Abstract
The essential micronutrient selenium (Se) is required for various systemic functions, but its beneficial range is narrow and overexposure may result in adverse health effects. Additionally, the chemical form of the ingested selenium contributes crucially to its health effects. While small Se species play a major role in Se metabolism, their toxicological effects, bioavailability and metabolic transformations following elevated uptake are poorly understood. Utilizing the tractable invertebrate Caenorhabditis elegans allowed for an alternative approach to study species-specific characteristics of organic and inorganic Se forms in vivo, revealing remarkable species-dependent differences in the toxicity and bioavailability of selenite, selenomethionine (SeMet) and Se-methylselenocysteine (MeSeCys). An inverse relationship was found between toxicity and bioavailability of the Se species, with the organic species displaying a higher bioavailability than the inorganic form, yet being less toxic. Quantitative Se speciation analysis with HPLC/mass spectrometry revealed a partial metabolism of SeMet and MeSeCys. In SeMet exposed worms, identified metabolites were Se-adenosylselenomethionine (AdoSeMet) and Se-adenosylselenohomocysteine (AdoSeHcy), while worms exposed to MeSeCys produced Se-methylselenoglutathione (MeSeGSH) and γ-glutamyl-MeSeCys (γ-Glu-MeSeCys). Moreover, the possible role of the sole selenoprotein in the nematode, thioredoxin reductase-1 (TrxR-1), was studied comparing wildtype and trxr-1 deletion mutants. Although a lower basal Se level was detected in trxr-1 mutants, Se toxicity and bioavailability following acute exposure was indistinguishable from wildtype worms. Altogether, the current study demonstrates the suitability of C. elegans as a model for Se species dependent toxicity and metabolism, while further research is needed to elucidate TrxR-1 function in the nematode.

Introduction
Selenium (Se) is an essential trace element with ambivalent characteristics in animals and humans. Though it is required for manifold physiological functions,1 overexposure can result in severe toxicity. The adequate range between deficient, essential and toxic Se supply is particularly narrow and of great concern.2,3 Nutritional supplementation of Se remains highly debatable, especially since epidemiological and laboratory studies gathered conflicting results for Se supplementation. While some studies indicated health-promoting effects and even suggested anti-cancer properties,4 other studies observed adverse outcomes, including increased risk for type 2 diabetes.3,5 The effects depend not only on dosage and baseline Se status, but also strongly on the administered Se species.6,7 Various organic and inorganic forms of Se occur in food or dietary supplements, including selenomethionine (SeMet), Se-methylselenocysteine (MeSeCys) and selenite. One of the most abundant Se species in plants and animals is SeMet, which is incorporated non-specifically into proteins as a substitute for methionine. The amino acid derivative MeSeCys is a plant metabolite mainly found in Allium and Brassicaceae species, while selenite is commonly used in dietary supplements in the European Union.6,8 However, the species-specific characteristics and metabolism of these Se species have yet to be fully understood.
While many studies in human in vitro models are available (e.g.9–14), in vivo studies on the species-dependent toxicity and bioavailability are scarce. Being less complex than mammalian systems, the tractable invertebrate Caenorhabditis elegans (C. elegans) is a complementary and alternative in vivo model system in toxicological research and a multipurpose tool to study the effect of trace elements and macromolecules in the context of a whole organism. Features that turn C. elegans into an invaluable model include the easy and cost-effective maintenance, rapid hermaphroditic life cycle, straightforward genetics and high amount of evolutionary conserved genes.15 In humans, the essential functions of Se are mediated by 25 selenoproteins that carry selenocysteine at their active site.16 Among them there are important components of the antioxidant defense system like three thioredoxin reductases (TrxR-1, TrxR-2 and TrxR-3)16,17, the deletion of two of which resulted in embryonic lethality in mice.18 With respect to Se biology, the nematode’s genome encodes the selenocysteine tRNA and all components of the insertion machinery in order to incorporate Se as selenocysteine into proteins.19 However, C. elegans contains only one selenoprotein, TrxR-1, which is the orthologue of the human gene. TrxR-2 is also present in C. elegans, but with cysteine instead of selenocysteine in the catalytic center, while TrxR-3 is altogether absent in the worm.20,21 Stenvall et al. showed that C. elegans trxr-1 deletion mutants grow normally and display no obvious defects in development, reproduction or locomotion.22 The function of this enzyme in the nematode is thus an open question. To date, its role in Se-induced toxicity is controversial; it remains to be clarified and is, therefore, addressed in this study.
Initial studies on Se toxicity in C. elegans have focused mainly on the effects of sodium selenite, demonstrating that high dose exposure induces an increase in reactive oxygen/nitrogen species (RONS), stress responses, impaired growth, as well as progressive movement impairment culminating in an irreversible paralysis phenotype.23,24 High dose selenite is also sufficient to induce neuronal damage and cell loss and to alter cholinergic signaling in the nematode.25 Furthermore, it was shown for different developmental stages and exposure scenarios that selenite causes a dose- and time-dependent lethality.23,26,27 These studies promise the suitability of C. elegans for investigating mechanisms of Se species toxicity. However, Se species other than selenite have not been investigated to our knowledge, and studies on the bioavailability and metabolic transformations of Se species in the nematode have yet to be carried out. These factors are of great importance for understanding the species-dependent effects and evaluating the toxic potential of inorganic and organic Se forms. Therefore, this study is addressing endpoints of Se toxicity, bioavailability and metabolic transformations of selenite and - for the first time - of the organic Se species SeMet and MeSeCys in C. elegans.
Experimental
C. elegans strains and maintenance
C. elegans strains were grown on plates containing 8P seeded with the Escherichia coli strain NA22 and propagated at 20 °C as described previously.28 The following strains were used: N2, wildtype (Caenorhabditis Genetics Center, Minneapolis, USA) and the trxr-1 deletion mutant strain VB1414, trxr-1(sv47). Genotypes were verified regularly using published primer pairs.22 For each experiment, age-synchronized populations were obtained by isolating eggs from gravid adults using a bleaching solution (1% NaOCl and 0.25 M NaOH), segregating eggs from worm and bacterial debris by flotation on a sucrose gradient and hatching the eggs overnight in M9 buffer.
Preparation of Se species stock solutions
Selenite (Na2SeO3 ∙ 5 H2O, ≥99%) and D/L-SeMet (≥99%) were obtained from Sigma-Aldrich (Steinheim, Germany). L-MeSeCys (>98%) was supplied from Abcam (Cambridge, UK). Stock solutions of the Se species (50 – 100 mM) were prepared and diluted in purified water (10 MΩcm, Elix® 15, Merck Millipore, used for all experiments) and stored at 4 °C. Selenite stock solutions were prepared freshly every week, while the organic Se species solutions were used for up to two months.
Acute Se species treatment and lethality/development assay
2000 synchronized L1 worms were acutely exposed to selenite, D/L-SeMet or L-MeSeCys in 1.5 mL conical tubes for 30 minutes on a tube rotator. Worms were then pelleted by centrifugation at 4000 rpm for 2 minutes and washed three times in M9 buffer. 30 – 50 worms were pre-counted and transferred onto Nematode Growth Medium (NGM) plates seeded with the E. coli strain OP50. 48 hours post-treatment, the number of surviving worms was scored as a percentage of the original plated worm count and their developmental stage was evaluated.
Total Se quantification in C. elegans
Total Se content was quantified with an ICP-QQQ-MS 8800 mass spectrometer (Agilent, Waldbronn, Germany) after microwave-assisted acid digestion and normalization to the protein content. Briefly, 40 000 synchronized L1 worms were acutely treated for 30 minutes with the Se species, pelleted and washed four times with M9 buffer. For the determination of basal Se levels, 100 000 L1 worms were used. Worms were pelleted in a defined volume and re-suspended by adding purified water to a total volume of 500 μL. After sonication (3 × 20 sec, 1 cycle, 100% amplitude) and centrifugation (14 000 rpm, 10 min, 4°C), an aliquot of the supernatant was taken for protein quantification using the bicinchoninic acid (BCA) assay (Sigma-Aldrich, Steinheim, Germany). Subsequently, the worm suspension was mixed again and transferred into a 20 mL TFA microwave vessel with additional 500 μL of water. 25 μL of a 100 μg Ge/L solution (diluted from 1 g/L Ge, High Purity Standards, Charleston, USA) as an internal standard and 500 μL of Suprapur® nitric acid (65%, Merck, Darmstadt, Germany) were added. Digestion was carried out in a closed microwave digestion system (Mars 6, CEM, Kamp-Lintfort, Germany) by heating to 200°C within 15 minutes applying 650 W and maintaining the temperature for additional 20 minutes. After cooling, samples were transferred into 15 mL tubes and filled up to a total volume of 2.5 mL. Calibration standards (0.1 – 20 μg Se/L) were prepared using a 1 g Se/L standard (Merck, Darmstadt, Germany) including Ge as internal standard to the same final concentration (1 μg/L) as in the samples. To increase Se signal intensity by exploiting the carbon enhancement effect,29 3% isopropanol (≥99.999%, Sigma-Aldrich, Steinheim, Germany) were added to the samples and calibration standards before ICP-QQQ-MS analysis. For Se quantification, the method by Marschall et al. was applied.9 Briefly, in this method a reaction and collision cell mixture of oxygen (0.4 mL/min) and hydrogen (1 mL/min), leading to a mass shift of the Se isotope 80Se+ (m/z 80) to 80Se16O+ (m/z 96), is used for optimal interference control.30 Besides, the transition of the isotope 78Se+ (m/z 78) to 78Se16O+ (m/z 94) was monitored. Additionally, both Se isotopes (78Se+ and 80Se+) were measured in ‘on mass’ mode as well. For quantification, the mass transition m/z 80 ⟶ 96 was used, with a limit of quantification (LOQ) of 0.075 μg/L. The internal standard Ge was detected at m/z 72. Determinations of blank and reference material (certified fish reference material (ERM® – BB422) (Joint Research Centre, European Commission, Geel, Belgium) were performed periodically.
Speciation analysis in C. elegans lysates
Worms were acutely treated with the respective Se species for 30 minutes, pelleted and sonicated as described above. After centrifugation at 14 000 rpm for 10 minutes at 4°C, the supernatant was collected and, if necessary, diluted with purified water into the linear range of the applied method. The lysates where subjected to isotope dilution HPLC-ICP-QQQ-MS analysis as described elsewhere.10 Briefly, chromatographic separation was carried out using a 1260 infinity HPLC system (Agilent, Waldbronn, Germany) with a YMC-TriartPFP column (3 μm, 3 × 250 mm, YMC, Dinslaken, Germany). The eluent consisted of 20 mM ammonium formate (≥99.995%, Sigma-Aldrich, Steinheim, Germany) with 3% methanol (LC-MS grade, VWR, Darmstadt, Germany) (pH 3). A flow rate of 250 μL/min, column temperature of 30°C and injection volume of 20 μL were used. The column eluate was transferred to the ICP-QQQ-MS by coupling the capillary online to a T-piece. For quantification, a 5 μg 77Se/L solution (Eurisotop SAS, Saarbruecken, Germany) was mixed to the eluate via the T-piece using a syringe pump (Fisher Scientific, Schwerte, Germany) with a 50 mL BFP syringe (Hamilton, Nevada, USA) at a flow rate of 50 μL/min. ICP-QQQ-MS detection and data analysis via isotope dilution have been extensively described before.10 The LOQ was determined to be 0.60 μg Se/L for the mass transitions , which were used for quantification.
Identification of metabolites
Worms were acutely exposed to 15 mM SeMet or MeSeCys for 30 minutes as described for the other experiments. To yield higher metabolite concentrations, 70 000 synchronized L1 worms were used and after washing, the worm pellets were sonicated in a total volume of 150 μL. Lysates were obtained as described before, shipped to Graz on dry ice and stored at −80°C until analysis. For selenium speciation analysis the lysates were thawed and centrifuged (21000 × g, 4°C, 5 min). The supernatants were subjected to analysis by HPLC/ICP-MS on an Agilent 1100 HPLC system consisting of a binary pump, a thermostated autosampler and a column oven coupled to an Agilent 7500ce ICP-MS operated in the hydrogen reaction mode (3.5 mL H2/min) using the chromatographic conditions detailed above with an injection volume of 5 μL. For identification of selenium metabolites the HPLC system was coupled to a Q-Exactive Orbitrap Mass Spectrometer (Thermo Scientific, Waltham, USA) equipped with a heated electrospray ionization (HESI2) source from the same manufacturer. MS was performed in the positive mode and source settings were: gas temp 400°C, flow rates 47 (sheath) and 11 (aux) instrument units, spray voltage 3500 V and capillary temp 250°C. The full scan range was m/z 170 – 600 and a resolution of 70 000 (full width half-maximum, FWHM), an automatic gain control (AGC) target of 3 × 106 and a maximum injection time (IT) of 500 ms were used. MS/MS (ddMS/MS) was performed after recording full scans. Collision energies of 10, 20, 30 (stepped) instrument units were used for the MS/MS experiments. MS/MS settings were: maximum injection time 150 msec, AGC 1 × 105, threshold 5.3 × 104, resolution 17 500 FWHM. An isolation window of 8 Thomson (mass/charge) was used.
Statistics
All histograms and dose-response lethality curves were created with GraphPad Prism (GraphPad Software Inc.). For lethality curves and calculation of the respective LD50 values (values representing the Se species concentrations that cause 50% reduction in survival), a sigmoidal dose-response model with a top constraint at 100% was used. The basal Se content of the strains was compared using an unpaired t-test. Two-way analysis of variance (ANOVA) was performed on the bioavailability data, followed by Sidak’s multiple comparison post-hoc tests.
Results and discussion
Toxicity and bioavailability of selenite, SeMet and MeSeCys
It has been recognized that an elevated uptake of Se can be toxic to animal species. With respect to C. elegans, only few studies have addressed the question as to whether worms ingest and utilize Se species, and to what extent they are toxic. As a first step in comparing the effects of organic and inorganic Se species after acute exposure we assessed the impact of various Se species on C. elegans survival. Selenite exhibited significant toxic effects and decreased survival rates in a dose-dependent manner, with an LD50 of 13.1 mM (Fig. 1). Additionally, with increasing toxicity, selenite exposure caused developmental retardation (Fig. 2). In contrast, SeMet and MeSeCys had only slight effects at the highest applied dose of 50 mM, causing approximately 20% lethality (Fig. 1). These data are in agreement with studies in other model systems in literature, corroborating greater toxicity of inorganic selenite compared to organic Se species.9,11,14,31 Studies in C. elegans on the toxicity of Se compounds are scarce. While a few studies have addressed the toxicity of selenite, SeMet and MeSeCys have yet to be investigated. However, due to different study designs a direct comparison with the published toxicity data is unfeasible. Morgan et al. observed a dose-dependent increase in selenite-induced lethality after 12-hour treatment of adult worms, with an LD50 of 3.47 mM.23 A dose- and time-dependent decrease in survival has also been reported by Li and colleagues,27 who monitored C. elegans during its life cycle on agar plates containing 1 – 3 mM sodium selenite. Boehler et al. studied long-term effects of selenite on worms grown in axenic media for 12 days and determined an LD50 of 0.20 mM.26 Additionally, they also observed developmental defects, from a growth delay caused by selenite concentrations ≤ LD50, to more severe developmental failure at higher doses.24,26
Figure 1.

Dose-response survival curves of L1 stage wildtype C. elegans following acute exposure (30 min) to selenite, SeMet or MeSeCys, respectively. Data are expressed as means ± SEM of at least three independent experiments.
Figure 2.
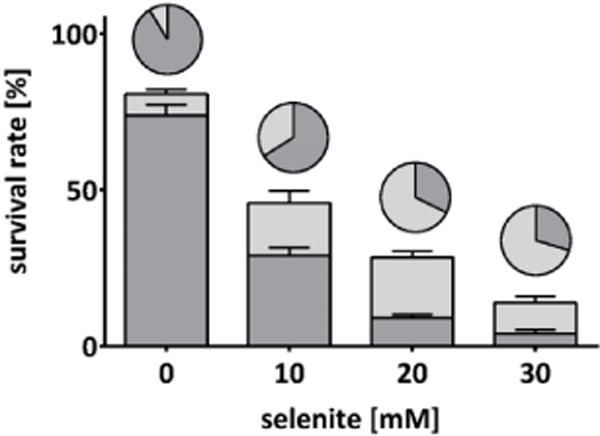
Development plots of wildtype C. elegans following acute exposure (30 min) to selenite. Stacked columns represent the percentages of worms with regular development (dark grey) and growth delay (light grey), based on the initial number of worms seeded. Pie charts represent the same groups, normalized to the number of surviving worms. Data are expressed as mean + SEM of at least four independent experiments.
To investigate whether the observed differences in toxicity might be related to a different bioavailability of the compounds, total Se content in worms incubated with the inorganic and organic Se species was quantified. Non-treated worms had a basal Se level of 2.1 ± 0.2 ng Se/mg protein. Normalized to the estimated worm volume,32 this approximates the Se concentration in human serum of 1 μM.33 The congruence between basal Se status in worms and humans points to the physiological relevance and suitability of the nematode model system. Treatment with the Se species resulted in increasing total Se with higher doses as shown in Table 1. Comparison of Se accumulation for the applied compounds showed that the more toxic inorganic Se species selenite was accumulated to a lower extent than the less toxic organic Se species. For example, incubating 5 mM of the most toxic species, selenite, caused about 2.5-fold lower total Se levels than the seleno-amino acids at the same dose. Therefore, the data indicate an inverse relationship between toxicity and bioavailability of the Se species. This is in accordance with previous observations in human cell lines9,11 and might be attributed to species-specific differences in uptake and metabolism. In zebrafish larvae, it was also shown that the organic forms SeMet and MeSeCys accumulate to a greater extent than the inorganic forms, selenite and selenate. But, in contrast to our findings, the organic species displayed considerably higher toxicity compared to the inorganic forms.34 Consequently, different mechanisms might be involved in the toxic modes of action.
Table 1.
Total Se contents of wildtype C. elegans following 30 min incubation with the respective Se species. Shown are mean values of at least three independent determinations ± SEM.
| Incubation [mM Se] | Total Se [ng Se/mg protein] | x-fold of control | |
|---|---|---|---|
| Control | 0 | 2.1 ± 0.2 | 1 x |
| Selenite | 0.5 | 32 ± 3 | 15 x |
| 1.0 | 49 ± 3 | 23 x | |
| 2.5 | 125 ± 23 | 58 x | |
| 5.0 | 221 ± 43 | 103 x | |
| SeMet | 5 | 612 ± 115 | 285 x |
| 15 | 1117 ± 180 | 519 x | |
| 25 | 1221 ± 201 | 568 x | |
| 50 | 1797 ± 105 | 836 x | |
| MeSeCys | 5 | 564 ± 94 | 262 x |
| 15 | 1326 ± 306 | 617 x | |
| 25 | 1514 ± 164 | 704 x | |
| 50 | 2258 ± 130 | 1050 x |
Se speciation
While incubation with SeMet and MeSeCys led to large increases in total Se content and therefore pointed towards a high bioavailability of these compounds, almost no toxic effects were observed. In contrast, the far less bioavailable selenite significantly decreased survival rates. Since no studies are available on the possible transformation of Se species in C. elegans, it remains unclear which factors might account for the lack of correlation between uptake and toxic effects. Thus, the question arose if and how the Se species are metabolized by C. elegans, in order to explain this seemingly inverse relationship between toxicity and bioavailability. To follow up on this question, we used isotope dilution HPLC-ICP-QQQ-MS to perform Se speciation analysis in C. elegans lysates after acute Se species treatment for screening of potentially formed Se species as an indication of metabolism of the applied Se compounds. After selenite treatment, only one Se species was detected in the water-soluble fractions of the incubated worms, and retention time matching with standard solutions indicated this species to be selenite (Fig. 3A, Table 2), thus indicating no direct metabolism of selenite in C. elegans after acute treatment. In contrast, in lysates of worms acutely treated with SeMet or MeSeCys, respectively, additional Se species were detected. In the case of SeMet, three additional peaks appeared in the ICP-QQQ-MS chromatograms besides SeMet itself (Fig. 3B, Table 2). The first might be a species known from speciation studies in human cell lines and is suspected to be selenomethionine-oxide (SeOMet), a supposed artefact from sample preparation.9,11 However, in this experiment its identity could not be verified by HPLC-ESI-Orbitrap-MS experiments due to the heavy sample matrix of the crude worm lysate co-eluting in this region of the chromatogram.
Figure 3.

RP-HPLC-ICP-QQQ-MS mass flow chromatograms of wildtype C. elegans lysates following acute exposure (30 min) to the respective Se species. (A) 1 mM selenite, (B) 15 mM SeMet diluted 1:4, (C) 15 mM MeSeCys, (D) 15 mM MeSeCys, shown enlarged. Unknown peaks are marked with I-V. Chromatographic separation was performed on a YMC-TriartPFP column (3 μm, 3 × 250 mm) at 30 °C with 250 μL min-1 ammonium formate (pH 3, 3% methanol) as mobile phase. .
Table 2.
Small Se species in C. elegans lysates as quantified by ID-HPLC-ICP-QQQ-MS. Shown are mean values of two independent experiments ± SEM.
| Incubated species | Detected species | Concentration in the lysate [μg/L] | Share on total Se in the lysate [%] | Sum of small Se species [%] |
|---|---|---|---|---|
| 1 mM selenite | Selenite | 8.1 ±0.3 | 89.6 ± 7.5 | 90 |
| 15 mM SeMet | SeOMet? | 130 ± 8 | 31.5 ± 1.5 | 98 |
| Unknown I | 29 ± 12 | 7.0 ± 2.8 | ||
| SeMet | 164 ± 43 | 43.2 ±6.2 | ||
| Unknown II | 13.6 ± 1.1 | 3.3 ±0.2 | ||
| 15 mM MeSeCys | MeSeCys | 249 ± 23 | 85.0 ±2.2 | 95 |
| Unknown III | 4.7 ± 1.3 | 1.7 ± 0.6 | ||
| Unknown IV | 12.3 ± 0.4 | 4.2 ± 0.2 | ||
| Unknown V | 13.8 ± 0.2 | 4.8 ± 0.4 |
After incubation with MeSeCys, multiple peaks with varying intensities were detected (Table 2, Fig. 3C, D). The three most abundant metabolites (metabolites III, IV, and V, Fig. 3D) eluted at retention times in the range similar to those of γ-glutamyl-MeSeCys (γ-Glu-MeSeCys) and Se-methylselenoglutathione (MeSeGSH) identified in HepG2 cells after treatment with MeSeCys in a previous study.10 Altogether, the speciation analyses point out the metabolic activity of the nematode in Se metabolism.
Identification of unknown Se metabolites
For identification of the Se metabolites formed upon exposure to SeMet or MeSeCys, HPLC-ESI-Orbitrap-MS experiments were performed. To achieve higher metabolite concentrations the worm number was increased for these experiments resulting in the occurrence of a number of additional unknown peaks albeit at low Se concentrations compared to the administered species SeMet and MeSeCys (Supplementary Fig. S-1A and S-1B). Due to the low sample volumes available, further clean-up steps were not tested and HPLC-ESI-Orbitrap-MS was performed on the crude lysates. Thus, the high matrix complicated/prevented identification of some of the unknown metabolites.
At least ten Se metabolites were formed when C. elegans was incubated with SeMet, with two of them representing major metabolites (species 2 and 8, Supplementary Fig. S-1A). One major signal eluted between the suspected SeOMet and SeMet at approximately the retention time of MeSeCys (6.3 min, species 2, Supplementary Fig. S-1A) and the other at about 14 minutes (species 8, Supplementary Fig. S-1A). A weak HPLC-ESI-Orbitrap-MS signal for MeSeCys heavily influenced by the co-eluting matrix indicated the presence of this selenium species after exposure of C. elegans to SeMet (Supplementary Fig. S-2, Table S-1). For species 8 (retention time 14 min, Supplementary Fig. S-1A) a mass of m/z 447.0890 was observed with an isotope pattern of a species containing one Se atom and matching the molecular formula for Se-adenosylselenomethionine (AdoSeMet, Fig. 4, Table S-1). Fragmentation of m/z 447.0890 and comparison with literature data35 confirmed this assignment (Fig. 4C).
Figure 4.

HPLC-ESI-Orbitrap-MS (background subtracted) of a C. elegans lysate following acute treatment (30 min) at L1 stage with SeMet: Identification of AdoSeMet (species 8, Supplementary Figure S-1A, Table S-1) via its isotope pattern, accurate mass and fragmentation; (A) calculated isotope pattern of AdoSeMet, (B) spectrum of AdoSeMet in the crude lysate (absolute intensity of the most intense peak in the isotope pattern of the species: 5.4 × 105), (C) fragmentation of m/z 447.0890 in the crude lysate. For chromatographic conditions see Supplementary Fig. S-1; injection volume 20 μL.
AdoSeMet is the Se analogue of the methyl donor S-adenosylmethionine (SAM). The incorporation of SeMet instead of methionine into SAM has already been indicated by in vitro experiments with methionine-activating enzyme prepared from rabbit liver or yeast36 as well as rabbit reticulocyte lysate.35 The presence of both AdoSeMet as well as its demethylated form Se-adenosylselenohomocysteine (AdoSeHcy) was suggested in selenized yeast and it was, thus, speculated that AdoSeMet might be involved in transmethylation reactions in the yeast.37 However, whereas the presence of AdoSeHcy in selenized yeast extracts has been repeatedly confirmed in the literature (e.g.38–45), the presence of both AdoSeMet and AdoSeHcy together has only been thoroughly demonstrated by molecular MS in wheat germ extracts.35
The possible involvement of AdoSeMet in transmethylation processes in C. elegans forced us to seek the lysates for the presence of its demethylated form AdoSeHcy. A signal at m/z 433.0731 (Fig. 5, Supplementary Table S-1) strongly indicated that the last small peak in the chromatogram (species 13, retention time 38 min, Supplementary Fig. S-1A) corresponded to AdoSeHcy.
Figure 5.
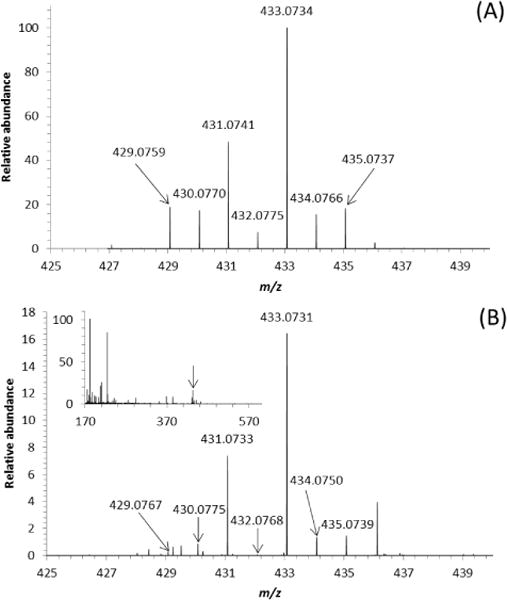
HPLC-ESI-Orbitrap-MS (background subtracted) of a C. elegans lysate following acute treatment (30 min) at L1 stage with SeMet: Identification of AdoSeHcy (species 13, Supplementary Figure S-1A, Table S-1) via its isotope pattern and accurate mass; (A) calculated isotope pattern of AdoSeHcy, (B) spectrum of AdoSeHcy in the crude lysate (absolute intensity of the most intense peak in the isotope pattern of the species: 1.2 × 104). Due to their low absolute intensities (ca. 5 × 101 to 1 × 103) the less intense isotope signals in the molecular pattern do not ideally match the theoretical abundances. For chromatographic conditions see Supplementary Fig. S-1; injection volume 20 μL.
While the in vitro study in wheat germ extract suggested that AdoSeMet formation may be the mechanism underlying SeMet toxicity,35 this couldn’t be confirmed in the nematodes in our study.
Incubation with MeSeCys resulted in the detection of at least eight Se metabolites in addition to MeSeCys in the concentrated lysates of C. elegans (Supplementary Fig. S-1B). Species 3 coeluted with SeMet; a definite identification by HPLC-ESI-Orbitrap-MS was, however, not possible. HPLC-ESI-Orbitrap-MS (Supplementary Fig. S-3 and S-4, Table S-1) confirmed the presence of γ-Glu-MeSeCys (species 4, retention time 9.8 min, Supplementary Fig. S-1B) and MeSeGSH (species 5, retention time 11 min, Supplementary Fig. S-1B) previously identified also in lysates of HepG2 cells after incubation with MeSeCys.10 Consecutive closer inspection of the Orbitrap spectra for further glutathione derivatives formerly detected in selenized yeast indicated that species 11 (retention time 22 min, Supplementary Fig. S-1B) might correspond to the previously reported S-selenomethyl-glutathione42–45 (Supplementary, Fig. S-5, Table S-1). Given that the lysates of C. elegans treated with SeMet also showed small peaks at the retention times of species 4 and 5 (Supplementary Fig. S-1A) their Orbitrap-MS spectra were analyzed to check for the presence of γ-Glu-MeSeCys and MeSeGSH. Weak signals with the corresponding accurate masses indicated their presence (data not shown), but did not allow a definite identification.
γ-glutamyl-MeSeCys constitutes a dietary source of Se and in vitro experiments reported in the literature suggested that it loses its glutamyl moiety during gastric digestion.46,47 However, data characterizing the effects of γ-Glu-MeSeCys are rare and up to now it remains uncertain whether it is a detoxification product of Se metabolism like in plants. While γ-Glu-MeSeCys might have contributed to the cytotoxic effects of MeSeCys in a previous study with hepatoma cells,10 it failed to exert toxic effects in the nematodes in this study. The second identified metabolite MeSeGSH has been detected before in selenium-rich yeast48,49 and recently for the first time in human cells.10 To date, the biological effects of MeSeGSH have yet to be characterized, indicating that further research is required. It is also noteworthy that AdoSeMet and AdoSeHcy were not detectable in the lysates of the worms exposed to MeSeCys supporting the assumption that they are specific metabolites of SeMet.
Comparison of wildtype and trxr-1 deletion mutant worms
While the human genome encodes 25 selenoproteins, C. elegans has only one selenocysteine-harbouring protein, thioredoxin reductase-1 (TrxR-1).20,21 Despite being the orthologue of human TrxR-1, the function of C. elegans TrxR-1 is poorly understood. Since selenite can be reduced by thioredoxin reductase,50 resulting in the highly reactive selenide, the enzyme might be involved in selenite toxicity. On the other hand, mammalian TrxR-1 is implicated in the antioxidant defense system,17 pointing towards a protective role of this enzyme in C. elegans. In our initial survival studies, selenite was the most potent Se species in wildtype C. elegans. Therefore, we characterized selenite disposition in trxr-1 deletion mutant worms, to investigate if TrxR-1 is likely to play a role in either toxification or detoxification processes. As shown in Fig. 6, the dose-response survival curve of trxr-1 deletion mutant worms after acute exposure to selenite had an analogous shape to the one observed for the wildtype worms. Statistical analysis of the LD50 values confirmed that there was no significant difference between the two strains. This is in agreement with results from Boehler et al., who observed no differences in susceptibility to Se toxicity between wildtype, trxr-1, trxr-2 and double-knockout worms after 12 days in axenic media supplemented with selenite, selenide or selenate.26 In addition, selenite exposure affected the growth of trxr-1 deletion mutant worms in a similar manner and to the same extent as observed in wildtype C. elegans (Fig. 7). Taken together, these results indicate that the loss of TrxR-1 does not alter the nematodes’ sensitivity towards selenite toxicity.
Figure 6.

Dose-response survival curve of VB1414 (trxr-1) worms following acute exposure (30 min) to selenite at L1 stage. Data are expressed as mean ± SEM of at least three independent experiments.
Figure 7.
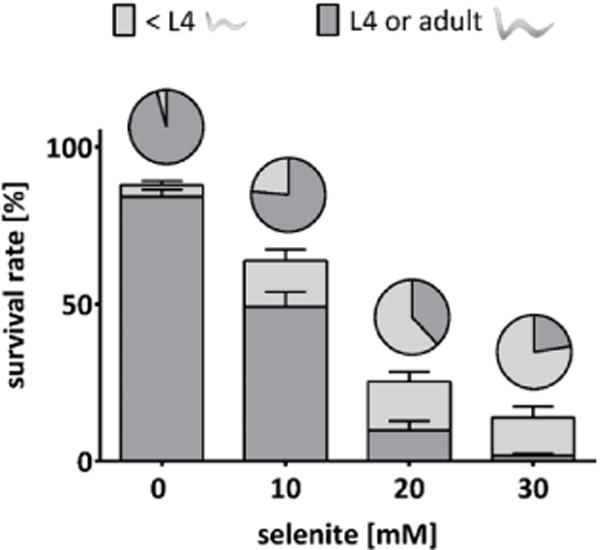
Development plots of VB1414 (trxr-1) worms following acute exposure (30 min) to selenite. Stacked columns represent the percentages of worms with regular development (dark grey) and growth delay (light grey), based on the initial number of worms seeded. Pie charts represent the same groups, normalized to the number of surviving worms. Data are expressed as mean + SEM of at least four independent experiments.
To further characterize the trxr-1 deletion mutant strain and the role of TrxR-1 in C. elegans, total Se was measured to assess the Se content in these worms in the basal state and following Se exposure. As shown in Table 3, trxr-1 mutants had a basal level of 1.3 ± 0.1 ng Se/mg protein, which is significantly lower than in wildtype worms (P value = 0.003). Upon incubation with selenite, a dose-dependent increase of the Se content was observed. Here, at equal incubation doses, no statistical differences between the two nematode strains where noted. Altogether, under basal conditions, trxr-1 mutants have less Se, but upon Se exposure, the bioavailability is indistinguishable from wildtype worms.
Table 3.
Total Se contents of trxr-1 deletion mutant worms following 30 min incubation with selenite. Data are expressed as mean values of at least three independent determinations ± SEM.
| Incubation [mM Se] | Total Se [ng Se/mg protein] |
|---|---|
| 0 | 1.3 ± 0.1 |
| 0.5 | 29 ± 1 |
| 1.0 | 48 ± 1 |
| 2.5 | 104 ± 5 |
| 5.0 | 238 ± 7 |
Conclusions
The current study demonstrates that the tractable invertebrate C. elegans is a promising and useful in vivo model system to study species-specific characteristics of organic and inorganic Se forms. Providing an alternative approach to otherwise time-consuming and costly vertebrate models, the nematode affords an alternative and complementary organismic model compared to in vitro systems. For the first time, a combined effort of toxicity testing and speciation analysis offers insights into the nematode’s Se metabolism after acute high-dose exposure to Se species. Moreover, the obtained speciation data underline the potential of C. elegans as a model for Se metabolism, due to its ability to metabolize Se species in the herein observed extent and complexity, in spite of the fact that these worms harbor only one selenoprotein. Thus, the identification of the remaining unknown Se species should be promoted in future studies, to enhance our understanding of the nematodes’ Se metabolism in order to establish its feasibility as a potential model for higher organisms. As the loss of TrxR-1 did not modulate Se toxicity and bioavailability, it might be worthwhile to study its possible implication in protective mechanisms related to low dose Se exposure.
Supplementary Material
Acknowledgments
This work was supported by the German Research Foundation (DFG) grant number SCHW 903/9-1 and the Austrian Science Fund (FWF), project number I 2262-N28, as well as the DFG Research Unit TraceAge (FOR 2558). We thank the German Research Foundation (DFG) further for the financial support of BO 4103/2-1. We would like to acknowledge the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), for providing the N2 strain used in this work. We thank the NAWI Graz Central Lab Metabolomics for HPLC-ESI-Orbitrap-MS. MA was supported in part by the National Institute of Environmental Health Science - NIH R01ES10563, R01ES07331.
Footnotes
Electronic Supplementary Information (ESI) available: [Supplementary Figures S-1 to S-5]. See DOI: 10.1039/x0xx00000x
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1.Margaret P. Rayman Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 2.European Commission. Opinion of the Scientific Committee on Food on the tolerable upper intake level of selenium. 2000 [Google Scholar]
- 3.Jablonska Ewa, Vinceti Marco. Selenium and human health: witnessing a Copernican Revolution? J Environ Sci Health. 2015;33:328–368. doi: 10.1080/10590501.2015.1055163. [DOI] [PubMed] [Google Scholar]
- 4.Duffield-Lillico Anna J, Reid Mary E, Turnbull Bruce W, Combs Gerald F, Slate Elizabeth H, Fischbach Lori A, Marshall James R, Larry C. Clark Baseline Characteristics and the Effect of Selenium Supplementation on Cancer Incidence in a Randomized Clinical Trial: A Summary Report of the Nutritional Prevention of Cancer Trial 1. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 5.Lippman Scott M, Klein Eric A, Goodman Phyllis J, Scott Lucia M, Thompson Ian M, Ford Leslie G, Parnes Howard L, Minasian Lori M, Gaziano Michael, Hartline Jo A, Parsons J Kellogg, Bearden James D, III, Crawford E David, Goodman Gary E, Claudio Jaime, Winquist Eric, Cook Elise D, Karp Daniel D, Walther Philip, Lieber Michael M, Kristal Alan R, Darke Amy K, Arnold Kathryn B, Ganz Patricia A, Santella Regina M, Albanes Demetrius, Taylor Philip R, Probstfield Jeffrey L, Jagpal TJ, Crowley John J, Meyskens Frank L, Baker Laurence H, Coltman Charles A., Jr Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayman Margaret P. Heidi Goenaga-Infante, Mike Sargent Food-chain selenium and human health: spotlight on speciation. Br J Nutr. 2008;100:238–253. doi: 10.1017/S0007114508922522. [DOI] [PubMed] [Google Scholar]
- 7.Gammelgaard Bente, Matthew I, Gabel-Jensen Charlotte. Surveying selenium speciation from soil to cell-forms and transformations. Anal Bioanal Chem. 2011;399:1743–1763. doi: 10.1007/s00216-010-4212-8. [DOI] [PubMed] [Google Scholar]
- 8.Directive 2002/46/EC of the European Parliament and the Council of 10 June 2002 on the approximation of the laws of the member states relating to food supplements. 2015:1–16. [Google Scholar]
- 9.Marschall Talke A, Bornhorst Julia, Kuehnelt Doris, Schwerdtle Tanja. Differing cytotoxicity and bioavailability of selenite, methylselenocysteine, selenomethionine, selenosugar 1 and trimethylselenonium ion and their underlying metabolic transformations in human cells. Mol Nutr Food Res. 2016;60:2622–2632. doi: 10.1002/mnfr.201400013. [DOI] [PubMed] [Google Scholar]
- 10.Marschall Talke A, Kroepfl Nina, Jensen Kenneth B, Bornhorst Julia, Meermann Bjoern, Kuehnelt Doris. Tanja Schwerdtle Tracing cytotoxic effects of small organic Se species in human liver cells back to total cellular Se and Se metabolites. Metallomics. 2017;9:268–277. doi: 10.1039/C6MT00300A. [DOI] [PubMed] [Google Scholar]
- 11.Lunøe Kristoffer, Gabel-Jensen Charlotte, Stürup Stefan, Andresen Lars, Skov Søren, Gammelgaard Bente. Investigation of the selenium metabolism in cancer cell lines. Metallomics. 2011;3:162–168. doi: 10.1039/c0mt00091d. [DOI] [PubMed] [Google Scholar]
- 12.Hoefig Carolin S, Renko Kostja, Köhrle Josef, Birringer Marc, Schomburg Lutz. Comparison of different selenocompounds with respect to nutritional value vs toxicity using liver cells in culture. J Nutr Biochem. 2011;22:945–955. doi: 10.1016/j.jnutbio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Kazuaki, Suzuki Noriyuki, Ogra Yasumitsu. Bioavailability Comparison of Nine Bioselenocompounds In Vitro and In Vivo. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lérida L, Flores Villavicencio Gustavo, Cruz-Jiménez Gloria, Barbosa-Sabanero Carlos, Kornhauser-Araujo M. Eugenia Mendoza-Garrido, Guadalupe de la Rosa, Myrna Sabanero-López. Human lung cancer cell line A-549 ATCC is differentially affected by supranutritional organic and inorganic selenium. Bioinorg Chem Appl. 2014;2014 doi: 10.1155/2014/923834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung Maxwell CK, Williams Philipp L, Benedetto Alexandre, Catherine Au, Helmcke Kirsten J, Aschner Michael, Meyer Joel N. Caenorhabditis elegans: An Emerging Model in Biomedical and Environmental Toxicology. Toxicol Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryukov Gregory V, Castellano Sergi, Novoselov Sergey V, Lobanov Alexey V, Zehtab Omid, Guigó Roderic, Vadim N. Gladyshev Characterization of Mammalian Seleno-proteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 17.Holmgren Arne. Antioxidant Function of Thioredoxin and Glutaredoxin Systems. Antioxid Redox Signal. 2000;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 18.Conrad Marcus. Transgenic mouse models for the vital selenoenzymes cytosolic thioredoxin reductase, mitochondrial thioredoxin reductase and glutathione peroxidase 4. Biochim Biophys Acta. 2009;1790:1575–1585. doi: 10.1016/j.bbagen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee Byeong J, Rajagopalan Malini, Kim Yeong S, You Kwang H, Bruce Jacobson K. Dolph Hatfield Selenocysteine tRNA [Ser] sec Gene Is Ubiquitous within the Animal Kingdom. Mol Cell Biol. 1990;10:1940–1949. doi: 10.1128/mcb.10.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladyshev Vadim N, Krause Michael, Xu Xue-Ming, Korotkov Konstantin V, Kryukov Gregory V, Sun Qi-An, Lee Byeong J, Wootton John C, Hatfield Dolph L. Selenocysteine-Containing Thioredoxin Reductase in C elegans. Biochem Biophys Res Commun. 1999;249:244–249. doi: 10.1006/bbrc.1999.0765. [DOI] [PubMed] [Google Scholar]
- 21.Buettner Christoph, Harney John W, Berry Marla J. The Caenorhabditis elegans Homologue of Thioredoxin Reductase Contains a Selenocysteine Insertion Sequence (SECIS) Element That Differs from Mammalian SECIS Elements but Directs Selenocysteine Incorporation. J Biol Chem. 1999;274:21598–21602. doi: 10.1074/jbc.274.31.21598. [DOI] [PubMed] [Google Scholar]
- 22.Stenvall Joergen, Fierro-González Juan C, Swoboda Peter, Saamarthy Karunakar, Cheng Qing, Cacho-Valadez Briseida, Arnér Elias SJ, Olof P. Persson, Antonio Miranda-Vizuete, Simon Tuck Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc National Acad Sci U S A. 2011;108:1064–1069. doi: 10.1073/pnas.1006328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan Kathleen L, Estevez Annette O, Mueller Catherine L, Cacho-Valadez Briseida, Miranda-Vizuete Antonio, Szewczyk Nathaniel J. Miguel Estevez The Glutaredoxin GLRX-21 Functions to Prevent Selenium-Induced Oxidative Stress in Caenorhabditis elegans. Toxicol Sci. 2010;118:530–543. doi: 10.1093/toxsci/kfq273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehler Christopher J, Raines Anna M, Sunde Roger A. Toxic-Selenium and Low-Selenium Transcriptomes in Caenorhabditis elegans: Toxic Selenium Up-Regulates Oxidoreductase and Down-Regulates Cuticle-Associated Genes. PLoS One. 2014:9. doi: 10.1371/journal.pone.0101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estevez Annette O, Mueller Catherine L, Morgan Kathleen L, Szewczyk Nathaniel J, Teece Luke, Miranda-Vizuete Antonio. Miguel Estevez Selenium induces cholinergic motor neuron degeneration in Caenorhabditis elegans. Neurotoxicology. 2012;33:1021–1032. doi: 10.1016/j.neuro.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehler Christopher J, Raines Anna M, Sunde Roger A. Deletion of Thioredoxin Reductase and Effects of Selenite and Selenate Toxicity in Caenorhabditis elegans. PLoS One. 2013:8. doi: 10.1371/journal.pone.0071525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen-Hsuan Li, Yun-Ru Ju, Chung-Min Liao, Vivian Hsiu-Chan Liao. Assessment of selenium toxicity on the life cycle of Caenorhabditis elegans. Ecotoxicology. 2014;23:1245–1253. doi: 10.1007/s10646-014-1267-x. [DOI] [PubMed] [Google Scholar]
- 28.Brenner Sidney. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gammelgaard Bente, Jøns Ole. Determination of selenium in urine by inductively coupled plasma mass spectrometry : interferences and optimization. J Anal At Spectrom. 1999;14:867–874. [Google Scholar]
- 30.Balcaen Lieve, Bolea-Fernandez Eduardo, Resano Martin, Vanhaecke Frank. Inductively coupled plasma – Tandem mass spectrometry (ICP-MS/MS): A powerful and universal tool for the interference-free determination of (ultra)trace elements – A tutorial review. Anal Chim Acta. 2015;894:7–19. doi: 10.1016/j.aca.2015.08.053. [DOI] [PubMed] [Google Scholar]
- 31.Ammar Elsayed M, Couri Daniel. Acute toxicity of sodium selenite and selenomethionine in mice after ICV or IV administration. Neurotoxicology. 1981;2:383–386. [PubMed] [Google Scholar]
- 32.Moore Brad T, Jordan James M, Ryan Baugh L. WormSizer: High-throughput Analysis of Nematode Size and Shape. Plos One. 2013:8. doi: 10.1371/journal.pone.0057142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen Naomi E, Appleby Paul N, Roddam Andrew W, Tjønneland Anne, Johnsen Nina F, Overvad Kim, Boeing Heiner, Weikert Steffen, Kaaks Rudolf, Linseisen Jakob, Trichopoulou Antonia, Misirli Gesthimani, Trichopoulos Dimitrios, Sacerdote Carlotta, Grioni Sara, Palli Domenico, Tumino Rosario, Bueno-de-mesquita H Bas, Kiemeney Lambertus A, Barricarte Aurelio, Larrañaga Nerea, Sánchez Maria-José, Agudo Antonio, Tormo María-José, Rodriguez Laudina, Stattin Paer, Hallmans Goeran, Bingham Sheila, Khaw Kay-Tee, Slimani Nadia, Rinaldi Sabina, Boffetta Paolo, Riboli Elio, J Timothy. Key Plasma selenium concentration and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Am J Clin Nutr. 2008;88:1567–75. doi: 10.3945/ajcn.2008.26205. [DOI] [PubMed] [Google Scholar]
- 34.Dolgova Natalia V, Hackett Mark J, MacDonald Tracy C, Nehzati Susan, James Ashley K, Krone Patrick H, George Graham N, Ingrid J. Pickering Distribution of selenium in zebrafish larvae after exposure to organic and inorganic selenium forms. Metallomics. 2016;8:305–312. doi: 10.1039/C5MT00279F. [DOI] [PubMed] [Google Scholar]
- 35.Ogra Yasumitsu, Kitaguchi Takashi, Ishiwata Kazuya, Suzuki Noriyuki, Toida Toshihiko, Suzuki Kazuo T. Speciation of selenomethionine metabolites in wheat germ extract. Metallomics. 2009;1:78–86. doi: 10.1039/b813118j. [DOI] [Google Scholar]
- 36.Mudd S Harvey, Cantoni GL. Selenomethionine in enzymatic transmethylations. Nature. 1957;180:1052. doi: 10.1038/1801052a0. [DOI] [PubMed] [Google Scholar]
- 37.Wrobel Kazimierz, Wrobel Katarzyna, Joseph A. Caruso Selenium speciation in low molecular weight fraction of Se-enriched yeasts by HPLC-ICP-MS: detection of selenoadenosylmethionine. J Anal At Spectrom. 2002;17:1048–1054. doi: 10.1039/B200920J. [DOI] [Google Scholar]
- 38.Casiot Corinne, Vacchina Veronique, Chassaigne Hubert, Szpunar Joanna, Potin-Gautier Martine, Lobinski Ryszard. An approach to the identification of selenium species in yeast extracts using pneumatically-assisted electrospray tandem mass spectrometry. Anal Commun. 1999;36:77–80. doi: 10.1039/A900319C. [DOI] [Google Scholar]
- 39.Kotrebai Mihály, Birringer Marc, Tyson Julian F, Block Eric, Peter C. Uden Identification of the principal selenium compounds in selenium-enriched natural sample extracts by ion-pair liquid chromatography with inductively coupled plasma- and electrospray ionization-mass spectrometric detection. Anal Commun. 1999;36:249–252. doi: 10.1039/A902770J. [DOI] [Google Scholar]
- 40.McSheehy Shona, Pohl Pawel, Szpunar Joanna, Potin-Gautier Martine, Lobinski Ryszard. Analysis for selenium speciation in selenized yeast extracts by two dimensional liquid chromatography with ICP-MS and electrospray MS-MS detection. J Anal At Spectrom. 2001;16:68–73. doi: 10.1039/B007427F. [DOI] [Google Scholar]
- 41.Garcia-Reyes Juan F, Dernovics Mihaly, Giusti Pierre, Lobinski Ryszard. Identification of new selenium non-peptide species in selenised yeast by nanoHPLC electrospray Q/time-of-flight-MS/MS. J Anal At Spectrom. 2006;21:655–665. doi: 10.1039/B518297B. [DOI] [Google Scholar]
- 42.Goenaga Infante Heidi, O’Connor Gavin, Rayman Margaret, Hearn Ruth, Cook Ken. Simultaneous identification of selenium-containing glutathione species in selenised yeast by on-line HPLC With ICP-MS and electrospray ionisation quadrupole time of flight (QTOF)-MS/MS. J Anal At Spectrom. 2006;21:1256–1263. doi: 10.1039/B601867J. [DOI] [Google Scholar]
- 43.Gil-Casal Sandra, Far Johann, Bierla Katarzyna, Ouerdane Laurent, Szpunar Joanna. Study of the Se-containing metabolomes in Se-rich yeast by size-exclusion-cation-exchange HPLC with the parallel ICP MS and electrospray orbital ion trap detection. Metallomics. 2010;2:535–548. doi: 10.1039/C0MT00002G. [DOI] [PubMed] [Google Scholar]
- 44.Preud’homme Hugues, Far Johann, Gil-Casal Sandra, Lobinski Ryszard. Large-scale identification of selenium metabolites by online size-exclusion-reversed phase liquid chromatography with combined inductively coupled plasma (ICP-MS) and electrospray ionization linear trap-Orbitrap mass spectrometry (ESI-MSn) Metallomics. 2012;4:422–432. doi: 10.1039/C2MT00172A. [DOI] [PubMed] [Google Scholar]
- 45.Arnaudguilhem Carine, Bierla Katarzyna, Ouerdane Laurent, Preud’homme Hugues, Yiannikouris Alexandros, Lobinski Ryszard. Selenium metabolomics in yeast using complementary reversed-phase/hydrophilic ion interaction (HILIC) liquid chromatography-electrospray hybrid quadrupole trap/Orbitrap mass spectrometry. Anal Chim Acta. 2012;757:26–38. doi: 10.1016/j.aca.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Fairweather-Tait Susan J, Bao Yongping, Broadley Martin R, Collings Rachel, Ford Dianne, Hesketh John E, Hurst Rachel. Selenium in human health and disease. Antioxid Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 47.Dumont Emmie, Ogra Yasumitsu, Vanhaecke Frank, Suzuki Kazuo, Cornelis Rita. Liquid chromatography-mass spectrometry (LC-MS): A powerful combination for selenium speciation in garlic (Allium sativum) Anal Bioanal Chem. 2006;384:1196–1206. doi: 10.1007/s00216-005-0272-6. [DOI] [PubMed] [Google Scholar]
- 48.Far Johann, Preud’homme Hugues, Lobinski Ryszard. Detection and identification of hydrophilic selenium compounds in selenium-rich yeast by size exclusion-microbore normal-phase HPLC with the on-line ICP-MS and electrospray Q-TOF-MS detection. Anal Chim Acta. 2010;657:175–190. doi: 10.1016/j.aca.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 49.Dernovics Mihaly, Far Johann, Lobinski Ryszard. Identification of anionic selenium species in Se-rich yeast by electrospray QTOF MS/MS and hybrid linear ion trap/orbitrap MSn. Metallomics. 2009;1:317–329. doi: 10.1039/b901184f. [DOI] [PubMed] [Google Scholar]
- 50.Kumar Sushil, Bjoernstedt Mikael, Holmgren Arne. Selenite is a substrate for calf thymus thioredoxin reductase and thioredoxin and elicits a large non-stoichiometric oxidation of NADPH in the presence of oxygen. Eur J Biochem. 1992;439:435–439. doi: 10.1111/j.1432-1033.1992.tb17068.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


