Abstract
The purpose of this study was to determine the involvement of the p42/p44 mitogen-activated protein kinase (MAPK) pathway and induction of p21waf1/cip1 in the antiproliferative effects of nitric oxide (NO) on rat aortic smooth muscle cells (RASMC). NO, like α-difluoromethylornithine (DFMO), interferes with cell proliferation by inhibiting ornithine decarboxylase (ODC) and, therefore, polyamine synthesis. S-nitroso-N-acetylpenicillamine or (Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)-amino]-diazen-1-ium-1,2-diolate inhibited RASMC growth at concentrations as low as 3 μM, and DFMO elicited effects at concentrations of 100 μM or greater. The cytostatic effect of NO and DFMO was prevented by the MAPK kinase 1/2 inhibitors PD 098,059 or U0126. This finding suggests that the p42/p44 MAPK pathway is involved in the inhibition of RASMC proliferation by NO. Western blot analysis revealed that treatment of RASMC with NO or DFMO leads to activation of p42/p44 MAPK and induction of p21waf1/cip1. This effect was prevented by MAPK kinase 1/2 inhibitors, suggesting that induction of p21waf1/cip1 depended on activation of p42/p44. Moreover, activation of p42/p44 and induction of p21waf1/cip1 were prevented by exogenous putrescine but not ornithine, suggesting this effect was due to the inhibition of ODC by NO or DFMO. Finally, activation of p42/p44 MAPK and induction of p21waf1/cip1 were cGMP-independent. Neither 1H-(1,2,4)oxadiazolo[4,3-α]quinoxalin-1-one nor zaprinast influenced the cytostatic effect of NO or DFMO or their ability to activate these signal transduction pathways. These observations suggest that inhibition of ODC and accompanying putrescine production are the underlying mechanisms by which NO and DFMO activate the MAPK pathway to promote induction of p21waf1/cip1 and consequent inhibition of cell proliferation.
Keywords: polyamines|ornithine decarboxylase|atherosclerosis|MAPK
Vascular smooth muscle cells (VSMC) are normally quiescent in the adult, rarely exhibiting mitogenic activity in their contractile state (1). However, vascular injury stimulates VSMC growth by disrupting the physiological balance between inhibition and stimulation. Over-proliferation of VSMC contributes to many clinical vascular pathologies, the causes of which vary from mechanical (restenosis after balloon angioplasty), to immunological (transplant arteriosclerosis), to multifactorial (atherosclerosis). Increased VSMC proliferation has been attributed to dysfunction of or damage to the endothelial lining in the area of intimal thickening and, more specifically, to decreased nitric oxide (NO) production. This effect is made evident by several studies in which NO donor agents or NO synthase gene transfer were shown to inhibit neointimal formation after balloon angioplasty in animal models (2–4). The effect inverse has been shown in studies where animals were subjected to balloon angioplasty and then treated with inhibitors of NO synthase (4). In these studies, intimal formation was enhanced by the inhibition of NO production.
The mechanism by which NO inhibits cell proliferation appears to be complex, in that both cGMP-dependent and -independent mechanisms may be involved (5–8). Experiments in this laboratory have consistently revealed cGMP-independent cytostatic effects of NO in various cell types, including Caco-2 tumor cells, murine macrophages, rat aortic endothelial cells, and rat aortic smooth muscle cells (RASMC) (refs. 5, 9 and unpublished data). One such cGMP-independent cytostatic mechanism is the inhibition of ornithine decarboxylase (ODC) (5, 9) and consequent inhibition of polyamine synthesis, as ODC is the initial and rate-limiting step in putrescine synthesis. ODC catalyzes the conversion of ornithine to putrescine and is essential for polyamine synthesis in mammalian cells. Despite the fact that polyamines are present in all mammalian cells, extensive research efforts have failed to fully elucidate their physiological functions. However, the requirement of polyamines for maintenance of cell growth and function is clearly established (10–12). Direct evidence for this view has been provided by experiments in which polyamine synthesis was prevented in mammalian cells in culture by mutations of key enzymes (such as ODC) or by the application of enzyme inhibitors (11, 13–15).
NO, like the enzyme-activated irreversible ODC inhibitor, α-difluoromethylornithine (DFMO), decreases intracellular polyamine levels in RASMC (9). The cytostatic effects of NO and DFMO on RASMC can be reversed by the addition of exogenous polyamines but not ornithine, supporting the view that NO, like DFMO, inhibits ODC (9). Therefore, NO and DFMO may share a common cytostatic mechanism, namely, inhibition of polyamine accumulation. Inhibition of cell proliferation via polyamine depletion may be mediated by increased expression of the cyclin-dependent kinase (cdk) inhibitor p21waf1/cip1 (16, 17), as a result of activation of the p42/p44 mitogen-activated protein kinase (MAPK) pathway (17). In separate independent studies, the cytostatic effect of NO was associated with the cGMP-independent up-regulation of p21waf1/cip1 (18–20). In addition, NO has been reported to activate the p42/p44 MAPK (Erk1/2) pathway in RASMC (18, 19, 21, 22). Recently, a novel paradigm was described in which Ras was reported to increase p21waf1/cip1 expression through activation of p42/p44 MAPK (23). On the basis of all these independent observations, we forward the hypothesis that both NO and DFMO inhibit cell proliferation by lowering cellular polyamine levels, which causes activation of the p42/p44 MAPK pathway and consequent up-regulation of p21waf1/cip1 and interference with cell cycle progression. Therefore, the purpose of this study was to determine the relationship among activation of the p42/p44 MAPK pathway, induction of p21waf1/cip1, inhibition of ODC and putrescine formation, and inhibition of RASMC proliferation by NO and DFMO.
Materials and Methods
Chemicals and Solutions.
Sources of cell culture media and supplements and reagents for protein determination have been described (5). Zaprinast and PD 098,059 were from Sigma. U0126 was purchased from Cell Signaling Technologies (Beverly, MA), and 1H-(1,2,4)oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) was purchased from Alexis Biochemicals (San Diego, CA). S-nitroso-N-acetylpenicillamine (SNAP) was synthesized as previously described (24). (Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)-amino]-diazen-1-ium-1,2-diolate (DETA/NO) was generously provided by David A. Wink, National Institutes of Health, Bethesda, MD. All other chemicals and reagents were purchased from Sigma, unless specified otherwise.
Cell Culture of RASMC.
RASMC were a kind gift from Steven Gross, Cornell Medical College, New York. Cells were plated, grown, subcultured, and cultured as described (25). Subcultured strains were used between passages 15 and 25. Exponentially growing RASMC were trypsinized and resuspended in fresh DMEM-Hepes medium supplemented with 10% (vol/vol) FBS, 2 mM glutamine/100 units/ml of penicillin/100 μg/ml of streptomycin. The cells were seeded in 12-well or 10-cm plates at a density of 3,000 cells/cm2 and incubated at 37°C in a humidified atmosphere of 5% CO2-95% air. After 24 h, the cells were washed twice with PBS, and the growth medium was replaced with arginine-free DMEM-Hepes supplemented with 5% FBS and 50 μM l-arginine and any test agents. To the 12-well plates, 0.1 μCi [3H]thymidine (6.7 Ci/mmol; NEN) was added and analyzed for [3H]thymidine incorporation after 24 h. Cells from 10-cm plates were harvested by scraping after 24 h and subjected to Western blot analysis.
Measurement of Cell Proliferation.
RASMC proliferation was determined by monitoring DNA synthesis by a modification of the [methyl-3H] thymidine incorporation procedure previously described (5). Briefly, cells in 12-well plates, which had been treated with test agents and 0.1 μCi [3H]thymidine for 24 h, were washed twice with ice-cold PBS, and 1 ml of ice-cold 10% trichloroacetic acid was added to each well. The plates were then incubated for 30 min at 4°C, after which each well was washed with 1 ml of ice-cold 10% trichloroacetic acid. To each well, 1 ml of 0.4 N NaOH, 0.1% (wt/vol) SDS was added, and the plates were incubated for 1 h at room temperature. The contents of each well were then transferred to 20-ml scintillation vials containing 10 ml of EcoLite scintillation mixture (ICN) and counted in a liquid scintillation spectrometer.
Determination of Protein Concentration.
Protein concentrations were determined by the Bradford Coomassie brilliant blue method (Bio-Rad) by using BSA as the standard.
Western Blot Analysis.
Cell lysates from RASMC were prepared after 24 h of exposure to different treatments by using ice-cold lysis buffer containing 50 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 μg/ml of leupeptin, 1 μg/ml of pepstatin, 1 μg/ml of aprotinin, 1 mM phenylmethylsulfonylfluoride, 1 mM sodium orthovanadate, and 1 mM sodium fluoride. Twenty micrograms of total protein from each sample was subjected to SDS/PAGE (10 or 15%), and proteins were transferred to a nitrocellulose membrane. The membrane was then probed with rabbit anti-p21 polyclonal antibody (1:750, Santa Cruz Biotechnology), mouse antiphospho-p42/p44 MAPK monoclonal antibody (1:500), or rabbit anti-p42/p44 MAPK, followed by anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution) (Cell Signaling Technologies, Beverly, MA). Blots were then visualized by using LumiGlo chemiluminescent detection reagent (Cell Signaling Technologies), as described by the manufacturer.
Results
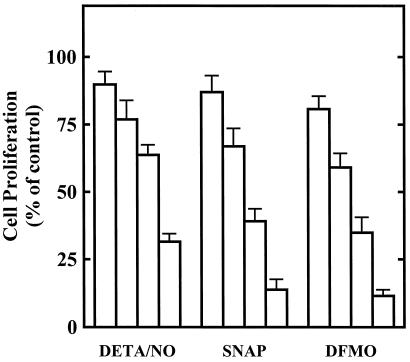
We previously reported that NO inhibits tumor cell and RASMC proliferation via inhibition of ODC (5, 9). Fig. 1 shows that NO and DFMO interfere with RASMC proliferation. DETA/NO, a NONOate NO donor agent, SNAP, an S-nitrosothiol NO donor agent, and DFMO, an enzyme-activated irreversible inhibitor of ODC, inhibited RASMC proliferation in a concentration-dependent manner. SNAP was slightly more effective than DETA/NO, particularly at higher concentrations. DFMO inhibited RASMC proliferation at relatively high concentrations ranging from 0.1 to 3 mM.
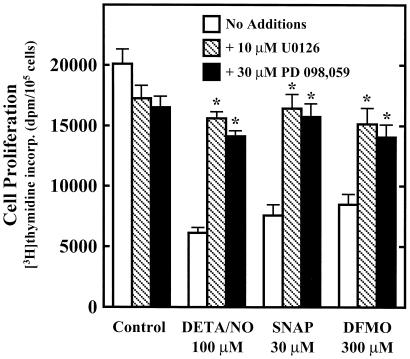
Figure 1.
Inhibition of RASMC proliferation by SNAP, DETA/NO, and DFMO. Cell proliferation was assessed by thymidine incorporation into DNA during a 24-h incubation, as described in Materials and Methods. The concentrations of test agents were as follows: DETA/NO, 3, 10, 30, 100 μM; SNAP, 3, 10, 30, 100 μM; and DFMO, 0.1, 0.3, 1, 3 mM. Data were calculated as dpm/105 cells and expressed as percent of control (assigned 100%), where control represents cells grown in the absence of added test agents. Data represent mean ± SE of duplicate determinations from three to four experiments.
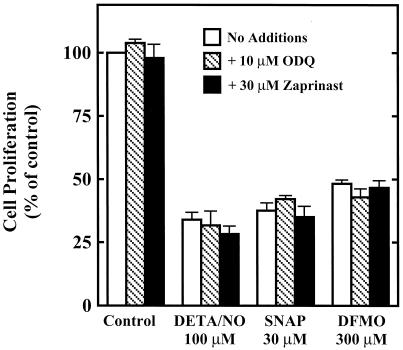
It is well established that NO is the endogenous ligand for soluble guanylate cyclase, and that many of the biological effects of NO are mediated through the second messenger cGMP. Increasingly, however, the cytostatic effects of NO are being attributed to cGMP-independent mechanisms. Therefore, experiments were conducted to determine the involvement of cGMP in the cytostatic effect of NO in RASMC. Fig. 2 illustrates that ODQ, a soluble guanylate cyclase inhibitor, and zaprinast, a cGMP phosphodiesterase inhibitor, did not affect control RASMC proliferation or the cytostatic action of NO on RASMC. These data suggest that NO inhibits RASMC proliferation by cGMP-independent mechanisms. In addition, the cytostatic effect of DFMO was unaffected by ODQ or zaprinast, which was expected, as DFMO increases neither NO nor cGMP production.
Figure 2.
Effect of ODQ or zaprinast on the cytostatic actions of DETA/NO, SNAP, and DFMO in RASMC. Cell proliferation was assessed by thymidine incorporation into DNA during 24-h incubation, as described in Materials and Methods. ODQ (10 μM) or zaprinast (30 μM) was added to cells at the same time as test agents. Data were calculated as dpm/105 cells and expressed as percent of control (assigned 100%), where control represents cells grown in the absence of added test agents. Values for DETA/NO, SNAP, and DFMO were not significantly (P > 0.05) different from corresponding values in the presence of ODQ or zaprinast. Data represent mean ± SE of duplicate determinations from three to four experiments.
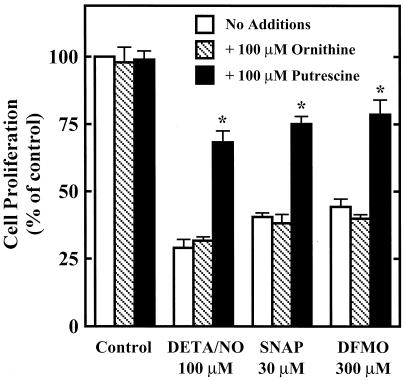
ODC inhibitors such as DFMO are known to cause cytostasis by depleting intracellular polyamines (11, 13–15). The cytostatic effect of DFMO is thus preventable by the addition of exogenous putrescine, the product of ODC, but not by ornithine, the substrate for ODC, as illustrated in Fig. 3. Putrescine, but not ornithine, also protected against the cytostatic effects of DETA/NO or SNAP. This suggests that NO inhibits RASMC proliferation by a similar mechanism to DFMO, that is, by inhibition of ODC (5, 26, 27) and therefore polyamine synthesis.
Figure 3.
Effect of putrescine or ornithine on the cytostatic actions of DETA/NO, SNAP, and DFMO in RASMC. Cell proliferation was assessed by thymidine incorporation into DNA during a 24-h incubation, as described in Materials and Methods. Putrescine (100 μM) or ornithine (100 μM) was added to cells at the same time as test agents. Data were calculated as dpm/105 cells and expressed as percent of control (assigned 100%), where control represents cells grown in the absence of added test agents. *, significantly different (P < 0.05) from corresponding control (no additions). Data represent mean ± SE of duplicate determinations from five experiments.
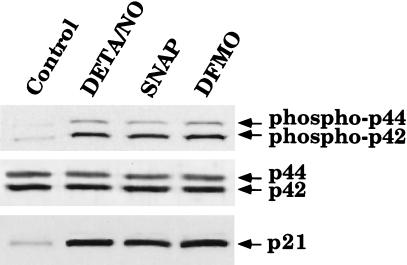
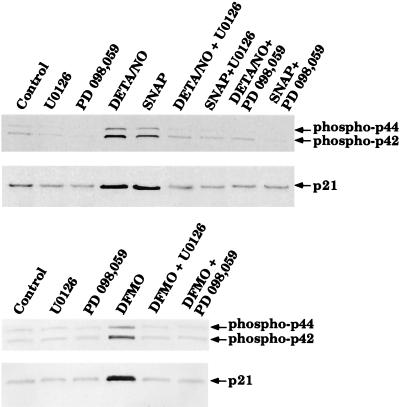
Activation of the MAPK pathway is sometimes associated with increased cell proliferation. However, more recently, studies have indicated that activation of the MAPK pathway can lead to growth arrest via induction of p21waf1/cip1 (18, 23). To determine whether this paradigm holds true in our system, we designed experiments to determine whether NO and DFMO cause activation of the p42/p44 MAPK pathway and induction of p21waf1/cip1 protein levels in RASMC. Cells were treated with or without 30 μM SNAP, 100 μM DETA/NO, or 300 μM DFMO for 24 h and then were subjected to Western blot analysis. By using antibodies specific for p42/p44 MAPK or phospho-p42/p44 MAPK, we show that SNAP, DETA/NO, and DFMO caused an increase in phospho-p42/p44 MAPK without affecting p42/p44 MAPK protein levels (Fig. 4). In addition, by using an antibody specific for p21waf1/cip1, we were able to observe induction of p21waf1/cip1 protein levels by SNAP, DETA/NO, and DFMO (Fig. 4). These observations indicate that NO and DFMO both activate MAPK and increase the expression of p21waf1/cip1.
Figure 4.
Activation of the p42/p44 MAPK pathway and induction of p21waf1/cip1 by SNAP, DETA/NO, or DFMO in RASMC. Whole-cell lysates of RASMC treated for 24 h with 30 μM SNAP, 100 μM DETA/NO, or 300 μM DFMO were subjected to Western blot analysis for p21waf1/cip1, phosphorylated p42/p44, or p42/p44, as described in Materials and Methods. Control represents RASMC grown in the absence of added test agents. Data are representative of three separate experiments.
The next step was to determine the involvement of the p42/p44 MAPK pathway in the inhibition of RASMC proliferation by NO and DFMO. MAPK kinase (MEK)1/2 are dual-specificity protein kinases with p42/p44 MAPK as their only known substrates. Activation of MEK1/2 leads to phosphorylation and activation of p42/p44 MAPK, which in turn leads to phosphorylation of downstream targets. Fig. 5 demonstrates that the MEK1/2 inhibitors U0126 and PD 098,059 protect against the cytostatic effects of NO and DFMO. This finding suggests a direct correlation between activation of the p42/p44 MAPK pathway and inhibition of cell proliferation by NO or DFMO. The MEK1/2 inhibitors also decreased control cell proliferation, which was not unexpected considering the involvement of this pathway in cell growth.
Figure 5.
Effect of MEK1/2 inhibitors on the cytostatic actions of DETA/NO, SNAP, and DFMO in RASMC. Cell proliferation was assessed by thymidine incorporation into DNA during 24-h incubation, as described in Materials and Methods. U0126 (10 μM) or PD 098,059 (30 μM) was added to cells 30 min before test agents. Data were calculated and expressed as dpm/105 cells. Control represents cells grown in the absence of added test agents. *, significantly different (P < 0.05) from corresponding control (no additions). Data represent mean ± SE of duplicate determinations from four experiments.
We next wished to determine whether activation of the p42/p44 MAPK pathway is responsible for induction p21waf1/cip1 by NO and/or DFMO in RASMC. Cells were pretreated for 30 min with or without the MEK1/2 inhibitors U0126 or PD 098,059. After preincubation, cells were treated with or without 30 μM SNAP, 100 μM DETA/NO, or 300 μM DFMO for 24 h and then examined by Western blot analysis. The MEK1/2 inhibitors blocked the phosphorylation of p42/p44 MAPK and the induction of p21waf1/cip1 by all three agents (Fig. 6). These data suggest that activation of the p42/p44 MAPK pathway is responsible for the induction of p21waf1/cip1 by NO and DFMO.
Figure 6.
Effect of MEK1/2 inhibitors on activation of the p42/p44 MAPK pathway and induction of p21waf1/cip1 by SNAP, DETA/NO, or DFMO in RASMC. U0126 (10 μM) or PD 098,059 (30 μM) was added to cells 30 min before test agents. Whole-cell lysates of RASMC treated for 24 h with 30 μM SNAP, 100 μM DETA/NO, or 300 μM DFMO were then subjected to Western blot analysis for p21waf1/cip1 or phosphorylated p42/p44, as described in Materials and Methods. Control represents RASMC grown in the absence of added test agents. Data are representative of three to four separate experiments.
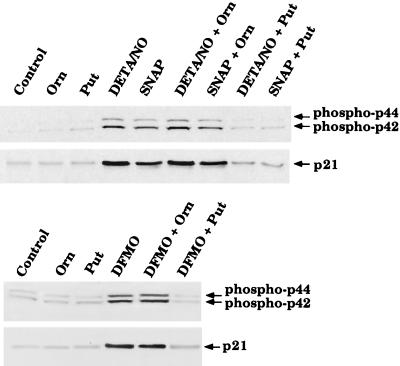
We have established that putrescine can protect against the cytostatic effect of NO and DFMO. Therefore, we next tested whether putrescine could prevent activation of p42/p44 MAPK and induction of p21waf1/cip1. Western blot analysis of cells treated with 30 μM SNAP, 100 μM DETA/NO, or 300 μM DFMO for 24 h in the presence or absence of ornithine or putrescine reveals that putrescine, but not ornithine, can prevent activation of these signal transduction pathways (Fig. 7). This observation supports the above view that inhibition of ODC and polyamine production is responsible for activation of p42/p44 MAPK and induction of p21waf1/cip1.
Figure 7.
Effect of putrescine or ornithine on activation of the p42/p44 MAPK pathway and induction of p21waf1/cip1 by SNAP, DETA/NO, or DFMO in RASMC. Putrescine (100 μM) or ornithine (100 μM) was added to cells at the same time as the test agents. Whole-cell lysates of RASMC treated for 24 h with 30 μM SNAP, 100 μM DETA/NO, or 300 μM DFMO were then subjected to Western blot analysis for p21waf1/cip1 or phosphorylated p42/p44, as described in Materials and Methods. Control represents RASMC grown in the absence of added test agents. Data are representative of four separate experiments.
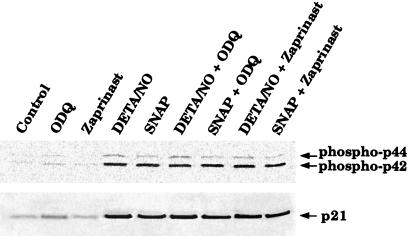
One additional experiment was conducted to verify that phosphorylation of p42/p44 and induction of p21waf1/cip1 are cGMP-independent. RASMC were treated with 30 μM SNAP or 100 μM DETA/NO in the presence or absence of zaprinast or ODQ for 24 h and then examined by Western blot analysis. Zaprinast and ODQ failed to affect the phosphorylation of p42/p44 or induction of p21waf1/cip1 by SNAP or DETA/NO, suggesting that activation of these signal transduction pathways by NO is a cGMP-independent process (Figs. 8 and 9).
Figure 8.
Effect of ODQ or zaprinast on activation of the p42/p44 MAPK pathway and induction of p21waf1/cip1 by SNAP, DETA/NO, or DFMO in RASMC. ODQ (10 μM) or zaprinast (30 μM) was added to cells at the same time as the test agents. Whole-cell lysates of RASMC treated for 24 h with 30 μM SNAP, 100 μM DETA/NO, or 300 μM DFMO were then subjected to Western blot analysis for p21waf1/cip1 or phosphorylated p42/p44, as described in Materials and Methods. Control represents RASMC grown in the absence of added test agents. Data are representative of three separate experiments.
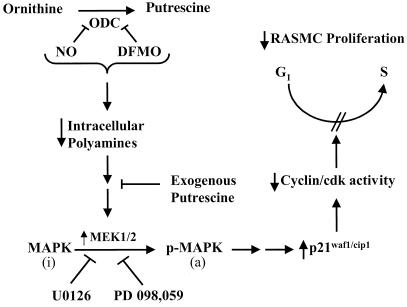
Figure 9.
Schematic illustration of the proposed mechanism by which NO inhibits RASMC proliferation. NO and DFMO inhibit (|—) ODC and reduce (↓) polyamine synthesis. Polyamine depletion results in the activation (↑) of MEK1/2 and consequent activation of MAPK via phosphorylation. The inactive (i) MAPK is converted to the active (a) phosphorylated (p) MAPK. Addition of exogenous putrescine can prevent (|—) activation of MEK 1/2 by polyamine depletion. U0126 and PD 098,059 (MEK 1/2 inhibitors) block (|—) activation of MAPK. MAPK activation causes up-regulation (↑) of p21waf1/cip1 and consequent inhibition (↓) of certain cdks, thereby leading to interference with cell cycle progression (blocks G1 to S-phase transition) and inhibition (↓) of RASMC proliferation.
Discussion
NO plays an important physiological role in the protection of the cardiovascular system against pathophysiological insults that can lead to chronic cardiovascular diseases (28). Endothelial dysfunction or damage to the vascular endothelium leading to a localized deficiency in NO production is likely involved in the development of atherosclerosis and restenosis after balloon injury (28–30). A hallmark of atherosclerosis and restenosis is migration of smooth muscle cells from the media to the intima and subsequent increased proliferation of the intimal smooth muscle cells leading to neointimal formation. Treatment of the injured area with NO donor agents or NOS gene transfer markedly reduces neointimal formation (2–4). In view of the known chemistry and biology of NO, it is not unreasonable to speculate that NO functions in normal healthy vascular endothelial cells to prevent the migration and proliferation of VSMC.
One important mechanism by which NO inhibits cell proliferation appears to be the direct inhibition of ODC and, therefore, polyamine synthesis (5, 9). This mechanism applies to RASMC, as demonstrated in this and in an earlier report (9). NO in the form of SNAP or DETA/NO, two chemically distinct NO donor agents, inhibited RASMC proliferation in a concentration-dependent manner. The concentrations at which the NO donor agents inhibited cell proliferation may greatly underestimate the potency of NO as a cytostatic agent. This is because the peak concentration of NO that is achieved by the release of NO from NO donor agents is far less than the concentration of the NO donor agent itself. For example, it has been reported that the peak NO concentration reached by using 1 mM DETA/NO in an aqueous solution is ≈3 μM (31). The cytostatic effect of NO was prevented by putrescine but not ornithine, suggesting that this effect of NO was caused by the inhibition of ODC. Recent studies by this laboratory revealed that NO inhibits purified ODC via S-nitrosylation of the active site cysteine 360 residue, which is critical for enzyme activity (26–27). We have suggested that S-nitrosylation of ODC by various chemical classes of NO donors may occur by different mechanisms. S-nitrosothiols, such as S-nitrosoglutathione, may act by participating in an S-transnitrosation reaction with cysteine residue(s) on ODC. NONOate NO donors, such as DEA/NO, likely act through the reaction of NO with O2 to form the nitrosating agents N2O3 or N2O4. Therefore, it is clear that NO inhibits ODC via a cGMP-independent mechanism. Consistent with this view, the inhibition of RASMC proliferation by NO in the present study was shown to be independent of cGMP.
DFMO is an enzyme-activated irreversible inhibitor of ODC that suppresses cell proliferation by interfering with polyamine synthesis. Like NO, DFMO inhibits cell proliferation by cGMP-independent mechanisms, and the cytostatic effect can be overcome by addition of excess putrescine, but not ornithine (5, 9). Therefore, although the molecular mechanisms by which NO and DFMO inhibit ODC are different, the ultimate effect is the same: polyamine depletion and cytostasis. Despite this effect, only little is known about the mechanism by which polyamine depletion leads to inhibition of cell proliferation. One mechanism that has been proposed, however, is up-regulation of the cdk inhibitor p21waf1/cip1.
The orderly progression through the cell cycle depends on the coordinated interaction of key cell cycle regulator proteins including cyclins, cdks, and cdk inhibitory proteins such as p21waf1/cip1 (32–35). The cdk inhibitor p21waf1/cip1 is regarded as a universal inhibitor of the cell cycle and has been shown to inhibit several cyclins and cdks, including cdk2, cyclin D, and cyclin E, which regulate the transition from G1 to S-phase (36). Recently, activation of p42/p44 MAPK by activation of Ras was reported to increase the protein expression of p21waf1/cip1 (23). In another study, NO was shown to induce expression of p21waf1/cip1 via the activation of p42/p44 MAPK in RASMC (18). Additionally, it has been suggested that the inhibition of cell proliferation via polyamine depletion by compounds such as DFMO is mediated through increased expression of p21waf1/cip1 (17). Further substantiating the role of p21waf1/cip1 in the inhibition of cell proliferation by polyamine depletion is a study by Singh et al., in which they report that inhibition of arginase activity results in the activation of p21waf1/cip1 and consequent inhibition of cell proliferation (37). Arginase catalyzes the conversion of arginine to ornithine and urea and, under conditions where ornithine is limiting for polyamine synthesis, arginase plays an important role in the regulation of polyamine synthesis. The induction of p21waf1/cip1 by polyamine depletion has, furthermore, been associated with the activation of the p42/p44 MAPK pathway, although no cause-and-effect relationship was established (17). These reports suggested to us that NO and DFMO inhibit cell proliferation by common mechanisms related to inhibition of polyamine production. That is, polyamine depletion may cause activation of the p42/p44 MAPK pathway, which results in the induction of p21waf1/cip1 and interference with cell cycle progression.
The present study reveals that NO and DFMO cause activation of the p42/p44 MAPK pathway and describes the direct involvement of this pathway in the cytostatic effects caused via inhibition of ODC by NO or DFMO. We demonstrate that activation of p42/p44 MAPK leads to the induction of p21waf1/cip1, a cyclin-dependent kinase inhibitor known to regulate cell cycle progression. Activation of MEK1/2 leads to phosphorylation and activation of p42/p44 MAPK. The MEK1/2 inhibitors U0126 and PD 098,059 reversed the cytostatic effects of both NO donor agents and DFMO. Furthermore, the MEK1/2 inhibitors prevented the up-regulation of p21waf1/cip1 protein levels, demonstrating the requirement of p42/p44 MAPK activation for the induction of p21waf1/cip1. Finally, putrescine, but not ornithine, was able to prevent both the cytostatic effects and the activation of p42/p44 MAPK and induction of p21waf1/cip1 caused by NO or DFMO. Taken together, these data indicate that inhibition of polyamine synthesis by NO or DFMO leads to activation of the p42/p44 MAPK pathway, which in turn causes induction of p21waf1/cip1, leading to decreased RASMC growth. Fig. 9 is a schematic illustration of the proposed mechanism by which NO inhibits RASMC proliferation.
Acknowledgments
This work was supported by National Institutes of Health Grants HL35014, HL40922, HL58433, and HL66999.
Abbreviations
- SNAP
S-nitroso-N-acetylpenicillamine
- DETA/NO
(Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)-amino]-diazen-1-ium-1,2-diolate
- ODQ
1H-(1,2, 4)oxadiazolo[4,3-α]quinoxalin-1-one
- RASMC
rat aortic smooth muscle cells
- VSMC
vascular smooth muscle cells
- DFMO
α-difluoromethylornithine
- MAPK
mitogen-activated protein kinase
- ODC
ornithine decarboxylase
- cdk
cyclin-dependent kinase
- MEK
MAPK kinase
References
- 1.Ross R. Nature (London) 1993;362:801–808. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Groves P H, Banning A P, Penny W J, Newby A C, Cheadle H A, Lewis M J. Cardiovasc Res. 1995;30:87–96. [PubMed] [Google Scholar]
- 3.Laitinen M, Ylä-Herttuala S. Curr Opin Lipidol. 1998;9:465–469. doi: 10.1097/00041433-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 4.De Meyer G R, Bult H, Üstünes L, Kockx M, Jordaens F H, Zonnekeyn L L, Herman A G. Br J Pharmacol. 1994;112:471–476. doi: 10.1111/j.1476-5381.1994.tb13097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buga G M, Wei L H, Bauer P M, Fukuto J M, Ignarro L J. Am J Physiol. 1998;275:R1256–R1264. doi: 10.1152/ajpregu.1998.275.4.R1256. [DOI] [PubMed] [Google Scholar]
- 6.Garg U C, Hassid A. Am J Physiol. 1989;257:F60–F66. doi: 10.1152/ajprenal.1989.257.1.F60. [DOI] [PubMed] [Google Scholar]
- 7.Garg U C, Hassid A. Biochem Biophys Res Commun. 1990;171:474–479. doi: 10.1016/0006-291x(90)91417-q. [DOI] [PubMed] [Google Scholar]
- 8.Blachier F, Robert V, Selamnia M, Mayeur C, Duee P H. FEBS Lett. 1996;396:315–318. doi: 10.1016/0014-5793(96)01122-2. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro L J, Buga G M, Wei L H, Bauer P M, Wu G, del Soldato P. Proc Natl Acad Sci USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. . (First Published March 20, 2001; 10.1073/pnas.071054698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams-Ashman H G, Canellakis Z N. Perspect Biol Med. 1979;22:421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- 11.Pegg A E, McCann P P. Am J Physiol. 1982;243:C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- 12.Tabor C W, Tabor H. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 13.Gamet L, Cazenave Y, Trocheris V, Denis-Plouxviel C, Murat J C. Int J Cancer. 1991;47:633–638. doi: 10.1002/ijc.2910470425. [DOI] [PubMed] [Google Scholar]
- 14.McCann P P, Pegg A E, Sjoerdsma A. Inhibition of Polyamine Metabolism: Biological Significance and Basis for New Therapies. Orlando, FL: Academic; 1987. pp. 1–371. [Google Scholar]
- 15.Pegg A E. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 16.Kramer D L, Vujcic S, Diegleman P, Alerfer J, Miller J T, Black J D. Cancer Res. 1999;59:1278–1286. [PubMed] [Google Scholar]
- 17.Ray R M, Zimmerman B J, McCormack S A, Patel T B, Johnson L R. Am J Physiol. 1999;276:C684–C691. doi: 10.1152/ajpcell.1999.276.3.C684. [DOI] [PubMed] [Google Scholar]
- 18.Kibbe M R, Li J, Nie S, Watkins S C, Lizonova A, Kovesdi I, Simmons R L, Billiar T R, Tzeng E. J Vasc Surg. 2000;31:1214–1228. doi: 10.1067/mva.2000.105006. [DOI] [PubMed] [Google Scholar]
- 19.Gu M, Lynch J, Brecher P. J Biol Chem. 2000;275:11389–11396. doi: 10.1074/jbc.275.15.11389. [DOI] [PubMed] [Google Scholar]
- 20.Ishida A, Sasaguri T, Miwa Y, Kosaka C, Taba Y, Abumiya T. Mol Pharmacol. 1999;56:938–946. doi: 10.1124/mol.56.5.938. [DOI] [PubMed] [Google Scholar]
- 21.Mii S, Khalil R A, Morgan K G, Ware J A, Kent K C. Am J Physiol. 1996;270:H142–H150. doi: 10.1152/ajpheart.1996.270.1.H142. [DOI] [PubMed] [Google Scholar]
- 22.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 23.Olson M F, Paterson H F, Marshall C J. Nature (London) 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro L J, Lippton H, Edwards J C, Baricos W H, Hyman A L, Kadowitz P J, Gruetter C A. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 25.Wei L H, Jacobs A T, Morris S M, Jr, Ignarro L J. Am J Physiol. 2000;279:C248–C256. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- 26.Bauer P M, Buga G M, Fukuto J M, Pegg A E, Ignarro L J. Biochem Biophys Res Commun. 1999;262:355–358. doi: 10.1006/bbrc.1999.1210. [DOI] [PubMed] [Google Scholar]
- 27.Bauer, P. M., Buga, G. M., Fukuto, J. M., Pegg, A. E. & Ignarro, L. J. (2001) J. Biol. Chem., in press. [DOI] [PubMed]
- 28.De Meyer G R Y, Hermann A G. In: Nitric Oxide Biology and Pathobiology. Ignarro L J, editor. San Diego: Academic; 2000. pp. 547–567. [Google Scholar]
- 29.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 30.Cooke J P. J Invest Med. 1998;46:377–380. [PubMed] [Google Scholar]
- 31.Keefer L K, Nims R W, Davies K M, Wink D A. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 32.Hengst L, Dulic V, Slingerland J M, Lees E, Reed S I. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffrey P D, Russo A A, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich N P. Nature (London) 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 34.Kiyokawa H, Kineman R D, Manova T K, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 35.Nigg E A. BioEssays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 36.Johnson D G, Walker C L. Annu Rev Pharmacol Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 37.Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G. Cancer Res. 2000;60:3305–3312. [PubMed] [Google Scholar]