Abstract
Objective
Extracorporeal Membrane Oxygenation (ECMO) is increasingly used as a bridge to lung transplantation. The impact of preoperative ECMO on health-related quality of life (HRQL) and depressive symptoms after lung transplant, however, remains unknown.
Methods
In a single-center prospective cohort study, we assessed HRQL and depressive symptoms before and at three, six, and twelve months after lung transplantation using the SF12-Physical and Mental Component Scores (SF12-PCS, SF12-MCS), Airway Questionnaire 20-Revised (AQ20R), EuroQol 5D (EQ5D), and the Geriatric Depression Scale (GDS). Changes in HRQL were quantified by segmented linear mixed effects models, controlling for age, sex, diagnosis, preoperative FEV1, six-minute walk distance, and Lung Allocation Score. We compared changes in HRQL between subjects bridged with ECMO, subjects hospitalized but not on ECMO, and subjects called in for transplant as outpatients.
Results
Of 189 subjects, 17 were bridged to transplant with ECMO. In all groups, improvements in HRQL following lung transplantation exceeded the minimally clinically important difference using the SF12-PCS, AQ20R, EQ5D and GDS. HRQL defined by SF12-MCS did not change after transplant. Improvements were generally similar between groups, except for in EQ5D where there was a trend for outpatients to benefit less, possibly due to their better HRQL before lung transplant.
Conclusions
Subjects ill enough to require ECMO as a bridge to lung transplantation appear to achieve similar improvements in HRQL and depressive symptoms as those who do not. It is reassuring to both providers and patients that lung transplant provides substantial improvements in HRQL, even for those patients who are critically ill in the run up to transplant.
Central Picture

Metal sculpture made by a survivor of ECMO as a bridge to lung transplantation.
Introduction
Lung transplantation aims to extend survival and to improve health-related quality of life (HRQL) for patients suffering from end-stage lung disease1–3. Historically, many lung transplant programs have been reluctant to offer transplantation to critically ill patients not only because of concern for poorer short-term survival but also compromised functional status and quality of life in those who do survive4–7. With increasing experience in the use of extracorporeal membrane oxygenation (ECMO) as a bridge to transplantation, critically ill patients are more readily being offered a chance at lung transplantation8. Although the first successful case of ECMO as a bridge to lung transplantation was reported in 1977, this practice remained relatively uncommon with only 24 patients being treated in this manner in the U.S. through 20009. From 2000 to 2014, however, the number of cases rose to 414, a remarkable 28-fold increase 10. Evidence supports that bridging patients to lung transplantation with ECMO is not only feasible, but that survival after transplant may be comparable to others who are transplanted while critically ill but not on ECMO 11–14. Understanding the impact of lung transplant on HRQL in this rapidly growing population of candidates is fundamental to defining transplant benefit.
Preoperative ECMO is associated with a lower likelihood of survival to lung transplantation, increased cost of hospitalization, and, if transplant is performed, a longer post-surgical length of stay 15–17. ECMO also confers added specific risks, including for hemorrhage, hemolysis, neurological injury, limb ischemia, and sepsis 18. The complications related to critical illness and ECMO are known to have a negative impact in non-transplant populations. For example, survivors of general critical illness have poorer physical functioning than healthy controls and have a high incidence of depressive symptoms 19,20. Furthermore, those who survived critical illness with the assistance of ECMO report poor subsequent HRQL and persistent physical and emotional health limitations 21–23. Since surviving to lung transplant through ECMO might be similar to surviving critical illness, it is plausible that the impact of lung transplant on HRQL benefit could be attenuated in this group.
Given the potential that bridging to lung transplant with ECMO may attenuate HRQL improvements, we sought to evaluate whether the impact of lung transplantation on HRQL within the first post-operative year was different in patients bridged to transplant with ECMO compared to those who were not. To do so, we performed a single center prospective cohort study utilizing multiple instruments to assess HRQL, including generic, respiratory-specific, and health utility instruments. Given the high incidence of depression following critical illness, we also measured depressive symptoms with a non-HRQL instrument.
Methods
Study design, participants, and setting
We performed this study amongst participants in the University of California, San Francisco “Breathe Again” study. The details of the study design, including exclusion criteria, have previously been described 3. In brief, Breathe Again is a prospective cohort study of subjects 18 years of age or older, who underwent first-time lung transplantation between February 2010 and January 2017; follow-up reported here continued until May 2017.
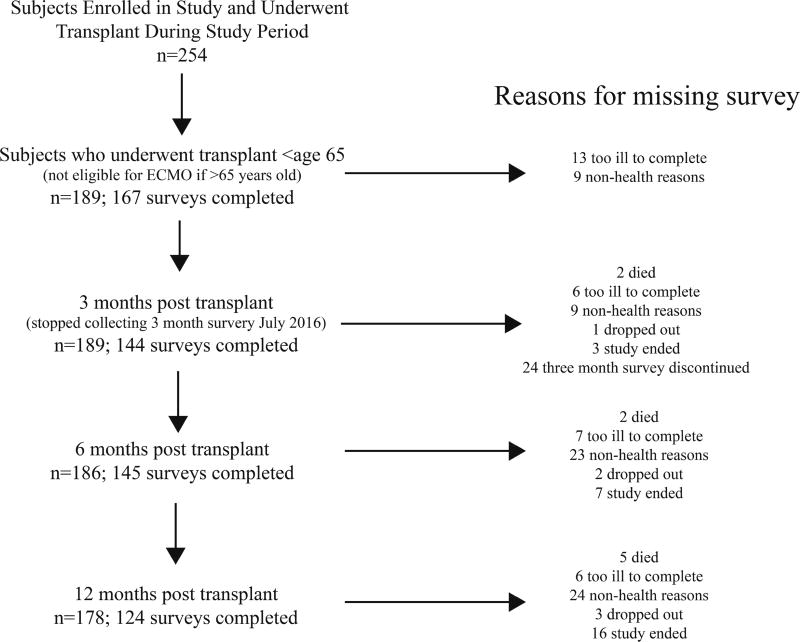
Subjects were enrolled at the time of placement on the lung transplant waiting list. At enrollment, subjects underwent a research visit that included functional assessments as well as a battery of HRQL measures and a depression scale. While waitlisted, assessments were repeated every three months or at the time of major changes in clinical status, including terminal hospitalization requiring transplantation, need for invasive mechanical ventilation, or implementation of ECMO. The same functional and HRQL assessments were administered at three, six, and twelve months after transplantation. 189 subjects comprised the cohort for this study (Figure 1).
Figure 1. Flow chart of subjects throughout the study.
Left-sided column represents number of subjects providing data for analysis at each time point. The number of subjects at the three-month post-transplant time point was less than six month time point because we stopped administering surveys at 3 months post-transplant after July 2016. Right-sided column explains reasons for missing surveys at each time point.
At our center, ECMO is utilized in patients with worsening clinical status that leads to cor pulmonale and/or profound hypoxemia, with oxygen needs exceeding what can be provided by mechanical ventilation. ECMO is utilized in both patients already listed for lung transplantation and patients who need extra support to complete their evaluation for transplantation. Patients eligible for ECMO must be under the age of 65. ECMO is typically deployed in an internal jugular dual lumen veno-venous cannula placement, unless the patient has pulmonary hypertension with a pulmonary artery systolic pressure greater than 50 mmHg. When patients have pulmonary hypertension, peripheral or central veno-arterial ECMO is utilized. Since we began our ECMO as a bridge to transplantation program in 2004, 61 patients have been placed on ECMO with the intent of facilitating survival to lung transplantation. Of the 61 patients, 52 (85%) were successfully bridged to lung transplantation. Seventeen of these patients were enrolled as subjects in the Breathe Again Cohort
Since our center does not offer ECMO to patients older than 65 years of age, we restricted our analysis to Breathe Again study participants aged 65 or younger. We defined three groups: subjects bridged to lung transplantation with ECMO; subjects hospitalized but not on ECMO at the time of transplantation; and subjects called in for transplantation as outpatients. This study was approved by the Committee on Human Research at the University of California, San Francisco, and written informed consent was obtained from all study participants. Some of the data have been previously been presented in an abstract and, in aggregate form, a manuscript 3,24.
Outcome variables: HRQL and Depressive symptoms
HRQL is, by definition, multidimensional 25,26. Existing HRQL instruments emphasize different conceptual health domains relevant to the population for which they were developed. Because various instruments provide complementary information, in order to more comprehensively measure conceptual HRQL domains relevant in lung transplantation, we utilized multiple measures (Supplemental Table 1). Specifically, we measured HRQL and depressive symptoms at pre-transplant baseline and at three, six, and twelve months after transplantation. To evaluate generic HRQL, we utilized the Medical Outcomes Survey Short Form-12 version 2 (SF12) Physical and Mental Component Summary scores (SF12-PCS and -MCS; range 0–100; higher scores denote better HRQL) 27. A change of five points in either of these measures is generally considered to meet the minimally clinically important difference (MCID) 28. To evaluate respiratory specific HRQL, we utilized the Airways Questionnaire 20-Revised (AQ20-R; range 0–20; lower scores denote better HRQL; ½ standard deviation used as distribution-based MCID: 1.75) 29,30. To evaluate health utility, we utilized the EuroQoL 5D (EQ5D; range −0.11 – 1.0; higher scores denote better HRQL and a score less than zero reflects a state “worse than death”; MCID: 0.06) 31.
It is estimated that as many as 63% of organ transplant recipients experience symptoms of depression or anxiety within the first few years after transplantation 32. Depressive symptoms following transplantation are also strongly associated with subsequent mortality 32,33. Similarly, survivors of critical illness experience high rates of depression 34. Thus, we were interested in whether subjects bridged with ECMO would report more depressive symptoms than other transplant recipients. Since HRQL instruments include only a limited number of items addressing depressive symptoms, we were not able to use a validated HRQL instrument to assess subjects for depressive symptoms. Thus, we employed the Geriatric Depression Scale Short Form (GDS; range 0–15; lower scores denote fewer depressive symptoms; ½ standard deviation used as distribution-based MCID: 1.65) 30,35.
Other variables
Baseline demographic and clinical variables were abstracted from our lung transplant center medical records. Variables collected included age, sex, diagnostic indication for transplant as categorized by groupings in the Lung Allocation Score 36, preoperative forced expiratory volume in 1 second (FEV1, liters), six minute walk distance (6MWD, meters), and the Lung Allocation Score. For this analysis, we considered data most proximal to the date of transplant.
Analytic approach
Baseline continuous and categorical variables were compared between our three groups using ANOVA and chi-squared test, as appropriate. For the SF12-PCS, SF12-MCS, and EQ5D, higher scores denote better HRQL. Lower scores denote better HRQL for the AQ20R and less depressive symptoms for the GDS. To facilitate interpretation, we reverse coded the AQ20R and GDS so that higher scores would represent better HRQL by AQ20R and less depressive symptoms by GDS. Changes in HRQL scores were plotted over time (Figure 2). Since our primary outcome was to quantify the magnitude of change in HRQL from before to after transplant between groups, we did not compare the individual absolute scores between groups at the three, six, and twelve-month time points. Visual inspection of the change in HRQL over time demonstrated two distinct periods with a different slope of change in HRQL; the early period of change from pre-transplant baseline to six months post-transplant (which accounted for most of the change in HRQL), and the late period of change from six to twelve months post-transplant (which had a relatively flat slope for all instruments). Because the change in HRQL over time was not linear, change in HRQL and depressive symptoms over time were estimated using segmented linear mixed effects models with a joint point set at six months after transplant. Subject and time were included as random effects, allowing for different slopes in the HRQL outcomes in the early and later time periods within each subject. Parameter estimates and the 95% confidence intervals for the other groups were derived by post-estimation commands (lincom). For each outcome, we performed unadjusted analyses as well as analyses adjusted for age, sex, diagnostic category, most recent preoperative FEV1, most recent preoperative 6MWD, and Lung Allocation Score. Since depressive symptoms are strongly associated with general HRQL, we also performed a secondary analysis in which we controlled for pre-operative GDS.
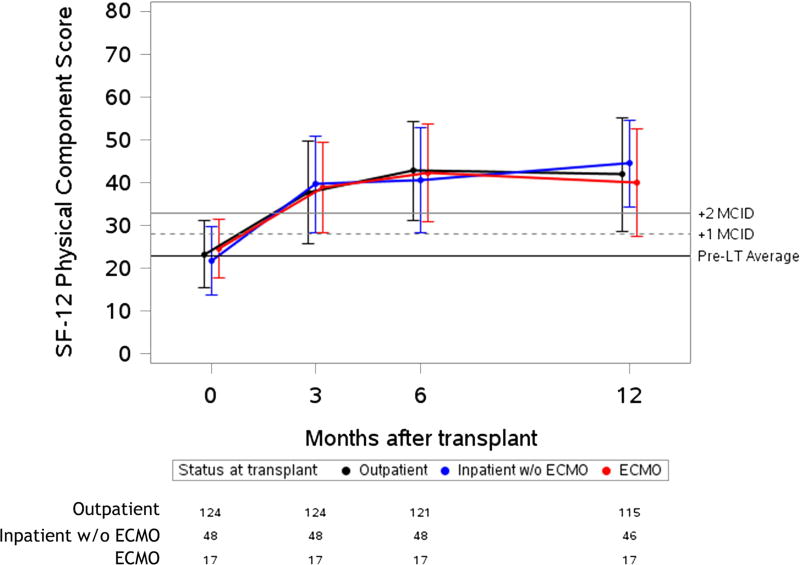
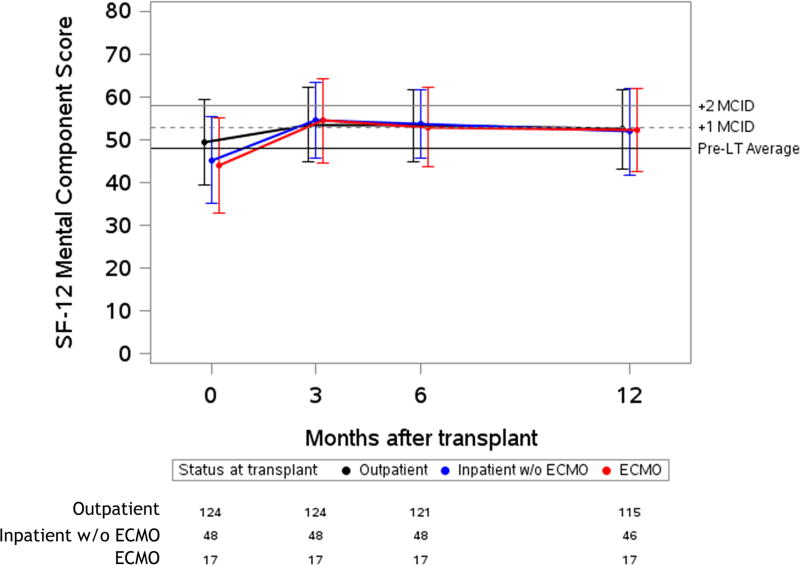
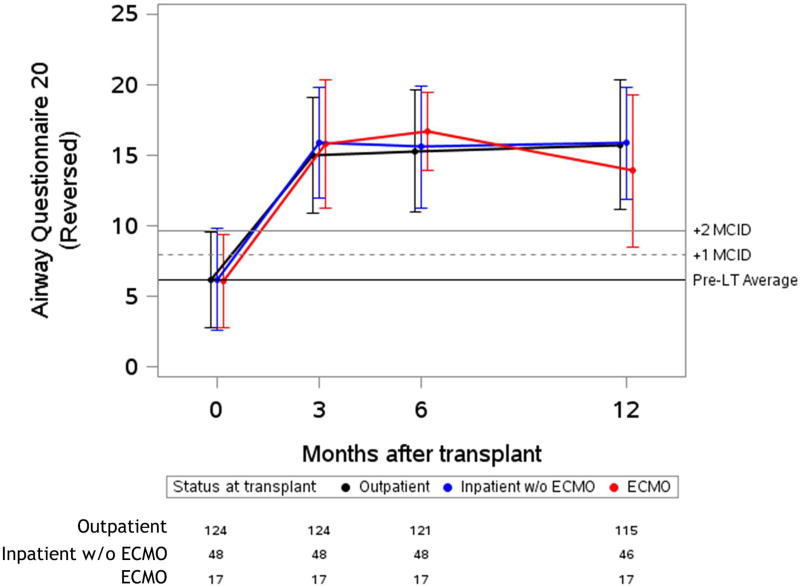
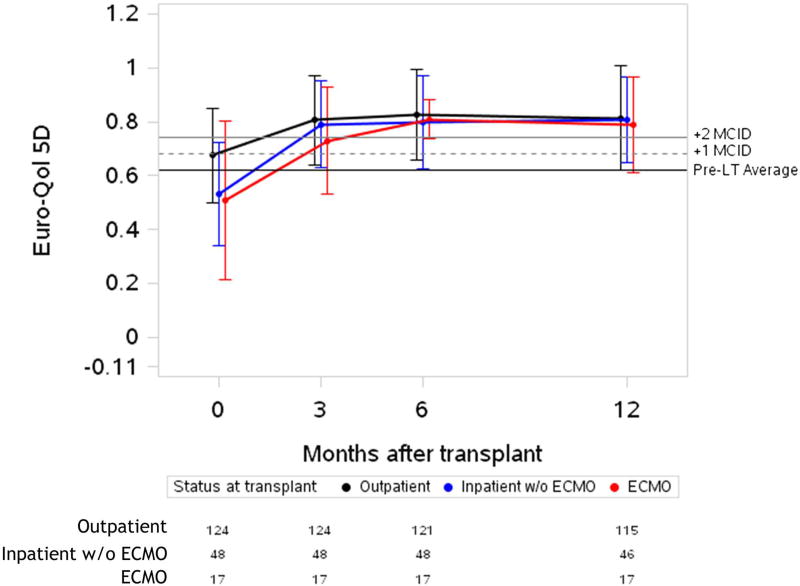
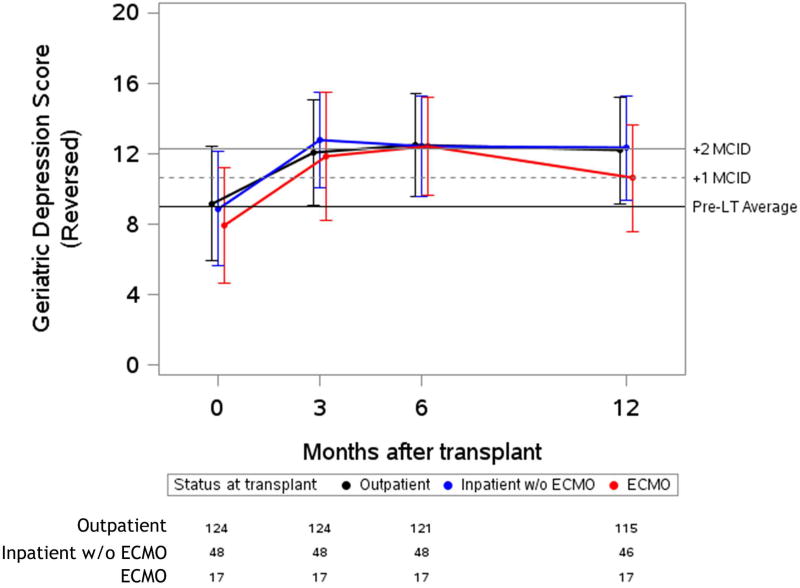
Figure 2. Unadjusted plots of average health-related quality of life from before to one-year after lung transplantation.
Unadjusted plots of average health-related quality of life (HRQL) from before to one-year after lung transplantation. (a) SF-12PCS (Generic-Physical HRQL); (b) SF-12MCS (Generic-Mental HRQL) (c); AQ20R (Respiratory Specific HRQL) (d); EQ5D (Health Utility HRQL); (e) GDS (Depression Scale). Red lines represent subjects who required ECMO as a bridge to transplant, blue lines represent subjects who were hospitalized but not on ECMO at the time of transplant and black lines represent subjects called in for transplant from the outpatient setting. Whiskers represent one standard deviation above and below the mean HRQL values for each time point. On the Y axis, the first horizontal black line denotes pre-transplant mean scores; the dashed and solid horizontal lines above denote a change in score equal to one- and two-times the minimally clinically important difference (MCID), respectively.
For all models, we tested whether HRQL or GDS changed from baseline to after transplant within each group and whether the magnitude of the change was different across groups. Since our primary aim was to estimate the within-subject change in HRQL across all time points (including baseline), we did not control for baseline HRQL.
Not all subjects completed all study visits. Overall, the number of missing surveys was small (104 of 742 potential timepoints, 14%). We did not impute these missing data because the maximum likelihood methods used to fit mixed effects models provide valid estimates for missing values 37. Nevertheless, it is possible that informative missingness could have impacted our effect estimates. As a result, we performed a sensitivity analysis in which we imputed missing values. Missing data were considered missing at random if subjects did not complete surveys for reasons other than their health (e.g., they missed their clinic appointment because of inclement weather or left clinic prior to completing their survey because of traffic). If data was believed to be missing at random, the clinical chart was reviewed to ensure that the subject had stable allograft function and was not dealing with an acute medical issue. Surveys were categorized as missing not at random if subjects were too ill to complete the survey. For data missing at random, we used 10-fold multiple imputation with chained equations 38. For data missing not at random, we imputed scores using the median of the lowest quartile of HRQL scores for all other participants at that time point, as we have done previously 3.
Analyses were conducted using Stata 15 (StataCorp, College Station, TX).
Results
Of the 259 subjects who completed pre-transplant assessments and underwent transplantation during the study period, the 189 subjects under the age of 65 comprised the cohort for this analysis (Figure 1). Of this cohort, 17 subjects (9%) were on ECMO support at the time of transplantation, 48 (25%) were inpatients but not on ECMO and 124 (66%) were outpatients. Of the patients placed on ECMO as a bridge to transplantation, eight were placed on veno-arterial ECMO and nine were placed on veno-venous ECMO. Ten of the patients were listed at the time of being placed on ECMO and seven of the patients were placed on ECMO to facilitate completion of lung transplant evaluation. Additionally, three of the patients bridged to transplant on ECMO required prolonged ECMO support after transplantation. In the whole cohort, 183 subjects underwent bilateral lung transplantation (including all those who were placed on ECMO as a bridge to transplantation), three underwent single lung transplantation, and three underwent combined heart and lung transplantation. The three groups were similar in age, gender, race, BMI, pre-transplant disease category, FEV1 and forced vital capacity (FVC) (Table 1). The groups were different in 6MWD and lung allocation score (p < 0.001).
Table 1.
Cohort Demographics and Clinical Variables
| ECMO n=17 |
Inpatient n=48 |
Outpatient n=124 |
Whole Cohort n=189 |
p-value | |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 46.1 ± 16.6 | 52.9 ± 9.0 | 51.8 ± 11.6 | 51.6 ± 11.6 | 0.11 |
|
| |||||
| Female | 9 (53) | 24 (50) | 52 (42) | 85 (45) | |
| Male | 8 (47) | 24 (50) | 72 (58) | 104 (55) | 0.50 |
|
| |||||
| White | 12 (80) | 26 (59) | 84 (70) | 122 (68) | |
| Black/African American | 1 (7) | 5 (11) | 11 (9) | 17 (9) | |
| Asian | 0 (0) | 2 (5) | 10 (8) | 12 (7) | |
| Hispanic | 2 (13) | 9 (20) | 16 (13) | 27 (15) | |
| Hawaiian/Pac. Islander | 0 (0) | 1 (2) | 0 (0) | 1 (1) | |
| Other | 0 (0) | 1 (2) | 0 (0) | 1 (1) | 0.42 |
|
| |||||
| LAS Group A:COPD | 1 (6) | 4 (8) | 21 (17) | 26 (14) | |
| LAS Group B: PAH | 1 (6) | 1 (2) | 8 (6) | 10 (5) | |
| LAS Group C: CF | 2 (11) | 4 (8) | 17 (14) | 23 (12) | |
| LAS Group D: IPF | 13 (76) | 39 (81) | 78 (63) | 130 (69) | 0.36 |
|
| |||||
| Expired at 1 year | 1 (6) | 2 (4) | 2 (2) | 5 (3) | 0.44 |
|
| |||||
| FEV1 (liters) | 1.7 ± 0.77 | 1.35 ± 0.74 | 1.41 ± 0.70 | 1.43 ± 0.72 | 0.17 |
| FVC (liters | 2.29 ± 0.74 | 1.84 ± 0.89 | 2.07 ± 0.78 | 2.03 ± 0.81 | 0.10 |
| BMI (kg/m2) | 24.5 ± 4.9 | 25.3 ± 4.1 | 25.4 ± 4.4 | 25.3 ± 4.4 | 0.75 |
| 6MWD (meter) | 212 ± 199 | 176 ± 133 | 283 ± 126 | 250 ± 143 | <0.001 |
| LAS | 67.8 ± 26.6 | 69.2 ± 19.4 | 44.5 ± 11.4 | 52.8 ± 19.3 | <0.001 |
Data presented as mean +/−standard deviation or as number of subjects (% of total) Diagnostic indication for transplant as categorized by groupings in the Lung Allocation Score37
Abbreviations: FEV1 (Forced Expiratory Volume, 1 second); FVC (Forced Vital Capacity); BMI (Body Mass Index); 6MWD (six minute walk distance); LAS (Lung Allocation Score); COPD (Chronic Obstructive Pulmonary Disease); PAH (Pulmonary Arterial Hypertension); CF (Cystic Fibrosis); IPF (Idiopathic Pulmonary Fibrosis)
Overall survival at one year after transplant was 97%, and was similar between groups (p = 0.44). One subject in the ECMO group (1/17; 6%), two subjects in the inpatient but not on ECMO group (2/48; 4%), and two subjects in the outpatient group (2/124; 2%) died within the first year.
Before transplant, HRQL was similar amongst the three groups by SF12-PCS and AQ20R. Depressive symptoms by GDS were also similar amongst the three groups (Table 2). Outpatients reported better baseline HRQL by SF12-MCS and EQ5D. After transplant, HRQL and depressive symptoms generally improved across all three groups (Figure 2). Overall, peak improvement in HRQL and depressive symptoms was seen in the early period, within six months post-transplant, and remained stable in the late period, through twelve months post-transplant. The magnitude of these early improvements, however, varied by instrument (Table 3). For example, in generic physical HRQL (SF12-PCS), early improvements exceeded three-fold the MCID for all groups (p-value for change < 0.001; p-value for difference among groups = 0.27).
Table 2.
Health-Related Quality of Life before Lung Transplantation
| ECMO n=17 |
Inpatient n=48 |
Outpatient n=124 |
Whole Cohort n=189 |
p-value | |
|---|---|---|---|---|---|
|
| |||||
| SF12-PCS (MCID=5) | 24.7 ± 6.8 | 21.7 ± 8.0 | 23.3 ± 7.9 | 23.0 ± 7.8 | 0.35 |
| SF12-MCS (MCID=5) | 43.9 ± 11.1 | 45.3 ± 10.0 | 49.5 ± 9.9 | 48.0 ± 10.3 | 0.01 |
| AQ20R (MCID=1.75) | 6.1 ± 3.3 | 6.2 ± 3.7 | 6.2 ± 3.4 | 6.2 ± 3.4 | 0.99 |
| EQ5D (MCID=0.06) | 0.51 ± 0.29 | 0.53 ± 0.19 | 0.68 ± 0.17 | 0.62 ± 0.21 | <0.001 |
| GDS (MCID=1.65) | 7.9 ± 3.3 | 8.9 ± 3.3 | 9.2 ± 3.3 | 9.0 ± 3.3 | 0.33 |
Data presented as unadjusted mean score ± standard deviation.
HRQL Instruments: Generic Physical Instrument: SF12-PCS (Short Form 12 – Physical Component Score) Range 0–100; Generic Mental Instrument: SF12-MCS (Short Form 12 – Mental Component Score) Range 0–100; Respiratory-Specific Instrument: AQ20R (Airways Questionnaire 20-Revised) Range 0–20 – reverse coded for analysis; Health-Utility Instrument: EQ5D (EuroQoL 5D) Range −1.11 – 1; Depression Scale: GDS (Geriatric Depression Scale) Range: 0–15 – reverse coded for analysis
Table 3.
Effect Estimates for change in HRQL over time from before through six months after lung transplantation
| Instrument | Instrument Type | ECMO n=17 |
Inpatient n=48 |
Outpatient n=124 |
p- value |
|---|---|---|---|---|---|
|
| |||||
| SF12-PCS (MCID=5) | Generic-Physical | 16.78 (10.65, 21.91) | 19.56 (15.62, 23.50) | 20.78 (18.50, 23.07) | 0.27 |
| SF12-MCS (MCID=5) | Generic-Mental | 8.78 (3.31, 14.26) | 7.48 (3.97, 10.99) | 4.48 (2.47, 6.49) | 0.01 |
| AQ20R (MCID=1.75) | Respiratory Specific | 10.76 (8.57, 12.96) | 9.84 (8.45, 11.23) | 9.76 (8.96, 10.56) | 0.59 |
| EQ5D (MCID=0.06) | Health Utility | 0.31 (0.20, 0.42) | 0.29 (0.22, 0.36) | 0.17 (0.13, 0.21) | 0.001 |
| GDS (MCID=1.65) | Depression Scale | 4.81 (3.15, 6.48) | 3.43 (2.38, 4.49) | 3.54 (2.94, 4.14) | 0.09 |
Data presented as mean effect estimates with 95% confidence intervals as quantified by segmented linear mixed effects models with a joint point at six months. Models were adjusted for age, sex, diagnosis, preoperative forced expiratory volume in 1 second, six-minute walk distance, and lung allocation score.
HRQL Instruments: Generic Physical Instrument: SF12-PCS (Short Form 12 – Physical Component Score) Range 0–100; Generic Mental Instrument: SF12-MCS (Short Form 12 – Mental Component Score) Range 0–100; Respiratory-Specific Instrument: AQ20R (Airways Questionnaire 20-Revised) Range 0–20 – reverse coded for analysis; Health-Utility Instrument: EQ5D (EuroQoL 5D) Range −1.11 – 1; Depression Scale: GDS (Geriatric Depression Scale) Range: 0–15 – reverse coded for analysis
All groups reported early improvement in respiratory-specific HRQL exceeding five-fold the MCID (p-value for change < 0.001; p value for difference among groups 0.59). In health utility (EQ5D), the ECMO and inpatient groups both had early improvements of four-fold the MCID, while the outpatient group improved by nearly three-fold the MCID (p-value for change < 0.001; p value for difference among groups=0.001). Of note, the baseline EQ5D scores were higher in the outpatient group, whereas after transplant, the absolute EQ5D scores were similar across groups. Depressive symptoms had early improvements which were greater than the MCID in all groups (p-value for change ≤ 0.001; p value for difference among groups=0.09). An exception to improvement in HRQL after transplant was the SF12-MCS, in which there was statistically significant early improvement that did not meet the MCID threshold in the outpatient group (p-value for change = 0.001). However, early improvements in HRQL by SF12-MCS did exceed the MCID in the inpatient and ECMO groups (p-value for change ≤ 0.002; p value for difference among groups = 0.01). The effect estimates for change in HRQL when controlled for baseline GDS or in unadjusted analysis were not substantially different than the primary analysis (Supplemental Table 2 and Supplemental Table 3). The effect estimate for change was also not substantially different when imputation methods were used for missing data (Supplemental Table 4).
Discussion
In a contemporary cohort of adults undergoing lung transplantation, we found that patients requiring ECMO as a bridge to transplant experience substantial early improvements in HRQL and depressive symptoms that are comparable to those undergoing transplant without bridging by ECMO. The improvements in generic-physical, respiratory-specific, and health utility metrics of HRQL, as well as depressive symptoms, exceeded one-to five-fold what is considered to be the minimally clinically important difference (MCID). The greatest improvement was seen in respiratory-specific HRQL, whereas the smallest improvement was in generic-mental HRQL (SF12-MCS). Our findings are consistent with prior studies demonstrating either no or minimal improvement in HRQL after lung transplantation when using a generic-mental HRQL instrument 2,3. It remains unclear whether the reason for this improvement is attributable to high baseline scores, the emergence of new problems after transplant that negatively impact mental health-related quality of life, or if the instrument itself is not sensitive enough to detect changes in our population.
It is surprising that patients bridged to lung transplantation with ECMO reported similar improvements in HRQL and depressive symptoms as other groups. Since surviving ECMO with resultant lung transplant might be similar to surviving critical illness more generally, we hypothesized that HRQL and depressive symptoms would be worse in this group relative to others. Indeed, survivors of critical illness across medical and surgical populations report marked impairments in HRQL 19,20,23. While speculative, differences between surviving general critical illness and the illness preceding lung transplantation might account for our findings. Generally, acute critical illness is an unexpected event, whereas most lung transplant recipients have suffered from advanced lung disease for some time. Thus, these patients may anticipate gradual, if not sudden, worsening. As a result, they may be more familiar with poor physical functioning, severe dyspnea, and the psychologic challenges of potential loss of life. Also, lung transplantation offers a potential reversal of chronic and progressive illness. This potential may help patients mitigate the stresses of critical illness in a way that other critically ill patients cannot. Another possibility is that lung transplant recipients represent a group highly enriched to have strong social support. This support network can help mitigate the influence of illness on HRQL. Finally, lung transplant candidates are also not likely to have significant co-morbidities because of the rigorous selection process which may facilitate their recovery from critical illness.
To date, most studies evaluating success of ECMO as a bridge to lung transplantation have defined efficacy by survival alone 39–41. In our clinical experience, patients – even those on ECMO - are keenly interested in their potential quality of life after lung transplantation. Given the substantial potential for morbidity inflicted by ECMO and its increasing use as a bridge to transplant, definitions of transplant efficacy should reflect the metrics most important to patients. Our study directly addresses this clinical need and complements the one other study of HRQL in recipients bridged to lung transplantation by ECMO 42. In that study, HRQL was assessed in seven subjects bridged to transplant with ECMO and 77 subjects transplanted without the use of ECMO bridging who survived the first post-operative year. HRQL in the non-ECMO group was assessed by the Visual Analog Scale and, in the ECMO group, both the Visual Analog Scale and EQ5D. The investigators found no difference in HRQL between the two groups by the Visual Analog Scale.
It should be noted that our study has limitations. Although our cohort represents the largest evaluation of the impact of lung transplantation on HRQL in patients bridged to transplant with ECMO, our sample size is, nonetheless, small. The impact of this sample size is reflected in the wide confidence intervals surrounding the effect estimates of change in HRQL and depressive symptoms in the ECMO group. For example, in the SF12-PCS the lower bound of the 95% confidence interval in the ECMO group falls nearly one-fold the MCID lower than for the inpatient and outpatient groups. Therefore, it is possible that lung transplant for patients bridged on ECMO may not lead to as large improvements as are seen in the other groups. Despite this possible difference, patients bridged on ECMO still derive substantial benefit in addition to a clear survival benefit. Also, although the types of patients undergoing transplantation at our center are similar to many others, our findings may not be generalizable. Further, HRQL outcomes and depressive symptoms beyond the first post-operative year were not addressed. Finally, although we chose a variety of HRQL instruments to perform a more comprehensive evaluation of HRQL, it is possible that some of the health domains impacted by ECMO, such as cognitive functioning, were not adequately queried 43,44.
Despite these limitations, our study features several strengths. This study represents the first prospective longitudinal cohort study to evaluate the impact of lung transplantation on HRQL and depressive symptoms in patients who were bridged to transplant with ECMO. Querying the same subjects before and repeatedly after transplant gives the ability to evaluate changes in HRQL on an individual subject level. Furthermore, our efforts to update preoperative assessments at the time of changes in clinical status and to address missing data through imputation approaches strengthen the validity of our effect estimates. By utilizing four instruments to assess HRQL as well as one depression scale, and employing two comparison groups, we were able to provide a more comprehensive picture of HRQL after transplant in those on ECMO in the run-up to transplant. Finally, that our study required seven years to amass 17 subjects bridged to transplant with ECMO with post-transplant follow-up suggests that larger sample sizes are likely to only result from multicenter studies or efforts from high ECMO volume centers.
In conclusion, we found that the impact of lung transplantation on HRQL and depressive symptoms for patients requiring preoperative ECMO is large and appears to be similar to non-critically ill transplant candidates. This knowledge may provide clinicians more tools to counsel critically ill patients and their families when considering ECMO as a bridge to transplant. Future work should focus on a multicenter approach to measure HRQL and investigate if our findings apply to the international transplant community.
Supplementary Material
Video of a UCSF lung transplant recipient with idiopathic pulmonary fibrosis who was placed on ECMO as a bridge to transplantation. With ECMO the patient was able to ambulate and maintain his strength before transplantation despite hypoxemic respiratory failure.
Central Message.
The impact of lung transplantation on HRQL and depressive symptoms for patients requiring preoperative ECMO is large and similar to the impact seen in non-critically ill transplant candidates.
Perspective Statement.
Lung transplantation improves Health-Related Quality of Life (HRQL) and depressive symptoms for patients with end-stage lung disease. Patients requiring ECMO prior to transplantation achieve the same magnitude of improvements as non-critically ill transplant candidates. This knowledge provides clinicians more tools to counsel critically ill patients when considering ECMO as a bridge to transplant.
Acknowledgments
Funding: NHLBI K23 HL111115 (JPS)
Glossary of Abbreviations
- 6MWD
six-minute walk distance
- AQ20R
Airway Questionnaire 20-Revised
- ECMO
Extra Corporeal Membrane Oxygenation
- EQ5D
EuroQol 5D
- FEV1
Forced Expiratory Volume in 1 second
- GDS
Geriatric Depression Scale
- HRQL
Health-Related Quality of Life
- LAS
Lung Allocation Score
- MCID
Minimally Clinically Important Difference
- SF12-MCS
Short form 12 Mental Component Score
- SF12-PCS
Short Form 12 Physical Component Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Justification for Greater than Seven Authors: Lung Transplantation is increasingly a multidisciplinary endeavor, especially in relation to the use of ECMO as a bridge to transplantation. The authors included in this study consist of our multidisciplinary team of physicians, surgeons, and researchers. Our team has regular research meetings where we discuss works in progress and how to improve them. Without the included authors, this study would not be possible at our institution. Additionally, this manuscript was previously published in abstract form with the above listed authors and was presented as an oral presentation. In addition to reviewing the manuscript and abstract, the authors above were involved in the oral presentation.
Disclosures: The authors have no disclosures and no relevant conflicts of interest.
References
- 1.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Singer LG, Chowdhury NA, Faughnan ME, et al. Effects of Recipient Age and Diagnosis on Health-related Quality-of-Life Benefit of Lung Transplantation. Am J Respir Crit Care Med. 2015;192(8):965–973. doi: 10.1164/rccm.201501-0126OC. [DOI] [PubMed] [Google Scholar]
- 3.Singer JP, Katz PP, Soong A, et al. Effect of Lung Transplantation on Health-Related Quality of Life in the Era of the Lung Allocation Score: A U.S. Prospective Cohort Study. Am J Transplant. 2017;17(5):1334–1345. doi: 10.1111/ajt.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb J, Warnecke G, Hadem J, et al. Outcome of critically ill lung transplant candidates on invasive respiratory support. Intensive Care Med. 2012;38(6):968–975. doi: 10.1007/s00134-012-2551-y. [DOI] [PubMed] [Google Scholar]
- 5.Kilic A, Beaty CA, Merlo CA, Conte JV, Shah AS. Functional status is highly predictive of outcomes after redo lung transplantation: an analysis of 390 cases in the modern era. Ann Thorac Surg. 2013;96(5):1804–11. doi: 10.1016/j.athoracsur.2013.05.080. discussion 1811. [DOI] [PubMed] [Google Scholar]
- 6.Moreno P, Alvarez A, Santos F, et al. Extended recipients but not extended donors are associated with poor outcomes following lung transplantation. Eur J Cardio-Thoracic Surg. 2014;45(6):1040–1047. doi: 10.1093/ejcts/ezt501. [DOI] [PubMed] [Google Scholar]
- 7.Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg. 2010;139(3):765–773.e1. doi: 10.1016/j.jtcvs.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Chiumello D, Coppola S, Froio S, Colombo A, Del Sorbo L. Extracorporeal life support as bridge to lung transplantation: a systematic review. Crit Care. 2015;19(1):19. doi: 10.1186/s13054-014-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Guzman E, Hoopes CW, Zwischenberger JB. The Evolution of Extracorporeal Life Support as a Bridge to Lung Transplantation. ASAIO J. 2013;59(1):3–10. doi: 10.1097/MAT.0b013e31827461c2. [DOI] [PubMed] [Google Scholar]
- 10.Hayanga JWA, Lira A, Aboagye JK, Hayanga HK, D’Cunha J. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg. 2016;22(4):406–410. doi: 10.1093/icvts/ivv379. [DOI] [PubMed] [Google Scholar]
- 11.Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg. 2013;145(4):1065–1071. doi: 10.1016/j.jtcvs.2012.12.067. [DOI] [PubMed] [Google Scholar]
- 12.Todd EM, Biswas Roy S, Hashimi AS, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: A single-center experience in the present era. J Thorac Cardiovasc Surg. 2017;154(5):1798–1809. doi: 10.1016/j.jtcvs.2017.06.063. [DOI] [PubMed] [Google Scholar]
- 13.Hoetzenecker K, Donahoe L, Yeung JC, et al. Extracorporeal life support as a bridge to lung transplantation - experience of a high-volume transplant center. J Thorac Cardiovasc Surg. 2017;0(0) doi: 10.1016/j.jtcvs.2017.09.161. [DOI] [PubMed] [Google Scholar]
- 14.Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg. 2013;145(3):862–868. doi: 10.1016/j.jtcvs.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Banga A, Mohanka M, Mullins J, et al. Hospital length of stay after lung transplantation: Independent predictors and association with early and late survival. J Heart Lung Transplant. 2017;36(3):289–296. doi: 10.1016/j.healun.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Oude Lansink-Hartgring A, van den Hengel B, van der Bij W, et al. Hospital Costs Of Extracorporeal Life Support Therapy. Crit Care Med. 2016;44(4):717–723. doi: 10.1097/CCM.0000000000001477. [DOI] [PubMed] [Google Scholar]
- 17.Inci I, Klinzing S, Schneiter D, et al. Outcome of Extracorporeal Membrane Oxygenation as a Bridge To Lung Transplantation: An Institutional Experience and Literature Review. Transplantation. 2015;99(8):1667–1671. doi: 10.1097/TP.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 18.Makdisi G, Wang I-W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166–76. doi: 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNelly AS, Rawal J, Shrikrishna D, et al. An Exploratory Study of Long-Term Outcome Measures in Critical Illness Survivors. Crit Care Med. 2016;44(6):e362–e369. doi: 10.1097/CCM.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 20.Wolters AE, Peelen LM, Welling MC, et al. Long-Term Mental Health Problems After Delirium in the ICU*. Crit Care Med. 2016;44(10):1808–1813. doi: 10.1097/CCM.0000000000001861. [DOI] [PubMed] [Google Scholar]
- 21.Fleck TPK, Dangel G, Bächle F, et al. Long-Term Follow-Up on Health-Related Quality of Life After Mechanical Circulatory Support in Children. Pediatr Crit Care Med. 2017;18(2):176–182. doi: 10.1097/PCC.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 22.Chandler HK, Teppa B, Johnson KA, McCracken C, Fortenberry JD, Paden ML. Determining comorbidities and quality of life among pediatric survivors of extracorporeal life support. J Crit Care. 2015;30(5):1085–1089. doi: 10.1016/j.jcrc.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolaitis NA, Huang D, Soong A, et al. ECMO Prior to Lung Transplantation Does Not Impact Health-Related Quality of Life (HRQL) After Transplant. J Hear Lung Transplant. 2017;36(4):S72–S73. [Google Scholar]
- 25.Singer JP, Chen J, Blanc PD, Leard LE, Kukreja J, Chen H. A thematic analysis of quality of life in lung transplant: the existing evidence and implications for future directions. Am J Transplant. 2013;13(4):839–850. doi: 10.1111/ajt.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seiler A, Klaghofer R, Ture M, Komossa K, Martin-Soelch C, Jenewein J. A systematic review of health-related quality of life and psychological outcomes after lung transplantation. J Heart Lung Transplant. 2016;35(2):195–202. doi: 10.1016/j.healun.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): a literature review and directions for future research. Curr Opin Rheumatol. 2002;14(2):109–114. doi: 10.1097/00002281-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Eisner MD, Katz PP, Yelin EH, Blanc PD. Measuring disease-specific quality of life in obstructive airway disease: validation of a modified version of the airways questionnaire 20. Chest. 2006;129(6):1644–1652. doi: 10.1378/chest.129.6.1644. [DOI] [PubMed] [Google Scholar]
- 30.Norman GR, Sloan JA, Wyrwich KW. Interpretation of Changes in Health-related Quality of Life. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 31.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 32.Dew MA, Rosenberger EM, Myaskovsky L, et al. Depression and Anxiety as Risk Factors for Morbidity and Mortality After Organ Transplantation. Transplantation. 2015;100(5):988–1003. doi: 10.1097/TP.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith PJ, Blumenthal JA, Snyder LD, et al. Depressive symptoms and early mortality following lung transplantation: A pilot study. Clin Transplant. 2017;31(2):e12874. doi: 10.1111/ctr.12874. [DOI] [PubMed] [Google Scholar]
- 34.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35(5):796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 36.Egan TM, Murray S, Bustami RT, et al. Development of the New Lung Allocation System in the United States. Am J Transplant. 2006;6(5p2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 37.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7(1–2):305–315. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]
- 38.Lee KJ, Simpson JA. Introduction to multiple imputation for dealing with missing data. Respirology. 2014;19(2):162–167. doi: 10.1111/resp.12226. [DOI] [PubMed] [Google Scholar]
- 39.Biscotti M, Gannon WD, Agerstrand C, et al. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann Thorac Surg. 2017;104(2):412–419. doi: 10.1016/j.athoracsur.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 40.Schechter MA, Ganapathi AM, Englum BR, et al. Spontaneously Breathing Extracorporeal Membrane Oxygenation Support Provides the Optimal Bridge to Lung Transplantation. Transplantation. 2016;100(12):2699–2704. doi: 10.1097/TP.0000000000001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes D, Tobias JD, Tumin D. Center Volume and Extracorporeal Membrane Oxygenation Support at Lung Transplantation in the Lung Allocation Score Era. Am J Respir Crit Care Med. 2016;194(3):317–326. doi: 10.1164/rccm.201511-2222OC. [DOI] [PubMed] [Google Scholar]
- 42.Lansink-Hartgring AO, van der Bij W, Verschuuren EA, et al. Extracorporeal Life Support as a Bridge to Lung Transplantation: A Single-Center Experience With an Emphasis on Health-Related Quality of Life. Respir Care. 2017;62(5):588–594. doi: 10.4187/respcare.05300. [DOI] [PubMed] [Google Scholar]
- 43.Smith PJ, Blumenthal JA, Carney RM, et al. Neurobehavioral Functioning and Survival Following Lung Transplantation. Chest. 2014;145(3):604–611. doi: 10.1378/chest.12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith PJ, Rivelli S, Waters A, et al. Neurocognitive Changes after Lung Transplantation. Ann Am Thorac Soc. 2014;11(10):1520–1527. doi: 10.1513/AnnalsATS.201406-232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of a UCSF lung transplant recipient with idiopathic pulmonary fibrosis who was placed on ECMO as a bridge to transplantation. With ECMO the patient was able to ambulate and maintain his strength before transplantation despite hypoxemic respiratory failure.