of Type 1 eosinophils coincides with an acute neutrophil influx (associated with Type I cytokine expression) into allergen-challenged lungs, is specific to the interstitium, and is gradually replaced by eosinophils acquiring a Type 2 phenotype and migrating to the airway lumens (1). Analogous to eosinophils, alveolar macrophages were recently found to exhibit a bimodal Siglec-Flow and Siglec-Fhigh phenotype (142). Specifically, Siglec-Flow monocyte-derived alveolar macrophages were found to drive fibrosis in bleomycin injury models (142). It remains to be determined whether Type 1 eosinophils also play a role in fibrosis and various cancers (143). Interestingly, a subset of CD11c− cells with Type 1-characteristic morphology in allergen-challenged mice expresses high levels of GR1 (144), which could indicate their more recent hematopoietic origin. It is yet to be determined whether Type 1 eosinophils are generated from steady state cells or HSC precursors, or whether these cells are recruited in this state from circulation.
Type 2 eosinophils
The best distinguishing characteristic of these cells in homeostasis (lung, thymus, Peyer’s patches) and mouse models of disease (asthma, chronic colitis) is the high expression of Siglec-F (1, 145, 146) and a very distinct morphology with highly segmented nuclei and presence of vacuoles (1). These cells are also characterized by the acquisition of CD11c (1), higher expression of CD101, and lack of CD62L (2). Type 2 eosinophils closely associate with the epithelium, are found on the luminal surface of the airway and intestine, and can be readily extracted from bronchoalveolar lavages in mouse models (1). Cells with this phenotype can also be found in lung lavages during postnatal development (our unpublished observations).
Different eosinophil subsets may co-exist and perform different functions in the same tissue. For example, although speculative at this moment, based on their location and association with immune and morphogenetic environments, Type 1 eosinophils may preferentially interact with fibroblasts and assist in building ECM scaffolds, while Type 2 eosinophils may directly interact with the epithelium and participate in scaffold removal and resolution of repair. Future studies employing single cell sequencing approaches and careful functional validation in relevant biological contexts will be needed to resolve origins and functions of different eosinophil subsets.
Targeting eosinophils in allergic disease
In situ proliferation of eosinophils or the recruitment of these cells to sites of inflammation is the target of several biologics (Benralizumab, Mepolizumab, Reslizumab). These biologics target either IL-5 or IL-5Rα, with the intent of inhibiting or ablating eosinophil survival and proliferation. Although these biologics successfully reduce blood and sputum eosinophil levels, the subtype of eosinophils targeted remains undefined and may be limited. For example, several studies in mice suggest that although IL-5 is highly contributory to eosinophil development, it is not critical for their tissue expansion or survival. Mesnil and colleagues (2) demonstrated that IL-5-independent eosinophils exist in the lung (described as steady state eosinophils). Moreover, IL-5 deficient mice still produce basal levels of functionally competent eosinophils which are comparable to wild type mice in the blood, but cannot elicit pronounced eosinophilia in response to inflammatory stimulation following an aeroallergen challenge (147, 148). Additionally, mepolizumab attenuates airway eosinophil numbers in asthma but not their functional phenotype (149). As such, targeting IL-5 or IL-5Rα may reduce the inflammatory state eosinophils (Type 1 and Type 2) but fail to have any effect on tissue progenitor and resident eosinophil populations. In clinical trials of asthma, eosinophil depletion strategies that target IL-5 (mepolizumab) or IL-5Rα (benralizumab) achieve only partial success, which emphasizes the need to recognize the heterogeneity of asthma and identify the specific patient populations where targeting eosinophils can be effective (150). In eosinophilic esophagitis, mepolizumab and reslizumab, two anti-IL-5 antibodies, resulted in the reduction of esophageal tissue and blood eosinophils in children and adults but showed no significant reduction in symptoms (151). This brings up a complicated issue of addressing eosinophil causality in development and the progression of allergic disease. If eosinophils are to be viewed as only responders to underlying causes of Th2 immune response (i.e., cells assisting in the restoration of a tissue homeostatic state), depletion of these cells may not be a sufficient therapeutic measure for allergic disease. Significant promise for future targeting of eosinophils in disease may lie in understanding the homeostatic versus pathogenic activation of eosinophils. Identifying novel targets and the processes underlying Th2 responses at the tissue level is equally important, among them underappreciated are changes in extracellular matrix and metabolic tissue microenvironment with regulatory activity for eosinophil phenotype and function.
Summary
Placing eosinophils within heterogeneous tissue contexts and acknowledging the significant regulatory potential of tissue factors supports the view of eosinophils as inherently homeostatic cells driven by complex immune and non-immune changes in their immediate tissue microenvironment. The epithelial-mesenchymal interface, extracellular matrix, and metabolism are emerging as novel tissue regulators of eosinophil biology. Understanding eosinophil-tissue interactions in homeostasis will be beneficial to identifying the tissue processes driving eosinophils in disease as well as understanding the return contribution of these cells to disease pathogenesis. Eosinophil phenotyping and classification is an emerging area in eosinophil research with many future challenges. Here, we demonstrate the diversity and complexity of the actual eosinophil tissue microenvironment, where each component has the potential to influence eosinophil phenotype and function. Addressing immune activation alone is not sufficient to explain disease-relevant eosinophil biology. To fully understand eosinophil heterogeneity within actual biological in vivo contexts, we need to reconcile the differential activation of eosinophils by the immune system with tissue-driven regulation. Single cell sequencing, novel in situ hybridization approaches, context-relevant functional assays, and lineage tracing studies will likely significantly advance our understanding of the true nature of these cells in heterogeneous tissue and immune contexts of development, homeostasis, and disease.
Acknowledgments
This work was supported by the National Institutes of Health (NIH/NIAID) grants R21AI115055 and R01AI127783 to Dr. Berdnikovs. Additionally, this study was supported by the Ernest S. Bazley Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
H.A.-V. and S.B. outlined and structured the concepts covered in this review. All authors participated in writing and editing the manuscript. M.E.C. and S.B. designed and prepared the figures. Final version of the manuscript was approved by S.B.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
References
- 1.Abdala Valencia H, Loffredo LF, Misharin AV, Berdnikovs S. Phenotypic plasticity and targeting of Siglec-F(high) CD11c(low) eosinophils to the airway in a murine model of asthma. Allergy. 2016;71(2):267–71. doi: 10.1111/all.12776. Epub 2015/09/29. [DOI] [PubMed] [Google Scholar]
- 2.Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, Schleich FN, Radermecker M, Thielemans K, Gillet L, Thiry M, Belvisi MG, Louis R, Desmet C, Marichal T, Bureau F. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279–95. doi: 10.1172/JCI85664. Epub 2016/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Need EF, Atashgaran V, Ingman WV, Dasari P. Hormonal regulation of the immune microenvironment in the mammary gland. J Mammary Gland Biol Neoplasia. 2014;19(2):229–39. doi: 10.1007/s10911-014-9324-x. Epub 2014/07/06. [DOI] [PubMed] [Google Scholar]
- 4.Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, Lee EH, Jang MH, Woo SY, Seoh JY, Miyasaka M, Rothenberg ME. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8(4):930–42. doi: 10.1038/mi.2014.123. Epub 2015/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Kleer IM, Kool M, de Bruijn MJ, Willart M, van Moorleghem J, Schuijs MJ, Plantinga M, Beyaert R, Hams E, Fallon PG, Hammad H, Hendriks RW, Lambrecht BN. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity. 2016;45(6):1285–98. doi: 10.1016/j.immuni.2016.10.031. Epub 2016/12/13. [DOI] [PubMed] [Google Scholar]
- 6.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–308. doi: 10.1016/j.cell.2014.03.066. Epub 2014/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SD, Tontonoz P. Eosinophils in fat: pink is the new brown. Cell. 2014;157(6):1249–50. doi: 10.1016/j.cell.2014.05.025. Epub 2014/06/07. [DOI] [PubMed] [Google Scholar]
- 8.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–91. doi: 10.1016/j.cell.2014.03.065. Epub 2014/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40(4):563–75. doi: 10.1111/j.1365-2222.2010.03484.x. Epub 2010/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Helmers RA, Lacy P, Moqbel R, Lee NA. Human versus mouse eosinophils: "that which we call an eosinophil, by any other name would stain as red". J Allergy Clin Immunol. 2012;130(3):572–84. doi: 10.1016/j.jaci.2012.07.025. Epub 2012/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo RC, Liu L, Xenakis JJ, Spencer LA. Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy. 2013;68(3):274–84. doi: 10.1111/all.12103. Epub 2013/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22. doi: 10.1038/nri3341. Epub 2012/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7(5):e1002003. doi: 10.1371/journal.ppat.1002003. Epub 2011/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13(8):607–14. doi: 10.1038/nri3476. Epub 2013/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Beiting DP, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, Lee JJ, Appleton JA. Eosinophils and IL-4 Support Nematode Growth Coincident with an Innate Response to Tissue Injury. PLoS Pathog. 2015;11(12):e1005347. doi: 10.1371/journal.ppat.1005347. Epub 2016/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer. 2015;136(8):1803–13. doi: 10.1002/ijc.29181. Epub 2014/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasari P, Sharkey DJ, Noordin E, Glynn DJ, Hodson LJ, Chin PY, Evdokiou A, Robertson SA, Ingman WV. Hormonal regulation of the cytokine microenvironment in the mammary gland. J Reprod Immunol. 2014;106:58–66. doi: 10.1016/j.jri.2014.07.002. Epub 2014/08/21. [DOI] [PubMed] [Google Scholar]
- 18.Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol Lung Cell Mol Physiol. 2002;283(3):L510–7. doi: 10.1152/ajplung.00144.2002. Epub 2002/08/10. [DOI] [PubMed] [Google Scholar]
- 19.Shi W, Xu J, Warburton D. Development, repair and fibrosis: what is common and why it matters. Respirology. 2009;14(5):656–65. doi: 10.1111/j.1440-1843.2009.01565.x. Epub 2009/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loffredo LF, Abdala-Valencia H, Anekalla KR, Cuervo-Pardo L, Gottardi CJ, Berdnikovs S. Beyond epithelial-to-mesenchymal transition: Common suppression of differentiation programs underlies epithelial barrier dysfunction in mild, moderate, and severe asthma. Allergy. 2017;72(12):1988–2004. doi: 10.1111/all.13222. Epub 2017/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schleimer RP, Berdnikovs S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J Allergy Clin Immunol. 2017;139(6):1752–61. doi: 10.1016/j.jaci.2017.04.010. Epub 2017/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69(8):760–5. doi: 10.1136/thoraxjnl-2013-204608. Epub 2013/12/18. [DOI] [PubMed] [Google Scholar]
- 23.Kagalwalla AF, Akhtar N, Woodruff SA, Rea BA, Masterson JC, Mukkada V, Parashette KR, Du J, Fillon S, Protheroe CA, Lee JJ, Amsden K, Melin-Aldana H, Capocelli KE, Furuta GT, Ackerman SJ. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol. 2012;129(5):1387–96. e7. doi: 10.1016/j.jaci.2012.03.005. Epub 2012/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minshall EM, Hamid QA. Fibroblasts: a cell type central to eosinophil recruitment? Clin Exp Allergy. 2000;30(3):301–3. doi: 10.1046/j.1365-2222.2000.00753.x. Epub 2000/02/26. [DOI] [PubMed] [Google Scholar]
- 25.Diny NL, Hou X, Barin JG, Chen G, Talor MV, Schaub J, Russell SD, Klingel K, Rose NR, Cihakova D. Macrophages and cardiac fibroblasts are the main producers of eotaxins and regulate eosinophil trafficking to the heart. Eur J Immunol. 2016;46(12):2749–60. doi: 10.1002/eji.201646557. Epub 2016/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barreto-Luis A, Corrales A, Acosta-Herrera M, Gonzalez-Colino C, Cumplido J, Martinez-Tadeo J, Carracedo A, Villar J, Carrillo T, Pino-Yanes M, Flores C. A pathway-based association study reveals variants from Wnt signalling genes contributing to asthma susceptibility. Clin Exp Allergy. 2017;47(5):618–26. doi: 10.1111/cea.12883. Epub 2017/01/13. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Xu F, Yu JJ, Zhang W. YAP is up-regulated in the bronchial airway smooth muscle of the chronic asthma mouse model. Int J Clin Exp Pathol. 2015;8(9):11132–9. Epub 2015/12/01. [PMC free article] [PubMed] [Google Scholar]
- 28.Lafkas D, Shelton A, Chiu C, de Leon Boenig G, Chen Y, Stawicki SS, Siltanen C, Reichelt M, Zhou M, Wu X, Eastham-Anderson J, Moore H, Roose-Girma M, Chinn Y, Hang JQ, Warming S, Egen J, Lee WP, Austin C, Wu Y, Payandeh J, Lowe JB, Siebel CW. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature. 2015;528(7580):127–31. doi: 10.1038/nature15715. Epub 2015/11/19. [DOI] [PubMed] [Google Scholar]
- 29.Furmanski AL, Saldana JI, Ono M, Sahni H, Paschalidis N, D'Acquisto F, Crompton T. Tissue-derived hedgehog proteins modulate Th differentiation and disease. J Immunol. 2013;190(6):2641–9. doi: 10.4049/jimmunol.1202541. Epub 2013/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu CH, Nguyen TT, Irvine KM, Sweet MJ, Frazer IH, Blumenthal A. Recombinant Wnt3a and Wnt5a elicit macrophage cytokine production and tolerization to microbial stimulation via Toll-like receptor 4. Eur J Immunol. 2014;44(5):1480–90. doi: 10.1002/eji.201343959. Epub 2014/03/20. [DOI] [PubMed] [Google Scholar]
- 31.Giannetti M, Schroeder HA, Zalewski A, Gonsalves N, Bryce PJ. Dysregulation of the Wnt pathway in adult eosinophilic esophagitis. Dis Esophagus. 2015;28(8):705–10. doi: 10.1111/dote.12273. Epub 2014/08/29. [DOI] [PubMed] [Google Scholar]
- 32.Gerarduzzi C, Di Battista JA. Myofibroblast repair mechanisms post-inflammatory response: a fibrotic perspective. Inflamm Res. 2017;66(6):451–65. doi: 10.1007/s00011-016-1019-x. Epub 2017/01/04. [DOI] [PubMed] [Google Scholar]
- 33.Batra V, Musani AI, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy. 2004;34(3):437–44. doi: 10.1111/j.1365-2222.2004.01885.x. Epub 2004/03/10. [DOI] [PubMed] [Google Scholar]
- 34.Phan SH. Genesis of the myofibroblast in lung injury and fibrosis. Proc Am Thorac Soc. 2012;9(3):148–52. doi: 10.1513/pats.201201-011AW. Epub 2012/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erle DJ, Pytela R. How do integrins integrate? The role of cell adhesion receptors in differentiation and development. Am J Respir Cell Mol Biol. 1992;6(5):459–60. doi: 10.1165/ajrcmb/6.5.459. Epub 1992/05/01. [DOI] [PubMed] [Google Scholar]
- 36.McGowan SE. Extracellular matrix and the regulation of lung development and repair. FASEB J. 1992;6(11):2895–904. Epub 1992/08/01. [PubMed] [Google Scholar]
- 37.Liesker JJ, Ten Hacken NH, Zeinstra-Smith M, Rutgers SR, Postma DS, Timens W. Reticular basement membrane in asthma and COPD: similar thickness, yet different composition. Int J Chron Obstruct Pulmon Dis. 2009;4:127–35. doi: 10.2147/copd.s4639. Epub 2009/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bains SN, Tourkina E, Atkinson C, Joseph K, Tholanikunnel B, Chu HW, Riemer EC, Martin R, Hoffman S. Loss of caveolin-1 from bronchial epithelial cells and monocytes in human subjects with asthma. Allergy. 2012;67(12):1601–4. doi: 10.1111/all.12021. Epub 2012/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts CR, Burke AK. Remodelling of the extracellular matrix in asthma: proteoglycan synthesis and degradation. Can Respir J. 1998;5(1):48–50. Epub 1998/06/20. [PubMed] [Google Scholar]
- 40.Laitinen A, Altraja A, Kampe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med. 1997;156(3 Pt 1):951–8. doi: 10.1164/ajrccm.156.3.9610084. Epub 1997/10/06. [DOI] [PubMed] [Google Scholar]
- 41.Lambropoulou M, Limberis V, Koutlaki N, Simopoulou M, Ntanovasilis D, Vandoros GP, Tatsidou P, Kekou I, Koutsikogianni I, Papadopoulos N. Differential expression of tenascin-C in the developing human lung: an immunohistochemical study. Clin Exp Med. 2009;9(4):333–8. doi: 10.1007/s10238-009-0057-x. Epub 2009/07/25. [DOI] [PubMed] [Google Scholar]
- 42.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2009;40(6):633–42. doi: 10.1165/rcmb.2008-0334OC. Epub 2008/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Udalova IA, Ruhmann M, Thomson SJ, Midwood KS. Expression and immune function of tenascin-C. Crit Rev Immunol. 2011;31(2):115–45. doi: 10.1615/critrevimmunol.v31.i2.30. Epub 2011/05/06. [DOI] [PubMed] [Google Scholar]
- 44.Young SL, Chang LY, Erickson HP. Tenascin-C in rat lung: distribution, ontogeny and role in branching morphogenesis. Dev Biol. 1994;161(2):615–25. doi: 10.1006/dbio.1994.1057. Epub 1994/02/01. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn C, Mason RJ. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol. 1995;147(6):1759–69. Epub 1995/12/01. [PMC free article] [PubMed] [Google Scholar]
- 46.Arron JR, Izuhara K. Asthma biomarkers: what constitutes a 'gold standard'? Thorax. 2015;70(2):105–7. doi: 10.1136/thoraxjnl-2014-206069. Epub 2014/11/30. [DOI] [PubMed] [Google Scholar]
- 47.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–8. doi: 10.1016/j.jaci.2003.10.041. Epub 2004/01/10. [DOI] [PubMed] [Google Scholar]
- 48.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S28–38. doi: 10.1164/ajrccm.164.supplement_2.2106061. Epub 2001/12/06. [DOI] [PubMed] [Google Scholar]
- 49.Homer RJ, Elias JA. Consequences of long-term inflammation. Airway remodeling. Clin Chest Med. 2000;21(2):331–43. ix. doi: 10.1016/s0272-5231(05)70270-7. Epub 2000/07/25. [DOI] [PubMed] [Google Scholar]
- 50.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029–36. doi: 10.1172/JCI17974. Epub 2003/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006;35(3):378–86. doi: 10.1165/rcmb.2006-0027OC. Epub 2006/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giblin SP, Midwood KS. Tenascin-C: Form versus function. Cell Adh Migr. 2015;9(1–2):48–82. doi: 10.4161/19336918.2014.987587. Epub 2014/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi JS, Harley BA. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci Adv. 2017;3(1):e1600455. doi: 10.1126/sciadv.1600455. Epub 2017/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohta M, Sakai T, Saga Y, Aizawa S, Saito M. Suppression of hematopoietic activity in tenascin-C-deficient mice. Blood. 1998;91(11):4074–83. Epub 1998/05/30. [PubMed] [Google Scholar]
- 55.Demange E, Kassim Y, Petit C, Buquet C, Dulong V, Cerf DL, Buchonnet G, Vannier JP. Survival of cord blood haematopoietic stem cells in a hyaluronan hydrogel for ex vivo biomimicry. J Tissue Eng Regen Med. 2013;7(11):901–10. doi: 10.1002/term.1482. Epub 2012/04/05. [DOI] [PubMed] [Google Scholar]
- 56.Klamer S, Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adh Migr. 2014;8(6):563–77. doi: 10.4161/19336918.2014.968501. Epub 2014/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103(12):1719–27. doi: 10.1172/JCI6560. Epub 1999/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, Wert SE, Rothenberg ME. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998;95(11):6273–8. doi: 10.1073/pnas.95.11.6273. Epub 1998/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci U S A. 2002;99(3):1479–84. doi: 10.1073/pnas.261462598. Epub 2002/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8(3):464–75. doi: 10.1038/mi.2015.2. Epub 2015/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–55. doi: 10.1016/B978-0-12-404717-4.00004-4. Epub 2013/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477(7363):229–33. doi: 10.1038/nature10329. Epub 2011/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schuijs MJ, Willart MA, Hammad H, Lambrecht BN. Cytokine targets in airway inflammation. Curr Opin Pharmacol. 2013;13(3):351–61. doi: 10.1016/j.coph.2013.03.013. Epub 2013/05/07. [DOI] [PubMed] [Google Scholar]
- 64.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43(3):305–15. doi: 10.1165/rcmb.2009-0168OC. Epub 2009/10/22. [DOI] [PubMed] [Google Scholar]
- 65.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185(6):3472–80. doi: 10.4049/jimmunol.1000730. Epub 2010/08/10. [DOI] [PubMed] [Google Scholar]
- 66.Bouffi C, Rochman M, Zust CB, Stucke EM, Kartashov A, Fulkerson PC, Barski A, Rothenberg ME. IL-33 markedly activates murine eosinophils by an NF-kappaB-dependent mechanism differentially dependent upon an IL-4-driven autoinflammatory loop. J Immunol. 2013;191(8):4317–25. doi: 10.4049/jimmunol.1301465. Epub 2013/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobsen EA, Doyle AD, Colbert DC, Zellner KR, Protheroe CA, LeSuer WE, Lee NA, Lee JJ. Differential activation of airway eosinophils induces IL-13-mediated allergic Th2 pulmonary responses in mice. Allergy. 2015;70(9):1148–59. doi: 10.1111/all.12655. Epub 2015/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121(6):1484–90. doi: 10.1016/j.jaci.2008.04.005. Epub 2008/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wen T, Besse JA, Mingler MK, Fulkerson PC, Rothenberg ME. Eosinophil adoptive transfer system to directly evaluate pulmonary eosinophil trafficking in vivo. Proc Natl Acad Sci U S A. 2013;110(15):6067–72. doi: 10.1073/pnas.1220572110. Epub 2013/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–8. doi: 10.1038/nature12526. Epub 2013/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas LL, Xu W, Ardon TT. Immobilized lactoferrin is a stimulus for eosinophil activation. J Immunol. 2002;169(2):993–9. doi: 10.4049/jimmunol.169.2.993. Epub 2002/07/05. [DOI] [PubMed] [Google Scholar]
- 72.Mahajan L, Gautam P, Dodagatta-Marri E, Madan T, Kishore U. Surfactant protein SP-D modulates activity of immune cells: proteomic profiling of its interaction with eosinophilic cells. Expert Rev Proteomics. 2014;11(3):355–69. doi: 10.1586/14789450.2014.897612. Epub 2014/04/05. [DOI] [PubMed] [Google Scholar]
- 73.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, Zhu Z, Tiemeyer M, Bochner BS. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135(5):1329–40. e9. doi: 10.1016/j.jaci.2014.10.027. Epub 2014/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6'-sulfated sialyl Lewis x. J Pharmacol Exp Ther. 2009;330(2):608–12. doi: 10.1124/jpet.109.152439. Epub 2009/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–7. doi: 10.1126/science.1201475. Epub 2011/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hussaarts L, Garcia-Tardon N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, Ozir-Fazalalikhan A, Berbee JF, Willems van Dijk K, van Harmelen V, Yazdanbakhsh M, Guigas B. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29(7):3027–39. doi: 10.1096/fj.14-266239. Epub 2015/04/09. [DOI] [PubMed] [Google Scholar]
- 77.Lee CT, Harris SB, Retnakaran R, Gerstein HC, Perkins BA, Zinman B, Hanley AJ. White blood cell subtypes, insulin resistance and beta-cell dysfunction in high-risk individuals--the PROMISE cohort. Clin Endocrinol (Oxf) 2014;81(4):536–41. doi: 10.1111/cen.12390. Epub 2014/01/01. [DOI] [PubMed] [Google Scholar]
- 78.Lin HH, Yen TH, Huang CC, Chiang YJ, Kuo HL. Blood eosinophilia, corticoadrenal insufficiency and eosinophilic cystitis. Urol Int. 2008;80(2):219–21. doi: 10.1159/000112618. Epub 2008/03/26. [DOI] [PubMed] [Google Scholar]
- 79.Neuwirth A, Dobes J, Oujezdska J, Ballek O, Benesova M, Sumnik Z, Vcelakova J, Kolouskova S, Obermannova B, Kolar M, Stechova K, Filipp D. Eosinophils from patients with type 1 diabetes mellitus express high level of myeloid alpha-defensins and myeloperoxidase. Cell Immunol. 2012;273(2):158–63. doi: 10.1016/j.cellimm.2011.12.001. Epub 2012/01/18. [DOI] [PubMed] [Google Scholar]
- 80.Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol. 2005;116(6):1228–34. doi: 10.1016/j.jaci.2005.09.003. Epub 2005/12/13. [DOI] [PubMed] [Google Scholar]
- 81.Tamaki M, Konno Y, Kobayashi Y, Takeda M, Itoga M, Moritoki Y, Oyamada H, Kayaba H, Chihara J, Ueki S. Expression and functional roles of G-protein-coupled estrogen receptor (GPER) in human eosinophils. Immunol Lett. 2014;160(1):72–8. doi: 10.1016/j.imlet.2014.03.012. Epub 2014/04/11. [DOI] [PubMed] [Google Scholar]
- 82.Ueki S, Mahemuti G, Oyamada H, Kato H, Kihara J, Tanabe M, Ito W, Chiba T, Takeda M, Kayaba H, Chihara J. Retinoic acids are potent inhibitors of spontaneous human eosinophil apoptosis. J Immunol. 2008;181(11):7689–98. doi: 10.4049/jimmunol.181.11.7689. Epub 2008/11/20. [DOI] [PubMed] [Google Scholar]
- 83.Ueki S, Nishikawa J, Yamauchi Y, Konno Y, Tamaki M, Itoga M, Kobayashi Y, Takeda M, Moritoki Y, Ito W, Chihara J. Retinoic acids up-regulate functional eosinophil-driving receptor CCR3. Allergy. 2013;68(7):953–6. doi: 10.1111/all.12175. Epub 2013/06/08. [DOI] [PubMed] [Google Scholar]
- 84.Peinhaupt M, Sturm EM, Heinemann A. Prostaglandins and Their Receptors in Eosinophil Function and As Therapeutic Targets. Front Med (Lausanne) 2017;4:104. doi: 10.3389/fmed.2017.00104. Epub 2017/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Konno Y, Ueki S, Takeda M, Kobayashi Y, Tamaki M, Moritoki Y, Oyamada H, Itoga M, Kayaba H, Omokawa A, Hirokawa M. Functional analysis of free fatty acid receptor GPR120 in human eosinophils: implications in metabolic homeostasis. PLoS One. 2015;10(3):e0120386. doi: 10.1371/journal.pone.0120386. Epub 2015/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Powell WS, Chung D, Gravel S. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J Immunol. 1995;154(8):4123–32. Epub 1995/04/15. [PubMed] [Google Scholar]
- 87.O'Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, Daniel LW. 5-Oxo-eicosatetraenoate is a broadly active, eosinophil-selective stimulus for human granulocytes. J Immunol. 1996;157(1):336–42. Epub 1996/07/01. [PubMed] [Google Scholar]
- 88.Langlois A, Ferland C, Tremblay GM, Laviolette M. Montelukast regulates eosinophil protease activity through a leukotriene-independent mechanism. J Allergy Clin Immunol. 2006;118(1):113–9. doi: 10.1016/j.jaci.2006.03.010. Epub 2006/07/04. [DOI] [PubMed] [Google Scholar]
- 89.Langlois A, Chouinard F, Flamand N, Ferland C, Rola-Pleszczynski M, Laviolette M. Crucial implication of protein kinase C (PKC)-delta, PKC-zeta, ERK-1/2, and p38 MAPK in migration of human asthmatic eosinophils. J Leukoc Biol. 2009;85(4):656–63. doi: 10.1189/jlb.0808492. Epub 2009/01/24. [DOI] [PubMed] [Google Scholar]
- 90.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. Epub 2012/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masterson JC, McNamee EN, Fillon SA, Hosford L, Harris R, Fernando SD, Jedlicka P, Iwamoto R, Jacobsen E, Protheroe C, Eltzschig HK, Colgan SP, Arita M, Lee JJ, Furuta GT. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut. 2015;64(8):1236–47. doi: 10.1136/gutjnl-2014-306998. Epub 2014/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19(6):676–80. doi: 10.1016/j.coi.2007.07.017. Epub 2007/08/28. [DOI] [PubMed] [Google Scholar]
- 93.Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19(6):681–6. doi: 10.1016/j.coi.2007.07.021. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 94.Munitz A, Bachelet I, Levi-Schaffer F. Reversal of airway inflammation and remodeling in asthma by a bispecific antibody fragment linking CCR3 to CD300a. J Allergy Clin Immunol. 2006;118(5):1082–9. doi: 10.1016/j.jaci.2006.07.041. Epub 2006/11/08. [DOI] [PubMed] [Google Scholar]
- 95.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. Epub 2004/03/23. [DOI] [PubMed] [Google Scholar]
- 96.Levi-Schaffer F, Garbuzenko E, Rubin A, Reich R, Pickholz D, Gillery P, Emonard H, Nagler A, Maquart FA. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta) Proc Natl Acad Sci U S A. 1999;96(17):9660–5. doi: 10.1073/pnas.96.17.9660. Epub 1999/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takeda K, Shiraishi Y, Ashino S, Han J, Jia Y, Wang M, Lee NA, Lee JJ, Gelfand EW. Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge. J Allergy Clin Immunol. 2015;135(2):451–60. doi: 10.1016/j.jaci.2014.08.014. Epub 2014/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gleich GJ, Klion AD, Lee JJ, Weller PF. The consequences of not having eosinophils. Allergy. 2013;68(7):829–35. doi: 10.1111/all.12169. Epub 2013/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mackie EJ, Tucker RP. The tenascin-C knockout revisited. J Cell Sci. 1999;112(Pt 22):3847–53. doi: 10.1242/jcs.112.22.3847. Epub 1999/11/05. [DOI] [PubMed] [Google Scholar]
- 100.Elovic A, Wong DT, Weller PF, Matossian K, Galli SJ. Expression of transforming growth factors-alpha and beta 1 messenger RNA and product by eosinophils in nasal polyps. J Allergy Clin Immunol. 1994;93(5):864–9. doi: 10.1016/0091-6749(94)90379-4. Epub 1994/05/01. [DOI] [PubMed] [Google Scholar]
- 101.Wong DT, Elovic A, Matossian K, Nagura N, McBride J, Chou MY, Gordon JR, Rand TH, Galli SJ, Weller PF. Eosinophils from patients with blood eosinophilia express transforming growth factor beta 1. Blood. 1991;78(10):2702–7. Epub 1991/11/15. [PubMed] [Google Scholar]
- 102.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44(2):127–33. doi: 10.1165/rcmb.2010-0027TR. Epub 2010/06/08. [DOI] [PubMed] [Google Scholar]
- 103.Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116(4):796–804. doi: 10.1016/j.jaci.2005.06.031. Epub 2005/10/08. [DOI] [PubMed] [Google Scholar]
- 104.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107(6):1034–8. doi: 10.1067/mai.2001.115626. Epub 2001/06/09. [DOI] [PubMed] [Google Scholar]
- 105.Liu Z, Lu X, Wang H, Gao Q, Cui Y. The up-regulated expression of tenascin C in human nasal polyp tissues is related to eosinophil-derived transforming growth factor beta1. Am J Rhinol. 2006;20(6):629–33. doi: 10.2500/ajr.2006.20.2918. Epub 2006/12/22. [DOI] [PubMed] [Google Scholar]
- 106.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997;17(4):519–28. doi: 10.1165/ajrcmb.17.4.2877. Epub 1997/11/05. [DOI] [PubMed] [Google Scholar]
- 107.Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997;16(3):212–9. doi: 10.1165/ajrcmb.16.3.9070604. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 108.Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T. Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: direct and synergistic effects with transforming growth factor beta1. Int J Cancer. 2003;105(1):53–60. doi: 10.1002/ijc.11037. Epub 2003/04/03. [DOI] [PubMed] [Google Scholar]
- 109.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194(6):809–21. doi: 10.1084/jem.194.6.809. Epub 2001/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hernnas J, Sarnstrand B, Lindroth P, Peterson CG, Venge P, Malmstrom A. Eosinophil cationic protein alters proteoglycan metabolism in human lung fibroblast cultures. Eur J Cell Biol. 1992;59(2):352–63. Epub 1992/12/01. [PubMed] [Google Scholar]
- 111.Zagai U, Dadfar E, Lundahl J, Venge P, Skold CM. Eosinophil cationic protein stimulates TGF-beta1 release by human lung fibroblasts in vitro. Inflammation. 2007;30(5):153–60. doi: 10.1007/s10753-007-9032-4. Epub 2007/06/26. [DOI] [PubMed] [Google Scholar]
- 112.Rochester CL, Ackerman SJ, Zheng T, Elias JA. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J Immunol. 1996;156(11):4449–56. Epub 1996/06/01. [PubMed] [Google Scholar]
- 113.Pegorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177(7):4861–9. doi: 10.4049/jimmunol.177.7.4861. Epub 2006/09/20. [DOI] [PubMed] [Google Scholar]
- 114.Temkin V, Aingorn H, Puxeddu I, Goldshmidt O, Zcharia E, Gleich GJ, Vlodavsky I, Levi-Schaffer F. Eosinophil major basic protein: first identified natural heparanase-inhibiting protein. J Allergy Clin Immunol. 2004;113(4):703–9. doi: 10.1016/j.jaci.2003.11.038. Epub 2004/04/22. [DOI] [PubMed] [Google Scholar]
- 115.Januskevicius A, Gosens R, Sakalauskas R, Vaitkiene S, Janulaityte I, Halayko AJ, Hoppenot D, Malakauskas K. Suppression of Eosinophil Integrins Prevents Remodeling of Airway Smooth Muscle in Asthma. Front Physiol. 2016;7:680. doi: 10.3389/fphys.2016.00680. Epub 2017/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fanat AI, Thomson JV, Radford K, Nair P, Sehmi R. Human airway smooth muscle promotes eosinophil differentiation. Clin Exp Allergy. 2009;39(7):1009–17. doi: 10.1111/j.1365-2222.2009.03246.x. Epub 2009/05/15. [DOI] [PubMed] [Google Scholar]
- 117.Ferland C, Guilbert M, Davoine F, Flamand N, Chakir J, Laviolette M. Eotaxin promotes eosinophil transmigration via the activation of the plasminogen-plasmin system. J Leukoc Biol. 2001;69(5):772–8. Epub 2001/05/19. [PubMed] [Google Scholar]
- 118.Uderhardt S, Ackermann JA, Fillep T, Hammond VJ, Willeit J, Santer P, Mayr M, Biburger M, Miller M, Zellner KR, Stark K, Zarbock A, Rossaint J, Schubert I, Mielenz D, Dietel B, Raaz-Schrauder D, Ay C, Gremmel T, Thaler J, Heim C, Herrmann M, Collins PW, Schabbauer G, Mackman N, Voehringer D, Nadler JL, Lee JJ, Massberg S, Rauh M, Kiechl S, Schett G, O'Donnell VB, Kronke G. Enzymatic lipid oxidation by eosinophils propagates coagulation, hemostasis, and thrombotic disease. J Exp Med. 2017;214(7):2121–38. doi: 10.1084/jem.20161070. Epub 2017/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, Ten Berg JM, Adriaenssens T, Guagliumi G, Godschalk TC, Neumann FJ, Trenk D, Feldman LJ, Steg PG, Desmet W, Alfonso F, Goodall AH, Wojdyla R, Dudek D, Philippi V, Opinaldo S, Titova A, Malik N, Cotton J, Jhagroe DA, Heestermans AA, Sinnaeve P, Vermeersch P, Valina C, Schulz C, Kastrati A, Massberg S, Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort I Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J. 2016;37(19):1538–49. doi: 10.1093/eurheartj/ehv419. Epub 2016/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, Lohse P, Patel KD, Engelmann B. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109(3):995–1002. doi: 10.1182/blood-2006-02-004945. Epub 2006/09/28. [DOI] [PubMed] [Google Scholar]
- 121.Hara K, Hasegawa T, Ooi H, Koya T, Tanabe Y, Tsukada H, Igarashi K, Suzuki E, Arakawa M, Gejyo F. Inhibitory role of eosinophils on cell surface plasmin generation by bronchial epithelial cells: inhibitory effects of transforming growth factor beta. Lung. 2001;179(1):9–20. doi: 10.1007/s004080000042. Epub 2001/08/02. [DOI] [PubMed] [Google Scholar]
- 122.Shimizu S, Kouzaki H, Ogawa T, Takezawa K, Tojima I, Shimizu T. Eosinophil-epithelial cell interactions stimulate the production of MUC5AC mucin and profibrotic cytokines involved in airway tissue remodeling. Am J Rhinol Allergy. 2014;28(2):103–9. doi: 10.2500/ajra.2014.28.4018. Epub 2014/04/11. [DOI] [PubMed] [Google Scholar]
- 123.Matsumoto K, Fukuda S, Nakamura Y, Saito H. Amphiregulin production by human eosinophils. Int Arch Allergy Immunol. 2009;149(Suppl 1):39–44. doi: 10.1159/000210652. Epub 2009/06/12. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Yang P, Cui R, Zhang M, Li H, Qian C, Sheng C, Qu S, Bu L. Eosinophils Reduce Chronic Inflammation in Adipose Tissue by Secreting Th2 Cytokines and Promoting M2 Macrophages Polarization. Int J Endocrinol. 2015;2015:565760. doi: 10.1155/2015/565760. Epub 2015/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chatzigeorgiou A, Chavakis T. Immune Cells and Metabolism. Handb Exp Pharmacol. 2016;233:221–49. doi: 10.1007/164_2015_8. Epub 2015/04/24. [DOI] [PubMed] [Google Scholar]
- 126.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. Epub 2015/11/11. [DOI] [PubMed] [Google Scholar]
- 127.Fang Y, Chen K, Jackson DA, Sharp GC, Braley-Mullen H. Eosinophils infiltrate thyroids, but have no apparent role in induction or resolution of experimental autoimmune thyroiditis in interferon-gamma(−/−) mice. Immunology. 2010;129(3):329–37. doi: 10.1111/j.1365-2567.2009.03187.x. Epub 2009/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–49. doi: 10.1038/nri3071. Epub 2011/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feltenmark S, Gautam N, Brunnstrom A, Griffiths W, Backman L, Edenius C, Lindbom L, Bjorkholm M, Claesson HE. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci U S A. 2008;105(2):680–5. doi: 10.1073/pnas.0710127105. Epub 2008/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Withers SB, Forman R, Meza-Perez S, Sorobetea D, Sitnik K, Hopwood T, Lawrence CB, Agace WW, Else KJ, Heagerty AM, Svensson-Frej M, Cruickshank SM. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep. 2017;7:44571. doi: 10.1038/srep44571. Epub 2017/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hui CC, McNagny KM, Denburg JA, Siracusa MC. In situ hematopoiesis: a regulator of TH2 cytokine-mediated immunity and inflammation at mucosal surfaces. Mucosal Immunol. 2015;8(4):701–11. doi: 10.1038/mi.2015.17. Epub 2015/03/19. [DOI] [PubMed] [Google Scholar]
- 132.Chu DK, Jimenez-Saiz R, Verschoor CP, Walker TD, Goncharova S, Llop-Guevara A, Shen P, Gordon ME, Barra NG, Bassett JD, Kong J, Fattouh R, McCoy KD, Bowdish DM, Erjefalt JS, Pabst O, Humbles AA, Kolbeck R, Waserman S, Jordana M. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med. 2014;211(8):1657–72. doi: 10.1084/jem.20131800. Epub 2014/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139–55. doi: 10.1034/j.1600-065x.2001.790114.x. Epub 2001/04/09. [DOI] [PubMed] [Google Scholar]
- 134.Brandt EB, Zimmermann N, Muntel EE, Yamada Y, Pope SM, Mishra A, Hogan SP, Rothenberg ME. The alpha4bbeta7-integrin is dynamically expressed on murine eosinophils and involved in eosinophil trafficking to the intestine. Clin Exp Allergy. 2006;36(4):543–53. doi: 10.1111/j.1365-2222.2006.02456.x. Epub 2006/04/25. [DOI] [PubMed] [Google Scholar]
- 135.Johansson MW. Eosinophil Activation Status in Separate Compartments and Association with Asthma. Front Med (Lausanne) 2017;4:75. doi: 10.3389/fmed.2017.00075. Epub 2017/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, Berdnikovs S, Bryce PJ. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J Immunol. 2016;197(9):3445–53. doi: 10.4049/jimmunol.1600611. Epub 2016/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith SG, Gugilla A, Mukherjee M, Merim K, Irshad A, Tang W, Kinoshita T, Watson B, Oliveria JP, Comeau M, O'Byrne PM, Gauvreau GM, Sehmi R. Thymic stromal lymphopoietin and IL-33 modulate migration of hematopoietic progenitor cells in patients with allergic asthma. J Allergy Clin Immunol. 2015;135(6):1594–602. doi: 10.1016/j.jaci.2014.12.1918. Epub 2015/02/07. [DOI] [PubMed] [Google Scholar]
- 138.Reece P, Baatjes AJ, Cyr MM, Sehmi R, Denburg JA. Toll-like receptor-mediated eosinophil-basophil differentiation: autocrine signalling by granulocyte-macrophage colony-stimulating factor in cord blood haematopoietic progenitors. Immunology. 2013;139(2):256–64. doi: 10.1111/imm.12078. Epub 2013/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tworek D, Heroux D, O'Byrne SN, Mitchell P, O'Byrne PM, Denburg JA. Toll-like receptor-induced expression of epithelial cytokine receptors on haemopoietic progenitors is altered in allergic asthma. Clin Exp Allergy. 2017;47(7):900–8. doi: 10.1111/cea.12913. Epub 2017/03/03. [DOI] [PubMed] [Google Scholar]
- 140.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4(4):155–64. doi: 10.1186/bcr441. Epub 2002/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142(10):4515–21. doi: 10.1210/endo.142.10.8459. Epub 2001/09/21. [DOI] [PubMed] [Google Scholar]
- 142.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, Abdala-Valencia H, Yacoub TJ, Chi M, Chiu S, Gonzalez-Gonzalez FJ, Gates K, Lam AP, Nicholson TT, Homan PJ, Soberanes S, Dominguez S, Morgan VK, Saber R, Shaffer A, Hinchcliff M, Marshall SA, Bharat A, Berdnikovs S, Bhorade SM, Bartom ET, Morimoto RI, Balch WE, Sznajder JI, Chandel NS, Mutlu GM, Jain M, Gottardi CJ, Singer BD, Ridge KM, Bagheri N, Shilatifard A, Budinger GRS, Perlman H. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214(8):2387–404. doi: 10.1084/jem.20162152. Epub 2017/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–17. doi: 10.1038/ni.3159. Epub 2015/04/29. [DOI] [PubMed] [Google Scholar]
- 144.Percopo CM, Brenner TA, Ma M, Kraemer LS, Hakeem RM, Lee JJ, Rosenberg HF. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol. 2017;101(1):321–8. doi: 10.1189/jlb.3A0416-166R. Epub 2016/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81(6):1434–44. doi: 10.1189/jlb.1106686. Epub 2007/03/07. [DOI] [PubMed] [Google Scholar]
- 146.Griseri T, Arnold IC, Pearson C, Krausgruber T, Schiering C, Franchini F, Schulthess J, McKenzie BS, Crocker PR, Powrie F. Granulocyte Macrophage Colony-Stimulating Factor-Activated Eosinophils Promote Interleukin-23 Driven Chronic Colitis. Immunity. 2015;43(1):187–99. doi: 10.1016/j.immuni.2015.07.008. Epub 2015/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4(1):15–24. doi: 10.1016/s1074-7613(00)80294-0. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 148.Matthaei KI, Foster P, Young IG. The role of interleukin-5 (IL-5) in vivo: studies with IL-5 deficient mice. Mem Inst Oswaldo Cruz. 1997;92(Suppl 2):63–8. doi: 10.1590/s0074-02761997000800010. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 149.Kelly EA, Esnault S, Liu LY, Evans MD, Johansson MW, Mathur S, Mosher DF, Denlinger LC, Jarjour NN. Mepolizumab Attenuates Airway Eosinophil Numbers, but Not Their Functional Phenotype, in Asthma. Am J Respir Crit Care Med. 2017;196(11):1385–95. doi: 10.1164/rccm.201611-2234OC. Epub 2017/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Corren J. Inhibition of interleukin-5 for the treatment of eosinophilic diseases. Discov Med. 2012;13(71):305–12. Epub 2012/05/01. [PubMed] [Google Scholar]
- 151.Ko E, Chehade M. Biological Therapies for Eosinophilic Esophagitis: Where Do We Stand? Clin Rev Allergy Immunol. 2018 doi: 10.1007/s12016-018-8674-3. Epub 2018/01/27. [DOI] [PubMed] [Google Scholar]