Abstract
The sharp increase in overdose deaths involving illicit opioid use has been declared a national crisis in the United States. This growing number of overdose deaths can in part be attributed to the increased frequency of fentanyl contamination in the United States heroin supply. To combat this growing trend, we designed a vaccine containing a mixture of heroin and fentanyl hapten-conjugates as a proof-of-concept immunotherapy targeting a combination of these drugs. Rodents immunized with the admixture vaccine showed drug retention in serum and reduced distribution in the brain after administration of an intravenous bolus of heroin coadministered with fentanyl (10% w/w). Moreover, the admixture vaccine performed as well as or better than individual immunoconjugate vaccines in antinociception behavioral models and recognized six other fentanyl analogues with nanomolar affinity. Taken together, these data highlight the potential of an admixture vaccine against heroin contaminated with fentanyl.
Keywords: Overdose, heroin, fentanyl, vaccine, hapten
Graphical Abstract
INTRODUCTION
The illicit use and demand of heroin has been increasing due to two primary factors: the growing availability of heroin in the United States and controlled prescription opioid abusers transitioning from drugs like oxycodone to more readily accessible heroin.1 This increase in heroin usage has consequently led to a growing trend in heroin-related deaths, which increased by 248% from 2011 to 2014.1 There are many underlying determinants prompting an increase in the mortality rates associated with heroin, which cannot be solely blamed on the increase in heroin usage. The primary culprit is thought to be highly toxic synthetic opioid adulterants such as fentanyl, exacerbating the opioid crisis and contributing to a sharp spike in opioid deaths.2 The precipitous rise of fentanyl disguised as heroin is due to its inexpensive cost of production and its enhanced potency, as fentanyl is 25–50 times more potent than heroin. By incorporating fentanyl, the same drug payload can be delivered for a fraction of the cost of heroin. Now, due to its infamous potency, fentanyl is appearing as a contaminant in other illicitly abused drugs as it becomes one of the most prominent killers linked to the nation’s drug crisis.3,4
Another contributor to fatal opioid overdose involves recovering substance abusers, who are at risk of overdose during periods of relapse due to unrealized decreased tolerance.5 Naloxone, a competitive opioid receptor antagonist, is currently the standard emergency treatment for opioid overdose. While buprenorphine and methadone, weak agonists at the μ-opioid receptor, are the most prevalent medications used for long-term opioid addiction management, these traditional pharmacotherapies, although vital for treating opioid substance use disorders, still suffer from side effects such as dysphoria for antagonists and risk of abuse for partial agonists.
We have previously published several examples of immunotherapy as an innovative treatment strategy for substance use disorders. This strategy provides immunoantagonistic protection from the drug without interacting with the drug’s target receptor(s).6–9 Moreover, antidrug vaccines produce a long-lasting blockade against the target drug without any of the side effects characteristic of traditional pharmacological therapies. The importance of this strategy was seen in preventing various hallmarks of heroin abuse in rodents, including heroin reward, drug-induced reinstatement of drug seeking behavior, and reescalation of compulsive heroin self-administration following abstinence in dependent rats.6 In a nonhuman primate study, the heroin vaccine was able to elicit the production of IgG antibodies targeting heroin, morphine, and mainly 6-acetylmorphine (6-AM), the primary mediator of heroin’s psychoactivity.10 Importantly, all nonhuman primates treated with this vaccine demonstrated blockade of acute heroin effects similar to naltrexone.11 Moreover, we have also disclosed a fentanyl immunoconjugate vaccine, which shifted the fentanyl dose–effect curve in antinociceptive testing 30-fold in mice.7 To our knowledge, this is the first and only report of a fentanyl conjugate vaccine, which demonstrated an ability to act as a “synthetic opioid pan-vaccine,” mitigating the effects of not only fentanyl, but also six other closely related fentanyl derivatives.
In our efforts to further combat the opioid crisis as well as tackle the rampant emergence of fentanyl in the United States heroin supply, we questioned whether a heroin–fentanyl dual vaccine could be feasible. A major reason for our addressing of heroin contaminated with fentanyl is that brain hypoxia is greatly potentiated by the drug mixture of heroin contaminated fentanyl compared to heroin or fentanyl alone.12 Admixture haptenic-conjugate vaccines are rare; however, one example utilizing structurally congruent opioids, morphine and oxy-codone, conjugated to an immunogenic carrier protein has been reported.13 Yet even with this disclosure, there were sound reasons for pessimism on the success of a heroin–fentanyl admixture vaccine. Thus, while all of these drugs target the μ-opioid receptors, an admixture vaccine addressing both heroin and fentanyl would require the targeting of two drastically different chemical scaffolds. If successful, this vaccine could lend itself as a proof-of-concept for protection against the unintentional fentanyl poisoning of heroin users. Herein, we detail an admixture immunization strategy vaccine that demonstrates efficacy against a combination of heroin and fentanyl.
RESULTS AND DISCUSSION
The frequency of fentanyl contamination appearing in the United States heroin supply is growing at an alarming rate in several parts of the US.1 In devising a vaccine to combat this dangerous trend, we were interested in testing an admixture vaccine and its ability to protect against both drugs (Figure 1).
Figure 1.
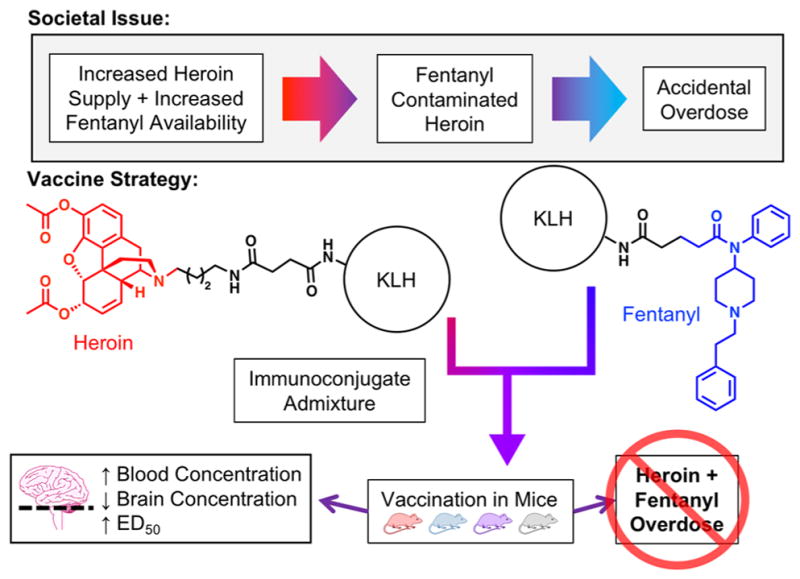
Illustration describing admixture vaccine strategy, including representative structures of each hapten immunoconjugate vaccine. Fentanyl and heroin are highlighted in blue and red, respectively.
To begin this campaign, we monitored conjugation numbers for each hapten on BSA over time (Figure S13) and found that the fentanyl hapten rapidly attaches within 30 min to BSA, whereas heroin hapten conjugation required extended incubation time. We designed and implemented a study to investigate the efficacy of an admixture vaccine after obtaining individual immunoconjugates with comparable copy numbers (Tables 1 and S1). Each conjugate vaccine was prepared separately and combined according as shown in Table 1. The amount of immunogenic carrier protein (T-cell epitope) was kept constant among all the vaccination groups to stimulate an equivalent adaptive immune response.
Table 1.
Immunoconjugate Formulation Parameters and Vaccine Schedule Used for Each Groupa
| Vaccination Group | Immunoconjugate (μg/dose) | Conjugation Number | Alum (mg/mL) | Adjuvant | Antinoc. Drug | BBB Drug |
|---|---|---|---|---|---|---|
| Fent-KLH | 50 μg Fent-KLH | 11.8 | 0.8 | 50 μg CpG | Fentanyl | Fentanyl + Heroin |
| Her-KLH | 50 μg Her-KLH | 7.3 | 0.8 | 50 μg CpG | Heroin | Fentanyl + Heroin |
| Fent-KLH + Her-KLH | 25 μg Fent-KLH + 25 μg Her-KLH | 11.8; 7.3 | 0.8 | 50 μg CpG | Fentanyl | Fentanyl + Heroin |
| Heroin | Fentanyl + Heroin | |||||
| Control | 50 μg KLH | n/a | 0.8 | 50 μg CpG | Fentanyl | Fentanyl + Heroin |
| Heroin | Fentanyl + Heroin | |||||
|
| ||||||
Antinoc. stands for antinociception; BBB stands for blood-brain barrier study. Blue corresponds to mice receiving only fentanyl conjugate vaccines; red corresponds to mice receiving only heroin conjugate vaccines; purple corresponds to mice receiving admixture of heroin and fentanyl conjugate vaccines; and white corresponds to the control group. In the admixture and control group, the 12 mice were split to test either heroin or fentanyl during an antinociception assay.
All sera collected from mice at week 3 and 5 were analyzed by ELISA against both Her-BSA and Fent-BSA to determine the presence of any anti-heroin or anti-fentanyl antibodies (Table 2). As expected, each individual vaccine exhibited robust midpoint titer levels. Heroin titers increased in vaccination groups containing Her-KLH after a subsequent boost between week 3 and week 5; however, fentanyl titers in the individual Fent-KLH vaccine decreased slightly over time, but exhibited increased antibody affinity by surface plasmon resonance (SPR, Table 2). We have observed this phenomenon for several antidrug vaccines, including a fentanyl vaccine,7 an oxycodone vaccine,14 and a methamphetamine vaccine,8 and this may be the result of a shortened vaccination schedule.15 Antibody titers detected in the admixture were approximately half the level detected in the individual Her-KLH vaccine, as predicted based on the amount of Her-KLH immunoconjugate employed in each group (Table 1), but antibody affinity remained at similar levels. This trend was also observed for fentanyl antibodies in the admixture vaccine group by week 5 (Table 2). Surprisingly, the admixture vaccine possessed more potent IC50 values for fentanyl by week 5, indicative of a polyclonal antibody response with increased drug affinity compared to the individual immunoconjugate vaccines (Table 2).
Table 2.
ELISA and SPR Results for Each Vaccination Group from Week 3 and Week 5 Bleedsa
| vaccination group | antigen | midpoint titersb | IC50 (μM) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| week 3 | week 5 | analyte | week 3 | week 5 | ||
| Fent-KLH | Her-BSA | 517 ± 253 | 3084 ± 1453 | 6-AMc | ||
| Fent-BSA | 35139 ± 8708 | 21158 ± 3134 | fentanyl | 0.40 | 7.34 nM | |
| Her-KLH | Her-BSA | 40600 ± 8893 | 55432 ± 11952 | 6-AMd | 0.20 | 0.22 |
| Fent-BSA | 728 ± 469 | 2141 ± 591 | fentanylc | |||
| Fent-KLH + Her-KLH | Her-BSA | 20100 ± 2779 | 25872 ± 3251 | 6-AMd | 0.28 | 0.27 |
| Fent-BSA | 7435 ± 1724 | 11303 ± 872 | fentanyl | 0.24 | 9.31 nM | |
| control | Her-BSA | 10 ± 8 | 13 ± 7 | 6-AMd | nde | nde |
| Fent-BSA | 0 ± 0 | 16 ± 8 | fentanyl | nde | nde | |
IC50 values are only reported for 6-acetylmorphine and fentanyl. The log IC50 values ± SEM table can be found in Table S2 in the Supporting Information.
Midpoint titers are reported as means ± SEM.
6-AM and fentanyl were not run for Fent-KLH and Her-KLH vaccination groups, respectively.
6-AM was the primary analyte used in the SPR studies due to its longer half-life at pH 7.4 at rt compared to heroin. Heroin rapidly hydrolyzed during the experimental time frame and therefore was not reported.
Control vaccine groups were run by SPR but did not converge due to lack of detected antibody binding.
In the current study, hot plate and tail flick antinociception was used as a surrogate for drug reward because it is mediated in the central nervous system and provides a relevant behavioral model of the vaccine’s ability to reduce drug access to brain and its subsequent effects. ED50 values reflect the effective dose of drug where half of the animals in a group experience the full antinociceptive effect of the opioid. Potency ratios were calculated by dividing the vaccine-shifted ED50 value from the control values in each antinociceptive test (Figure 2). The calculated immunoconjugate dosage for the admixture vaccine was reduced by half (i.e., 25 μg immunoconjugate each instead of 50 μg), yet the vaccine still attenuated both heroin’s and fentanyl’s nociception effects at comparable levels to the singular Her-KLH or Fent-KLH vaccines (Figure 2). Based on potency ratios, both the admixture and the individual vaccines shifted the heroin ED50 curves by 2–6 times for both drugs and antinociceptive tests compared to controls (Figure 2C,D).
Figure 2.
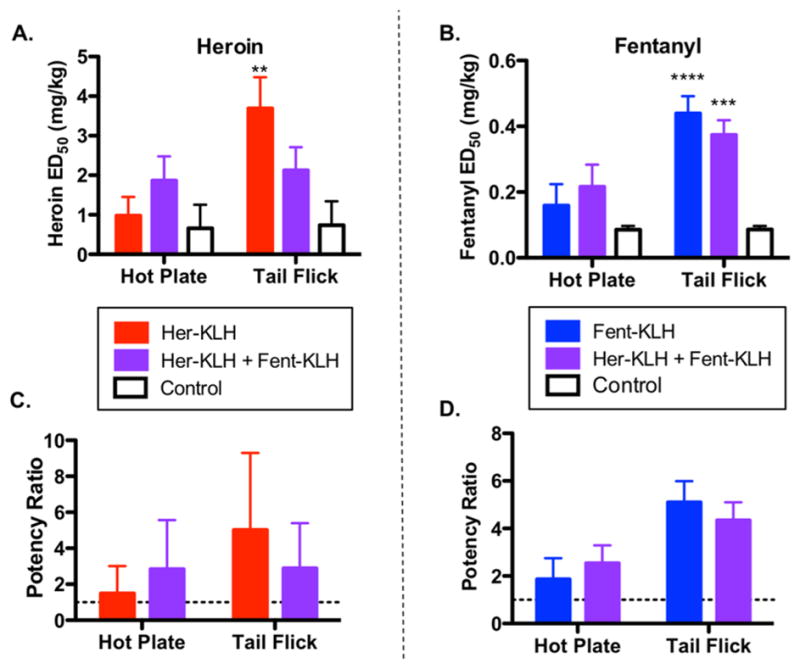
Admixture vaccine is effective in protecting against both i.p. heroin and fentanyl in antinociception assays compared to mice. (A) Results from antinociceptive tests using heroin. (B) Results from antinociceptive tests using fentanyl. (C) Potency ratios for vaccine groups against heroin. (D) Potency ratios for vaccine groups against fentanyl. Significance is denoted by an asterisk from a two-way ANOVA and a Dunnett post hoc test when comparing vaccinated groups to controls. **P < 0.01, *** P < 0.001, ****P < 0.0001 versus control. Dashed line in potency panels denotes control levels.
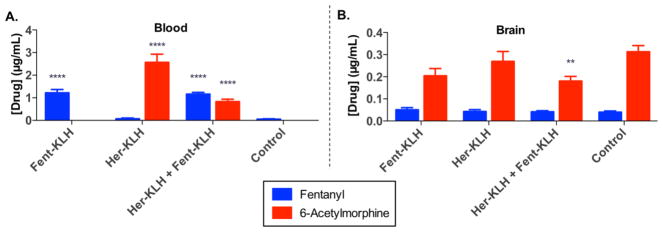
Administering heroin intravenously (i.v.) is an aspect that we considered when evaluating our vaccines, especially in terms of method of choice for drug users and clinical relevancy. However, in a murine model, we are limited to maximal doses of 200 μL through i.v. administration.16 In addition, cumulative dosing is not an option for either route of i.v. administration (retro-orbital or tail vein) as they only allow for 1–2 infusions per session. Despite these limitations, we were keenly interested in studying the efficacy of our vaccine in mice after coadministration of heroin and fentanyl as an intravenous bolus. Although nociception assays precluded this type of administration, a solution to this challenge would be blood-brain distribution experiments as here only a single infusion of drug prior to analysis would be required. Accordingly, mice received an intravenous bolus of 10% (w/w) fentanyl in heroin, and then 15 min after administration, their brain tissue and trunk blood were harvested and analyzed for drug concentrations (Figure 3).
Figure 3.
Admixed vaccine blood-brain distribution experiment. Mice were intravenously administered a 10% fentanyl in heroin bolus (i.e., 0.9 mg/ kg heroin and 0.1 mg/kg fentanyl, i.v.). (A) Concentration of drug found in the blood 15 min after administration. (B) Concentration of drug found in the brain 15 min after administration. Bars show means + SEM. Significance is denoted by an asterisk from a two-way ANOVA and a Dunnett post hoc test when comparing vaccinated groups to vaccinated controls. Only trace amounts of 6-AM were detected in sera for Fent-KLH (6 ± 6 ng/ mL, n = 5) and control (20 ± 6 ng/mL, n = 12). **P < 0.01 and ****P < 0.0001 versus control.
The pharmacokinetics and pharmacodynamics of a mixture of heroin and fentanyl most likely influence and impact their individual actions on opioid receptors in vivo. Typically fentanyl exhibits a faster rate of transfer from the plasma to the CNS (4.7–6.6 min),17 higher affinity for the μ-opioid receptor (fentanyl > morphine > heroin),18 and a more rapid rate of metabolism than compared to heroin and its psychoactive metabolites (t1/2,brain = 4.9 min vs 74 min, fentanyl vs morphine in mouse).19 Due to the rapid hydrolysis of the heroin in serum, heroin and all of its related psychoactive intermediates (i.e., 6-AM and morphine) were analyzed during the blood-brain distribution experiment. Heroin and morphine levels found in blood and brain are shown in Figure S17. The blood-brain distribution experiments are in agreement with the findings from the antinociception assays in that the individual vaccines retained their respective parent drug (or intermediates) in serum, while the admixture vaccine retained both 6-AM and fentanyl (Figure 3). This retention of both drugs reflects the specific IgG antibodies circulating in the periphery and trapping free drug immediately following i.v. administration. Of note, the Her-KLH + Fent-KLH combination vaccine had significantly lower 6-AM levels than compared to controls (Figure 3).
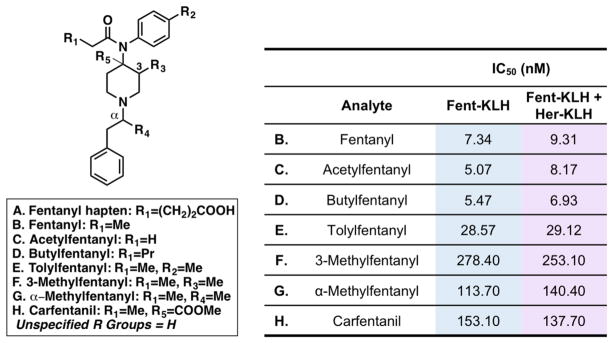
In some cases, it is highly likely that the fentanyl laced in heroin may be a clandestine analogue of fentanyl.4,20,21 To test the utility of our admixture vaccine in this scenario, we ran the admixture vaccine (week 5) against six analogues of fentanyl by SPR, including the highly toxic large animal tranquilizer, carfentanil (Figure 4). Indeed, our fentanyl vaccine can also target six other fentanyl congeners with nanomolar affinity. Based on the hapten structure, it is not surprising that the antibodies generated from the admixture and Fent-KLH had similar levels of affinity to acetylfentanyl and butylfentanyl when compared to fentanyl (Figure 4). The fentanyl component of our admixture vaccine enables the vaccine to function almost like a “pan-fentanyl” vaccine.
Figure 4.
Cross-reactivity of Fent-KLH and admixture vaccines to derivatives of fentanyl. The left-hand panel shows the structures of relevant fentanyl analogues. The right-hand panel shows the cross-reactivity of the corresponding fentanyl analogues on a Fent-BSA-loaded sensor chip incubated with pooled and diluted mouse sera from either Fent-KLH or Fent-KLH + Her-KLH from week 5.
Opioid-induced respiratory depression occurs when the μ-opioid receptors expressed on the neurons (located on the brainstem) responsible for breathing are overstimulated.17 Heroin and fentanyl work together to potentiate the effects of respiratory depression, namely potentially fatal brain hypoxia.12 Our heroin–fentanyl admixture vaccine produces antibodies that effectively sequester each drug creating an antibody-drug complex that is too large to penetrate the blood- brain barrier. By partially blocking access to the CNS, the vaccine inhibits the stimulation of μ-opioid receptors necessary to reach the critical threshold for respiratory depression. While naloxone still remains the standard treatment in opioid overdose rescue, this vaccine fills a current void in specialized prevention therapy directed toward recovering opioid users at risk of relapsing. This risk is intensified by the recent exponential growth of drug poisoning deaths involving fentanyl. By employing the vaccine, users would be protected against both drugs in the event of a relapse, reducing the likelihood of unintended overdose.
To summarize, a proof of concept admixture vaccine was constructed to address the growing prevalence of heroin contaminated with fentanyl. Based on our preliminary findings, an opioid vaccine-cocktail containing structural incongruent immunoconjugates can be readily applied to target vastly different drug species. The admixture vaccine bound both drugs and demonstrated efficacy in antinociception animal models in comparison to our singular opioid drug vaccines. Importantly, blood-brain drug quantification of heroin contaminated fentanyl with the admixture vaccine showed that the vaccine generated antibodies could sequester and greatly reduce both fentanyl and 6-AM from brain penetration. Future plans will be to increase copy numbers of both immunoconjugates,22 and with more efficacious carrier proteins, like tetanus toxoid, the admixture vaccine should approach efficacy seen with the singular vaccines.7,11 Nonetheless, the current admixture vaccine protects against both heroin and fentanyl and may provide a future therapy to combat accidental exposure to fentanyl-contaminated heroin.
METHODS
Animals
All studies were performed in compliance with the Scripps Institutional Animal Care and Use Committee, and all protocols adhered to the National Institute of Health Guide for the Care and Use of Laboratory Animals. Female Balb/c mice (Taconic Farms, Germantown, NY; 6–8 weeks old; 17–22 g) were immunized subcutaneously (s.c.) with 50 μg of immunoconjugate vaccines on days 0, 14, and 28 (Table 1). The overall vaccination schedule and behavioral experiments can be seen in Table 1. Groups were composed of 6 or 12 mice. Mice were group-housed in an AAALAC-accredited vivarium containing temperature and humidity controlled rooms, and kept on a reverse light cycle (lights on: 9PM to 9AM). Mice were bled on day 24 and 38 using retro-orbital puncture in order to collect approximately 100–150 μL of whole blood. One mouse from the Fent-KLH and one mouse from the Fent-KLH + Her-KLH vaccination group were euthanized due to proptosed eyes after bleeds. Submandibular bleeds may be optimal for future studies with these mice to avoid loss of vaccinated subjects. Mouse weights were measured every week (Figure S14) and showed no significant difference compared to control mice by repeated measures ANOVA (F(2.306, 11.53) = 3.346, p = 0.067). Injection site reactions were measured on the day of antinociception (Figure S15) and also showed no significant difference when compared to vaccinated control mice by one-way ANOVA (F(3, 68) = 2.163, p = 0.100).
Vaccine Preparation, Formulation, and Schedule
Heroin was obtained from the National Institute on Drug Abuse, and fentanyl was purchased from Sigma-Aldrich. The heroin11 and fentanyl hapten7 were synthesized according to literature methods. Briefly, after preparation of the hapten, the carboxylic acid was activated with NHS using EDC-mediate coupling for several hours at rt and then mixed with BSA or KLH (1 mg/mL) in a 1:1 w/w ratio of hapten to protein. Immunoconjugates were allowed to react at rt for 20 h using gentle end-over-end mixing. Following conjugation, the solutions were dialyzed against PBS (pH 7.4) using a 10k MW cut off dialysis cassettes, and buffer was exchanged several times. Immunoconjugates were diluted to 50% (v/v) with glycerol, formulated with 50 μg CpG/ dose, and stored at –80 °C. On the day of vaccination, vaccines were defrosted and formulated with 0.8 mg alum/dose.
Copy Number by MALDI
The heroin and fentanyl hapten density for immunoconjugates prepared in this study were quantified using MALDI-ToF and ESI-ToF MS analysis and compared to BSA, using the following formula: hapten density = (MWhapten-protein –MWprotein)/ (MWHer/Fent – MWwater). Heroin or fentanyl immuno-conjugated to BSA (Her-BSA or Fent-BSA) was used as a surrogate for KLH to quantify the number of heroin or fentanyl haptens (Her/Fent) due to the tendency of KLH to form large aggregates (4.5 × 105–1.3 × 107 Da).23 The immunoconjugates were run through a PD MiniTrap G-10 desalting column (GE Healthcare) and then analyzed by MS. Spectra can be found in the Supporting Information (Figures S1–12). A summary of the hapten density results is listed in Tables 1 and S1.
ELISA
Costar 3690 plates with half-area, high-binding 96-well microtiter plates were coated with 25 ng of Her-BSA or Fent-BSA (antigen) per well overnight at 37 °C. The plates were blocked with 5% skim milk in PBS buffer (pH 7.4) for 40 min at rt. Mouse serum was serially diluted 1:1 in 2% BSA in PBS buffer (pH 7.4) across 12 columns starting at 1:200. After 2 h incubation at rt, the plates were washed 10 times with PBS, and donkey anti-mouse IgG horseradish peroxidase (HRP) secondary antibody (Jackson ImmunoResearch) at 1:10000 dilution in 2% BSA in PBS buffer (pH 7.4) was added and incubated for 1 h. After washing 10 times with PBS buffer, a 1:1 solution of 3,3′,5,5′-tetramethylbenzidine (TMB) and H2O2 substrate (Thermo Pierce) was added. Plates were incubated for 12 min and then quenched with 2.0 M H2SO4. Plates were read at 450 nm. Using GraphPad PRISM 6, absorbance values were normalized to the highest absorbance value per set, and a curve was fit using the log(inhibitor) vs normalized response–variable slope equation to determine midpoint titer and standard error. Serum from nonvaccinated mice did not contain any detectable or very minor anti-heroin or anti-fentanyl titers (Table 2). Data were tested for statistical outlier evaluation using Grubbs test and significant outliers were removed.
SPR
The binding IC50 for mouse serum IgGs and free heroin (and intermediates) or fentanyl (and analogues) was determined by competitive binding assay via surface plasmon resonance using a Biacore 3000 instrument (GE Healthcare) equipped with a research-grade CM5 sensor chip according to literature methods.8 Diluted mouse serum IgGs from day 24 (week 3) and 38 (week 5) were incubated with serial dilutions of fentanyl (and analogues), heroin, 6-AM, and morphine and injected into a Biacore 3000 containing a Her-BSA-loaded or Fent-BSA-loaded sensor chip. The ligand, BSA immunoconjugate, was immobilized using NHS, EDC coupling reaction. The surface of flow cells 1 and 2 were activated for 7 min with a 1:1 mixture of 0.1 M NHS and 0.1 M EDC at a flow rate of 5 μL/min. The ligand resuspended in 10 mM sodium acetate (pH 4.0) was immobilized at a density of 5000 RU on flow cell 2; whereas flow cell 1 was immobilized with BSA at the same density to serve as a reference surface. All the surfaces were blocked with a 7 min injection of 1.0 M ethanolamine-HCl (pH 8.5). The mouse serum IGs were diluted in running buffer (HBS-EP + buffer) and titrated on both coated flow cells, so as to give a response of ~100 RU within 3 min of injection and 2.5 min dissociation at a flow rate of 30 μL/min. The mouse serum IGs prepared in HBS-EP + buffer at determined concentration was incubated with a series concentration of compounds for 20 min at rt before conducting the competitive binding assay. The chip surface was regenerated by injection of 10 mM Gly-HCl (pH 1.5) for 30 s before the next round of assays. IC50 values for heroin could not be detected due to the rapid hydrolysis of heroin to 6-AM during the experimental time frame. IC50 values were determined from a 12-point 6-AM dilution and fentanyl (and analogues) curve and derived from a nonlinear fit of the binding curves in PRISM 6.
Antinociception Assays
Mice were tested for cumulative heroin response in primarily suprapinal (hot plate) and spinal (tail flick) behavioral tests as previously described.24 The hot plate test was measured by placing the mouse in an acrylic cylinder (14 cm diameter × 22 cm) on a 54 °C surface and timing latency to perform one of the following nociceptive responses: licking of hind paw, shaking/ withdrawal of hind paw or jumping. Typical baseline latency was between 10 to 20 s with a cutoff of 35 s to prevent tissue damage. After response or reaching the cutoff time, mice were immediately removed from the hot plate. The tail flick test was administered by lightly restraining mice in a small pouch constructed from absorbent laboratory underpads and using an IITC Life Science Tail Flick Analgesia Meter to measure time of withdrawal from a heated beam of light (active intensity, 45%). Typical baseline response was 0.2–1.1 s, and an automatic 10 s cutoff was used to prevent tissue damage. Since tail flick immersion is a more reflexive behavior, testing order was always hot plate first followed by tail immersion. Immediately following completion of both antinociceptive assays, heroin (0.4 mg/kg in saline) or fentanyl (0.025 mg/kg) was immediately injected intraperitoneally. The heroin doses tested were 2, 4, 6, 8, 10, 14, and 18 mg/kg and the fentanyl doses tested were 0.025, 0.05, 0.1, 0.15, 0.25, 0.45, and 0.85 mg/kg to generate a full dose–response curve. Testing was repeated at roughly 15 min intervals, following each injection and this cycle of testing and injections was repeated with increase cumulative dosing until full antinociception (cut off times surpassed) was observed in both assays. Antinociception data were transformed from time to percent maximum possible effect (%MPE), which is calculated as
These data were then fit using a log(agonist) vs normalized response nonlinear regression in GraphPad PRISM 6. The ED50 values and 95% confidence intervals were determined for each antinociception test and individual treatment groups to determine ED50 values.
Blood-Brain Distribution Assay
Blood-brain biodistribution was determined according to literature procedure with minor modifications.6,25 A calibration curve for using standard solutions of fentanyl, heroin, 6-acetylmorphine, and morphine was constructed (Figure S16). On week 7 (3 weeks from last boost), vaccine groups and mice (n = 12, respectively) were injected intravenously with 10% fentanyl in heroin (0.1 and 0.9 mg/kg, respectively) through orbital injection to the venous sinus.26 At 15 min following injection the animals were fully anesthetized and then rapidly decapitated using a sharp guillotine. The brain and trunk blood were collected. The trunk blood was collected in a 1:1 ratio with acetate buffer (0.1 M sodium acetate/0.1 M acetic acid/50 g/L NaF, pH 6.0), placed on ice for several hours, centrifuged at 10000 rpm for 10 min. Brain tissue was immediately diluted with acetate buffer (1:1) and homogenized using a Bullet Blender with zirconium oxide beads (0.5 mm diameter, Thomas Scientific) and then centrifuged at 10000 rpm for 10 min. A 100 μL aliquot of the homogenate or plasma was added to 100 μL of spiked fentanyl, heroin, 6-acetylmorphine, and morphine concentrations (for standard curve, made up in 85:15 ACN/MeOH) or 100 μL of 85:15 ACN/MeOH (for samples), 100 μL of d5-fentanyl, d9-heroin, d3-6-acetylmorphine, and d3-morphine (1 μg/mL in ACN), and 300 μL of ice-cold acetonitrile/methanol (85:15). The mixture was vortexed for a 30 s, followed by centrifugation at 2500 rpm for 10 min. A 450 μL aliquot was transferred to another test tube, and the samples was evaporated using GENEVAC. The dried sample was taken up in acetonitrile, centrifuged at 10000 rpm for 5 min, and then transferred to vials for LCMS analysis.
Supplementary Material
Acknowledgments
Funding
This work was supported by National Institutes of Health Grants UH3DA041146 (K.D.J.) and F32AI126628 (C.S.H.).
ABBREVIATIONS
- 6-AM
6-acetylmorphine
- CpG ODN
cytosine-phosphodiester-guanine oligodeoxynucleotide
- TT
tetanus toxoid
- KLH
keyhole limpet hemocyanin
- PBS
phosphate buffered saline
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- MALDI-ToF MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- s.c
subcutaneous
- i.p
intraperitoneal
- i.v
intravenous
- SPR
surface plasmon resonance
Footnotes
Author Contributions
All authors have given approval to the final version of the manuscript. C.S.H. and K.D.J. designed the research strategy. Y.N. synthesized all chemical reagents. C.S.H, L.C.S., and B.E. performed behavioral experiments. B.Z. performed SPR experiments. C.S.H., L.C.S., and B.Z. analyzed the data. C.S.H., L.C.S, and K.D.J. wrote the paper.
Notes
This is manuscript #29655 from The Scripps Research Institute.
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneur-o.8b00079.
Detailed data including immunoconjugate characterization by MALDI-ToF, mouse body weight measurements, injection site reactions, standard calibration curves, and results of the blood-brain distribution study with all primary drug metabolites (PDF)
References
- 1.United States Drug Enforcement Administration. DEA Intelligence Report No. DEA-DCT-DIR-031-16. 2016. National Heroin Threat Assessment Summary-Updated. [Google Scholar]
- 2.CDC National Center for Injury Prevention and Control. Prescription Behavior Surveillance System (PBSS) 2017. Increase in overdose deaths involving synthetic opioids other than methadone linked to increase in supply of fentanyl in PBSS states. [Google Scholar]
- 3.Hull MJ, Juhascik M, Mazur F, Flomenbaum MA, Behonick GS. Fatalities associated with fentanyl and co-administered cocaine or opiates. J Forensic Sci. 2007;52:1383–1388. doi: 10.1111/j.1556-4029.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 4.Arens AM, van Wijk XM, Vo KT, Lynch KL, Wu AH, Smollin CG. Adverse Effects From Counterfeit Alprazolam Tablets. JAMA Intern Med. 2016;176:1554–1555. doi: 10.1001/jamainternmed.2016.4306. [DOI] [PubMed] [Google Scholar]
- 5.Binswanger IA, Nowels C, Corsi KF, Glanz J, Long J, Booth RE, Steiner JF. Return to drug use and overdose after release from prison: a qualitative study of risk and protective factors. Addiction Science & Clinical Practice. 2012;7:3–3. doi: 10.1186/1940-0640-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AAK, Stowe GN, Edwards S, Janda KD, Koob GF. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A. 2013;110:9036–9041. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD. Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew Chem, Int Ed. 2016;55:3772–3775. doi: 10.1002/anie.201511654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooyit M, Miranda PO, Wenthur CJ, Ducime A, Janda KD. Influencing Antibody-Mediated Attenuation of Methamphetamine CNS Distribution through Vaccine Linker Design. ACS Chem Neurosci. 2017;8:468–472. doi: 10.1021/acschemneuro.6b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimishima A, Wenthur CJ, Eubanks LM, Sato S, Janda KD. Cocaine Vaccine Development: Evaluation of Carrier and Adjuvant Combinations That Activate Multiple Toll-Like Receptors. Mol Pharmaceutics. 2016;13:3884–3890. doi: 10.1021/acs.molpharmaceut.6b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raleigh MD, Pentel PR, LeSage MG. Pharmacokinetic Correlates of the Effects of a Heroin Vaccine on Heroin Self-Administration in Rats. PLoS One. 2014;9:e115696. doi: 10.1371/journal.pone.0115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, Janda KD. Development of a Clinically-Viable Heroin Vaccine. J Am Chem Soc. 2017;139:8601–8611. doi: 10.1021/jacs.7b03334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solis E, Cameron-Burr KT, Kiyatkin EA. Heroin Contaminated with Fentanyl Dramatically Enhances Brain Hypoxia and Induces Brain Hypothermia. eNeuro. 2017;4 doi: 10.1523/ENEURO.0323-17.2017. ENEURO.0323-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, Pentel PR. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine. 2012;30:4617–4624. doi: 10.1016/j.vaccine.2012.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimishima A, Wenthur CJ, Zhou B, Janda KD. An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chem Biol. 2017;12:36–40. doi: 10.1021/acschembio.6b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Peña A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity. 2017;46:1073–1088. e1076. doi: 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, Vorstenbosch CVD. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 17.Boom M, Niesters M, Sarton E, Aarts L, Smith WT, Dahan A. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18:5994–6004. doi: 10.2174/138161212803582469. [DOI] [PubMed] [Google Scholar]
- 18.Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther. 2006;316:1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- 19.Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: assessment of unbound brain EC50, μ and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther. 2007;323:346–355. doi: 10.1124/jpet.107.119560. [DOI] [PubMed] [Google Scholar]
- 20.Macmadu A, Carroll JJ, Hadland SE, Green TC, Marshall BDL. Prevalence and correlates of fentanyl-contaminated heroin exposure among young adults who use prescription opioids non-medically. Addictive Behaviors. 2017;68:35–38. doi: 10.1016/j.addbeh.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stogner JM. The Potential Threat of Acetyl Fentanyl: Legal Issues, Contaminated Heroin, and Acetyl Fentanyl “Disguised” as Other Opioids. Ann Emerg Med. 2014;64:637–639. doi: 10.1016/j.annemergmed.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Jalah R, Torres OB, Mayorov AV, Li F, Antoline JF, Jacobson AE, Rice KC, Deschamps JR, Beck Z, Alving CR, Matyas GR. Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjugate Chem. 2015;26:1041–1053. doi: 10.1021/acs.bioconjchem.5b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurokawa T, Wuhrer M, Lochnit G, Geyer H, Markl J, Geyer R. Hemocyanin from the keyhole limpet Megathura crenulata (KLH) carries a novel type of N-glycans with Gal(β1–6)Man-motifs. Eur J Biochem. 2002;269:5459–5473. doi: 10.1046/j.1432-1033.2002.03244.x. [DOI] [PubMed] [Google Scholar]
- 24.Bremer PT, Schlosburg JE, Lively JM, Janda KD. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharmaceutics. 2014;11:1075–1080. doi: 10.1021/mp400631w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karinen R, Andersen JM, Ripel Å, Hasvold I, Hopen AB, Mørland J, Christophersen AS. Determination of heroin and its main metabolites in small sample volumes of whole blood and brain tissue by reversed-phase liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2009;33:345–350. doi: 10.1093/jat/33.7.345. [DOI] [PubMed] [Google Scholar]
- 26.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim (NY) 2011;40:155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.